Abstract
The network formed by the amygdala (AMG) and the medial Prefrontal Cortex (mPFC), at the interface between our internal and external environment, has been shown to support some important aspects of behavioral adaptation. Whether and how the anatomo-functional organization of this network evolved across primates remains unclear. Here, we compared AMG nuclei morphological characteristics and their functional connectivity with the mPFC in humans and macaques to identify potential homologies and differences between these species. Based on selected studies, we highlight two subsystems within the AMG-mPFC circuits, likely involved in distinct temporal dynamics of integration during behavioral adaptation. We also show that whereas the mPFC displays a large expansion but a preserved intrinsic anatomo-functional organization, the AMG displays a volume reduction and morphological changes related to specific nuclei. We discuss potential commonalities and differences in the dialogue between AMG nuclei and mPFC in humans and macaques based on available data.
Keywords: Amygdala, Medial prefrontal cortex, Anatomo-functional connectivity, Human, Non-human primate
Graphical abstract
Abbreviations:
- AB
accessory basal nucleus
- ACC
anterior cingulate cortex
- AMG
amygdala
- B
basal nucleus
- CE
central nucleus
- CM
centromedial subdivision
- DTI
diffusion tensor imaging
- DWI
diffusion weighting imaging
- FC
functional connectivity
- LA
lateral nucleus
- LB
laterobasal subdivision
- MCC
midcingulate cortex
- ME
medial nucleus
- mPFC
medial prefrontal cortex
- NHP
non-human primates
- rs-fMRI
resting-state functional MRI
- vmPFC
ventro-medial prefrontal cortex
1. Introduction
From the exploration of the environment to the regulation of mood and decision making, the amygdala (AMG) and its dynamic interactions with the medial prefrontal cortex (mPFC) encompass a wide range of functions that support behavioral adaptation in primates (see for review Gangopadhyay et al., 2021; Murray and Fellows, 2021). These interactions are thought to allows us to react to relevant salient information from our environment and to regulate, control, and adjust these reactions when necessary (Kim et al., 2011b). Accordingly, clinical studies in humans (Johnstone et al., 2007; Price and Drevets, 2010; Likhtik and Paz, 2015; Mukherjee et al., 2016; Dong et al., 2019; Paul et al., 2019; Li et al., 2021) and lesion studies in the most studied model of the human brain, i.e. the macaque rhesus (Málková et al., 1997; Bechara et al., 1999; Rudebeck et al., 2013; Wellman et al., 2016; Elorette et al., 2020; Taswell et al., 2021), have shown that such behavioral adaptation abilities depend, at least in part, on the integrity of this network. In particular, a dysregulation of the top-down control of the mPFC onto the AMG, present in a wide range of pathologies, leads to inappropriate and maladaptive behavioral reactions (Johnstone et al., 2007; Dong et al., 2019).
Over the course of primate evolution, behavioral adaptation has evolved to permit the proper navigation of each species in their respective ecological niches. In humans, this ability reaches its highest level of complexity to face highly complex environments and social interactions (Henke-von der Malsburg et al., 2020). However, whether and how the anatomo-functional interactions of this AMG-mPFC network change in the primate order to subserve behavioral adaptation with increasing complexity is still currently poorly understood. In the present review article, we aimed at providing insights toward that question by identifying, in humans and macaques, homologies and differences of the morphological characteristics of the AMG nuclei and their functional connectivity with the mPFC, two aspects that are often considered separately. Regarding the functional organization of these networks, we deliberately focus on resting-state functional Magnetic Resonance Imaging (rs-fMRI), a powerful tool increasingly used to study functional networks in comparative neuroscience. By doing so, we sought to identify the potential relationships between anatomical and functional organizations in AMG-mPFC circuitries across species, despite their difference in spatial resolution.
First, although the frontal cortex displays an increase of volume from the last common ancestor of humans and old-world monkeys to humans (Semendeferi et al., 2002; Smaers et al. 2011, 2017; Barrett et al., 2020), a large body of evidences point towards a preserved anatomo-functional organization of the mPFC (Petrides and Pandya, 2002; Petrides et al., 2012; Neubert et al., 2015; Procyk et al., 2016; Amiez et al., 2019). Specifically, the mPFC is composed of several regions arranged on the medial part of the brain along the corpus callosum (Fig. 1B). A similar topographical organization can be found along the anterior/ventral-postero/dorsal axis in both macaques and humans, including the ventromedial prefrontal cortex (vmPFC), the anterior cingulate cortex (ACC), the anterior midcingulate cortex (aMCC), and the posterior mid-cingulate cortex (pMCC) (Procyk et al., 2016; Vogt, 2016; Lopez-Persem et al., 2019). From vmPFC to MCC in both macaques and humans, the literature points toward a functional organization sustaining different aspects of behavioral adaptation, i.e., from the evaluation of both our internal and external environment, the evaluation and update of our goals, to the evaluation of our decisions and of their outcomes (Quilodran et al., 2008; Grabenhorst and Rolls, 2011; Amiez et al., 2012; Boorman et al., 2013; Scholl et al., 2015; Procyk et al., 2016; Wittmann et al., 2016; Juechems et al., 2019). Comparatively, as detailed more thoroughly below, the AMG displays the reverse pattern, i.e., a decreased brain occupation volume from the last common ancestor of humans and old-world monkeys to humans that is accompanied by morphological changes of the nuclei forming the AMG (Barger et al. 2007, 2014; Chareyron et al., 2011).
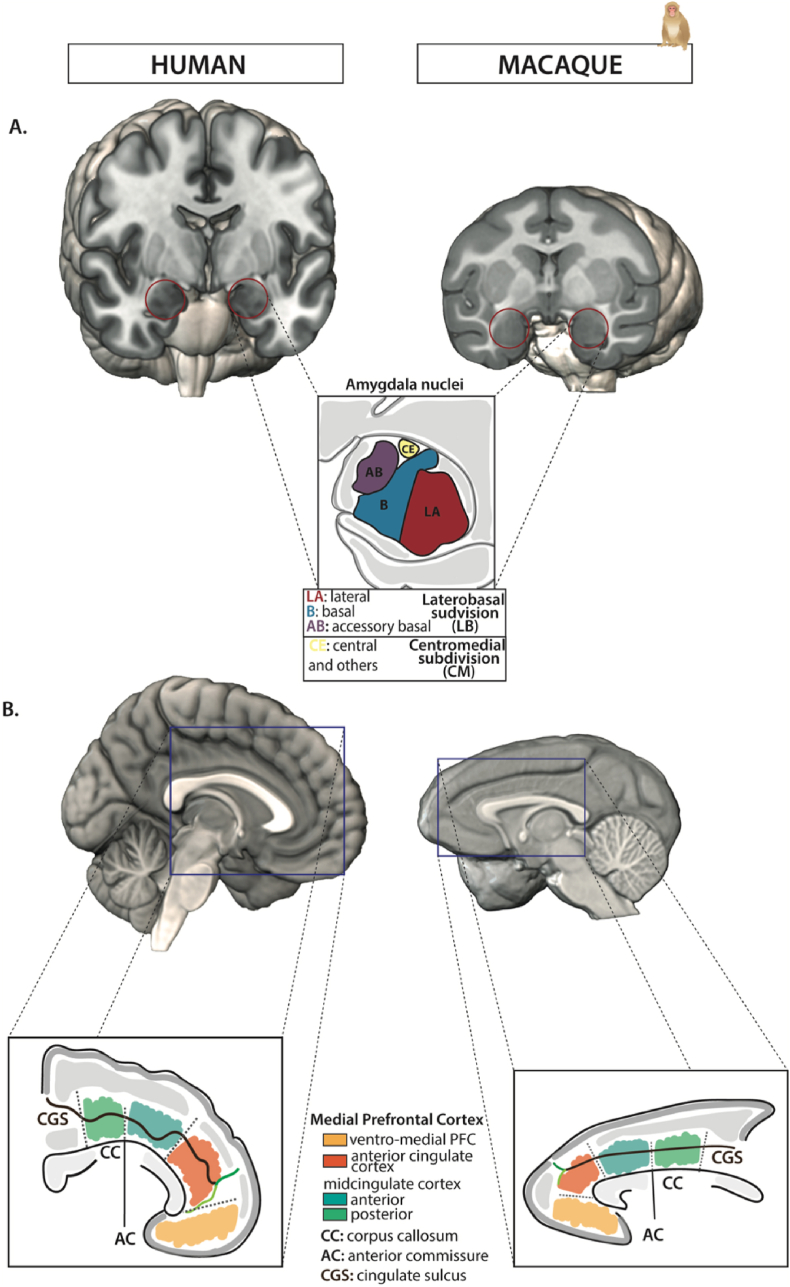
Fig. 1.
Amygdala and medial prefrontal cortex organization in humans and macaques. Human brain sections, from the MNI averaged ICBM152 brain, are displayed in the left column. Macaque rhesus brain sections, from the NMT macaque atlas, are displayed in the right column. A. The amygdala (AMG) is outlined (red circle) on the coronal sections of both species. A schematic representation of the AMG main nuclei subdivisions are represented in the center of the figure: Lateral (LA, in red), Basal (B or BL, in blue), Accessory Basal (AB or BM, in purple), and Central (CE, in yellow) nuclei. B. The mPFC organization is displayed on sagittal sections in both species. The mPFC encompasses the ventro-medial Prefrontal Cortex (vmPFC, which includes area 25, and parts of area 14m, 10m, and 32, yellow area), the Anterior Cingulate Cortex (ACC, i.e., which includes areas 32 and 24 abc, orange area), the anterior and posterior Mid-Cingulate Cortex (MCC, which includes areas 24a'b’c’, 32′, anterior MCC: teal area, posterior MCC: green area). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2. Morphological comparison of the amygdala nuclei volumes in primates
In both macaques and humans, the AMG is an almond-shaped structure nested deep in the medial temporal lobe of the brain, and composed of an ensemble of nuclei displaying distinct anatomical and connectivity features (see details in next sections) (Stephan et al., 1987; Aggleton, 2000; Amunts et al., 2005). In primates, AMG nuclei are broadly parcellated into a deep and a superficial group. The deep group is composed of the lateral (LA), basal (B) and accessory basal (AB) nuclei (Aggleton, 2000). In the literature, the latter two nuclei are also found under the abbreviations BL and BM, respectively. LA nucleus is situated on the lateral part of the AMG complex and is ventrally and caudally bounded by the temporal horn of the lateral ventricle (red, Fig. 1A). B nucleus (blue, Fig. 1A) is bounded laterally by LA and medially by AB nucleus (purple, Fig. 1A). The superficial group is composed of the medial (ME) and cortical nuclei (CO), while excluding the central (CE) nucleus. The CE nucleus, also part of the extended AMG (Fox and Shackman, 2019; Holley and Fox, 2022), lies dorsally and caudally within the AMG complex above the AB nucleus (yellow, Fig. 1A). All these AMG nuclei are strongly interconnected, creating a micro-circuit within the AMG itself, where LA is considered as a sensory gateway, receiving inputs from sensory association cortices, then the flux of information circulates to the other AMG nuclei. Therein, as discussed below, B and AB are more heavily connected to mPFC while CE is connected to autonomic centers nuclei (Aggleton, 2000), and the amygdalar inhibitory intercalated masses contribute to regulatory processes within the AMG (Royer et al., 1999).
