Abstract
Background
Prior work has suggested relationships between prenatal intake of certain nutrients and autism.
Objectives
We examined a broad set of prenatal nutrients and foods using a Bayesian modeling approach.
Methods
Participants were drawn from the Early Autism Risks Longitudinal Investigation (n = 127), a cohort following women with a child with autism through a subsequent pregnancy. Participants were also drawn from the Nurses’ Health Study II (NHSII, n = 713), a cohort of United States female nurses, for comparison analyses. In both studies, information on prospectively reported prenatal diet was drawn from food frequency questionnaires, and child autism-related traits were measured by the Social Responsiveness Scale (SRS). Bayesian kernel machine regression was used to examine the combined effects of several nutrients with neurodevelopmental relevance, including polyunsaturated fatty acids (PUFAs), iron, zinc, vitamin D, folate, and other methyl donors, and separately, key food sources of these, in association with child SRS scores in crude and adjusted models.
Results
In adjusted analyses, the overall mixture effects of nutrients in Early Autism Risks Longitudinal Investigation and foods in both cohorts on SRS scores were not observed, though there was some suggestion of decreasing SRS scores with increasing overall nutrient mixture in NHSII. No associations were observed with folate within the context of this mixture, but holding other nutrients fixed, n–6 PUFAs were associated with lower SRS scores in NHSII. In both cohorts, lower SRS scores were observed with higher intake of some groupings of vegetables, though for differing types of vegetables across cohorts, and some vegetable groups were associated with higher SRS scores in NHSII.
Conclusions
Our work extends prior research and suggests the need to further consider prenatal dietary factors from a combined effects perspective. In addition, findings here point to potential differences in nutrient associations based on a family history of autism, which suggests the need to consider gene interactions in future work.
Keywords: autism, diet, Social Responsiveness Scale, Bayesian mixture modeling, EARLI, NHSII
Introduction
Prenatal nutrition is known to play a critical role in fetal neurodevelopment, as demonstrated most classically by relationships between periconceptional folic acid and neural tube defects and between extreme nutrient deprivation during pregnancy and schizophrenia [1,2]. Over roughly the past decade, the examination of maternal dietary factors in relationship to child outcomes has expanded to consider potential relationships with autism spectrum disorder (ASD, hereafter referred to as “autism”) and related traits that capture the autism phenotype. This research has been motivated by evidence of autism’s prenatal origins and knowledge of the roles that many nutrients play in key neurodevelopmentally relevant pathways, including immune functioning, methylation, and direct roles in neurodevelopmental processes such as neurogenesis and synaptogenesis [[3], [4], [5]]. Yet, work to date examining the role of dietary factors during early neurodevelopment has primarily taken a single-nutrient approach and has focused on a small set of nutrients, with most work focused on folic acid/folate, vitamin D, and PUFA.
Overall, the literature supports an inverse association of autism or autism-related traits with higher folic acid intake around the time of conception, but several null findings have also been reported [6]. Vitamin D also has evidence for an inverse association with autism, as supported in several studies relying on measured 25(OH)D in prenatal samples; although the primary source is sunlight, diet represents a key secondary source [7,8]. Other prenatal dietary factors, including PUFAs and fish (as a key source of PUFAs), iron, and zinc, have been examined only in a handful of studies, with conflicting and/or insufficient evidence for strong links [[9], [10], [11], [12], [13]]. Yet, a wider set of dietary factors may also be relevant, including not only other methyl donor nutrients beyond folate that may influence DNA methylation but also those involved in immune disruption/inflammation or oxidative stress—each implicated pathway in autism etiology [[14], [15], [16]]. Furthermore, it is not known the extent to which observed associations reflect confounding from the numerous other dietary factors not assessed, as many nutrients (and their food sources) are correlated and act in antagonistic and synergistic ways in overlapping biological processes [17,18]. Accounting for combined exposures and such interactions may therefore reveal novel effects, as supported by evidence from other fields [19].
One approach to address combined dietary effects is utilizing advanced statistical models capable of handling a large number of potentially correlated exposures while allowing for interactions and complex exposure-response relationships [20]. To our knowledge, no prior study has implemented such methods in a study of prenatal maternal diet and child autism-related traits. Thus, the goal of this work was to take a more comprehensive approach to examining the relationship between prenatal diet and child autism-related outcomes by considering a broader set of neurodevelopmentally relevant nutrients and their food sources. We utilized Bayesian mixture models in order to estimate the overall combined effects of nutrients and foods, adjust for potential co-nutrient confounding, allow for interactions between pairs of nutrients, and assess the strength of associations with individual nutrients and foods within the context of a broader diet. We also used data from 2 separate studies with differing background likelihood of autism to allow for comparisons to be made by family history of autism and to assess the replicability of findings.
Methods
Study population
Participants were drawn from 2 United States cohort studies. Our primary study population, owing to the timing of dietary data to pregnancy, was the Early Autism Risks Longitudinal Investigation (EARLI). Briefly, EARLI is a prospective cohort following women who had already had a child with autism through a subsequent pregnancy and that child’s early development. Due to the increased recurrence risk of autism in families [21], there is a higher probability of autism and a shifted distribution of autism-related traits in the children followed in EARLI than in the general population, making it an efficient design for studying factors that may contribute to the development of autism and autism-related outcomes. EARLI families were recruited at 4 network sites (Drexel/Children's Hospital of Philadelphia; Johns Hopkins/Kennedy Krieger Institute; University of California Davis; and Northern California Kaiser Permanente) from 2009–2012. In addition to having a biological child with an ASD confirmed by EARLI study clinicians, participants must have met the following inclusion criteria: able to communicate in English or Spanish; be 18 y or older; live within 2 h of a study site; and be <29 wk pregnant. Children born into the cohort were followed until the age of 3 years. Two hundred fifty-six children have been born into EARLI; this includes 8 pairs of twins not included here due to potential differences in diet and the likelihood of autism in multiple births. To be included in the analyses here, women must have nutrient intake calculated from a dietary questionnaire completed during pregnancy and have autism-related outcome information available on their child (n = 127) (Supplemental Figure 1).
Given the small sample size of EARLI, and the desire to examine potentially differing associations related to the familial history of autism, we compared results from primary analyses in EARLI to a second, larger United States cohort, whose source population was not based on family history of autism: the Nurses’ Health Study II (NHSII). The NHSII is a large ongoing longitudinal study of 116,429 female, registered nurses from the United States aged 25–42 y beginning in 1989 [22]. Questionnaires (available online: https://nurseshealthstudy.org/researchers) are mailed to participants every other year and capture information about lifestyle practices, reproductive events, and medical conditions. Participants were eligible for this analysis if they had index births between 1991 and 2007 in order to allow for prospective reporting of diet and to include participants from the nested case-control study of autism that collected the outcome measure used here. The details of this nested case-control study have been previously described [23]. After excluding women without eligible dietary or child information, 10,314 women completed a questionnaire during pregnancy or lactation. Of these, 727 women were part of the nested case-control study and returned a Social Responsiveness Scale (SRS) form for their child, and 713 were from singleton pregnancies and were used in these analyses. Further details on both cohorts and their use in this comparative approach have been published previously [[24], [25], [26]]. The EARLI study was reviewed and approved by the Drexel University Institutional Review Board (project no. 71109; protocol no. 17862), and all EARLI study sites obtained local institutional review board approvals. The study protocol for NHSII was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, and the completion and return of questionnaires sent by United States mail constitute implied consent.
