Abstract
Post stroke depression (PSD) is a common neuropsychiatric complication following stroke closely associated with the immune system. The development of medications for PSD remains to be a considerable challenge due to the unclear mechanism of PSD. Multiple researches agree that the functions of gene ontology (GO) are efficient for the investigation of disease mechanisms, and DeepPurpose (DP) is extremely valuable for the mining of new drugs. However, GO terms and DP have not yet been applied to explore the pathogenesis and drug treatment of PSD. This study aimed to interpret the mechanism of PSD and discover important drug candidates targeting risk proteins, based on immune-related risk GO functions and informatics algorithms. According to the risk genes of PSD, we identified 335 immune-related risk GO functions and 37 compounds. Based on the construction of the GO function network, we found that STAT protein may be a pivot protein in underlying the mechanism of PSD. Additionally, we also established networks of Protein-Protein Interaction as well as Gene-GO function to facilitate the evaluation of key genes. Based on DP, a total of 37 candidate compounds targeting 7 key proteins were identified with a potential for the therapy of PSD. Furthermore, we noted that the mechanisms by which luteolin and triptolide acting on STAT-related GO function might involve three crucial pathways, including specifically hsa04010 (MAPK signaling pathway), hsa04151 (PI3K-Akt signaling pathway) and hsa04060 (Cytokine-cytokine receptor interaction). Thus, this study provided fresh and powerful information for the mechanism and therapeutic strategies of PSD.
Keywords: Post-stroke depression (PSD), Text mining, Network, DeepPurpose, GO function, Compound
1. Introduction
Post-stroke depression (PSD) is the most common psychiatric complication in patients survived a stroke [1], which is the second leading cause of death worldwide [2]. It results in severe disability, higher mortality, and lower ability for rehabilitation therapies [3]. With the improvement of diagnostic technology and the general extension of human lifespan, the incidence of PSD has gradually increased in recent years [4]. In clinical practice, drug therapy is still the vital method for PSD. However, current treatments have presented with a limited efficacy with certain side effects [5].
Networks, such as the Protein-Protein Interaction Network (PPIN) and the gene ontology Functional Network (GOFN), have been widely used in various fields of researches [6,7]. Gene ontology (GO) describes the functional knowledge of genes in biological systems through ontology [8]. Researchers concluded that the gene ontology could greatly promote the investigation of the cardiac conduction system [9]. GO usually analyzes the interpretation of genes in the following three parts: cellular component (CC), biological process (BP), and molecular function (MF). GO-BP has been extensively analyzed on a wide range of projects, returning 2,460,741 results as of February 2023 with the PubMed searching for ‘Biological process'. These projects have built models that reveal the multilevel regulatory mechanisms of biological processes in cells [10], suggesting that GO-BP has a potential in explaining the onset and progression of the diseases to some extent. Moreover, the disease could be caused by abnormal expression of genes that disturb the BP involved. Compounds could bind to target proteins to weaken these abnormalities, creating new possibilities for the treatment of diseases.
Given the huge number of drug-like molecules, to discover new drugs in traditional forms is not only time-consuming but also expensive. With the emergence of various computational methods, there have been great potential opportunities to integrate existing resources to explore therapeutic drugs [11]. Drug discovery involves the extraction of compounds that act specifically and closely on target proteins, for which deep learning (DL) is one of such powerful artificial intelligence (AI) tools [12]. DL has built prediction models of protein-protein interaction (PPI), compound property, and drug-target interaction et al. DeepPurpose (DP), one of the deep learning algorithms, has been used in numerous studies in the field of drug research and development [[13], [14], [15]]. A study used this algorithm to unearth 11 drugs targeting ERBB2 that have potential in treating drug-resistant melanoma [11]. Other researchers have used the same bioinformatic approach to identify 14 potential drugs for keloids and hypertrophic scars [14]. However, PSD has not been fully explored in this area currently.
Our study recognized GO functions and compounds based on immune-related risk genes of PSD. Furthermore, we constructed networks of PPI, GO terms, and gene-GO function, identifying nine key genes and important immune-related GO functions for PSD. Pleasantly surprised, signal transducer and activator of transcription (STAT) protein might be a pivot protein that mediated multiple pathways to induce PSD. We discovered some new PSD candidate compounds by calculating affinity scores (AS) between compounds and key proteins. Subsequently, we identified that the GO function might interact with three risk-related pathways regulated by two important potential compounds through key proteins. We proposed for the first time that the STAT protein is related to the mechanisms of PSD. It is also the first time that we performed DP analysis to explore the potential PSD drugs, possibly providing references for the mechanism and treatments of PSD.
2. Materials and methods
2.1. Text mining
PubMed data for risk genes of PSD, published before September 1st, 2022, were manually mined in PubMed (https://pubmed.ncbi.nlm.nih.gov/). The search terms were “((“Depression” [Mesh]) and (“Stroke” [Mesh]) OR “post stroke depression” OR “post-stroke depression”), and we filtered the results for “English (language)". The criteria for collecting eligible genes: The RNA and protein expression levels of the risk genes vary significantly (P-value <0.05) in no less than 5 PSD patients, verified by PCR, ELISA, or other reliable experimental methods.
Genecards (www.genecards.com) [16] is a comprehensive database that publicly provides human genetic information. OMIM (https://www.omim.org/) [17] is a database that focuses on the relationship between disease phenotypes and their pathogenic genes. Besides, the additional databases were also used to obtain PSD data. The ImmPort Portal database [18] shared resources related to immunology, which were used to annotate immune genes.
2.2. Enrichment analysis
Databases of Gene ontology (GO, www.geneontology.org), Kyoto Encyclopedia of Genes and Genomes (KEGG, www.genome.ad.jp/kegg/) and Disgenet (http://www.disgenet.org/) were applied for annotation with the clusterProfiler package [19] in R software, based on PSD risk genes. The adjusted P value of <0.05 was considered to be statistically significant. Further, we used R package “ggplot” [20] to visualized the annotated results. GO functions containing at least five PSD immune-related risk genes were extracted for the construction of the GO function network (GOFN).
2.3. Protein-protein interaction (PPI) network
We acquired PPI information from the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org/) and generated the network with Cytoscape software, which was a software platform for the visualization of complex networks and the mining of data attributes. We uploaded risk genes of PSD to the STRING platform and took the “Homo sapiens” as the organism. We used the MCODE app as well as Cytohubba plug-in [21] to calculate the relevant parameters of the network. In our study, “Betweenness”, “Degree”, and “Maximal Clique Centrality (MCC)" were taken as important parameters for the identification of key nodes.
2.4. Cumulative hypergeometric distribution
The cumulative hypergeometric test was performed to measure the correlations in the GO function network (GOFN) and the gene-GO function network (GGOFN). The risk genes of PSD in the network were immune genes, among which at least five were related with the GO function in the network. The p value was computed with the following formula:
When evaluating the correlation of pairs between random GO functions, we assumed that the entire human genome had M genes; two GO functions had N genes and K genes, respectively; x represented the number of genes shared between functions.