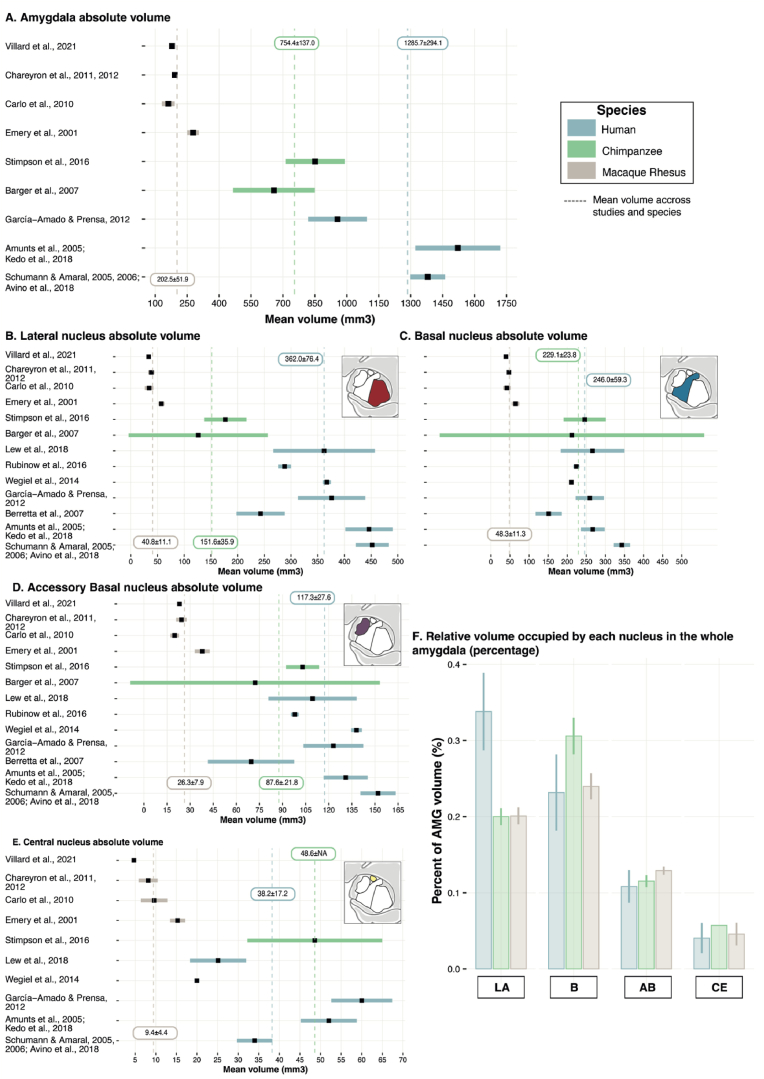
The first question we addressed here is whether and how AMG main nuclei, LA, B, AB and CE, have evolved after the split of humans and macaques from their last common ancestor. In that goal, we gathered studies that have examined the volume of AMG and AMG main nuclei in humans and rhesus macaques (see Fig. 2, Table S1 and methods in supplementary material for the studies selected and associated references). As an intermediate specie between humans and macaques, we selected studies including chimpanzees (great apes) to understand whether any changes between humans and macaques are proper to the “homo” genus or to the Hominidae family (comprising great apes and humans’ genii) (Pozzi et al., 2014). In the 3 species, we only considered ex-vivo stereological studies that specifically reported the volume of the whole AMG and AMG main nuclei. We excluded MRI volumetry studies given the lack of consensus and precision in particular regarding the identification of AMG nuclei on MRI images. Note that potential lateralization, sex and age effects were not assessed because 1) several studies reported non-significant volume variations across hemispheres (Brabec et al., 2010; Kedo et al., 2018), and 2) most studies included only one hemisphere. We first created forest plots comprising each of the selected studies for the whole AMG (AMG, Fig. 2A) and its nuclei separately: LA (Fig. 2B), B (Fig. 2C), AB (Fig. 2D) and CE (Fig. 2E).
Fig. 2.
Volumetric analysis of whole amygdala and amygdala nuclei (LA, B, AB, and CE) in primates. Humans in blue and non-human primates (chimpanzees and macaques) in green and brown, respectively. The analysis was performed exclusively on ex-vivo stereological studies in which we extracted mean volumes, standard deviations and sample size. The majority of studies either include only one hemisphere or did not specify; for the few studies indicating AMG volumes in both hemispheres, we calculated the average volume across hemispheres. A. Forest plots displaying the mean volume of the whole AMG in each selected study (black square) and 95% confidence interval around the mean (wide line) for each specie. Mean ± sd volume across species and studies are represented by dashed vertical lines and displayed in rectangles. B.C.D.E. Mean absolute volumes of LA (B), B (C), AB (D) and CE (E) nuclei across studies and species. Note that the studies included in C are those included in B. F. Because the absolute volume cannot be used to compare the 3 primate species, we calculated the percentage of volume occupied by each nucleus in the whole AMG volume (volume nuclei/volume AMG*100) in each study indicating the volume of the whole AMG. Results are displayed on a bar plot representing the relative volume of LA, B/BL, AB/BM and CE within the AMG for each species (error bars represent interstudy variability). The LA nucleus displays a large expansion in humans compared to macaques and chimpanzees. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Results showed that the absolute AMG volume is higher in humans (mean across studies, blue dotted line, 1285.7 ± 294.1 mm3), compared to chimpanzees (green dotted line, 754.4 ± 137 mm3, 1.8 times smaller than humans), and macaques (brown dotted line, 202.5 ± 51.9 mm3, 6 times smaller than humans). When accounting for differential brain size between species using telencephalic absolute volume as a reference (Semendeferi et al., 1997: humans (1 125 492 mm3), chimpanzees (305 521 mm3), and macaques (62 737 mm3)), we identified that the volume occupied by the AMG in the whole brain is 0.11%, 0.24%, and 0.34% in human, chimpanzee, and macaque brains, respectively. In other words, although the absolute volume of the AMG increased in humans compared to the other species, the percentage of volume it represents in the whole brain decreased compared to the other species.
Importantly, among the AMG nuclei, the LA displays the largest expansion relative to the other nuclei in humans, compared to chimpanzees and macaques (Fig. 2B and F). It represents 34% of the whole AMG volume in humans whereas it represents 20% and 21% in chimpanzees and macaques, respectively. These results are in agreement with previous findings that either compared humans and great apes or humans and macaques (Barger et al., 2007; Chareyron et al., 2011) suggesting that the expansion of LA appeared after the split of humans and chimpanzees from their last common ancestor. In the next sections, we will successively summarize the current state of knowledge regarding the anatomical and functional relationships of the different AMG nuclei in humans and macaques before discussing the potential functional significance of the expansion of the LA nucleus in humans (see last section).
3. Amygdala and medial prefrontal cortex anatomical connections in humans and macaques
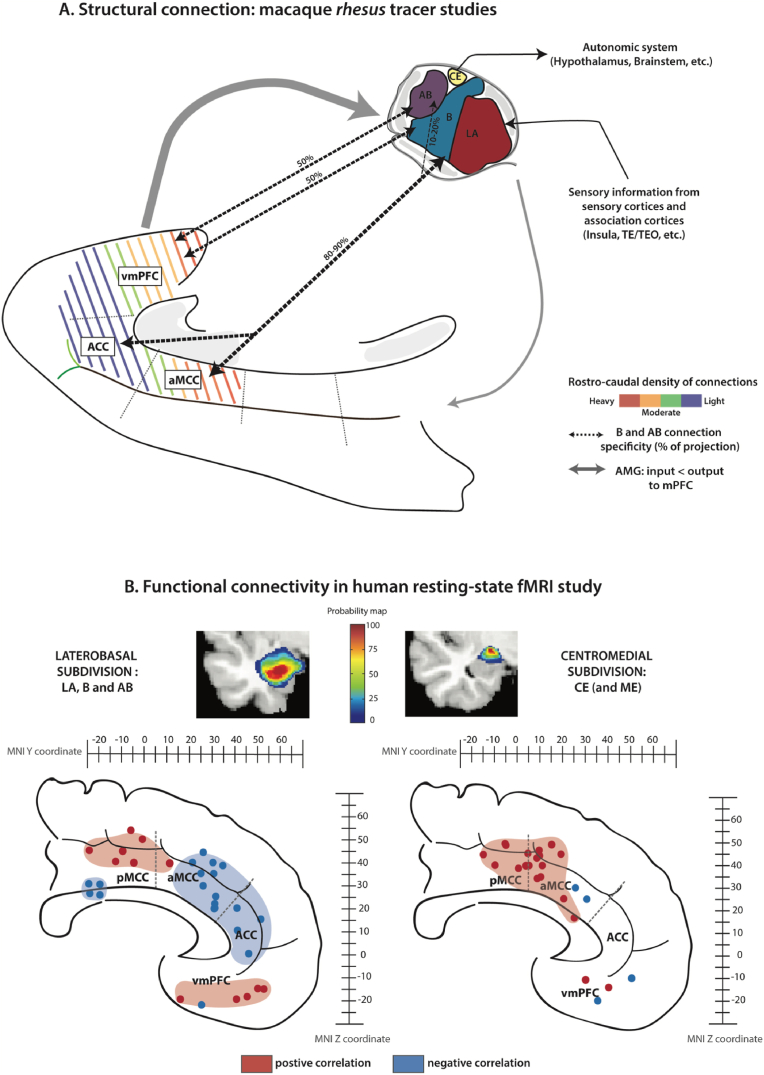
Pioneer lesion studies in macaques showed causal evidences of bidirectional anatomical connections between AMG and mPFC: 1) broad lesions of AB and LA nuclei induce an axonal degeneration in the rostral cingulate cortex, and, 2) reciprocally, lesions in various areas of the cingulate cortex and vmPFC are associated with degenerated cells in B nucleus (Pandya et al., 1973; Nauta, 1993). Tracers’ studies in NHP further refined the topological organization of the anatomical connections between AMG and mPFC. First, they demonstrate that these connections are strictly ipsilateral. Second, AMG efferent fibers preferentially terminate in the deep layer II and I of mPFC regions while mPFC efferences towards AMG arose mainly from layer V (Jacobson and Trojanowski, 1975; Aggleton et al., 1980; Porrino et al., 1981; Amaral and Price, 1984; Vogt and Pandya, 1987; Barbas and de Olmos, 1990; Carmichael and Price, 1995; Stefanacci and Amaral, 2000; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007; Morecraft et al., 2007; Cho et al., 2013; Zikopoulos et al., 2017; Kim et al., 2018; Sharma et al., 2020; Calderazzo et al., 2021; Kelly et al., 2021). In addition, mPFC projections toward the AMG are denser compared to AMG projections toward mPFC, qualifying the mPFC as a “sender” region (Fig. 3A) (Ghashghaei et al., 2007). These connections display a peculiar rostro-caudal organization from low to high density in mPFC regions along the corpus callosum: the connection density is stronger between the most caudal part of vmPFC (area 25), then decreases rostrally in ACC (area 32), and increases with MCC regions (area 24; Fig. 3A) (Ghashghaei et al., 2007; Sharma et al., 2020; Calderazzo et al., 2021).