Dietary assessment
Dietary information in both cohorts was collected via comparable FFQs. EARLI used a modified version of the NCI Dietary History Questionnaire II modified for the National Children’s Study to cover the pregnancy period (first and second half) and to include foods and preparation/packaging questions relevant to toxicant exposures. The Dietary History Questionnaire consists of 124 food and supplement items and has been validated [27]; in EARLI, a separate supplement questionnaire was used to capture prenatal supplements. Responses were converted into servings per day of selected food groups based on the amount and frequency of intake.
In the NHSII, dietary intake was collected in 1991 and every 4 y thereafter, using a previously validated semiquantitative 131-item FFQ [28]. Participants were asked about the frequency of food consumption of standard portion size of each food on average over the preceding year. An open-ended question asking about the regular consumption of foods not listed is also included. Dietary information from FFQs 1 y prior to the birth year of the index child, as well as that 1–2 y after the birth of the index child from women reporting breastfeeding, were utilized for dietary data in analyses here. Diet in NHSII thus covered a broader time period than in EARLI, though prior work in the NHSII has supported the stability of diet over time, including before and during pregnancy [10,29].
Nutrient intake was derived from the questionnaires based on standard data sources and adjusted for total EI using the nutrient residual energy adjustment method [30]. Intake from supplements, based on questions querying frequency and, in some cases, dose, and brand, was included for all NHSII nutrients. In EARLI, values from supplements were not calculated for all nutrients, but intake from supplements was included for the majority of nutrients assessed in primary analyses (including folate, omega-3 PUFAs, iron, zinc, vitamin D, vitamin E, vitamin B12, vitamin B6, as well as vitamins A and C). In both studies, total EI was calculated by summing energy from all foods. We confirmed that individuals in each study did not have implausible EI values.
Child autism-related trait assessment
Autism-related traits were captured by the SRS. The SRS is a parent-report questionnaire generating a raw score ranging from 0 to 195, with higher values indicating greater expression of the autism-related phenotype and greater deficits in social reciprocity. The SRS is the most widely-used quantitative measure of autism-related phenotype. The SRS has well-established psychometric properties in both the general population and autistic families, with high internal validity, reliability, reproducibility, and score stability across ages. The SRS has been validated against the “gold standard” for diagnosis, the Autism Diagnostic Interview-Revised (ADI-R), with strong results (r = 0.7 for SRS scores and ADI-R algorithm scores for Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria) [31]. Established SRS score thresholds reliably distinguish children with an autism diagnosis from both non-affected children and those with other conditions, such as intellectual disability [31,32]. In EARLI, SRS forms were completed by mother participants about their child at 36 mo, whereas mothers completed SRS forms on their child aged approximately 4–18 y in the NHSII. Prior work has supported score stability across age and test-retest reliability [33,34].
Statistical analyses
Basic characteristics of the study populations, including descriptive statistics of food and nutrient intake, were examined. All nutrients were adjusted for total EI via the nutrient residual method [30]. All primary analyses were conducted in EARLI and the NHSII separately.
Given the variability in the distribution across different nutrients (e.g., differing ranges across the nutrients included), nutrient values were first standardized using a z-score prior to including in combined effects analyses. As noted, we utilized a Bayesian approach capable of accomplishing our goal of assessing a range of dietary factors and their combined “mixture” effect and addressing limitations that can occur under other approaches. Unlike frequentist approaches, Bayesian approaches rely on inferences based on prior and posterior probabilities rather than P values [35]. In addition, Bayesian approaches can help address the potential for invalid conclusions when using standard statistical approaches that consider exposures 1 at a time and corresponding overly conservative traditional approaches (such as Bonferroni) for guarding against false positive findings, as well as potential confounding by correlated exposures and model convergence problems when attempting adjustment under conventional approaches [36]. Bayesian kernel machine regression (BKMR) [37] was therefore used to examine the combined effects of nutrients and, separately, foods in mixture analyses in association with total raw SRS scores as the outcome. BKMR was used because this method can capture high dimensional and complex exposure-response relationships by flexibly modeling the relationship between a large number of variables and an outcome.
Covariates in adjusted models were selected a priori on the basis of known or potential associations with both diet and autism (and/or our outcome measure, SRS) and included: maternal pre-pregnancy BMI (in kg/m2), maternal age, household income, total EI, prenatal vitamin use (for EARLI, in the first month of pregnancy; for NHSII, anytime during pregnancy), and child sex. Sensitivity analyses tested adjustment for additional covariates, including breastfeeding status, household smoking, maternal race, maternal ethnicity, and maternal education.
In primary analyses, key nutrients of interest were selected a priori based on associations with autism and/or established roles in pathways relevant to neurodevelopment (including inflammation, oxidative stress, methylation, and direct influences on neurodevelopmental process). These included ω-3 PUFAS (n–3 PUFAs), ω-6 PUFAS (n–6 PUFAs), iron, zinc, vitamin D, folate, vitamin B12, vitamin B6, choline, and betaine. In addition, mixture analyses using an expanded set of nutrients were performed for comparison to our primary model (including, in addition to the 10 primary nutrients, vitamins A, C, and E; flavonoids; methionine; and carotenoids). Due to moderate to high correlations between several nutrients, we used hierarchical variable selection, grouping nutrients based on the hypothesized shared pathway (inflammation: iron, zinc, n–6 PUFAs, vitamin D, n–3 PUFAs; 1 carbon metabolism: folate, vitamin B12, vitamin B6, choline, betaine). Secondary analyses examining an expanded set of nutrients added vitamin E to the inflammation pathway, methionine to the 1 carbon metabolism pathway, and included vitamin A, vitamin C, flavonoids, and carotenoids in a third pathway, oxidative stress. Given the known overlap in nutrient roles in these pathways, and to examine the effect of such grouping, we also conducted secondary analyses using component-wise variable selection, which does not group exposures, for comparison. Several visual contrasts related to understanding both the combined effect and individual nutrient’s contribution to this effect relative to the other nutrients were generated based on BKMR estimates, including the overall mixture effect, the individual nutrient effects within the context of other nutrients, and joint effects.
Parallel strategies were used for key food sources of nutrients, though foods were not grouped into pathways before input into the model. Initially, 14 food groups that are key sources of these nutrients were included in BKMR models; final models were pared down to 10 food groups based on those with the highest posterior inclusion probabilities (PIPs). In both cohorts, these models included groupings for meat, fish, vegetables, fruits, nuts, and sugar-sweetened beverages, though specific food groups included varied slightly across cohorts, given slight differences in groupings of certain foods within individual item questions (see Appendix A). As for nutrient analyses, secondary models tested the inclusion of a broader set of foods, and we also explored a model that separated fish into component groups (fried fish, shellfish, salmon, and other large fatty fish) based on differences in associations with these outcomes by fish type indicated in our prior work [38].