Similarly, to identify the correlations of pairs between GO functions and risk genes, we considered that human had M PSD-risk genes; the given GO function had K genes; the given gene had N interacting genes (the confidence score was seted as 0.9); and x genes existed in both the GO function and the interaction genes.
2.5. Drug-gene interaction and compound-gene interaction
The TCMSP database [22] (http://tcmspw.com/tcmsp.php), a pharmacological platform that provides the relationship between Chinese herbal medicine, target, and disease, was used to obtain the same compound components in herbs for the treatment of stroke and depression. The criteria for compound screening were as follows: (1) oral bioavailability (OB) ≥ 30%, (2) drug similarity (DL) ≥ 0.18, (3) half-life (HL) ≥4 [23,24]. Ultimately, we extracted compounds that potentially target hub proteins.
The Bioinformatics Analysis Tool for Molecular mechANism of TCM (BATMAN-TCM) database [25] is the first online platform designed for the study of molecular mechanisms of traditional Chinese medicine. We inquired about all compounds targeting risk genes of PSD from it and extracted compounds with validated or predictive score greater than 100 (targeting hub genes) and interaction scores greater than 30 with at least three risk proteins.
We filtered potential drugs targeting risk genes of PSD by Interaction Score (IC) and Query Score (QC) from the DGIdb database [26] (http://dgidb.genome.wustl.edu/), providing information about the relationship between genes and their known or potential drugs. According to IC and QC, we extracted the top ten as the potential targeted drugs separately. In addition, we also determined the druggability of genes using DGIdb.
2.6. DP
We assessed the affinity between key proteins and their potential compounds using DP [13]. Based on the protein-compound interaction of the DAVIS, BindingDB, or KIBA databases, we selected 14 models for affinity calculation, consisting of a combination of five compound codes (CNN, MPNN, Morgan, Daylight, and Transformer) and two protein coding models (CNN and AAC). We obtained amino acid sequences of the selected targets from Uniprot [27] (http://www.uniprot.org/), which was a world-leading database of protein sequence and function information. The SMILES of each compound were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), while drugs without SMILES structures were removed. Subsequently, we summarized the AS based on the amino acid sequence and the SMILES structure that inputted into the pretrained models. Finally, the potential compounds were filtered based on AS≥12.1 by KIBA dataset and AS≥7.0 by BindingDB or DAVIS datasets [11,14].
3. Result
3.1. Identification of risk genes of human PSD
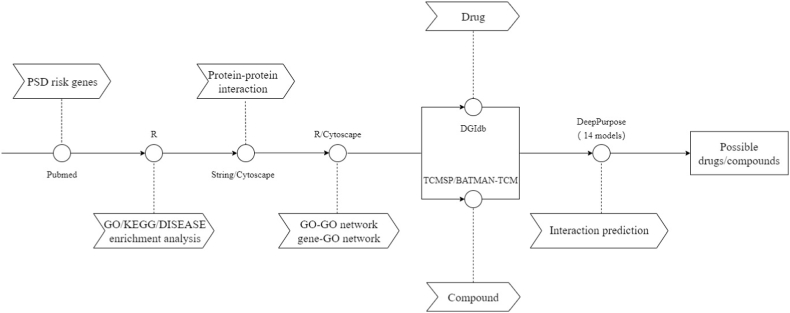
A catalog of 71 PSD risk genes corresponding to 69 proteins was exported by text mining(Table S1). Nearly half of the risk genes were immune genes (35/71). The general operation procedure in this article is shown in Fig. 1.
Fig. 1.
Flow chart for PSD bioinformatics.
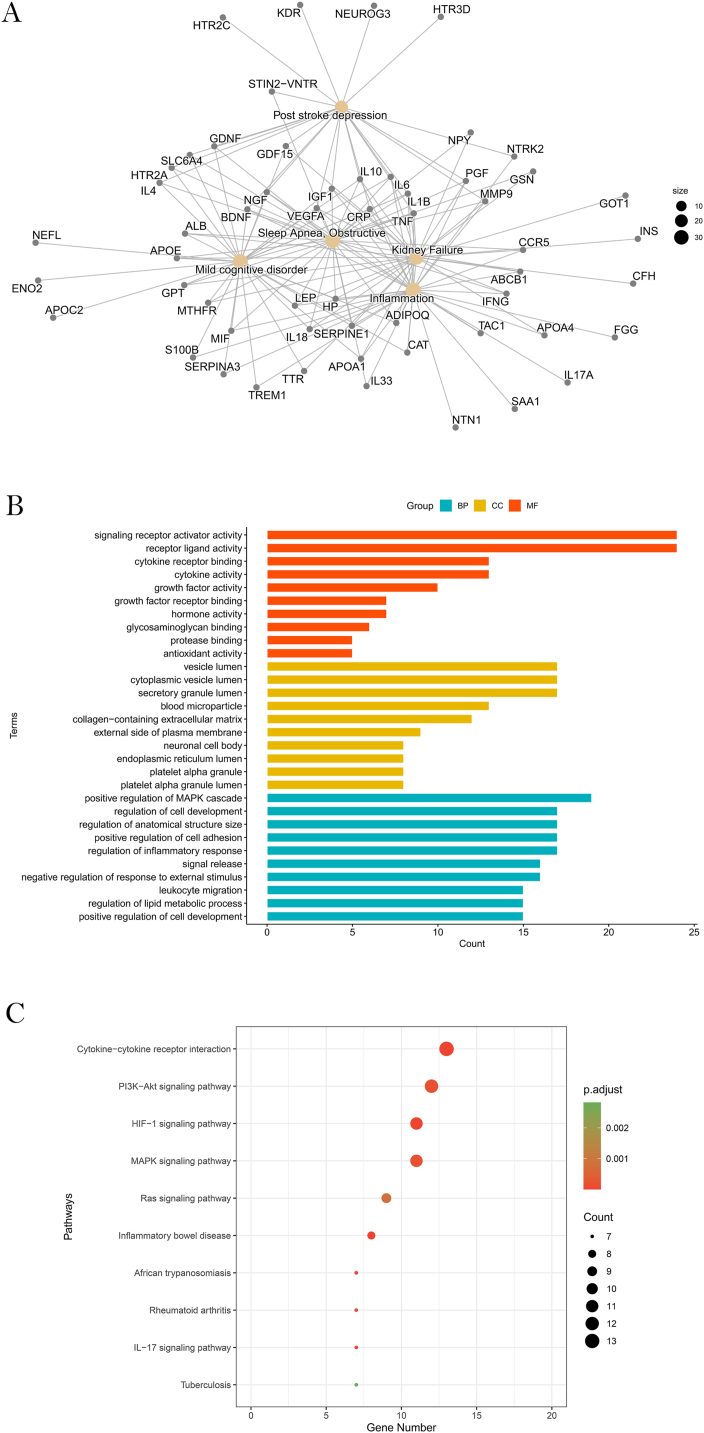
Based on the significant enrichment of these genes in PSD, it could be inferred that the genes we mined should be correct (Fig. 2A; Table S2). Furthermore, the enrichment results(Fig. 2; Tables S2,S3,S4) were also significant in terms of inflammation, which coincided with the hypothesis of the inflammatory mechanism of PSD [28].