Fig. 3.
AMG-mPFC dialogue: two different levels of analysis between macaque structural connectivity and human functional connectivity. A. Diagram representing the structural connectivity based on tract-tracing studies in macaques between the AMG nuclei, i.e., LA, B/BL, AB/BM, CE, and the various mPFC regions. AMG and mPFC share strictly ipsilateral and bidirectional connections. The mPFC sends more projections to the AMG than it received (i.e., “senders”, wide gray arrows). The colored dotted lines in the mPFC represent the rostro-caudal gradient of density of structural connections between AMG and mPFC, i.e., high density (red-orange) with vmPFC and aMCC to low density with ACC (green-blue). The dotted arrows in black represent the preference of connectivity between B/BL and MCC/ACC on one hand, and between AB/BM with vmPFC on the other hand. B. Correlation peaks for each selected study between mPFC and the laterobasal (LB, which includes LA, B/BL, and AB/BM nuclei) and centromedial (CM, which includes CE and ME nuclei) AMG subdivisions as defined by Amunts et al. (2005) in humans. We selected studies identifying significant peaks of activation with MNI coordinates and we focused on ipsilateral connectivity. Note that some studies did not directly use Amunts et al. (2005) parcellation, they instead used it as a reference for their own clustering of the AMG. Location (Y, and Z MNI stereotaxic coordinates values) of significant correlation peak values are displayed on a medial sagittal section of the human brain. Negative correlations are represented in blue and positive correlations in red. Results show a differential functional connectivity pattern between the 2 AMG subdivisions. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Finally, with their fine-grained spatial resolution, these latter studies also revealed a gradient with a varying density of connections between AMG nuclei and mPFC regions. Specifically, among the AMG nuclei, LA and CE share few direct connections with mPFC (Barbas and de Olmos, 1990; Zikopoulos et al., 2017; Kim et al., 2018; Kelly et al., 2021) as they are mainly connected to sensory association cortices (inferior temporal areas TE and TEO, Superior Temporal Sulcus, etc.) and autonomic centers (hypothalamus or brainstem), respectively (Aggleton, 2000; Stefanacci and Amaral, 2000). By contrast, the B and AB nuclei present the densest reciprocal anatomical connections with mPFC regions. Quantitative histological studies further revealed a differential pattern of efferent connections from these two AMG nuclei (AB and B) toward mPFC regions. the rostral and dorsal mPFC regions, namely ACC and aMCC, receive more projections from B nucleus compared to AB nucleus with a proportional ratio of 90-80% and 10–20%, respectively. Note that pMCC appears to receive sparser projections from the AMG (Morecraft et al., 2007). By contrast, vmPFC receives a similar proportion of projections from both B and AB nuclei (Barbas and de Olmos, 1990; Morecraft et al., 2007; Kim et al., 2018; Sharma et al., 2020), yet, relative to ACC and MCC, its inputs from nucleus AB are denser (Sharma et al., 2020). A similar topographical organization is also present when considering efferences from mPFC toward those AMG nuclei: whereas both MCC and ACC project heavily to B nucleus compared to AB nucleus (Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007; Morecraft et al., 2007; Cho et al., 2013; Zikopoulos et al., 2017; Kelly et al., 2021), vmPFC sends efferences to both B and AB nuclei (Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007; Cho et al., 2013).
Knowledge about the structural connectivity in humans comes from either post-mortem dissection or from non-invasive diffusion MRI performed in-vivo or ex-vivo (Diffusion tensor imaging -DTI). DTI refers to analytic methods using diffusion weighting imaging (DWI) MRI sequence that provides information about the orientation, the strength and integrity of white fiber tracts at the macroscopic level (Basser and Pierpaoli, 1996). DTI can inform on the orientation and pathway of white fiber tracts and the strength and integrity of fiber tracts (Basser and Pierpaoli, 1996). DTI is an indirect measure with limited spatial resolution and accuracy compared to ex-vivo tracer studies in animal studies (Sarwar et al., 2021). Direct comparisons in macaques between anatomical connectivity as estimated with ex-vivo tracer studies and DTI studies using at 3T show that these connectivity measures display only moderate correspondances (e.g. Grier et al., 2020; Yendiki et al., 2022). This becomes even trickier when considering the intricate structural connectivity pattern of each individual amygdala nucleus with mPFC. However, DTI has the advantage of allowing the comparison of structural connections in both human and non-human primates using the same approach. These studies have shown that the principal white matter tracts connecting AMG with mPFC, i.e., the uncinate fasciculus, the amygdalofugal pathway, and the cingulum, are highly conserved between humans and macaques (Thiebaut de Schotten et al., 2012; Folloni et al., 2019a; Barrett et al., 2020). In addition, Mars et al. (2018) have developed a connectional blueprint based on the main bundles of white matter tracts that can be anatomically matched across species (i.e., the uncinate fasciculus) to identify homologous brain areas (Mars et al., 2018). In humans, DTI has also been used to delineate the AMG in two subdivisions based on their differential structural connections with other brain regions, i.e. the basolateral and the centromedial subdivision (defined by Amunts et al., 2005 in humans, see next section), coherently with macaque ex-vivo tracer studies (Aggleton, 2000; Solano-Castiella et al., 2010; Bzdok et al., 2013; Balderston et al., 2015). Finally, more recent development in the field provides a finer segmentation of the AMG nuclei at different times during adolescence (Azad et al., 2021). While DTI is a promising tool, future developments using for instance ultra-high-resolution MRI and advances in analytical tools will undoubtedly help overcome the current limitations and hopefully provide more finer-grained cross-species comparisons (Sotiropoulos and Zalesky, 2019; Grier et al., 2022).
In summary, although the literature suggests preserved fiber tracts between macaques and humans at the macroscopic level, differential connectivity profiles between the various AMG nuclei and mPFC regions are observed in macaques at the microscopic level. Importantly, whether these latter highly specific patterns do exist in humans remains to be elucidated. In the next section, we tackle the question of the functional dialogue between AMG nuclei and mPFC regions, as measured at rest using functional Magnetic Resonance Imaging (rs-fMRI).
4. Functional connectivity in the AMG-mPFC network in humans and macaques
Rs-fMRI measures the temporal correlation of spontaneous low-frequency fluctuations of the BOLD signals between different brain regions in the absence of any specific task or stimulus (Biswal et al., 1995). It has the great advantage to provide in-vivo information on brain network functional connectivity (FC) as well as the connectivity profile of a particular brain region. While the spatial resolution of fMRI does not permit direct comparisons with the tract-tracing anatomical studies described above, it can provide key information about the functional relationships between anatomically interconnected regions (Greicius et al., 2009).
Numerous studies in humans have described the functional dialogue between AMG and mPFC while considering the whole extent of the AMG or focusing on AMG subdivisions (Amunts et al., 2005). These studies have shown that the fluctuations of activity in the whole AMG is positively correlated with those in more ventral mPFC regions and negatively correlated with those in more dorsal mPFC regions (Roy et al., 2009; Kim et al., 2011a). To identify the respective FC of AMG nuclei with mPFC, we focused on human rs-fMRI studies (see Table S2 and methods in supplementary materials with the associated references) that relied on the most widely used nomenclature in the field: laterobasal subdivision (LB, composed of LA, B, AB nuclei), and centromedial subdivision (CM, composed of CE and ME) (Amunts et al., 2005; Eickhoff et al., 2005). Our intent was to identify the FC organization pattern and the regulatory interactions between AMG nuclei and mPFC focusing on the sign of correlation (positive vs negative) (Gopinath et al., 2015). Note that we only targeted correlations within hemispheres and we did not take into consideration any lateralization effect as this was outside the scope of the present review (see Table S2). The corresponding correlation peaks for each selected study is displayed on sagittal brain diagrams between Y MNI coordinates from −26 to 52 and Z coordinates from −22 to 50 for LB and CM nuclei (blue and red correspond to negative and positive correlations, respectively; Fig. 3B). Not surprisingly, as LB subdivision occupies the major portion of AMG, we confirm that it displays a similar FC pattern with mPFC subregions than when considering the whole AMG (Roy et al., 2009; Kim et al., 2011a): positive correlations with vmPFC, negative correlations with ACC and aMCC, and positive correlations with pMCC. A recent study that used a finer data-driven parcellation of AMG and high-resolution data further suggested that the strongest FC was observed between AB/B nuclei -as compared to LA nucleus-with mPFC regions, with a trend toward a preferential functional coupling between the AB nucleus and vmPFC (area 25) on one hand and between the B nucleus and aMCC on the other hand (Klein-Flügge et al., 2022). By contrast, the activity of CM subdivision appeared to display a very distinct functional connectivity pattern with mPFC: positive correlations with mPFC regions, more specifically with the MCC regions (both aMCC and pMCC). These patterns of connectivity between the various AMG nuclei and mPFC regions point toward the existence of complex relationships that might be dynamically and differentially adjusted depending on the environmental context.
How are the functional relationships organized between AMG and mPFC in macaques at rest? To the best of our knowledge, only a handful of studies have examined this interplay in macaques (Neubert et al., 2015; Grayson et al., 2016; Folloni et al., 2019b; Morin et al., 2020; Reding et al., 2020). Two points need to be raised. Firstly, these studies have been carried out under anaesthesia (e.g., isoflurane). Yet, several studies have demonstrated that anaesthesia strongly affects brain activity, and in particular the functional dialogue within the frontal cortex (Hutchison et al., 2014; Barttfelda et al., 2015; Uhrig et al., 2018; Giacometti et al., 2022). Anaesthesia notably causes a global decrease of negative correlations in the brain (Hutchison et al., 2014; Barttfelda et al., 2015; Uhrig et al., 2018; Hori et al., 2020; Giacometti et al., 2022), thus calling for some caution when directly comparing studies conducted in anaesthetized macaques versus in awake humans using similar parameters. Furthermore, to the best of our knowledge, so far macaques rs-fMRI studies have only examined the FC of the whole extent of AMG. These studies showed 1) positive correlations between the AMG, and the vmPFC, the ACC, and the ACC/aMCC limit, and 2) negative correlation between the AMG and aMCC (Neubert et al., 2015; Folloni et al., 2019b; Reding et al., 2020).