All analyses were performed using Copyright © SAS Institute Inc version 9.4 and R Core Team version 4.2.1. BKMR was modeled using the bkmr package [20], and BKMR fit was assessed using the bkmrhat package. The number of iterations was adjusted until convergence was achieved, defined as a German Rubin (rhat) value of 1.0 (≤1.05).
Finally, while the focus of this work was to employ mixture methods to address potential combined effects and enable examination across a wider set of nutrients and foods than possible using more traditional methods, we also secondarily tested select nutrients and foods with prior associations with autism and/or signals in our BKMR models in more standard regression models. Specifically, quantile regression modeled at the 50th percentile (to account for the non-normality of the SRS distribution) was used to examine associations between select nutrients (folate, n–3 PUFAs, n–6 PUFAs, and vitamin D) and foods (fish, meat, and vegetables) and SRS scores.
Results
Basic characteristics of the study populations are shown in Table 1. In EARLI, approximately 20% of the study group was of Hispanic ethnicity and nearly 30% of multiple or non-White races; diversity was low in NHSII, with the majority of participants of non-Hispanic ethnicity and White race. By design, all mothers in EARLI had a previous child (proband) with autism, and 23% of EARLI follow-up children received an ASD diagnosis (consistent with ASD recurrence rates for younger siblings of children with ASD [39]). Similarly, consistent with evidence of trait shifts in families with a history of autism [40,41], the mean SRS score was higher in EARLI than in NHSII.
TABLE 1.
Basic characteristics of the study populations
| EARLI (n = 127) |
NHSII (n = 713) | |
|---|---|---|
| n (%) | ||
| Child’s sex | ||
| M | 69 (54.3) | 414 (58.1) |
| F | 58 (45.7) | 299 (41.9) |
| Maternal ethnicity | ||
| Hispanic/Latino | 24 (18.9) | 13 (1.8) |
| Not Hispanic/Latino | 103 (81.1) | 700 (98.2) |
| Maternal race | ||
| White | 87 (68.5) | 694 (97.3) |
| Black/African American | 7 (5.5) | 1 (0.1) |
| Native American or Native Alaskan | 2 (1.6) | 0 |
| Asian | 13 (10.2) | 9 (1.3) |
| Multiple/other race | 13 (10.2) | 7 (1.0) |
| Missing | 5 (3.9) | 2 (0.3) |
| Household income1 | ||
| Low | 25 (19.7) | 26 (3.7) |
| Medium | 51 (40.2) | 308 (43.2) |
| High | 51 (40.2) | 244 (34.2) |
| Missing | 0 (0) | 135 (18.9) |
| Prenatal smoking | ||
| Active | 4 (3.2) | 51 (7.2) |
| Passive | 2 (1.6) | - |
| None | 98 (77.2) | 662 (92.9) |
| Missing | 23 (18.1) | 0 (0) |
| Birth weight | ||
| ≤2500 g | 5 (3.9) | 7 (1.0) |
| >2500 g | 121 (95.3) | 433 (60.7) |
| Missing | 1 (0.8) | 273 (38.3) |
| Ever breastfeed | ||
| Yes | 80 (63.0) | 678 (95.1) |
| No | 33 (26.0) | 34 (4.8) |
| Missing | 14 (11.0) | 1 (0.1) |
| Prenatal vitamin use2 | ||
| Yes | 73 (57.5) | 524 (73.5) |
| No | 53 (41.7) | 189 (26.5) |
| Missing | 1 (0.8) | 0 (0) |
| ASD diagnosis | ||
| Yes | 29 (22.8) | 102 (14.3)3 |
| No | 96 (75.6) | 611 (85.7) |
| Missing | 2 (1.6) | 0 (0) |
| Mean (SD) | ||
|---|---|---|
| Maternal age, y | 33.9 (4.4) | 34.2 (4.2) |
| Parity4 | 1.7 (0.9) | 1.3 (1.2) |
| Pre-pregnancy BMI, kg/m2 | 28.2 (7.4) | 23.4 (4.2) |
| Physical activity, METs/wk | 5.6 (9.4) | 20.3 (26.2) |
| Total calorie intake, kcal | 1833.8 (793.4) | 1945.6 (549.3) |
| Total SRS raw score | 36.0 (26.4) | 27.9 (33.6) |
ASD, autism spectrum disorder; EARLI, Early Autism Risks Longitudinal Investigation; MET, metabolic equivalent; NHSII, Nurses’ Health Study II; SRS, Social Responsiveness Scale.
Low-income category defined as <$50,000 in EARLI and <$40,000 in NHSII; medium category as $50,000–$100,000 in EARLI and $40,000–$100,000 in NHSII; high as >$100,000 in both studies.
For the EARLI sample, this is defined as use initiated in the first month of pregnancy; for the NHSII sample, this was prenatal vitamin use during pregnancy in general; the use of prenatal vitamins overall was high in both cohorts.
Note that due to the case-control design from which NHSII data for this analysis are drawn, the prevalence of ASD is skewed higher than the general population. The overall prevalence of ASD in the NHSII is approximately 2%.
Parity value does not include the study child in EARLI; by design, all children in EARLI were 2nd or later birth order.
A summary of nutrient and food intake in the study populations is shown in Supplemental Tables 1 and 2. Intake was similar for most nutrients and foods across cohorts, though intake of a few nutrients, including n–6 PUFAs, folate, and vitamin D, was higher on average in EARLI. Many nutrients and some food groups demonstrated high correlations, including folate and other B vitamins, vitamin D with other B vitamins, folate, and iron, and n–3 and n–6 PUFAs, among others; vegetable groups were also highly correlated (>0.5; Supplemental Figure 2).
Nutrient analyses
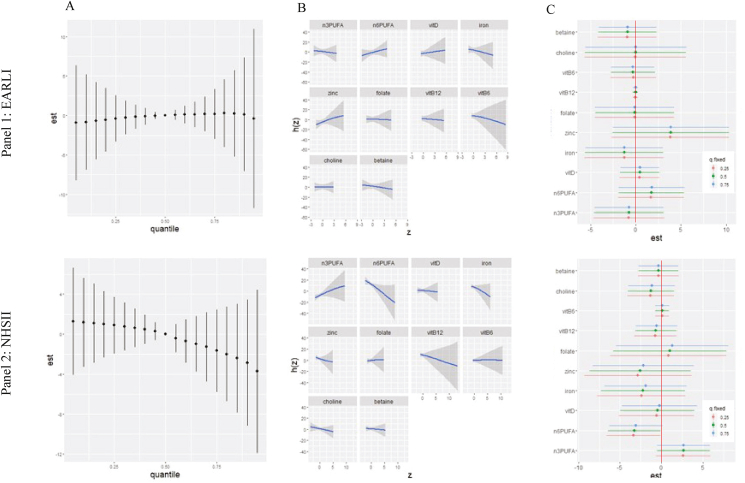
No overall mixture effects on child SRS scores were observed in crude or adjusted BKMR analyses in EARLI, and credible intervals crossed the null (Figure 1A, Panel 1; crude results shown in Supplemental Figure 3, with results similar to adjusted). Examining the univariate exposure-response function (Figure 1B, Panel 1), which displays individual nutrient associations with child SRS scores, whereas all other nutrients are fixed at the 50th percentile, zinc and n–6 PUFAs demonstrated weakly positive associations with SRS scores, whereas B6 and iron showed inverse associations, while other nutrients displayed null associations. Examining the individual nutrient contribution to the overall effect of the nutrient mixture on child SRS scores (Figure 1C, Panel 1), illustrated by the change in SRS score when a nutrient is at its 25th compared to its 75th percentile, whereas all other nutrients are fixed at a specific percentile (either 25th, 50th, or 75th), single-exposure effects were null with wide credible intervals for most nutrients. There was some signal for zinc, with evidence for a positive association (an increase from the 25th to 75th percentile, holding all other nutrients at the median, associated with a ∼4-point increase in SRS score). These analyses suggested no interaction between mixture components, given the similarity in estimates across percentiles of other nutrients (Figure 1C, Panel 1). Additional contrasts from adjusted analyses also suggested no clear interaction effects among components of the mixture (Supplemental Figure 4). Group PIPs from the adjusted analysis suggested a stronger association with SRS scores for the inflammation pathway than the 1 carbon metabolism pathway (group PIPs 0.51 and 0.34, respectively). Within the inflammation pathway group, zinc had the highest PIP (conditional PIP = 0.28), whereas, within the 1 carbon metabolism group, vitamin B6 had the highest PIP value (conditional PIP = 0.36, followed by betaine with PIP = 0.18; Supplemental Table 3).