Fig. 2.
Enrichment results. (A)Diseases with the most significant enrichment of risk genes. (B) The most statistically significant GO functions in PSD. (C) Top 10 risk gene enrichment pathways.
3.2. Construction of the PPI network
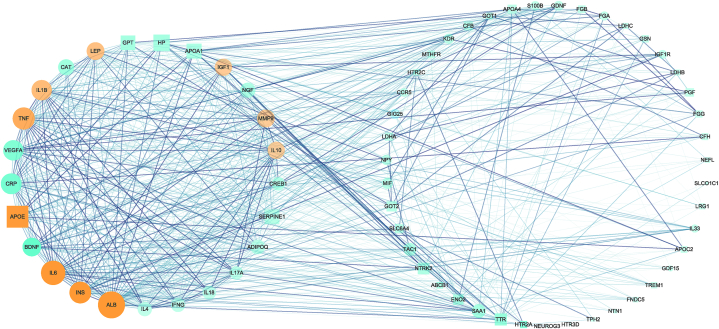
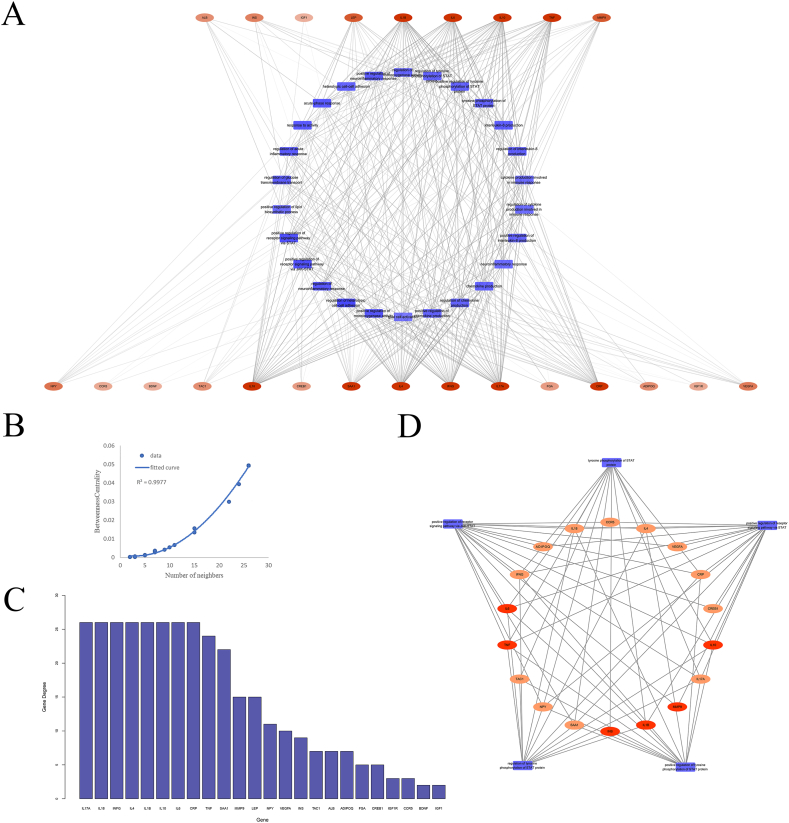
The PPI network consisted of 68 nodes and 705 edges (Fig. 3). The circular module on the left contained the 25 most tightly connected genes obtained by Cytoscape's MCODE plugin, among which 21 were immune genes. The Cytohubba plug-in selected the “MCC” method to obtain hub genes (TOP10), including INS, APOE, IL-10, ALB, IL-6, TNF, IL-1β, IGF1, LEP, and MMP9, all presenting in the left module. All of these genes belonged to immune genes. Therefore, we focused on the exploration of these genes and immune-related GO functions.
Fig. 3.
The PPI network. The circular nodes represent immune genes, while the square ones represent others. Similarly, nodes for hub genes are orange, while nodes for others are green. The betweenness centrality (BC) value of the nodes in each circle decreases clockwise. The size of the node is positively correlated with degree. The circle on the left is the tightest module in PPIN obtained by the MCODE plug-in.
3.3. Enrichment analysis of PSD-related GO functions
GO analysis in BP showed that genes were significantly enriched in areas related to inflammation, such as regulation of the inflammatory response and leukocyte migration (Fig. 2B). The immune system of stroke survivors is often activated to produce more inflammatory cytokines, which may suppress neurotrophic factors in the brain and result in symptoms of depression [29]. Numerous studies have shown that the alteration of the immune response was the mechanism that promoted PSD [3,[30], [31], [32]], which allowed the immune-related GO function to potentially modulate the pathogenesis of PSD.
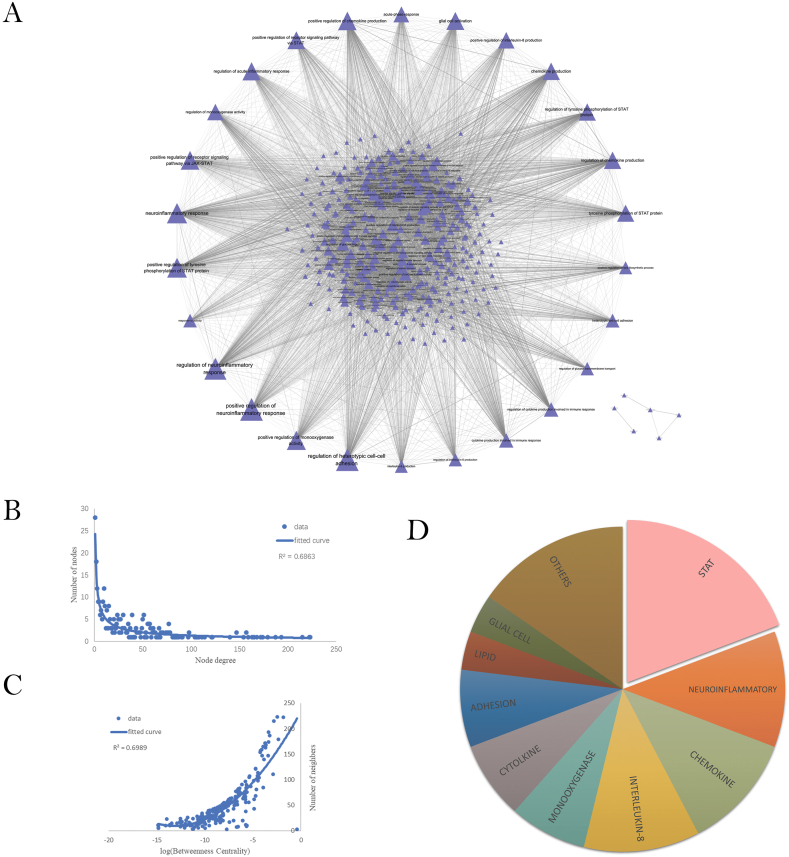
362 GO-BP functions with no less than five immune-risk genes were identified. Based on the GO functions obtained above, we constructed a GOFN with 335 nodes and 6176 edges (Fig. 4A). The pairs of GO functions were significantly correlated (adjusted p < 0.01).