Overall, the current available evidences from anaesthetized macaques suggests that the FC between vmPFC with AMG share similarities with awake humans while the FC between ACC and AMG displays some differences between the two species. Whether these difference are driven by the state (anaesthesia vs awake) or represent mere interspecies differences is further discussed in the last section of the review.
5. Multiple routes of communication within the amygdala-mPFC network in macaques and humans: functional significance and future directions
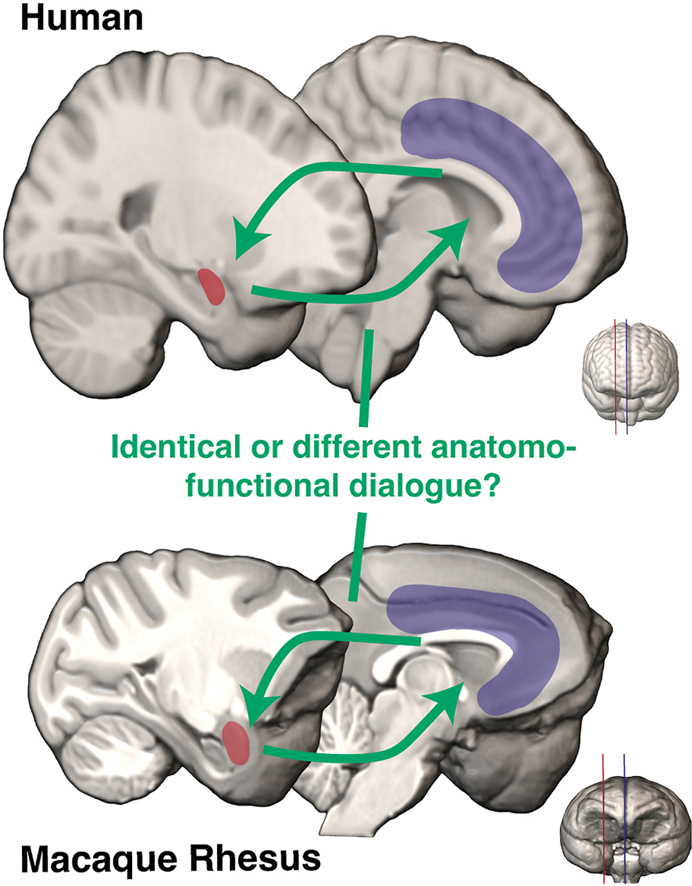
The goal of the review was to scrutinize anatomical and functional relationships within AMG-mPFC circuitries and identify potential homologies and differences between macaques and humans. We highlighted in particular: 1) differences between AB and B nuclei structural connectivity with mPFC regions from tract tracing studies in macaques (Fig. 3A), and 2) differential pattern of functional connectivity between the mPFC regions and the laterobasal (LA, B and AB) or centromedial AMG subdivisions (CE and ME) from rs-fMRI studies in humans (Fig. 3B). These evidences suggest multiple routes of communications between AMG nuclei -or subdivisions- and mPFC. Below we discuss how these different AMG-mPFC routes might enable the integration of information from the internal and external environments to support flexible behavior (Saez et al., 2015). Specifically, we propose that two routes within the AMG-mPFC network support distinct temporal dynamics of integration necessary for behavioral adaptation, the former dealing with long-term contextual adaptation (vmPFC-LB) and the latter dealing with online monitoring of actions (LB-aMCC and CM-aMCC). In addition, we highlight an expansion of the LA nucleus in humans compared to macaques and discuss potential commonalities and differences between both species in AMG-mPFC circuits.
5.1. Two routes with distinct behavioral adaptation temporalities within AMG-mPFC
Among the LB subdivision, B and AB nuclei share strong bidirectional structural connections with mPFC regions, especially with vmPFC and aMCC (Fig. 3A). Of note, the projections from mPFC towards AMG nuclei are denser that their counterparts (Ghashghaei et al., 2007), suggesting a moderating role of mPFC onto the AMG, that is gradually setup from the end of infancy to adolescence (Gee et al. 2013, 2022; Tottenham, 2015). While evidence of the functional relationships between AMG nuclei and mPFC using rs-fMRI remain elusive to date in macaque, evidence from human studies show a differential functional relationship with two particular regions of mPFC in humans: 1) a positive versus negative functional coupling at rest between the LB subdivision and the vmPFC versus the aMCC, respectively, and 2) a positive functional coupling between the CM subdivision and the aMCC (Fig. 3B). Interestingly, vmPFC and MCC sustain different and complementary aspects of flexible decision-making necessary for behavioral adaptation (Quilodran et al., 2008; Grabenhorst and Rolls, 2011; Amiez et al., 2012; Boorman et al., 2013; Scholl et al., 2015; Procyk et al., 2016; Wittmann et al., 2016; Juechems et al., 2019), that might in part be reflected in their differential dialogue with the LB and CM subdivisions.
On one hand, vmPFC is thought to integrate contextual and value information with previous knowledge to update decisions accordingly (Grabenhorst and Rolls, 2011; Boorman et al., 2013; Vassena et al., 2014; Scholl et al., 2015; Wittmann et al., 2016; Schneider and Koenigs, 2017; Juechems et al., 2019; Dal Monte et al. 2020, 2022; Gangopadhyay et al., 2021). This integration depends, at least in part, on information processed within the LB subdivision associated with choices, value and rewards evaluation in both social and non-social contexts coded in abstract conceptual format (Gupta et al., 2011; Wellman et al., 2016; O'Neill et al., 2018; Grabenhorst et al., 2019; Dal Monte et al., 2020; Elorette et al., 2020; Jezzini and Padoa-Schioppa, 2020; Grabenhorst and Schultz, 2021; Dal Monte et al., 2022). For instance, the level of complexity in the social network enhances the strength of connectivity between vmPFC and LB (Bickart et al., 2012). Beyond its interactions with the LB subdivision (B and AB nuclei), vmPFC also received inputs from the entorhinal cortex (EC), a gateway to the hippocampus, mediating long-term contextual and episodic memory functions (Joyce and Barbas, 2018; Calderazzo et al., 2021). Through these connections, the LB-vmPFC circuit might thus be in an ideal position to update decisions based on stored information and support longer term (as opposed to short-term) behavioral adaptation. Previous studies have suggested strong interactions between another region of the PFC (the orbitofrontal cortex) and the LB subdivision in shaping behavioral responses depending on the environment (Saez et al., 2017; Zikopoulos et al., 2017). Here, we further suggest that the circuit formed by the vmPFC and the LB subdivision also participate in these processes and it would be interesting in future studies to disentangle the contribution of these pathways in behavioral adaptation.
On the other hand, MCC (often referred as dACC) strongly interacts with the premotor cortex and the dorso-lateral PFC (Morecraft et al., 2012; Calderazzo et al., 2021) involved in action planning, cognitive control, and feedback-based decision, critical to rapidly adapt behaviors when required by the environment (Amiez et al., 2012; Boorman et al., 2013; Vassena et al., 2014; Procyk et al., 2016; Wittmann et al., 2016; Juechems et al., 2019). The evidences based on rs-fMRI in humans that we presented above suggest two distinct ways by which AMG and MCC might interact: 1) a negative functional coupling between the activity of MCC and the LB subdivision and 2) a positive functional coupling between the activity of MCC and the CM subdivision.
While the LB-MCC route is sustained by massive direct anatomical projections, characterized in particular by dense projections between the B nucleus and the MCC (Sharma et al., 2020), the CM-MCC route presumably reflects indirect interactions. Yet, both are likely to participate in functions that require fast stimulus-response adaptations to face immediate changes in the environment (Bickart et al., 2012; Klavir et al., 2013; Aryeh Hai Taub et al., 2018; Aryeh H. Taub et al., 2018; Terburg et al., 2018; Leitão et al., 2022). For example, during aversive learning, electrophysiological recordings in monkeys have shown that stimulus processing rapidly occurs within LB before reaching the MCC and propagates back to the LB (Klavir et al., 2013; Aryeh Hai Taub et al., 2018; Aryeh H. Taub et al., 2018). In the CM subdivision, the CE nucleus is mainly connected with autonomic centers such as the hypothalamus and brainstem regions (Aggleton, 2000; Cardinal et al., 2002). Lesion study in non-human primates has demonstrated its implication in defensive and physiological responses in anxiety-related and stress-related situations (Kalin, 2004). In humans, studies showed strong coupling between CE and MCC at rest (Bickart et al., 2012; Tillman et al., 2018). The MCC also participates in sympathetic and parasympathetic responses (Amiez and Procyk, 2019). One might thus hypothesize that the MCC, through its functional interactions with the CM, including CE, and more largely the central extended AMG, may shape avoidance-approach responses based on previous negative and/or positive feedback-based experiences that might have been previously integrated by LB-vmPFC circuit. Such regulation likely depends on dynamic and flexible interactions between AMG nuclei involving inhibitory neurons such as the intercalated interneurons masses and top-down regulation from the mPFC (Mcdonald and Augustine, 1993; Zikopoulos et al., 2017).
It is therefore possible that these two routes within the AMG-mPFC network support distinct temporal dynamics of integration, the former dealing with long-term contextual adaptation (vmPFC-LB) and the latter dealing with online monitoring of actions (LB-MCCa anc CM-MCCa). A balance between these different circuits within the AMG-mPFC network would be essential to support decision-making flexibility in various environmental contexts in light to our internal state to promote behavioral adaptation. The AMG, formed by a collection of nuclei, is often viewed as a hub sustaining a high degree of information integration coordinating multiple brain circuits during behavior adaptation (Morrow et al., 2019; Putnam and Gothard, 2019). Accordingly, in humans, a disruption within these AMG-mPFC circuits leads to various mental illnesses characterized by maladaptive behavioral responses, i.e. exaggerated, reduced, or even an absence, and/or an atypical behavior (Blair, 2008; Kim et al., 2011a; Murray et al., 2011; Swartz et al., 2013; Likhtik and Paz, 2015; Nicholson et al., 2015; Mukherjee et al., 2016; Dong et al., 2019; Li et al., 2021; Cao et al., 2022; Gao et al., 2022). In particular, the LA nucleus in humans is a very sensible target to a large number of neuropsychiatric diseases. Several postmortem studies comparing patients with healthy subjects have reported morphological alterations of the LB with a special focus on the LA nucleus (e.g. volume and neurons numbers) in autism spectrum disorder, William syndrome, major depression disorder, panic disorder, schizophrenia, and bipolar disorders (Schumann and Amaral, 2006; Berretta et al., 2007; Bezchlibnyk et al., 2007; Kreczmanski et al., 2007; Schumann et al., 2011; Wegiel et al., 2014; Rubinow et al., 2016; Asami et al., 2018; Avino et al., 2018; Lew et al., 2018). The studies highlighted above deciphering the AMG-mPFC circuitries at the nuclei subdivisions level will undoubtedly help us to better understand these maladaptive behaviors and hopefully help to refine therapeutic strategies to help these patients.