FIGURE 1.
Adjusted associations between prenatal nutrient intake and child Social Responsiveness Scale (SRS) scores in Early Autism Risks Longitudinal Investigation (EARLI) (Panel 1) and Nurses’ Health Study II (Panel 2).1 Results of adjusted Bayesian kernel machine regression (BKMR) analyses including energy-adjusted nutrient intake for n–3 PUFAs, n–6 PUFAs, iron, zinc, vitamin D, folate, vitamin B12, vitamin B6, choline, and betaine and adjusted for the following covariates: pre-pregnancy BMI, maternal age, child sex, household income, total EI, and prenatal vitamin use (for EARLI, in the first month of pregnancy). Panel 1 shows results in EARLI (n = 127), and Panel 2 results in Nurses’ Health Study II (n = 713). Nutrients were grouped by shared biological pathways (1 carbon metabolism: folate, vitamin B12, vitamin B6, betaine, choline; inflammation: iron, zinc, n–6 PUFAs, vitamin D, n–3 PUFAs). Plots show (A) the overall mixture effect or the relationship between the nutrient mixture and child SRS scores when covariates are held constant. (B) Individual nutrient associations with SRS scores holding all other nutrients at their 50th percentile and all other covariates constant. (C) Single-exposure effects; plot shows the impact on a child’s SRS score when each exposure increases from its 25th to 75th percentile (whereas other exposures are fixed at their 25th, 50th, or 75th percentiles) and covariates are held constant. Additional contrasts of these associations (interaction effects, contour plots, and bivariate exposure-response functions) are shown in Supplemental Figures 4 and 6. BKMR models testing adjustment for additional covariates (birthweight, breastfeeding status, household smoking, maternal race, maternal ethnicity, and maternal education) in EARLI yielded similar results (Data not shown). BKMR models testing adjustment for alternate covariates that may be considered downstream or a potential causal pathway (preterm birth and low birth weight) in EARLI yielded similar results (data not shown). A model including an expanded set of nutrients in EARLI is provided in Supplemental Figure 7 (additional nutrients: vitamin A, vitamin C, vitamin E, flavonoids, methionine, and carotenoids); the primary set here was selected as described in text based on a priori interest for neurodevelopmental relevance. Results were similar when included folate from food only compared with supplemental folic acid (data not shown).
In our comparison analyses conducted in NHSII, there was more of a downward SRS trend within increasing nutrient mixture in NHSII than in EARLI in both crude and adjusted analyses (Figure 1A, Panel 2; crude results shown in Supplemental Figure 5 and as for EARLI, were similar to adjusted). Assessing the univariate exposure-response function (Figure 1B, Panel 2), vitamin B12, iron, and n–6 PUFAs showed inverse relationships, and n–3 PUFAs a positive relationship with SRS scores in the context of the mixture, though credible intervals were wide; other nutrients showed mostly null associations. In analyses assessing nutrient interquartile range increases holding other nutrients at the 25th, 50th, or 75th percentiles, although credible intervals included the null for several estimates, overall patterns suggested a positive association with n–3 PUFAs regardless of the percentile at which other nutrients were fixed, and an inverse association with n–6 PUFAs with little impact of percentile at whereas other nutrients were held fixed (Figure 1C, Panel 2). Zinc and iron also showed inverse trends, but with credible intervals more widely overlapping in the null. As in EARLI, we did not observe strong interactive effects across nutrients (Supplemental Figure 6). Group PIPs from the adjusted analysis suggested a stronger association with SRS scores for the inflammation pathway than the 1 carbon metabolism pathway (group PIPs = 0.63 and 0.34, respectively). Within the inflammation pathway group, zinc had the highest PIP (conditional PIP = 0.34). Within the 1 carbon metabolism group, folate had the highest PIP value (conditional PIP = 0.36; Supplemental Table 4).
Secondary nutrient analyses
Secondary analyses conducted in EARLI incorporating additional nutrients showed similar results and did not suggest signals with other nutrients (Supplemental Figure 7), though nutrients from the oxidative stress pathway (carotenoids, vitamin A, vitamin C, and flavonoids) were most likely to be associated with child SRS scores according to PIP values (group PIP = 0.43; highest conditional PIP for vitamin C at 0.38) (Supplemental Table 5). In comparison, component-wise analyses, which did not group nutrients, results were very similar to primary analyses (Supplemental Figure 8). Sensitivity analysis excluding nutrient outliers (n = 56) yielded similar trends as in primary analyses (data not shown).
Food groups
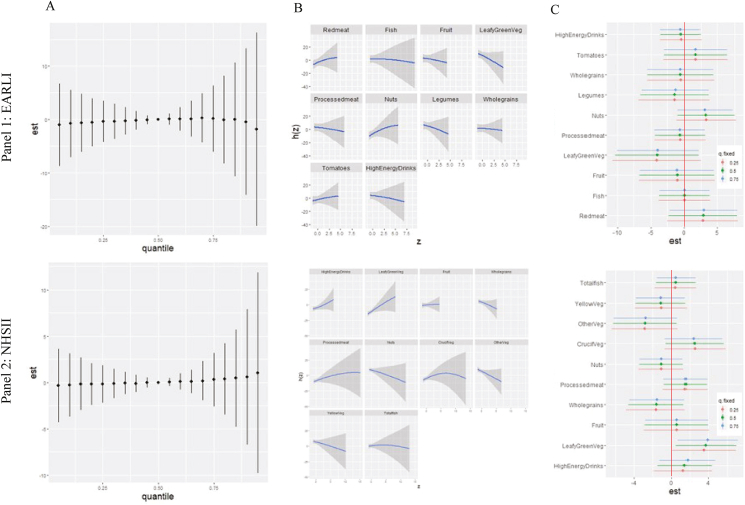
Food groups with the highest PIPs out of the 14 assessed (Supplemental Table 6A) and used in primary food group mixture analyses are shown in Figure 2; these included several categories of meats, vegetables, and other foods. Overall mixture effects of foods in adjusted BKMR analyses in EARLI yielded estimates with credible intervals crossing the null in both crude and adjusted analyses (Figure 2A, Panel 1; crude results in Supplemental Figure 9, similar to adjusted). However, some signals were indicated for individual foods within the mixture when all other foods were fixed at the 50th percentile (Figure 2B, Panel 1), including an inverse association with leafy green vegetables and suggested positive associations with nuts. Examining the single-exposure effects (Figure 2C, Panel 1), nuts and red meat had modest positive associations, and leafy green vegetables had an inverse association with SRS scores, though the percentile at which the other food groups in the mixture were fixed appeared to have little impact. Leafy green vegetables, followed closely by red meat, had the highest PIP in adjusted analyses (Supplemental Table 7). We did not observe strong evidence of interaction across food groups according to interaction effects, contour plots, or bivariate exposure-response functions (Supplemental Figure 10).