Fig. 4.
Relationship among GO-BPs. (A) PSD Immune-Related GO Function Network (GOFN). The triangles represent the GO functions; the larger the node is, the greater the degree would be; There is a significant correlation between two GO functions at both ends of the line. (B) Degree distribution for all nodes in the GOFN. (C) Correlation between betweenness centrality (BC) and degree. (D) Classification of GO terms with neighbors greater than 100. The area ratio of each color represents the proportion of the relevant GO terms.
As in the results of the topological analysis (Fig. 4B), the degree distribution of nodes in GOFN follows a power-law distribution (y = 24.254x−0.631). Some GO functions had high connectivity. The GO function with higher degree had higher betweenness(Fig. 4C), which means that it was closely connected to other nodes in the network. Therefore, this kind of GO function was more important for the occurrence of PSD. Those correlated with less than other 100 GO functions were excluded from this study. We obtained a total of 26 GO functions that were most relevant to PSD. As shown (Fig. 4D), STAT related GO functions accounted for the highest proportion (5/26), respectively, positive regulation of the receptor signaling pathway via STAT (GO:1,904,894), positive regulation of the receptor signaling pathway via JAK-STAT (GO:0046427), positive regulation of tyrosine phosphorylation of STAT protein (GO:0042531), regulation of tyrosine phosphorylation of STAT protein (GO:0042509) and tyrosine phosphorylation of STAT protein (GO:0007260). They contained eight risk genes, occupying more than half of the hub genes, namely TNF, IL-6, LEP, IGF1, IL-1β and IL-10. Experiments have reported that activation of the JAK/STAT pathway induced hypoxic neuronal apoptosis after ischemic stroke, while alterations in JAK3 and STAT1 expression levels were associated with the occurrence of PSD [33,34].
3.4. Construction and analysis of GGOFN
To understand the immune-risk genes most closely associated with the above 26 GO functions, we identified 355 pairs of gene and GO function (value of adjusted p < 0.01) and established GGOFN (Fig. 5A). We obtained 27 significantly associated risk genes, including ADIPOQ, ALB, BDNF, CCR5, CREB1, CRP, FGA, GDF-15, IFNG, IGF1, IGF1R, IL-10, IL-17A, IL-18, IL-1β, IL-33, IL-4, IL-6, INS, LEP, MMP9, NPY, SAA1, SERPINA3, TAC1, TNF, VEGFA. Intersecting the above genes with the hub genes filtered in the PPI network, we obtained the following 9 genes: ALB, IL-6, TNF, IL-1β, INS, MMP9, IL-10, LEP, and IGF1. The nine genes which were considered as key and druggable genes, were used to explore potential compounds and drugs.
Fig. 5.
Associations between GO-BPs and genes. (A) Gene - GO function network (GGOFN). The orange oval represents the gene and those in the first row are key genes. The higher the degree is, the fuller the color would be. The blue rectangle represents the GO function. There is a significant correlation between genes and GO function at both ends of the line. (B) Fitting curve of gene degree and betweenness centrality. (C) Degree distribution of genes. (D) The dissection between STAT-associated GO functions and immune-related risk genes. Orange ovals represent genes, and darker ones represent key genes. The blue rectangle represents the GO function.
We analyzed the degree distribution of the genes in GGOFN (Fig. 5C). As IL-6, IL-1β, IL-10 and TNF were significantly correlated with almost all GO terms. The nodes with higher degree had higher betweenness(Fig. 5B), which means that these genes were closely connected to GO functions in this network. Eighteen immune-related risk genes, including six key genes, were significantly correlated with STAT-associated GO functions (Fig. 5D). Therefore, the compounds held promise for the treatment of PSD by targeting these genes.
3.5. Acquisition of potential compounds or drugs
In TCMSP, 24 compounds targeted on selected proteins met the requirements. We screened 5 predicted compounds and 41 validated compounds targeting key proteins in BATMAN-TCM. From DGIdb, we obtained 20 drugs that met the criteria, of which only two had a SMILES structure.
3.6. Identification of candidate compounds or drugs using DP analysis
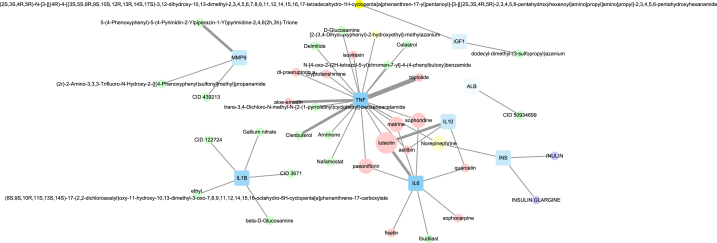
The AS list calculated by the pre-trained model in DP represented the binding probability between the compound and the target (Table 1). To identify the high-affinity compounds, the threshold was set to 7.0 for BindingDB or DAVIS and 12.1 for KIBA. We screened out 35 compounds and 2 drugs with verification value (Fig. 6). According to the results(Table S5), luteolin might closely interact with the most key proteins (TNF, IL-6, and IL-10). The AS of triptolide and TNF based on three databases were high, suggesting that these two compounds were highly reliable in the treatment of PSD.
Table 1.
Total potential compounds targeting key proteins.