5.2. Towards a comparison of AMG-mPFC networks in macaques and humans
Our objective was to identify potential homologies and differences in AMG-mPFC circuitries between macaques and humans. First, the CE nucleus, part of the CM and central extended AMG together with the bed nucleus of the stria terminalis (BST), is thought to have been conserved throughout the course of evolution, with limited differences between rodents and primates, hence between macaques and humans (Chareyron et al., 2011), as confirmed with the volumetric analysis presented above.
Comparatively, we highlighted in humans a selective expansion of the LA nucleus, part of the LB subdivision, and tightly connected with sensory and higher order association cortices (Stefanacci and Amaral, 2000). This volume expansion has also been associated with an increase of neuron numbers in humans (Barger et al., 2012). It is possible that this expansion allows the integration of a higher amount of multi-sensorial information that humans are facing in their daily life including their highly complex social interactions compared to macaques (see also Barger et al., 2012). Several neuroimaging studies reported an increase in AMG volume related to complex social networks (Dziura and Thompson, 2014) and the size of the social group (Sallet et al., 2011; Kanai et al., 2012). Although these studies refer to the whole AMG, and confirmation needs to be brought by, it is possible that this increase concerns in particular the LA nucleus. A recent study using electrical stimulation in epileptic patients showed that stimulation of the LA nucleus evoked an earlier response in ACC/aMCC compared to that of the B or AB nucleus (Sawada et al., 2022). While in macaques, LA only shares few direct projections with mPFC compared to AB and B, it remains to be determined whether the LA expansion might have led to differences in the organization of anatomical projections between AMG and mPFC in humans.
Regarding the rs-fMRI functional connectivity of AMG nuclei and/or subdivisions with mPFC regions, the current state of the art, especially in light with very limited evidences focusing essentially on the whole AMG functional connectivity under anaesthesia in macaques, does not allow to draw firm conclusions about potential homologies or differences between humans and macaques. Nevertheless, one could relate the FC involving vmPFC and aMCC with the whole AMG in anaesthetized macaques and whole AMG and LB subdivision in awake humans and the similarities between the two species in terms of structural connectivity of the CE nucleus. It is possible thus that the two routes described above are present in both humans and macaques to support distinct temporal dynamics of integration necessary for behavioral adaptation, yet with some differences. Indeed, the FC between the region located between the vmPFC and aMCC, namely the ACC, and the AMG displays some differences between the two species: the activity of this region is mainly negatively correlated with that of the AMG in humans while the available evidence in macaques points toward a positive correlational relationship between these two regions. While these conclusions await further evidences, we propose tentative interpretations that might be related to these functional differences across species. One possibility is that this difference is driven by the state (anaesthesia vs awake). Alternatively, this difference might reflect mere differences between species: although the macaque mPFC possesses all the sulcal precursors of the human mPFC, the region interfacing with vmPFC and MCC (which contains ACC) is the one displaying the most notable sulcal changes across primate species (the fork composed by two sulci situated at the rostral of the cingulate sulcus 1) faces downwards in humans versus forward in macaques and baboons, and 2) is located more dorsal in non-human than in human primates; Amiez et al., 2019). Whether and how these morphological changes would affect the functional relationships between mPFC and AMG nuclei is yet to be determined. With novel tools giving access to the fine-grained parcellation of the AMG in macaque (Hartig et al., 2021), it would be possible for future studies to further refine our knowledge about the AMG nuclei FC profiles with mPFC in awake macaques, therefore providing more direct comparisons with available evidences in humans.
5.3. Limitation and further perspectives
We mainly focused the scope of the review in the adult brain, not taking into account several factors that might also affect the anatomical and functional interplay within AMG-mPFC circuits such as sex, age or personality traits (see for instance Tottenham, 2015; Reber and Tranel, 2017; Kenwood and Kalin, 2021; Ferrara and Opendak, 2023). Another limitation of our hypotheses is that evidences come from different species (humans vs macaques) and from different approaches with different spatial scales: a microscopic scale providing structural connectivity of each AMG nuclei in macaques versus a macroscopic scale in humans using rs-fMRI or DWI. In comparative neurosciences, the gap between invasive -but highly precise-techniques used in non-human primates and non-invasive -but less precise-techniques used in humans is called the “macroscopic–microscopic divide” (Barron et al., 2021). Cross-species neuroimaging comparison approaches have emerged to bridge this gap as they have the advantage to be applicable in both human and non-human primates and in particular in macaques (Barron et al., 2021; Friedrich et al., 2021). With higher field strength at 7T or even 10.5 T in macaques (Thanh Vu et al., 2017; Yacoub et al., 2020), this approach can provide a finer-grained description of brain networks. As such, future works may apply this strategy to consider the respective whole-brain functional coupling of each AMG nuclei within and across species (e.g. Torrisi et al., 2015; Elvira et al., 2022). The implementation of effective connectivity in these studies might also provide important insights about the directionality of the interplay within these networks (Liu et al., 2016; Berboth and Morawetz, 2021). Our further understanding of the temporal and dynamic interactions between the AMG nuclei and the mPFC in primates may also benefit from interventional optogenetic and electrophysiological approaches similar to those carried out in mice (e.g. Kopell et al., 2014; Felix-Ortiz et al., 2016). Some electrophysiological studies, although rare, have already provided key information about similarities and differences of the efficiency and robustness of the functioning of the mPFC-AMG network in humans versus macaques (Pryluk et al., 2019). Combining these different and complementary techniques in future studies will be essential to understand how the different AMG nuclei interact with the mPFC and other brain networks such as the fronto-amygdala-striatal circuitry (Cho et al., 2013) to subserve differential behavioral adaptation capacities in humans and monkeys and shed light on the evolution of this network (Henke-von der Malsburg et al., 2020).
CRediT authorship contribution statement
C. Giacometti: Conceptualization, Investigation, Analyzing, Writing – original draft. C. Amiez: Conceptualization, Writing – review & editing. F. Hadj-Bouziane: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the French National Research Agency (ANR-18-CE37-0012 to C.A, ANR-15-CE37-0003 to F.H.-B.). C.A. and F.H.-B. are employed by the Centre National de la Recherche Scientifique (CNRS). C.A. and C.G. were also supported by Laboratoire d’excellence (LabEx) CORTEX ANR-11-LABX-0042 of Université de Lyon. Note that a CC-BY public copyright license has been applied by the authors to the present document and will be applied to all subsequent versions up to the Author Accepted Manuscript arising from this submission, in accordance with the grant's open access conditions. Conflict of Interest: None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2023.100103.
Contributor Information
C. Giacometti, Email: camille.giacometti@inserm.fr.
C. Amiez, Email: celine.amiez@inserm.fr.
F. Hadj-Bouziane, Email: fadila.hadj-bouziane@inserm.fr.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
No data was used for the research described in the article.
References
- Aggleton J.P. In: The Amygdala: a Functional Analysis. second ed. Aggleton J.P., editor. Oxford University Press; Oxford, OX ; New York: 2000. https://global.oup.com/academic/product/the-amygdala-9780198505013?cc=us&lang=en& [Google Scholar]
- Aggleton J.P., Burton M.J., Passingham R.E. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190(2):347–368. doi: 10.1016/0006-8993(80)90279-6. https://linkinghub.elsevier.com/retrieve/pii/0006899380902796 [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amiez C., Procyk E. vol. 166. 2019. Midcingulate somatomotor and autonomic functions; pp. 53–71.https://linkinghub.elsevier.com/retrieve/pii/B9780444641960000042 (Handbook of Clinical Neurology). [DOI] [PubMed] [Google Scholar]
- Amiez C., Sallet J., Procyk E., Petrides M. Modulation of feedback related activity in the rostral anterior cingulate cortex during trial and error exploration. Neuroimage. 2012;63(3):1078–1090. doi: 10.1016/j.neuroimage.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Amiez C., Sallet J., Hopkins W.D., Meguerditchian A., Hadj-Bouziane F., Ben Hamed S., Wilson C.R.E., Procyk E., Petrides M. Sulcal organization in the medial frontal cortex provides insights into primate brain evolution. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-11347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., Habel U., Schneider F., Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 2005;210(5–6):343–352. doi: 10.1007/s00429-005-0025-5. http://link.springer.com/10.1007/s00429-005-0025-5 [DOI] [PubMed] [Google Scholar]
- Asami T., Nakamura R., Takaishi M., Yoshida H., Yoshimi A., Whitford T.J., Hirayasu Y. Smaller volumes in the lateral and basal nuclei of the amygdala in patients with panic disorder. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207163. https://dx.plos.org/10.1371/journal.pone.0207163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avino T.A., Barger N., Vargas M.V., Carlson E.L., Amaral D.G., Bauman M.D., Schumann C.M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. 2018;115(14):3710–3715. doi: 10.1073/pnas.1801912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A., Cabeen R.P., Sepehrband F., Kim R., Campbell C.E., Lynch K., Tyszka J.M., Herting M.M. Microstructural properties within the amygdala and affiliated white matter tracts across adolescence. Neuroimage. 2021;243 doi: 10.1016/j.neuroimage.2021.118489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston N.L., Schultz D.H., Hopkins L., Helmstetter F.J. Functionally distinct amygdala subregions identified using DTI and high-resolution fMRI. Soc. Cognit. Affect Neurosci. 2015;10(12):1615–1622. doi: 10.1093/scan/nsv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H., de Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J. Comp. Neurol. 1990;300(4):549–571. doi: 10.1002/cne.903000409. https://onlinelibrary.wiley.com/doi/10.1002/cne.903000409 [DOI] [PubMed] [Google Scholar]
- Barger N., Stefanacci L., Semendeferi K. A comparative volumetric analysis of the amygdaloid complex and basolateral division in the human and ape brain. Am. J. Phys. Anthropol. 2007;134(3):392–403. doi: 10.1002/ajpa.20684. [DOI] [PubMed] [Google Scholar]