FIGURE 2.
Adjusted associations between prenatal food intake and child Social Responsiveness Scale (SRS) scores in Early Autism Risks Longitudinal Investigation (EARLI) (Panel 1) and Nurses’ Health Study II (NHSII) (Panel 2). Results of adjusted Bayesian kernel machine regression (BKMR) analyses, including food groups reported from prenatal FFQ, adjusted for the following covariates: pre-pregnancy BMI, maternal age, child sex, household income, total EI, and prenatal vitamin use (for EARLI, in the first month of pregnancy). Panel 1 shows results in EARLI (n = 127), and Panel 2 results in NHSII (n = 713). In EARLI, food groups included red meat, processed meat, fish, leafy green vegetables, tomatoes, whole grains, legumes, nuts, fruit, and high-energy drinks. In NHSII, food groups included high-energy drinks, leafy green vegetables, fruit, whole grains, processed meat, nuts, cruciferous vegetables, other vegetables (corn, mixed vegetables, onion, celery, beets, eggplant, zucchini, and peppers), yellow vegetables, and fish. Plots show (A) the overall mixture effect, or the relationship between the food group mixture and child SRS scores when covariates are held constant. (B) Individual food group associations with SRS scores, holding all other food groups at their 50th percentile and all other covariates constant. (C) Single-exposure effects; plot shows the impact on a child’s SRS score when each exposure increases from its 25th–75th percentile (whereas other exposures are fixed at their 25th, 50th, or 75th percentiles) and covariates are held constant. Additional contrasts of these associations (interaction effects, contour plots, and bivariate exposure-response functions) are shown in Supplemental Figures 9 and 11. BKMR models testing adjustment for additional covariates (birthweight, breastfeeding status, household smoking, maternal race, maternal ethnicity, and maternal education) in EARLI yielded similar results (data not shown). BKMR models testing adjustment for alternate covariates that may be considered downstream or a potential causal pathway (preterm birth and low birth weight) in EARLI yielded similar results (data not shown). A model including an expanded set of food groups in EARLI is provided in Supplemental Figure 12 [additional food groups: dairy, dark yellow vegetables, cruciferous vegetables, and other vegetables (celery, cucumbers, mushrooms, corn, onions, peppers, etc.)]; the primary set here was selected as described in text based on a priori interest for neurodevelopmental relevance and variable selection parameters.
In NHSII comparison analyses, a slightly differing set of foods was included, owing to PIP selection (see Figure 2, Panel 2 and Supplemental Table 6B) as well as some minor differences in how food items were grouped in questions (Appendix A). No overall mixture effect was observed across the food groups (Figure 2A, Panel 2; crude results in Supplemental Figure 11). More of an increase in SRS scores with the food mixture was suggested in crude analyses than in adjusted, though signals for other comparisons were generally similar to analyses including covariates. In adjusted analyses of individual food groups holding other groups at the 50th percentile (Figure 2B, Panel 2), leafy green vegetables and nuts displayed effects in opposing directions to those observed for EARLI. In analyses examining interquartile range increases in food group intake, overall, large differences in effects by quantile of other mixture components were not observed. However, “other vegetables” (which included a range of vegetables listed in Figure 2) were associated with decreases in SRS scores, whereas cruciferous and leafy green vegetables and, to a lesser extent, processed meat were associated with increases in SRS scores (Figure 2C, Panel 2). High-energy drinks and whole grains had the highest PIPs in adjusted analyses (0.19 and 0.15, respectively; Supplemental Table 8). Examining additional contrasts, overall, clear, interactive effects were not observed (Supplemental Figure 12).
Secondary food group analyses
Secondary analyses conducted in EARLI incorporating additional food groups demonstrated overall similar trends. In this expanded model, additional food groups showed overall null relationships with child SRS scores (Supplemental Figure 13). PIPs from the adjusted model demonstrated that red meat was most likely to be associated with child SRS scores (PIP = 0.16; Supplemental Table 9). Overall, we did not observe large differences by fish type in analyses including separate groupings for types of fish, though there was more of an inverse signal with fried fish than other categories (Supplemental Figure 14).
Secondary traditional regression results comparison
In comparative regression models of a subset of individual nutrients and foods in EARLI, no significant associations were observed, similar to mixture models (Supplemental Table 10). In traditional regression models for NHSII, we observed evidence for decreasing SRS scores with increasing folate and vitamin D; however, these effects were attenuated and were not statistically significant when adjusting for one another (Supplemental Table 11).
Discussion
This study examined the relationship between maternal prenatal diet and child autism-related traits by implementing a more comprehensive approach to capture diet using Bayesian mixture methods. We were able to compare findings for both nutrients and foods across a cohort with increased likelihood of autism due to family history and a cohort with a likelihood of autism consistent with the general population rate. We focused on a range of nutrients with neurodevelopmental relevance, including folic acid, vitamin D, n–3 PUFAs, n–6 PUFAs, iron, zinc, vitamin B12, vitamin B6, choline, and betaine, as well as their primary food sources. Overall, we did not observe strong effect sizes across the nutrients or foods assessed here in relationship to child SRS scores. However, modest signals with autism-related traits for individual nutrients or foods within the context of the mixture were suggested, and some differences across the 2 cohorts were also noted. Though credible intervals included the null value, there was also a suggested association with the mixture of nutrients selected for neurodevelopmental relevance, given the observed trend of lower child autism-related traits with an increasing nutrient mixture in the NHSII. Taken together, these findings suggest potential benefits to considering the mixture effects of dietary factors and present opportunities for future work.
Prior work has not examined prenatal nutrients from a mixture perspective in association with autism or SRS scores. Instead, the majority of prior work focused on maternal diet and autism has primarily investigated associations with single nutrients or foods. Here we examined a set of a-priori-defined nutrients with evidence for associations with autism and/or established roles in neurodevelopment. We observed some evidence suggestive of modest reductions in SRS scores with a greater intake of this “neurodevelopmentally relevant” nutrient mixture in the NHSII but not in EARLI. Given the high overlap in autism and cognitive deficits, including intellectual disability, this may be seen as broadly consistent with a study suggesting increases in scores on neurodevelopmental scales (and therefore lower likelihood of neurodevelopmental deficits) in relation to a “good nutrition index” including many of the nutrients examined here, and reductions in scores in relation to a “poor nutrition index,” in weighted quantile sums analyses [42].