| Target | ID | Compound/Drug | Database |
|---|---|---|---|
| TNF | 7124 | luteolin | TCMSP |
| TNF | 7124 | piperine | TCMSP |
| TNF | 7124 | fisetin | TCMSP |
| TNF | 7124 | matrine | TCMSP |
| TNF | 7124 | paeoniflorin | TCMSP |
| TNF | 7124 | wogonin | TCMSP |
| TNF | 7124 | sophocarpine | TCMSP |
| TNF | 7124 | sophoridine | TCMSP |
| TNF | 7124 | quercetin | TCMSP |
| TNF | 7124 | astilbin | TCMSP |
| TNF | 7124 | dl-praeruptorin a | TCMSP |
| TNF | 7124 | kaempferol | TCMSP |
| TNF | 7124 | cryptotanshinone | TCMSP |
| TNF | 7124 | triptolide | TCMSP |
| TNF | 7124 | aloe-emodin | TCMSP |
| TNF | 7124 | rutaecarpine | TCMSP |
| TNF | 7124 | isovitexin | TCMSP |
| TNF | 7124 | irisolidone | TCMSP |
| TNF | 7124 | ginsenoside rh2 | TCMSP |
| TNF | 7124 | [2-(3,4-Dihydroxyphenyl)-2-hydroxyethyl]-methylazanium | BATMAN(no evidence) |
| TNF | 7124 | Racephedrine | BATMAN(no evidence) |
| TNF | 7124 | Norepinephrine | BATMAN(no evidence) |
| TNF | 7124 | (1R)-2-(methylamino)-1-phenylpropan-1-ol; hydrochloride | BATMAN(no evidence) |
| TNF | 7124 | (−)-Synephrine | BATMAN(no evidence) |
| TNF | 7124 | Chloroquine | BATMAN (evidence) |
| TNF | 7124 | Clenbuterol | BATMAN (evidence) |
| TNF | 7124 | Amrinone | BATMAN (evidence) |
| TNF | 7124 | Epinephrine | BATMAN (evidence) |
| TNF | 7124 | Pseudoephedrine | BATMAN (evidence) |
| TNF | 7124 | Ethyl pyruvate | BATMAN (evidence) |
| TNF | 7124 | N-[4-oxo-2-(2H-tetrazol-5-yl)chromen-7-yl]-4-(4-phenylbutoxy)benzamide | BATMAN (evidence) |
| TNF | 7124 | d-Glucosamine | BATMAN (evidence) |
| TNF | 7124 | Thalidomide | BATMAN (evidence) |
| TNF | 7124 | Lenalidomide | BATMAN (evidence) |
| TNF | 7124 | Nafamostat | BATMAN (evidence) |
| TNF | 7124 | Pentoxifylline | BATMAN (evidence) |
| TNF | 7124 | Urapidil | BATMAN (evidence) |
| TNF | 7124 | Pirfenidone | BATMAN (evidence) |
| TNF | 7124 | Celastrol | BATMAN (evidence) |
| TNF | 7124 | Delmitide | BATMAN (evidence) |
| TNF | 7124 | trans-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzeneacetamide | BATMAN (evidence) |
| TNF | 7124 | 3-Hydroxypyridine-2-carbonyloxy-bis(3-chloro-4-methylphenyl)borane | BATMAN (evidence) |
| INS | 3630 | oleic acid | TCMSP |
| INS | 3630 | Norepinephrine | BATMAN(no evidence) |
| INS | 3630 | m-Cresol | BATMAN (evidence) |
| INS | 3630 | Myristic acid | BATMAN (evidence) |
| INS | 3630 | 3-Pyridinecarboximidamide, N-(2-hydroxy-3-(1-piperidinyl)propoxy)-, hydrochloride (1:2) | BATMAN (evidence) |
| INS | 3630 | INULIN | DGIdb |
| INS | 3630 | INSULIN GLARGINE | DGIdb |
| Il-6 | 3569 | luteolin | TCMSP |
| Il-6 | 3569 | piperine | TCMSP |
| Il-6 | 3569 | fisetin | TCMSP |
| IL6 | 3569 | matrine | TCMSP |
| Il-6 | 3569 | paeoniflorin | TCMSP |
| Il-6 | 3569 | wogonin | TCMSP |
| Il-6 | 3569 | sophocarpine | TCMSP |
| Il-6 | 3569 | sophoridine | TCMSP |
| Il-6 | 3569 | oroxylin a | TCMSP |
| Il-6 | 3569 | quercetin | TCMSP |
| Il-6 | 3569 | Ibudilast | TCMSP |
| ALB | 213 | linolenic acid | TCMSP |
| ALB | 213 | beta-carotene | TCMSP |
| ALB | 213 | Erythromycin | BATMAN (evidence) |
| ALB | 213 | Vancomycin | BATMAN (evidence) |
| ALB | 213 | Ebselen | BATMAN (evidence) |
| ALB | 213 | Iodipamide | BATMAN (evidence) |
| ALB | 213 | Irium | BATMAN (evidence) |
| ALB | 213 | Multihance | BATMAN (evidence) |
| ALB | 213 | CID 50934699 | BATMAN (evidence) |
| Il-10 | 3586 | luteolin | TCMSP |
| Il-10 | 3586 | quercetin | TCMSP |
| Il-10 | 3586 | astilbin | TCMSP |
| Il-10 | 3586 | sesamin | TCMSP |
| MMP9 | 4318 | Captopril | BATMAN (evidence) |
| MMP9 | 4318 | Marimastat | BATMAN (evidence) |
| MMP9 | 4318 | d-Glucosamine | BATMAN (evidence) |
| MMP9 | 4318 | L-tert-Leucine Methylamide | BATMAN (evidence) |
| MMP9 | 4318 | 2-{[Formyl(hydroxy)amino]methyl}-4-methylpentanoic acid | BATMAN (evidence) |
| MMP9 | 4318 | 5-(4-Phenoxyphenyl)-5-(4-Pyrimidin-2-Ylpiperazin-1-Yl)pyrimidine-2,4,6(2 h,3 h)-Trione | BATMAN (evidence) |
| MMP9 | 4318 | (2r)-2-Amino-3,3,3-Trifluoro-N-Hydroxy-2-{[(4-Phenoxyphenyl)sulfonyl]methyl}propanamide | BATMAN (evidence) |
| MMP9 | 4318 | (3r)-4,4-Difluoro-3-[(4-Methoxyphenyl)sulfonyl]butanoic Acid | BATMAN (evidence) |
| IGF1 | 3479 | dodecyl-dimethyl-(3-sulfopropyl)azanium | BATMAN (evidence) |
| IGF1 | 3479 | (2S,3S,4R,5R)-N-[3-[[(4R)-4-[(3S,5S,8R,9S,10S,12R,13R,14S,17S)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]-[3-[[(2S,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoyl]amino]propyl]amino]propyl]-2,3,4,5,6-pentahydroxyhexanamide | BATMAN (evidence) |
| Il-1β | 3553 | Ethyl (8S,9S,10R,11S,13S,14S)-17-(2,2-dichloroacetyl)oxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-17-carboxylate | BATMAN (evidence) |
| Il-1β | 3553 | Gallium nitrate | BATMAN (evidence) |
| Il-1β | 3553 | 2-methyl-1-(2-propan-2-ylpyrazolo [1,5-a]pyridin-3-yl)propan-1-one | BATMAN (evidence) |
| Il-1β | 3553 | Celastrol | BATMAN (evidence) |
| Il-1β | 3553 | beta-d-Glucosamine | BATMAN (evidence) |
Fig. 6.
The interaction of candidate compounds and target proteins. The blue square represents the key protein. The weak transparency of the proteins indicates a large degree. The circle represents compound. The pink and purple parts come from TCMSP, and DGIdb, respectively; while the green and yellow parts represent the verified and predicted compounds from BATMAN-TCM, respectively. The width of the line indicates the number of databases (DAVIS, BindingDB, and KIBA) that the AS has exceeded the threshold. The size of the circle represents the number of the target.
3.7. Mechanism dissection of PSD candidate compounds and relevant GO functions in pathways
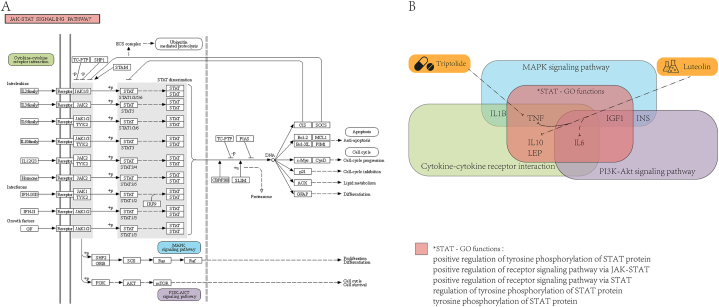
Finally, to investigate the potential mechanism of these two most promising compounds and risk GO functions, we further analyzed the potential association among candidate compounds, GO functions, and KEGG pathways enriched with risk genes. Pathways of cytokine-cytokine receptor interaction (hsa04060), PI3K-Akt signaling pathway (hsa04151) and MAPK signaling pathway (hsa04010) ranked first, second, and fourth in the results of KEGG enrichment (Fig. 2C). Interestingly, these three pathways had close crosstalk with the JAK-STAT signaling pathway. Therefore, STAT protein had the ability to participate in the PSD mechanism. In addition, the other three pathways and the JAK-STAT signaling pathway were regulated by many same genes, and there were common key genes in all four pathways (Fig. 7). Therefore, the drug candidates would also be similar. These results demonstrated the importance of GO terms in PSD mechanisms. Simultaneously, this study would provide a new perspective to clarify the pathogenesis and therapy of PSD.