- Barger N., Stefanacci L., Schumann C.M., Sherwood C.C., Annese J., Allman J.M., Buckwalter J.A., Hof P.R., Semendeferi K. Neuronal populations in the basolateral nuclei of the amygdala are differentially increased in humans compared with apes: a stereological study. J. Comp. Neurol. 2012;520(13):3035–3054. doi: 10.1002/cne.23118. https://onlinelibrary.wiley.com/doi/10.1002/cne.23118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger N., Hanson K.L., Teffer K., Schenker-Ahmed N.M., Semendeferi K. Evidence for evolutionary specialization in human limbic structures. Front. Hum. Neurosci. 2014;8(MAY) doi: 10.3389/fnhum.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett R.L.C., Dawson M., Dyrby T.B., Krug K., Ptito M., D'Arceuil H., Croxson P.L., Johnson P.J., Howells H., Forkel S.J., et al. Differences in frontal network anatomy across primate species. J. Neurosci. 2020;40(10):2094–2107. doi: 10.1523/JNEUROSCI.1650-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron H.C., Mars R.B., Dupret D., Lerch J.P., Sampaio-Baptista C. Cross-species neuroscience: closing the explanatory gap. Phil. Trans. Biol. Sci. 2021;376(1815) doi: 10.1098/rstb.2019.0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfelda P., Uhriga L., Sitta J.D., Sigmane M., Jarrayaa B., Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. U. S. A. 2015;112(3):887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R., Lee G.P. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19(13):5473–5481. doi: 10.1523/jneurosci.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berboth S., Morawetz C. Amygdala-prefrontal connectivity during emotion regulation: a meta-analysis of psychophysiological interactions. Neuropsychologia. 2021;153 doi: 10.1016/j.neuropsychologia.2021.107767. [DOI] [PubMed] [Google Scholar]
- Berretta S., Pantazopoulos H., Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol. Psychiatr. 2007;62(8):884–893. doi: 10.1016/j.biopsych.2007.04.023. https://linkinghub.elsevier.com/retrieve/pii/S0006322307003575 [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk Y.B., Sun X., Wang J.-F., MacQueen G.M., McEwen B.S., Young L.T. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J. Psychiatry Neurosci. 2007;32(3):203–210. http://www.ncbi.nlm.nih.gov/pubmed/17476367 [PMC free article] [PubMed] [Google Scholar]
- Bickart K.C., Hollenbeck M.C., Barrett L.F., Dickerson B.C. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J. Neurosci. 2012;32(42):14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. https://onlinelibrary.wiley.com/doi/10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. Review. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Phil. Trans. Biol. Sci. 2008;363(1503):2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman E.D., Rushworth M.F., Behrens T.E. Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. J. Neurosci. 2013;33(6):2242–2253. doi: 10.1523/JNEUROSCI.3022-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec J., Rulseh A., Hoyt B., Vizek M., Horinek D., Hort J., Petrovicky P. Volumetry of the human amygdala — an anatomical study. Psychiatry Res. Neuroimaging. 2010;182(1):67–72. doi: 10.1016/j.pscychresns.2009.11.005. https://linkinghub.elsevier.com/retrieve/pii/S0925492709002716 [DOI] [PubMed] [Google Scholar]
- Bzdok D., Laird A.R., Zilles K., Fox P.T., Eickhoff S.B. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp. 2013;34(12):3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderazzo S.M., Busch S.E., Moore T.L., Rosene D.L., Medalla M. Distribution and overlap of entorhinal, premotor, and amygdalar connections in the monkey anterior cingulate cortex. J. Comp. Neurol. 2021;529(4):885–904. doi: 10.1002/cne.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Li H., Liu J., Jiang J., Li B., Li X., Zhang S., Gao Y., Liang K., Hu Xinyue, et al. Disorganized functional architecture of amygdala subregional networks in obsessive-compulsive disorder. Commun Biol. 2022;5(1) doi: 10.1038/s42003-022-04115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. https://linkinghub.elsevier.com/retrieve/pii/S0149763402000076 [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363(4):615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chareyron L.J., Banta Lavenex P., Amaral D.G., Lavenex P. Stereological analysis of the rat and monkey amygdala. J. Comp. Neurol. 2011;519(16):3218–3239. doi: 10.1002/cne.22677. https://onlinelibrary.wiley.com/doi/10.1002/cne.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.T., Ernst M., Fudge J.L. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J. Neurosci. 2013;33(35):14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O., Chu C.C.J., Fagan N.A., Chang S.W.C. Specialized medial prefrontal–amygdala coordination in other-regarding decision preference. Nat. Neurosci. 2020;23(4):565–574. doi: 10.1038/s41593-020-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O., Fan S., Fagan N.A., Chu C.-C.J., Zhou M.B., Putnam P.T., Nair A.R., Chang S.W.C. Widespread implementations of interactive social gaze neurons in the primate prefrontal-amygdala networks. Neuron. 2022;110(13):2183–2197.e7. doi: 10.1016/j.neuron.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M., Xia L., Lu M., Li C., Xu K., Zhang L. A failed top-down control from the prefrontal cortex to the amygdala in generalized anxiety disorder: evidence from resting-state fMRI with Granger causality analysis. Neurosci. Lett. 2019;707 doi: 10.1016/j.neulet.2019.134314. https://linkinghub.elsevier.com/retrieve/pii/S0304394019303945 [DOI] [PubMed] [Google Scholar]
- Dziura S.L., Thompson J.C. Social-network complexity in humans is associated with the neural response to social information. Psychol. Sci. 2014;25(11):2095–2101. doi: 10.1177/0956797614549209. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Elorette C., Aguilar B.L., Novak V., Forcelli P.A., Malkova L. Dysregulation of behavioral and autonomic responses to emotional and social stimuli following bidirectional pharmacological manipulation of the basolateral amygdala in macaques. Neuropharmacology. 2020;179 doi: 10.1016/j.neuropharm.2020.108275. https://linkinghub.elsevier.com/retrieve/pii/S0028390820303439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvira U.K.A., Seoane S., Janssen J., Janssen N. Contributions of human amygdala nuclei to resting-state networks. Cristofori I. PLoS One. 2022;17(12) doi: 10.1371/journal.pone.0278962. https://dx.plos.org/10.1371/journal.pone.0278962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz A.C., Burgos-Robles A., Bhagat N.D., Leppla C.A., Tye K.M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N.C., Opendak M. Amygdala circuit transitions supporting developmentally-appropriate social behavior. Neurobiol. Learn. Mem. 2023;201 doi: 10.1016/j.nlm.2023.107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folloni D., Sallet J., Khrapitchev A.A., Sibson N., Verhagen L., Mars R.B. Dichotomous organization of amygdala/temporal-prefrontal bundles in both humans and monkeys. Elife. 2019;8 doi: 10.7554/eLife.47175. https://elifesciences.org/articles/47175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folloni D., Verhagen L., Mars R.B., Fouragnan E., Constans C., Aubry J.F., Rushworth M.F.S., Sallet J. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron. 2019;101(6):1109–1116.e5. doi: 10.1016/j.neuron.2019.01.019. https://linkinghub.elsevier.com/retrieve/pii/S0896627319300467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Shackman A.J. The central extended amygdala in fear and anxiety: closing the gap between mechanistic and neuroimaging research. Neurosci. Lett. 2019;693:58–67. doi: 10.1016/j.neulet.2017.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich P., Forkel S.J., Amiez C., Balsters J.H., Coulon O., Fan L., Goulas A., Hadj-Bouziane F., Hecht E.E., Heuer K., et al. Imaging evolution of the primate brain: the next frontier? Neuroimage. 2021;228 doi: 10.1016/j.neuroimage.2020.117685. https://linkinghub.elsevier.com/retrieve/pii/S1053811920311708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangopadhyay P., Chawla M., Dal Monte O., Chang S.W.C. Prefrontal–amygdala circuits in social decision-making. Nat. Neurosci. 2021;24(1):5–18. doi: 10.1038/s41593-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Sun J., Cheng L., Yang Q., Li J., Hao Z., Zhan L., Shi Y., Li M., Jia X., et al. Altered resting state dynamic functional connectivity of amygdala subregions in patients with autism spectrum disorder: a multi-site fMRI study. J. Affect. Disord. 2022;312:69–77. doi: 10.1016/j.jad.2022.06.011. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Hare T.A., Bookheimer S.Y., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Hanson C., Caglar L.R., Fareri D.S., Gabard-Durnam L.J., Mills-Finnerty C., Goff B., Caldera C.J., Lumian D.S., Flannery J., et al. Experimental evidence for a child-to-adolescent switch in human amygdala-prefrontal cortex communication: a cross-sectional pilot study. Dev. Sci. 2022:1–16. doi: 10.1111/desc.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. https://linkinghub.elsevier.com/retrieve/pii/S0306452202004463 [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. https://linkinghub.elsevier.com/retrieve/pii/S105381190600989X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti C., Dureux A., Autran-Clavagnier D., Wilson C.R.E., Sallet J., Dirheimer M., Procyk E., Hadj-Bouziane F., Amiez C. Frontal cortical functional connectivity is impacted by anaesthesia in macaques. Cerebr. Cortex. 2022;32(18):4050–4067. doi: 10.1093/cercor/bhab465. [DOI] [PubMed] [Google Scholar]
- Gopinath K., Krishnamurthy V., Cabanban R., Crosson B.A. Hubs of anticorrelation in high-resolution resting-state functional connectivity network architecture. Brain Connect. 2015;5(5):267–275. doi: 10.1089/brain.2014.0323. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cognit. Sci. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. https://linkinghub.elsevier.com/retrieve/pii/S1364661310002561 [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Schultz W. Functions of primate amygdala neurons in economic decisions and social decision simulation. Behav. Brain Res. 2021;409 doi: 10.1016/j.bbr.2021.113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Báez-Mendoza R., Genest W., Deco G., Schultz W. Primate amygdala neurons simulate decision processes of social partners. Cell. 2019;177(4):986–998.e15. doi: 10.1016/j.cell.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Bliss-Moreau E., Machado C.J., Bennett J., Shen K., Grant K.A., Fair D.A., Amaral D.G. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 2016;91(2):453–466. doi: 10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebr. Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. https://academic.oup.com/cercor/article-lookup/doi/10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier M.D., Zimmermann J., Heilbronner S.R. Estimating brain connectivity with diffusion-weighted magnetic resonance imaging: promise and peril. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(9):846–854. doi: 10.1016/j.bpsc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier M.D., Yacoub E., Adriany G., Lagore R.L., Harel N., Zhang R.Y., Lenglet C., Uğurbil K., Zimmermann J., Heilbronner S.R. Ultra-high field (10.5T) diffusion-weighted MRI of the macaque brain. Neuroimage. 2022;255 doi: 10.1016/j.neuroimage.2022.119200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Koscik T.R., Bechara A., Tranel D. The amygdala and decision-making. Neuropsychologia. 2011;49(4):760–766. doi: 10.1016/j.neuropsychologia.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig R., Glen D., Jung B., Logothetis N.K., Paxinos G., Garza-Villarreal E.A., Messinger A., Evrard H.C. The subcortical atlas of the rhesus macaque (SARM) for neuroimaging. Neuroimage. 2021;235 doi: 10.1016/j.neuroimage.2021.117996. https://linkinghub.elsevier.com/retrieve/pii/S1053811921002731 [DOI] [PubMed] [Google Scholar]