The majority of studies of prenatal nutrients and autism (mainly focused on folate, vitamin D, and, to a lesser extent, PUFAs) have either suggested reductions in the likelihood of autism or autism-related traits with higher nutrient concentrations or suggested null effects, with the strongest consistency for folate (particularly when based on reported supplement use) and vitamin D (according to measured concentrations in maternal plasma or serum samples) [5]. In our work, we did not observe signals with reported folate or vitamin D intake within the context of the mixture, though PIPs did support folate as a key player in this pathway for the NHSII sample. Several factors may explain differences in our results compared with prior work, including confounding in prior studies not considering the roles of these other nutrients. Alternatively, our estimates could have been impacted by sample size or, for vitamin D, the use of reported intake rather than measured concentrations. Nonetheless, given the key roles of vitamin D and folate in immune functioning and methylation pathways, continued investigation of their relationship with autism or autism-related traits within combined effects frameworks is needed, respectively.
Our analyses suggested some associations with PUFAs. Although n–3 PUFAs play a greater role in neurodevelopmental processes hypothesized to underlie associations with autism and neurodevelopmental outcomes, there is also some support for the potential roles of n–6 PUFAs. Prior work in NHSII has suggested an inverse association with n–6 PUFAs and autism [10], including the essential FA LA. In addition, n–6 PUFAs are involved in the regulation of inflammatory markers that could mediate associations with autism [43,44]. Both classes of PUFAs, as well as individual FAs within these groupings, are highly correlated, and thus, although the approach used here is capable of handling correlated exposures, we cannot rule out the correlation between n–3 and n–6 PUFAs influencing the results. Teasing out the effects of individual players remains challenging when the sources of these are overlapping. It should also be noted that in supplemental analyses (data not shown) including only n–3 and not n–6 in the model, n–3 showed an inverse association. The results for individual nutrients should therefore be interpreted cautiously and subject to replication, as findings are also related to the contents of the mixture included. Our models did not include all potential nutrients that could relate to these outcomes but instead focused on an a priori subset.
Strong associations were not seen with other nutrients, though there was a suggestion of a positive association with SRS scores for zinc in EARLI only. This is in contrast to prior work suggesting links between prenatal zinc deficiency, or inverse associations with zinc, and autism or other neurodevelopmental delays in human [45] and mouse studies [46,47]. In both cohorts, there was some suggestion of an inverse association (of small effect size and with wide credible intervals) for iron. Only a few prior studies have examined iron in association with autism and have suggested increases in autism risk with iron deficiency that may be seen as consistent with our results [13,48]. Although our sensitivity analyses that included an expanded set of nutrients in EARLI did not reveal strong signals with additional nutrients, particularly given our small sample size, future work should further examine these and other nutrients.
In analyses of foods, overall mixture effects were primarily null across cohorts. In contrast to the nutrient mixture analysis, food groups had a greater degree of differing anticipated direction of effects, with some healthful, anti-inflammatory, or antioxidant-promoting foods and some unhealthful or proinflammatory foods. In this setting, the benefits of the model in addressing potential confounding and joint effects may be more useful than attempting to estimate a single “mixture effect” of foods in this way. Nonetheless, future work might utilize and compare other mixture methods, such as weighted quantile sums, to further address sets of food groups whose hypothesized directionality is aligned. Dietary patterns may be considered as an alternate approach to examine combined effects of foods. Only a handful of prior studies have examined dietary patterns in association with autism or related traits, including work in these cohorts that did not find strong associations but did suggest modest increases in SRS scores with a Western or more proinflammatory diet [48].
When examining individual foods within the context of the mixture, in EARLI, a higher intake of leafy green vegetables was associated with reductions in SRS scores, a finding consistent with our prior work examining fruit and vegetable intake in more traditional analyses [49]. In contrast, increases in SRS scores were seen for leafy green and cruciferous vegetable groupings in NHSII, whereas decreases were seen with yellow and other vegetables (which included corn, mixed vegetables, onion, celery, beets, eggplant, zucchini, and peppers). It is possible that pesticide residues across these different foods contribute to these findings [50] and that the effects of prenatal exposure to pesticides differ by background and familial risk of autism. Prior work in EARLI did not suggest increases in SRS scores with pesticide residues in vegetables and fruits [49], but pesticide residues have been associated with other birth outcomes in other studies [51]. It is also worth noting that across cohorts, there were decreases in SRS scores with some groupings of vegetables and, to a lesser extent, increases with some types of meat intake. Although the FFQs used in these cohorts addressed the same major components of the diet, minor differences in the grouping of some foods within questions could have contributed to differing signals observed.
Although we were able to conduct novel analyses in 2 cohorts that allowed for comparisons, several limitations and considerations should be weighed when placing our findings in context. Although we hypothesized that cohort differences may relate to background genetic risk for autism, other differences may influence these comparisons. As noted, there were some differences across cohort FFQs, and the results of food group analyses may have been more sensitive to this. In addition, there were differences in the timing of exposure and outcome measurement across studies, with dietary data collected at approximately 20 weeks of gestation in EARLI and FFQs capturing prior year diet overlapping with pregnancy and/or lactation in NHSII, though results did not materially change when excluding participants with lactation-based diet in NHSII. Children from EARLI were younger than children from NHSII on average; however, prior work has supported the stability of SRS scores across these ages [33,34]. We also had a relatively small sample size, and while the approach employed has been successfully implemented in samples as small as n = 100 [37], this may have influenced our ability to detect signals. Our study populations also had limited diversity and may have reduced generalizability. However, despite the high use of prenatal vitamins in these cohorts, the distribution of intake across key nutrients and foods is broadly comparable to other samples of United States women of childbearing age, including the nationally representative cohort, NHANES [52]. Across analyses, we relied on reported diet only, and observed effect sizes were small, with most foods or nutrients with suggested signals showing increases or decreases of 3–5 raw total points (or approximately a 1/6 SD change in SRS score or less), and credible intervals for most comparisons included the null value. We did not conduct analyses of autism diagnosis here because of the limited sample size, but it is worth noting that there is strong consistency between high SRS scores and autism diagnosis [31,34]. Although score increases or decreases of effect sizes observed here may not be clinically meaningful at the individual level, a population-level shift in the distribution of these traits could translate into a meaningful increase in case load or symptom severity [53]. Furthermore, the potential for interactive effects between prenatal nutrients and foods with other autism risk factors [54] suggests the need for continued study in this area.
As noted, we focused on nutrients (and their food sources) with “neurodevelopmental relevance” as determined by playing key roles in immune functioning, oxidative stress, DNA methylation, or neurodevelopmental processes. Multiple lines of evidence link these broad pathways and autism [[14], [15], [16]]. For example, vitamin D and PUFAs influence immune markers, and the developing immune system is known to impact the developing nervous system [55,56]. The role of maternal inflammation and immune activation in autism is well-supported via human studies showing associations between maternal infections, fevers, and immune-mediated conditions and increased risk of autism, as well as animal models that use maternal immune activation by poly I:C to induce autism-like behaviors in rodents [57]. Meanwhile, a large number of genes have been linked with autism, and epigenetic modulation by folate and other nutrients is also supported [[58], [59], [60]]. However, additional mechanistic-focused work is needed to better understand pathways.