Fig. 7.
Relationship among compounds, GO functions, and pathways. (A) JAK-STAT signaling pathway. (B) Analysis of mechanisms between latent compounds and GO functions in pathways. The pink rectangle represents the STAT-related GO functions/pathway; the green, blue, purple, and orange rectangle represents hsa04060, hsa04010, hsa04151 and the compound, respectively. The genes marked in the figure are key genes of PSD that make up GO functions or pathways.
4. Discussion
This study evaluated the underlying mechanism of GO functions in PSD immunity and repurposed existing compounds as novel options to mitigate PSD for the first time. We carefully collected candidate differentially expressed genes of PSD and focused on enriching immune-related risk GO functions of PSD. We constructed GOFN and found 26 GO functions closely related to the immune mechanism of PSD, identifying a vital protein, STAT. We also built PPIN and GGOFN, screening key genes that may act on critical GO functions. Furthermore, we focused on selecting the most potential compounds using DP to analyze drug-target interactions (DTI). Finally, we investigate the underlying mechanism between compounds and GO functions, revealing the potential therapeutic axis “compound-risk proteins - STAT pathway".
Many scholars recognized that the excessive immune response was an important mechanism for inducing PSD. The change in the level of inflammatory cytokines was one of the main hypotheses on the mechanism of PSD, and there was an extensive cross-talk relationship with other hypotheses [29]. The 71 PSD risk genes we collected contained 35 immune genes, and the PPIN-extracted hub genes all belonged to the immune category, echoing the significance of the immune mechanism of PSD. Studies have shown that there was a positive correlation between serum MMP9 level and PSD in the acute stage of cerebral ischemia [35]. MMP9 overexpression promoted the degradation of the extracellular basal layer, thereby destroying the blood-brain barrier, and inducing the production of inflammatory cytokines (such as IL-6, CRP), finally resulting in neuronal apoptosis [35,36]. Acute stroke rapidly activated the general immune system, accompanied by a rapid increase in the expression of pro-inflammatory cytokines, such as TNF, IL-6, IL-1, and IL-10, which was closely associated with the dysfunctioned hypothalamic pituitary adrenal (HPA) axis and norepinephric system [32,37]. It accelerated the decline of the serotonin level in related regions of the brain, thereby promoting the process of depression [29], which were consistent with the findings of our research.
The GOFN showed that immune-related GO functions were significantly correlated with each other. On the basis of the topological properties of it, we were concerned about the potentially significant value of STAT in the PSD process. The STAT protein family consists of complex components. The phosphorylation of STAT protein is involved in the pathogenesis of inflammation and autoimmune diseases, and it is indispensable in the signal transduction of the interleukin family, such as IL-6 and IL-10 [38]. Flavonoids have been shown to play an anti-neuroinflammatory role by inhibiting the JAK/STAT signal pathway [39]. Furthermore, STAT expression in brain tissue increased after ischemia, and activated STAT phosphorylation led to overexpression of the p-STAT protein, which aggravated brain edema and neurological disorder [40]. Through deepening the analysis of STAT-related GO functions, we found that STAT interacted with other GO functions through some PSD key genes. Therefore, we speculated that the STAT protein was one of the most important proteins in the pathogenesis of PSD.
The JAK-STAT signaling pathway had close crosstalk with the cytokine-cytokine receptor interaction, the PI3K-Akt signaling pathway and the MAPK signaling pathway, which were top pathways in the results of KEGG enrichment. Cytokines were almost universally recognized as important components of neuroimmunology and neuroinflammatory responses [41,42]. Both bioinformatics and experimental studies have shown that activation of the PI3K/Akt signaling pathway tended to induce PSD [43]. Activation of the PI3K/Akt pathway could serve as a biomarker for PSD, which could protect nerves during cerebral ischemia/perfusion by inducing proliferation and differentiation of neural stem cells [40,44]. Various compounds, such as resveratrol, kaempferol (KPF), and NYT, have been shown to play a neuroprotective role by regulating the PI3K/Akt related pathways to reduce inflammatory factors and weaken microglial polarization [40,45,46]. MAPK activation could affect pathophysiological pathways such as monoamine neurotransmission, which was one of the four major mechanisms hypotheses of PSD [47]. Furthermore, the MAPK pathway was involved in biological processes such as oxidative stress, neuronal apoptosis, and regulation of oligodendrocyte survival and differentiation [[47], [48], [49]]. Research showed that PKM2 improves post-stroke depression behavior by activating the VEGF-mediated MAPK/ERK pathway [48]. Furthermore, a previous study has identified that hsa04151 and hsa04010 may be deeply involved in the pathogenesis of PSD [50]. On the basis of the above literature, STAT was probably the key protein that mediated the pathway to induce PSD. We extrapolated that the mechanism with the most potential impact was risk proteins→ STAT → pathways →PSD.
Small-molecule drugs have the following characteristics: easy to take orally, high stability, adjustable half-life, and easy penetration through the cell membrane into the desired tissue [51]. These characteristics make it a potential market in the pharmaceutical field. By analyzing the interaction between compounds and targets, we have obtained 37 candidate compounds, of which luteolin and triptolide are the most valuable. Luteolin could be found in many vegetables, fruits and herbs, with plenty of biological properties, such as inhibiting neuroinflammation, antioxidants, and nerve protection [52]. Luteolin has been reported to inhibit the production of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α) and inflammation-related proteins (STAT3) in cells [53]. Therefore, the potential mechanism of luteolin in the treatment of PSD was likely to be luteolin→ IL-6/IL-10/TNF→STAT→hsa04060/hsa04151/hsa04010. The application of triptolide in neurodegenerative diseases, cerebral ischemia, and other brain diseases reflected the effects of inhibiting the production of inflammatory cytokines (TNF-a, etc.) and inhibiting the activation of the MAPK/NF-kB pathway to protect nerves [54,55]. More importantly, triptolide had the characteristics of high lipophilicity and small molecular weight, making it easy to cross the blood-brain barrier and treat encephalopathy [56]. Therefore, we inferred the potential mechanism of triptolide was as follow: triptolide→ TNF→ STAT →hsa04060/hsa04151/hsa04010→ PSD.