- Henke-von der Malsburg J., Kappeler P.M., Fichtel C. Linking ecology and cognition: does ecological specialisation predict cognitive test performance? Behav. Ecol. Sociobiol. 2020;74(12):154. http://link.springer.com/10.1007/s00265-020-02923-z [Google Scholar]
- Holley D., Fox A.S. The central extended amygdala guides survival-relevant tradeoffs: implications for understanding common psychiatric disorders. Neurosci. Biobehav. Rev. 2022;142 doi: 10.1016/j.neubiorev.2022.104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y., Schaeffer D.J., Yoshida A., Cléry J.C., Hayrynen L.K., Gati J.S., Menon R.S., Everling S. 2020. Cortico-Subcortical Functional Connectivity Profiles of Resting-State Networks in Marmosets and Humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Hutchison M., Manning K.Y., Menon R.S., Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain's functional architecture. Hum. Brain Mapp. 2014;35(12):5754–5775. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Trojanowski J.Q. Amygdaloid projections to prefrontal granular cortex in rhesus monkey demonstrated with horseradish peroxidase. Brain Res. 1975;100(1):132–139. doi: 10.1016/0006-8993(75)90248-6. https://linkinghub.elsevier.com/retrieve/pii/0006899375902486 [DOI] [PubMed] [Google Scholar]
- Jezzini A., Padoa-Schioppa C. Neuronal activity in the primate amygdala during economic choice. J. Neurosci. 2020;40(6):1286–1301. doi: 10.1523/JNEUROSCI.0961-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., Van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M.K.P., Barbas H. Cortical connections position primate area 25 as a keystone for interoception, emotion, and memory. J. Neurosci. 2018;38(7):1677–1698. doi: 10.1523/JNEUROSCI.2363-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juechems K., Balaguer J., Herce Castañón S., Ruz M., O'Reilly J.X., Summerfield C. A network for computing value equilibrium in the human medial prefrontal cortex. Neuron. 2019;101(5):977–987.e3. doi: 10.1016/j.neuron.2018.12.029. [DOI] [PubMed] [Google Scholar]
- Kalin N.H. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.0292-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Bahrami B., Roylance R., Rees G. Online social network size is reflected in human brain structure. Proc. Biol. Sci. 2012;279(1732):1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedo O., Zilles K., Palomero-Gallagher N., Schleicher A., Mohlberg H., Bludau S., Amunts K. Receptor-driven, multimodal mapping of the human amygdala. Brain Struct. Funct. 2018;223(4):1637–1666. doi: 10.1007/s00429-017-1577-x. [DOI] [PubMed] [Google Scholar]
- Kelly E.A., Thomas V.K., Indraghanty A., Fudge J.L. Perigenual and subgenual anterior cingulate afferents converge on common pyramidal cells in amygdala subregions of the macaque. J. Neurosci. 2021;41(47):9742–9755. doi: 10.1523/jneurosci.1056-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenwood M.M., Kalin N.H. Nonhuman primate models to explore mechanisms underlying early-life temperamental anxiety. Biol. Psychiatr. 2021;89(7):659–671. doi: 10.1016/j.biopsych.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Gee D.G., Loucks R.A., Davis F.C., Whalen P.J. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebr. Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. https://academic.oup.com/cercor/article-lookup/doi/10.1093/cercor/bhq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J, Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Sakata H., Nejime M., Konoike N., Miyachi S., Nakamura K. Afferent connections of the dorsal, perigenual, and subgenual anterior cingulate cortices of the monkey: amygdalar inputs and intrinsic connections. Neurosci. Lett. 2018;681:93–99. doi: 10.1016/j.neulet.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Klavir O., Genud-Gabai R., Paz R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron. 2013;80(5):1290–1300. doi: 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Klein-Flügge M.C., Jensen D.E.A., Takagi Y., Priestley L., Verhagen L., Smith S.M., Rushworth M.F.S. Relationship between nuclei-specific amygdala connectivity and mental health dimensions in humans. Nat. Human Behav. 2022;6:1705–1722. doi: 10.1038/s41562-022-01434-3. https://www.nature.com/articles/s41562-022-01434-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N.J., Gritton H.J., Whittington M.A., Kramer M.A. Beyond the connectome: the dynome. Neuron. 2014;83(6):1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczmanski P., Heinsen H., Mantua V., Woltersdorf F., Masson T., Ulfig N., Schmidt-Kastner R., Korr H., Steinbusch H.W.M., Hof P.R., et al. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130(3):678–692. doi: 10.1093/brain/awl386. https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/awl386 [DOI] [PubMed] [Google Scholar]
- Leitão J., Burckhardt M., Vuilleumier P. Amygdala in action: functional connectivity during approach and avoidance behaviors. J. Cognit. Neurosci. 2022;34(5):729–747. doi: 10.1162/jocn_a_01800. [DOI] [PubMed] [Google Scholar]
- Lew C.H., Groeniger K.M., Bellugi U., Stefanacci L., Schumann C.M., Semendeferi K. A postmortem stereological study of the amygdala in Williams syndrome. Brain Struct. Funct. 2018;223(4):1897–1907. doi: 10.1007/s00429-017-1592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., He C., Jian T., Guo X., Xiao J., Li Y., Chen Heng, Kang X., Chen Huafu, Duan X. Attenuated link between the medial prefrontal cortex and the amygdala in children with autism spectrum disorder: evidence from effective connectivity within the “social brain.”. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;111 doi: 10.1016/j.pnpbp.2020.110147. [DOI] [PubMed] [Google Scholar]
- Likhtik E., Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 2015;38(3):158–166. doi: 10.1016/j.tins.2014.12.007. https://linkinghub.elsevier.com/retrieve/pii/S0166223614002355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Hadj-Bouziane F., Moran R., Ungerleider L.G., Ishai A. Facial expressions evoke differential neural coupling in macaques. Cerebr. Cortex. 2016:bhv345. doi: 10.1093/cercor/bhv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Persem A., Verhagen L., Amiez C., Petrides M., Sallet J. The human ventromedial prefrontal cortex: sulcal morphology and its influence on functional organization. J. Neurosci. 2019;39(19):3627–3639. doi: 10.1523/JNEUROSCI.2060-18.2019. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.2060-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L., Gaffan D., Murray E.A. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J. Neurosci. 1997;17(15):6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.17-15-06011.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R.B., Sotiropoulos S.N., Passingham R.E., Sallet J., Verhagen L., Khrapitchev A.A., Sibson N., Jbabdi S. Whole brain comparative anatomy using connectivity blueprints. Elife. 2018;7 doi: 10.7554/eLife.35237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald A.J., Augustine J.R. 1993. LOCALIZATION OF GABA-LIKE IMMUNOREACTIVITY IN THE MONKEY AMYGDALA. [DOI] [PubMed] [Google Scholar]
- Morecraft R.J., Mcneal D.W., Stilwell-Morecraft K.S., Gedney M., Ge J., Schroeder C.M., Van Hoesen G.W. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J. Comp. Neurol. 2007;500(1):134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morecraft R.J., Stilwell-Morecraft K.S., Cipolloni P.B., Ge J., McNeal D.W., Pandya D.N. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res. Bull. 2012;87(4–5):457–497. doi: 10.1016/j.brainresbull.2011.12.005. https://linkinghub.elsevier.com/retrieve/pii/S0361923011003650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin E.L., Howell B.R., Feczko E., Earl E., Pincus M., Reding K., Kovacs-Balint Z.A., Meyer J.S., Styner M., Fair D., et al. Developmental outcomes of early adverse care on amygdala functional connectivity in nonhuman primates. Dev. Psychopathol. 2020;32(5):1579–1596. doi: 10.1017/S0954579420001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J., Mosher C., Gothard K. Multisensory neurons in the primate amygdala. J. Neurosci. 2019;39(19):3663–3675. doi: 10.1523/JNEUROSCI.2903-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P., Sabharwal A., Kotov R., Szekely A., Parsey R., Barch D.M., Mohanty A. Disconnection between amygdala and medial prefrontal cortex in psychotic disorders. Schizophr. Bull. 2016;42(4):1056–1067. doi: 10.1093/schbul/sbw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A., Fellows L.K. Prefrontal cortex interactions with the amygdala in primates. Neuropsychopharmacology. 2021;(July):1–17. doi: 10.1038/s41386-021-01128-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A., Wise S.P., Drevets W.C. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol. Psychiatr. 2011;69(12):e43–e54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta W.J.H. Fibre degeneration following lesions of the amygdaloid complex in the monkey. Neuroanatomy. 1993:202–219. doi: 10.1007/978-1-4684-7920-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F.-X., Mars R.B., Sallet J., Rushworth M.F.S. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. USA. 2015;112(20):E2695–E2704. doi: 10.1073/pnas.1410767112. http://www.pnas.org/lookup/doi/10.1073/pnas.1410767112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W., McKinnon M.C., Lanius R.A. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology. 2015;40(10):2317–2326. doi: 10.1038/npp.2015.79. http://www.nature.com/articles/npp201579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P.-K., Gore F., Salzman C.D. Basolateral amygdala circuitry in positive and negative valence. Curr. Opin. Neurobiol. 2018;49:175–183. doi: 10.1016/j.conb.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya D.N., Van Hoesen G.W., Domesick V.B. A cingulo-amygdaloid projection in the rhesus monkey. Brain Res. 1973;61:369–373. doi: 10.1016/0006-8993(73)90540-4. https://linkinghub.elsevier.com/retrieve/pii/0006899373905404 [DOI] [PubMed] [Google Scholar]
- Paul S., Beucke J.C., Kaufmann C., Mersov A., Heinzel S., Kathmann N., Simon D. Amygdala-prefrontal connectivity during appraisal of symptom-related stimuli in obsessive-compulsive disorder. Psychol. Med. 2019;49(2):278–286. doi: 10.1017/S003329171800079X. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M., Tomaiuolo F., Yeterian E.H., Pandya D.N. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48(1):46–57. doi: 10.1016/j.cortex.2011.07.002. https://linkinghub.elsevier.com/retrieve/pii/S0010945211002279 [DOI] [PubMed] [Google Scholar]
- Porrino L.J., Crane A.M., Goldman-Rakic P.S. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J. Comp. Neurol. 1981;198(1):121–136. doi: 10.1002/cne.901980111. https://onlinelibrary.wiley.com/doi/10.1002/cne.901980111 [DOI] [PubMed] [Google Scholar]