Overall, though we did not find strong evidence for mixture and interactive effects among nutrients and foods here, there were several suggestive signals, including a trend for less autism-related traits with greater intake of a mixture of nutrients that are important for neurodevelopment. Across cohorts, we also observed associations with vegetable intake within the context of the mixture, though both decreases and increases were seen for different groups of vegetables, which could relate to pesticide residues. Mixtures approaches are capable of addressing combined and higher-order effects. Using models such as those used here, but also perhaps others that incorporate biologically and toxicologically-informed approaches (e.g., grouping factors that may act in similar biological pathways or have similar toxicologic effects), may prove useful to better understanding the large number of factors influencing autism. Expanding the approach here to consider interactive effects with other categories of exposures may serve fruitful not only to better understand combined effects but also to potentially identify mitigating factors. Furthermore, the cross-cohort comparative approach employed here suggests the need to further consider not only the effects of multiple nutrients or foods but also their interactions with genetic background.
Funding
This work was supported by funding from the Eagles Autism Foundation (to KL). The Early Autism Risks Longitudinal Investigation study was funded by National Institute of Environmental Health Sciences (NIEHS), NIMH, NICHD, and the National Institute of Neurologic Disease and Stroke (R01 ES016443; to CN), with additional funding from Autism Speaks (AS 5938) and the Eagles Autism Challenge Grant. The NHSII study was funded by grant U01 CA176726 from the NIMH and grant U01 HL145386 from National Institute of Health (NIH). Funding agencies did not have any involvement in the study design, collection, analysis, or interpretation of the data, nor restrictions on submission for publication.
Author contributions
The authors’ responsibilities were as follows–CN, IH-P, LAC, MDF: conducted research related to the original collection of Early Autism Risks Longitudinal Investigation data and design; KL: conceptualized the analyses, CN: designed the EARLI research study; KL: wrote the paper and had primary responsibility for final content; JR, SW: analyzed data and performed statistical analyses; GBH, JC, MW, LAC, MDF, IH-P, HEV, RJS, CN: assisted with paper edits and writing; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Data availability
Data from the Early Autism Risks Longitudinal Investigation study are deposited to the National Database for Autism Research. Data described in the manuscript, code book, and analytic code are available upon request pending application, approval, and (for NHSII data) payment if direct access is required.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdnut.2023.101978.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Susser E., Neugebauer R., Hoek H.W., Brown A.S., Lin S., Labovitz D., et al. Schizophrenia after prenatal famine. Further evidence. Arch. Gen. Psychiatry. 1996;53(1):25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 2.Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm. Rep. 1992;41(RR–14):1–7. [PubMed] [Google Scholar]
- 3.Lyall K., Schmidt R.J., Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol. 2014;43(2):443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong C., Tessing J., Lee B.K., Lyall K. Maternal dietary factors and the risk of autism spectrum disorders: A systematic review of existing evidence. Autism. Res. 2020;13(10):1634–1658. doi: 10.1002/aur.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Zou M., Sun C., Wu L., Chen W.-X. Prenatal folic acid supplements and offspring’s autism spectrum disorder: A meta-analysis and meta-regression. J. Autism Dev. Disord. 2022;52(2):522–539. doi: 10.1007/s10803-021-04951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T., Shan L., Du L., Feng J., Xu Z., Staal W.G., et al. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry. 2016;25(4):341–350. doi: 10.1007/s00787-015-0786-1. [DOI] [PubMed] [Google Scholar]
- 8.Vinkhuyzen A.A.E., Eyles D.W., Burne T.H.J., Blanken L.M.E., Kruithof C.J., Verhulst F., et al. Gestational vitamin D deficiency and autism spectrum disorder. B. J. Psych. Open. 2017;3(2):85–90. doi: 10.1192/bjpo.bp.116.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julvez J., Méndez M., Fernandez-Barres S., Romaguera D., Vioque J., Llop S., et al. Maternal consumption of seafood in pregnancy and child neuropsychological development: A longitudinal study based on a population with high consumption levels. Am. J. Epidemiol. 2016;183(3):169–182. doi: 10.1093/aje/kwv195. [DOI] [PubMed] [Google Scholar]
- 10.Lyall K., Munger K.L., O’Reilly É.J., Santangelo S.L., Ascherio A. Maternal dietary fat intake in association with autism spectrum disorders. Am. J. Epidemiol. 2013;178(2):209–220. doi: 10.1093/aje/kws433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steenweg-de Graaff J., Tiemeier H., Ghassabian A., Rijlaarsdam J., Jaddoe V.W., Verhulst F.C., et al. Maternal fatty acid status during pregnancy and child autistic traits: the generation R Study. Am. J. Epidemiol. 2016;183(9):792–799. doi: 10.1093/aje/kwv263. [DOI] [PubMed] [Google Scholar]
- 12.Surén P., Roth C., Bresnahan M., Haugen M., Hornig M., Hirtz D., et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309(6):570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt R.J., Tancredi D.J., Krakowiak P., Hansen R.L., Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol. 2014;180(9):890–900. doi: 10.1093/aje/kwu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goines P., Van de Water J. The immune system’s role in the biology of autism. Curr. Opin. Neurol. 2010;23(2):111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson P.H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain. Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Lyall K., Ashwood P., Van de Water J., Hertz-Picciotto I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J. Autism. Dev. Disord. 2014;44(7):1546–1555. doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friso S., Udali S., De Santis D., Choi S.W. One-carbon metabolism and epigenetics. Mol. Aspects. Med. 2017;54:28–36. doi: 10.1016/j.mam.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Spencer J.P. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4(4):243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int. J. Androl. 2008;31(2):233–240. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 20.Bobb J.F., Claus Henn B., Valeri L., Coull B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health. 2018;17(1):67. doi: 10.1186/s12940-018-0413-y. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risch N., Hoffmann T.J., Anderson M., Croen L.A., Grether J.K., Windham G.C. Familial recurrence of autism spectrum disorder: evaluating genetic and environmental contributions. Am. J. Psychiatry. 2014;171(11):1206–1213. doi: 10.1176/appi.ajp.2014.13101359. [DOI] [PubMed] [Google Scholar]