5. Conclusion
In this study, 71 risk genes of PSD were sorted out based on text mining. We constructed the GOFN and discovered that STAT-associated GO-BP seemed to play an important role in the regulation of risk-related pathways of PSD. We also identified key genes by constructing PPI and GGOFN, screening out 37 potential compounds for targeted therapy using DP. We have deeply dissected the regulatory mechanism of two potential compounds that are most significant for the targeted treatment of PSD through the risk-related pathway. These results allowed us to provide a reference for the exploration of novel mechanisms and therapies of PSD. Certainly, more confirmatory experiments are also indispensable in future.
Funding
Liyan is funded by the National Natural Science Foundation of China (grant number 82001386). Qi sihua is funded by the National Natural Science Foundation of China (grant number 82271207).
Author contribution statement
Tianyang Zhao: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Siqi Sun: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yueyue Gao, Yuting Rong, Hanwenchen Wang: Contributed reagents, materials, analysis tools or data.
Sihua Qi, Yan Li: Conceived and designed the experiments.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18622.
Contributor Information
Sihua Qi, Email: sihuaqi_2012@163.com.
Yan Li, Email: liyan_8809@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Medeiros G.C., Roy D., Kontos N., Beach S.R. Post-stroke depression: a 2020 updated review. Gen. Hosp. Psychiatr. 2020;66:70–80. doi: 10.1016/j.genhosppsych.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Campbell B.C.V., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., Donnan G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 3.Wijeratne T., Sales C. Understanding why post-stroke depression may Be the norm rather than the exception: the anatomical and neuroinflammatory correlates of post-stroke depression. J. Clin. Med. 2021;10 doi: 10.3390/jcm10081674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J.L., Wang J.J., Sun W., Liu X.F. The advances of post-stroke depression: 2021 update. J. Neurol. 2022;269:1236–1249. doi: 10.1007/s00415-021-10597-4. [DOI] [PubMed] [Google Scholar]
- 5.Frank D., Gruenbaum B.F., Zlotnik A., Semyonov M., Frenkel A., Boyko M. Pathophysiology and current drug treatments for post-stroke depression: a review. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232315114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Q.Q., Lu Q.W., Wang G.Y., Zhu W.J., Teng L.X., Chen W.P., Bi L. Optimizing component formula suppresses lung cancer by blocking DTL-mediated PDCD4 ubiquitination to regulate the MAPK/JNK pathway. J. Ethnopharmacol. 2022;299 doi: 10.1016/j.jep.2022.115546. [DOI] [PubMed] [Google Scholar]
- 7.Qiu S., Yu G., Lu X., Domeniconi C., Guo M. Isoform function prediction by Gene Ontology embedding. Bioinformatics. 2022;38:4581–4588. doi: 10.1093/bioinformatics/btac576. [DOI] [PubMed] [Google Scholar]
- 8.Thomas P.D., Hill D.P., Mi H.Y., Osumi-Sutherland D., Van Auken K., Carbon S., Balhoff J.P., Albou L.P., Good B., Gaudet P., Lewis S.E., Mungall C.J. Gene Ontology Causal Activity Modeling (GO-CAM) moves beyond GO annotations to structured descriptions of biological functions and systems. Nat. Genet. 2019;51:1429–1433. doi: 10.1038/s41588-019-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chloe Li K.Y., Cook A.C., Lovering R.C. GOing forward with the cardiac conduction system using gene ontology. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.802393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L., Chen K., Wu Y., Xiang G., Liu X. Epigenome-Metabolome-Epigenome signaling cascade in cell biological processes. J. Genet. Genomics. 2022;49:279–286. doi: 10.1016/j.jgg.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Liu M., Xu Y. Gene identification and potential drug therapy for drug-resistant melanoma with bioinformatics and deep learning technology. Dis. Markers. 2022;2022 doi: 10.1155/2022/2461055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Y.C., Xu Z.H., Wang J., Yu W.B. Uncovering the pharmacology of Ginkgo biloba folium in the cell-type-specific targets of Parkinson's disease. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1007556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang K., Fu T., Glass L.M., Zitnik M., Xiao C., Sun J. DeepPurpose: a deep learning library for drug-target interaction prediction. Bioinformatics. 2021;36:5545–5547. doi: 10.1093/bioinformatics/btaa1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y., Chen Z., Qi F., Liu J. Identification of drug compounds for keloids and hypertrophic scars: drug discovery based on text mining and DeepPurpose. Ann. Transl. Med. 2021;9:347. doi: 10.21037/atm-21-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M., Yang F., Xu Y. Identification of potential drug therapy for dermatofibrosarcoma protuberans with bioinformatics and deep learning technology. Curr. Comput. Aided Drug Des. 2022;18:393–405. doi: 10.2174/1573409918666220816112206. [DOI] [PubMed] [Google Scholar]
- 16.Rebhan M., Chalifa-Caspi V., Prilusky J., Lancet D. GeneCards: integrating information about genes, proteins and diseases. Trends Genet. 1997;13:163. doi: 10.1016/s0168-9525(97)01103-7. [DOI] [PubMed] [Google Scholar]
- 17.Amberger J.S., Bocchini C.A., Schiettecatte F., Scott A.F., Hamosh A. OMIM.org: online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–D798. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya S., Dunn P., Thomas C.G., Smith B., Schaefer H., Chen J., Hu Z., Zalocusky K.A., Shankar R.D., Shen-Orr S.S., Thomson E., Wiser J., Butte A.J. ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci. Data. 2018;5 doi: 10.1038/sdata.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Mi C., Guo Y. Satellite tracking reveals a new migration route of black-necked cranes (Grus nigricollis) in Qinghai-Tibet Plateau. PeerJ. 2020;8 doi: 10.7717/peerj.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen M., Duan C., Xie C., Wang H., Li Z., Li B., Wang T. Identification of key interferon-stimulated genes for indicating the condition of patients with systemic lupus erythematosus. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.962393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminf. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W., Chen Y., Jiang H., Yang J., Wang Q., Du Y., Xu H. Integrated strategy for accurately screening biomarkers based on metabolomics coupled with network pharmacology. Talanta. 2020;211 doi: 10.1016/j.talanta.2020.120710. [DOI] [PubMed] [Google Scholar]