- Pozzi L., Hodgson J.A., Burrell A.S., Sterner K.N., Raaum R.L., Disotell T.R. Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes. Mol. Phylogenet. Evol. 2014;75(1):165–183. doi: 10.1016/j.ympev.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. http://www.nature.com/articles/npp2009104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyk E., Wilson C.R.E., Stoll F.M., Faraut M.C.M., Petrides M., Amiez C. Midcingulate motor map and feedback detection: converging data from humans and monkeys. Cerebr. Cortex. 2016;26(2):467–476. doi: 10.1093/cercor/bhu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryluk R., Kfir Y., Gelbard-Sagiv H., Fried I., Paz R. A tradeoff in the neural code across regions and species. Cell. 2019;176(3):597–609.e18. doi: 10.1016/j.cell.2018.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam P.T., Gothard K.M. Multidimensional neural selectivity in the primate amygdala. eNeuro. 2019;6(5):1–13. doi: 10.1523/ENEURO.0153-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilodran R., Rothé M., Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57(2):314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Reber J., Tranel D. Sex differences in the functional lateralization of emotion and decision making in the human brain. J. Neurosci. Res. 2017;95(1–2):270–278. doi: 10.1002/jnr.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reding K.M., Grayson D.S., Miranda-Dominguez O., Ray S., Wilson M.E., Toufexis D., Fair D.A., Sanchez M.M. Effects of social subordination and oestradiol on resting-state amygdala functional connectivity in adult female rhesus monkeys. J. Neuroendocrinol. 2020;32(2) doi: 10.1111/jne.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. https://linkinghub.elsevier.com/retrieve/pii/S1053811908012214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S., Martina M., Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J. Neurosci. 1999;19(23):10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.19-23-10575.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow M.J., Mahajan G., May W., Overholser J.C., Jurjus G.J., Dieter L., Herbst N., Steffens D.C., Miguel-Hidalgo J.J., Rajkowska G., et al. Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct. Funct. 2016;221(1):171–184. doi: 10.1007/s00429-014-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Mitz A.R., Chacko R.V., Murray E.A. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron. 2013;80(6):1519–1531. doi: 10.1016/j.neuron.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A., Rigotti M., Ostojic S., Fusi S., Salzman C.D. Abstract context representations in primate amygdala and prefrontal cortex. Neuron. 2015;87(4):869–881. doi: 10.1016/j.neuron.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez R.A., Saez A., Paton J.J., Lau B., Salzman C.D. Distinct roles for the amygdala and orbitofrontal cortex in representing the relative amount of expected reward. Neuron. 2017;95(1):70–77.e3. doi: 10.1016/j.neuron.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J., Mars R.B., Noonan M.P., Andersson J.L., O'Reilly J.X., Jbabdi S., Croxson P.L., Jenkinson M., Miller K.L., Rushworth M.F.S. Social network size affects neural circuits in macaques. Science. 2011;334(6056):697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Sarwar T., Ramamohanarao K., Zalesky A. A critical review of connectome validation studies. NMR Biomed. 2021;34(12) doi: 10.1002/nbm.4605. [DOI] [PubMed] [Google Scholar]
- Sawada M., Adolphs R., Dlouhy B.J., Jenison R.L., Rhone A.E., Kovach C.K., Greenlee J.D.W., Howard M.A., III, Oya H. Mapping effective connectivity of human amygdala subdivisions with intracranial stimulation. Nat. Commun. 2022;13(1) doi: 10.1038/s41467-022-32644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B., Koenigs M. Human lesion studies of ventromedial prefrontal cortex. Neuropsychologia. 2017;107(July):84–93. doi: 10.1016/j.neuropsychologia.2017.09.035. [DOI] [PubMed] [Google Scholar]
- Scholl J., Kolling N., Nelissen N., Wittmann M.K., Harmer C.J., Rushworth M.F.S. The good, the bad, and the irrelevant: neural mechanisms of learning real and hypothetical rewards and effort. J. Neurosci. 2015;35(32):11233–11251. doi: 10.1523/JNEUROSCI.0396-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann C.M., Amaral D.G. Stereological analysis of amygdala neuron number in autism. J. Neurosci. 2006;26(29):7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.1285-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann C.M., Bauman M.D., Amaral D.G. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49(4):745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. https://linkinghub.elsevier.com/retrieve/pii/S0028393210004185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K., Damasio H., Frank R., Van Hoesen G.W. Academic Press; 1997. The Evolution of the Frontal Lobes: a Volumetric Analysis Based on Three-Dimensional Reconstructions of Magnetic Resonance Scans of Human and Ape Brains. [DOI] [PubMed] [Google Scholar]
- Semendeferi K., Lu A., Schenker N., Damasio H. Humans and great apes share a large frontal cortex. Nat. Neurosci. 2002;5(3):272–276. doi: 10.1038/nn814. http://www.nature.com/articles/nn814 [DOI] [PubMed] [Google Scholar]
- Sharma K.K., Kelly E.A., Pfeifer C.W., Fudge J.L. Translating fear circuitry: amygdala projections to subgenual and perigenual anterior cingulate in the macaque. Cerebr. Cortex. 2020;30(2):550–562. doi: 10.1093/cercor/bhz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaers J.B., Steele J., Case C.R., Cowper A., Amunts K., Zilles K. Primate prefrontal cortex evolution: human brains are the extreme of a lateralized ape trend. Brain Behav. Evol. 2011;77(2):67–78. doi: 10.1159/000323671. https://www.karger.com/Article/FullText/323671 [DOI] [PubMed] [Google Scholar]
- Smaers J.B., Gómez-Robles A., Parks A.N., Sherwood C.C. Exceptional evolutionary expansion of prefrontal cortex in great apes and humans. Curr. Biol. 2017;27(5):714–720. doi: 10.1016/j.cub.2017.01.020. [DOI] [PubMed] [Google Scholar]
- Solano-Castiella E., Anwander A., Lohmann G., Weiss M., Docherty C., Geyer S., Reimer E., Friederici A.D., Turner R. Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage. 2010;49(4):2958–2965. doi: 10.1016/j.neuroimage.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos S.N., Zalesky A. Building connectomes using diffusion MRI: why, how and but. NMR Biomed. 2019;32(4) doi: 10.1002/nbm.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L., Amaral D.G. 2000. Topographic Organization of Cortical Inputs to the Lateral Nucleus of the Macaque Monkey Amygdala: A Retrograde Tracing Study. [DOI] [PubMed] [Google Scholar]
- Stephan H., Frahm H.D., Baron G. Comparison of brain structure volumes in Insectivora and primates. VII. Amygdaloid components. J. Hirnforsch. 1987;28(5):571–584. http://www.ncbi.nlm.nih.gov/pubmed/3693895 [PubMed] [Google Scholar]
- Swartz J.R., Wiggins J.L., Carrasco M., Lord C., Monk C.S. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C.A., Costa V.D., Basile B.M., Pujara M.S., Jones B., Manem N., Murray E.A., Averbeck B.B. Effects of amygdala lesions on object-based versus action-based learning in macaques. Cerebr. Cortex. 2021;31(1):529–546. doi: 10.1093/cercor/bhaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub Aryeh Hai, Perets R., Kahana E., Paz R. Oscillations synchronize amygdala-to-prefrontal primate circuits during aversive learning. Neuron. 2018;97(2):291–298.e3. doi: 10.1016/j.neuron.2017.11.042. [DOI] [PubMed] [Google Scholar]
- Taub Aryeh H., Shohat Y., Paz R. Long time-scales in primate amygdala neurons support aversive learning. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-07020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D., Scheggia D., Triana del Rio R., Klumpers F., Ciobanu A.C., Morgan B., Montoya E.R., Bos P.A., Giobellina G., van den Burg E.H., et al. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell. 2018;175(3):723–735.e16. doi: 10.1016/j.cell.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh Vu A., Jamison K., Glasser M.F., Smith S.M., Coalson T., Moeller S., Auerbach E.J., Uğurbil K., Yacoub E. Tradeoffs in pushing the spatial resolution of fMRI for the 7T Human Connectome Project. Neuroimage. 2017;154:23–32. doi: 10.1016/j.neuroimage.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell'Acqua F., Valabregue R., Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Tillman R.M., Stockbridge M.D., Nacewicz B.M., Torrisi S., Fox A.S., Smith J.F., Shackman A.J. Intrinsic functional connectivity of the central extended amygdala. Hum. Brain Mapp. 2018;39(3):1291–1312. doi: 10.1002/hbm.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S., O'Connell K., Davis A., Reynolds R., Balderston N., Fudge J.L., Grillon C., Ernst M. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Hum. Brain Mapp. 2015;36(10):4076–4088. doi: 10.1002/hbm.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Social scaffolding of human amygdala-mPFCcircuit development. Soc. Neurosci. 2015;10(5):489–499. doi: 10.1080/17470919.2015.1087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig L., Sitt J.D., Jacob A., Tasserie J., Barttfeld P., Dupont M., Dehaene S., Jarraya B. Resting-state dynamics as a cortical signature of anesthesia in monkeys. Anesthesiology. 2018;129(5):942–958. doi: 10.1097/ALN.0000000000002336. [DOI] [PubMed] [Google Scholar]
- Vassena E., Krebs R.M., Silvetti M., Fias W., Verguts T. Dissociating contributions of ACC and vmPFC in reward prediction, outcome, and choice. Neuropsychologia. 2014;59:112–123. doi: 10.1016/j.neuropsychologia.2014.04.019. https://linkinghub.elsevier.com/retrieve/pii/S0028393214001419 [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Midcingulate cortex: structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 2016;74:28–46. doi: 10.1016/j.jchemneu.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Pandya D.N. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Wegiel J., Flory M., Kuchna I., Nowicki K., Ma S.Y., Imaki H., Wegiel J., Cohen I.L., London E., Wisniewski T., Brown W. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol Commun. 2014;2(1):141. doi: 10.1186/s40478-014-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman L.L., Forcelli P.A., Aguilar B.L., Malkova L. Bidirectional control of social behavior by activity within basolateral and central amygdala of primates. J. Neurosci. 2016;36(33):8746–8756. doi: 10.1523/JNEUROSCI.0333-16.2016. https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.0333-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M.K., Kolling N., Akaishi R., Chau B.K.H., Brown J.W., Nelissen N., Rushworth M.F.S. Predictive decision making driven by multiple time-linked reward representations in the anterior cingulate cortex. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E., Grier M.D., Auerbach E.J., Lagore R.L., Harel N., Adriany G., Zilverstand A., Hayden B.Y., Heilbronner S.R., Uğurbil K., et al. Ultra-high field (10.5 T) resting state fMRI in the macaque. Neuroimage. 2020;223 doi: 10.1016/j.neuroimage.2020.117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A., Aggarwal M., Axer M., Howard A.F.D., van Cappellen van Walsum A.-M., Haber S.N. Post mortem mapping of connectional anatomy for the validation of diffusion MRI. Neuroimage. 2022;256 doi: 10.1016/j.neuroimage.2022.119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B., Höistad M., John Y., Barbas H. Posterior orbitofrontal and anterior cingulate pathways to the amygdala target inhibitory and excitatory systems with opposite functions. J. Neurosci. 2017;37(20):5051–5064. doi: 10.1523/JNEUROSCI.3940-16.2017. http://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.3940-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.