- 22.Bao Y., Bertoia M.L., Lenart E.B., Stampfer M.J., Willett W.C., Speizer F.E., et al. Origin, methods, and evolution of the three nurses’ health studies. Am. J. Public Health. 2016;106(9):1573–1581. doi: 10.2105/AJPH.2016.303338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyall K., Pauls D.L., Spiegelman D., Santangelo S.L., Ascherio A. Fertility therapies, infertility and autism spectrum disorders in the nurses’ health Study II. Paediatr. Perinat. Epidemiol. 2012;26(4):361–372. doi: 10.1111/j.1365-3016.2012.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newschaffer C.J., Croen L.A., Fallin M.D., Hertz-Picciotto I., Nguyen D.V., Lee N.L., et al. Infant siblings and the investigation of autism risk factors. J. Neurodev. Disord. 2012;4(1):7. doi: 10.1186/1866-1955-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecchione R., Wang S., Rando J., Chavarro J.E., Croen L.A., Fallin M.D., et al. Maternal dietary patterns during pregnancy and child autism-related traits: results from two US cohorts. Nutrients. 2022;14(13):2729. doi: 10.3390/nu14132729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavarro J.E., Rich-Edwards J.W., Gaskins A.J., Farland L.V., Terry K.L., Zhang C., et al. Contributions of the nurses’ health studies to reproductive Health Research. Am. J. Public Health. 2016;106(9):1669–1676. doi: 10.2105/AJPH.2016.303350. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subar A.F., Thompson F.E., Kipnis V., Midthune D., Hurwitz P., McNutt S., et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am. J. Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 28.Willett W.C., Sampson L., Stampfer M.J., Rosner B., Bain C., Witschi J., et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 29.Rifas-Shiman S.L., Rich-Edwards J.W., Willett W.C., Kleinman K.P., Oken E., Gillman M.W. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr. Perinat. Epidemiol. 2006;20(1):35–42. doi: 10.1111/j.1365-3016.2006.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997;65(4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. Suppl. discussion 1229S. [DOI] [PubMed] [Google Scholar]
- 31.Constantino J.N., Davis S.A., Todd R.D., Schindler M.K., Gross M.M., Brophy S.L., et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism. Dev. Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Lee L.C., Chen Y.S., Hsu J.W. Assessing autistic traits in a Taiwan preschool population: cross-cultural validation of the Social Responsiveness Scale (SRS) J. Autism. Dev. Disord. 2012;42(11):2450–2459. doi: 10.1007/s10803-012-1499-7. [DOI] [PubMed] [Google Scholar]
- 33.Braun J.M., Yolton K., Stacy S.L., Erar B., Papandonatos G.D., Bellinger D.C., et al. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology. 2017;62:192–199. doi: 10.1016/j.neuro.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantino J.N., Gruber C. 2nd ed. Western Psychological Services; Torrance, CA: 2012. Social responsiveness scale. [Google Scholar]
- 35.Hackenberger B.K. Bayes or not Bayes, is this the question? Croat Med. J. 2019;60(1):50–52. doi: 10.3325/cmj.2019.60.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perneger T.V. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobb J.F., Valeri L., Claus Henn B.C., Christiani D.C., Wright R.O., Mazumdar M., et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecchione R., Vigna C., Whitman C., Kauffman E.M., Braun J.M., Chen A., et al. The association between maternal prenatal fish intake and child autism-Related Traits in the EARLI and HOME studies. J. Autism. Dev. Disord. 2021;51(2):487–500. doi: 10.1007/s10803-020-04546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozonoff S., Young G.S., Carter A., Messinger D., Yirmiya N., Zwaigenbaum L., et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyall K., Constantino J.N., Weisskopf M.G., Roberts A.L., Ascherio A., Santangelo S.L. Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2014;71(8):936–942. doi: 10.1001/jamapsychiatry.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virkud Y.V., Todd R.D., Abbacchi A.M., Zhang Y., Constantino J.N. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2009;150B(3):328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malin A.J., Busgang S.A., Cantoral A.J., Svensson K., Orjuela M.A., Pantic I., et al. Quality of prenatal and childhood diet predicts neurodevelopmental outcomes among children in Mexico City. Nutrients. 2018;10(8):1093. doi: 10.3390/nu10081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viladomiu M., Hontecillas R., Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016;785:87–95. doi: 10.1016/j.ejphar.2015.03.095. [DOI] [PubMed] [Google Scholar]
- 44.Willett W.C. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J. Cardiovasc. Med. (Hagerstown). 2007;8(Suppl 1):S42–S45. doi: 10.2459/01.JCM.0000289275.72556.13. [DOI] [PubMed] [Google Scholar]
- 45.Skogheim T.S., Weyde K.V.F., Engel S.M., Aase H., Surén P., Øie M.G., et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021;152 doi: 10.1016/j.envint.2021.106468. [DOI] [PubMed] [Google Scholar]
- 46.Sauer A.K., Hagmeyer S S., Grabrucker A.A.-O. Prenatal zinc deficient mice as a Model for autism spectrum disorders. Int. J. Mol. Sci. 2022;23(11):6082. doi: 10.3390/ijms23116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyas Y., Lee K., Jung Y., Montgomery J.A.-O. Influence of maternal zinc supplementation on the development of autism-associated behavioural and synaptic deficits in offspring Shank3-knockout mice. Mol. Brain. 2020;13(1):110. doi: 10.1186/s13041-020-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiegersma A.M., Dalman C., Lee B.K., Karlsson H., Gardner R.M. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. 2019;76(12):1294–1304. doi: 10.1001/jamapsychiatry.2019.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joyce E., Chavarro J.E., Rando J., Song A.Y., Croen L.A., Fallin M.D., et al. Prenatal exposure to pesticide residues in the diet in association with child autism-related traits: results from the EARLI study. Autism Res. 2022;15(5):957–970. doi: 10.1002/aur.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y., Chiu Y.H., Hauser R., Chavarro J., Sun Q. Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: A validation study. Environ. Int. 2016;92–93:294–300. doi: 10.1016/j.envint.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu Y.H., Williams P.L., Gillman M.W., Gaskins A.J., Mínguez-Alarcón L., Souter I., et al. Association between pesticide residue intake from consumption of fruits and vegetables and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technology. JAMA Intern. Med. 2018;178(1):17–26. doi: 10.1001/jamainternmed.2017.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.USDA, What we eat in America/NHANES data tables 2001-2012 [Internet] 2017 Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/. (Accessed 26 May 2017).
- 53.Lanphear B.P., Hornung R., Khoury J., Yolton K., Baghurst P., Bellinger D.C., et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ. Health. Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bragg M., Chavarro J.E., Hamra G.A.-O., Hart J.A.-O., Tabb L.A.-O., Weisskopf M.G., et al. Prenatal diet as a modifier of environmental risk factors for autism and related neurodevelopmental outcomes. Curr. Environ. Health Rep. 2022;9(2):324–338. doi: 10.1007/s40572-022-00347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bilbo S.D., Schwarz J.M. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33(3):267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez-Celis A., Croen L.A., Yoshida C.K., Alexeeff S.E., Schauer J., Yolken R.H., et al. Maternal autoantibody profiles as biomarkers for ASD and ASD with co-occurring intellectual disability. Mol. Psychiatry. 2022;27(9):3760–3767. doi: 10.1038/s41380-022-01633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haddad F.L., Patel S.V., Schmid S. Maternal Immune Activation by poly I:C as a preclinical Model for neurodevelopmental Disorders: A focus on autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Zhang N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015;1(3):144–151. doi: 10.1016/j.aninu.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ladd-Acosta C., Hansen K.D., Briem E., Fallin M.D., Kaufmann W.E., Feinberg A.P. Common DNA methylation alterations in multiple brain regions in autism. Mol. Psychiatry. 2014;19(8):862–871. doi: 10.1038/mp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xue J., Schoenrock S.A., Valdar W., Tarantino L.M., Ideraabdullah F.Y. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin. Epigenetics. 2016;8:107. doi: 10.1186/s13148-016-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Early Autism Risks Longitudinal Investigation study are deposited to the National Database for Autism Research. Data described in the manuscript, code book, and analytic code are available upon request pending application, approval, and (for NHSII data) payment if direct access is required.