- 24.Pan B., Fang S., Zhang J., Pan Y., Liu H., Wang Y., Li M., Liu L. Chinese herbal compounds against SARS-CoV-2: puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor. Comput. Struct. Biotechnol. J. 2020;18:3518–3527. doi: 10.1016/j.csbj.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z., Guo F., Wang Y., Li C., Zhang X., Li H., Diao L., Gu J., Wang W., Li D., He F. BATMAN-TCM: a bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Sci. Rep. 2016;6 doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner A.H., Coffman A.C., Ainscough B.J., Spies N.C., Skidmore Z.L., Campbell K.M., Krysiak K., Pan D., McMichael J.F., Eldred J.M., Walker J.R., Wilson R.K., Mardis E.R., Griffith M., Griffith O.L. DGIdb 2.0: mining clinically relevant drug-gene interactions. Nucleic Acids Res. 2016;44:D1036–D1044. doi: 10.1093/nar/gkv1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UniProt C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank D., Gruenbaum B.F., Zlotnik A., Semyonov M., Frenkel A., Boyko M. Pathophysiology and current drug treatments for post-stroke depression: a review. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232315114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang C., Zhang Z., Xu H., Liu Y., Wang X., Yuan L., Xu Y., Zhu Z., Zhang A., Shao A., Lou M. Natural products for the treatment of post-stroke depression. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.918531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Y., Yang Y.T., You H.M., Cao D., Liu C.Y., Zhou C.J., Wang Z.Y., Bai S.J., Mu J., Wu B., Zhan Q.L., Xie P. Plasma-based proteomics reveals lipid metabolic and immunoregulatory dysregulation in post-stroke depression. Eur. Psychiatr. 2014;29:307–315. doi: 10.1016/j.eurpsy.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Villa R.F., Ferrari F., Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol. Ther. 2018;184:131–144. doi: 10.1016/j.pharmthera.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Chen H., Liu F., Sun D., Zhang J., Luo S., Liao Q., Tian F. The potential risk factors of early-onset post-stroke depression from immuno-inflammatory perspective. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rayasam A., Kijak J.A., Kissel L., Choi Y.H., Kim T., Hsu M., Joshi D., Laaker C.J., Cismaru P., Lindstedt A., Kovacs K., Vemuganti R., Chiu S.Y., Priyathilaka T.T., Sandor M., Fabry Z. CXCL13 expressed on inflamed cerebral blood vessels recruit IL-21 producing T(FH) cells to damage neurons following stroke. J. Neuroinflammation. 2022;19:125. doi: 10.1186/s12974-022-02490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galecka M., Szemraj J., Su K.P., Halaris A., Maes M., Skiba A., Galecki P., Blizniewska-Kowalska K. Is the JAK-STAT signaling pathway involved in the pathogenesis of depression? J. Clin. Med. 2022;11 doi: 10.3390/jcm11072056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che B., Zhong C., Ge J., Li R., Zhu Z., Bu X., Xu T., Ju Z., Liu J., Zhang J., Chen J., Zhang Y., He J. Serum matrix metalloproteinase-9 is associated with depression after acute ischemic stroke. Circ. J. 2019;83:2303–2311. doi: 10.1253/circj.CJ-19-0376. [DOI] [PubMed] [Google Scholar]
- 36.Horstmann S., Su Y., Koziol J., Meyding-Lamade U., Nagel S., Wagner S. MMP-2 and MMP-9 levels in peripheral blood after subarachnoid hemorrhage. J. Neurol. Sci. 2006;251:82–86. doi: 10.1016/j.jns.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L., Wang T., Yu Y., Li M., Sun X., Song W., Wang Y., Zhang C., Fu F. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113146. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D.M. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Peng F., Xing Z., Chen J., Peng C., Li D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X.H., Yin F.T., Zhou X.H., Zhang A.H., Sun H., Yan G.L., Wang X.J. The signaling pathways and targets of natural compounds from traditional Chinese medicine in treating ischemic stroke. Molecules. 2022;27 doi: 10.3390/molecules27103099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becher B., Spath S., Goverman J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017;17:49–59. doi: 10.1038/nri.2016.123. [DOI] [PubMed] [Google Scholar]
- 42.Pape K., Tamouza R., Leboyer M., Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat. Rev. Neurol. 2019;15:317–328. doi: 10.1038/s41582-019-0174-4. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z., Sun Y., Bian L., Zhang Y., Zhang Y., Wang C., Tian J., Lu T. The crosstalk signals of Sodium Tanshinone ⅡA Sulfonate in rats with cerebral ischemic stroke: insights from proteomics. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113059. [DOI] [PubMed] [Google Scholar]
- 44.Li M., Ding R., Yang X., Ran D. Study on biomarkers related to the treatment of post-stroke depression and alternative medical treatment methods. Neuropsychiatric Dis. Treat. 2022;18:1861–1873. doi: 10.2147/NDT.S370848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva Dos Santos J., Goncalves Cirino J.P., de Oliveira Carvalho P., Ortega M.M. The pharmacological action of kaempferol in central nervous system diseases: a review. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.565700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tani A., Sakakima H., Otsuka S., Mizuno K., Nakanishi K., Norimatsu K., Takada S., Matsuoka T., Matsuzaki R., Nakakogawa T., Maruyama I. Stimulation of functional recovery via neurorepair mechanisms by the traditional Japanese Kampo medicine, Ninjin'yoeito, and physical exercise in a rat ischemic stroke model. J. Ethnopharmacol. 2023;302 doi: 10.1016/j.jep.2022.115927. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y., Wang L., Hu K., Yu C., Zhu Y., Zhang S., Shao A. Mechanisms and therapeutic targets of depression after intracerebral hemorrhage. Front. Psychiatr. 2018;9:682. doi: 10.3389/fpsyt.2018.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng Y., Li X., Wang J., Huang X., Meng L., Huang J. Pyruvate kinase M2 (PKM2) improve symptoms of post-ischemic stroke depression by activating VEGF to mediate the MAPK/ERK pathway. Brain Behav. 2022;12 doi: 10.1002/brb3.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubay J.E., de Halleux C., Jaumin P., Moulin D., Kestens-Servaye Y., Lintermans J., Stijns M., Vliers A., Chalant C.H. Long-term follow-up of the Senning operation for transposition of the great arteries in children under 3 months of age. J. Thorac. Cardiovasc. Surg. 1987;94:75–81. [PubMed] [Google Scholar]
- 50.Li Y., Wang Z.C., Zhu M.X., Fan G.B., Xu G.S., Zhao T.Y., Zhao A.Y., Ning S.W., Qi S.H. Network and pathway-based integrated analysis identified a novel "rs28457673-miR-15/16/195/424/497 family-igf1r-MAPK signaling pathway" Axis associated with post-stroke depression. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.622424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamali A., Ziadlou R., Lang G., Pfannkuche J., Cui S., Li Z., Richards R.G., Alini M., Grad S. Small molecule-based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11:27–47. doi: 10.7150/thno.48987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordaro M., Cuzzocrea S., Crupi R. An update of palmitoylethanolamide and luteolin effects in preclinical and clinical studies of neuroinflammatory events. Antioxidants. 2020;9 doi: 10.3390/antiox9030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratheeshkumar P., Son Y.O., Divya S.P., Roy R.V., Hitron J.A., Wang L., Kim D., Dai J., Asha P., Zhang Z., Wang Y., Shi X. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol. Appl. Pharmacol. 2014;281:230–241. doi: 10.1016/j.taap.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao M., Li X., Feng J., Pan N. Triptolide protects against ischemic stroke in rats. Inflammation. 2015;38:1617–1623. doi: 10.1007/s10753-015-0137-x. [DOI] [PubMed] [Google Scholar]
- 55.Cui Y., Jiang X., Feng J. The therapeutic potential of triptolide and celastrol in neurological diseases. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1024955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H., Zhu W., Su X., Wu S., Lin Y., Li J., Wang Y., Chen J., Zhou Y., Qiu P., Yan G., Zhao S., Hu J., Zhang J. Triptolide inhibits proliferation and invasion of malignant glioma cells. J. Neuro Oncol. 2012;109:53–62. doi: 10.1007/s11060-012-0885-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.