Summary
Background
Haemophagocytic lymphohistiocytosis (HLH) is a rare and life-threatening syndrome characterized by an excessive inflammatory response. Limited data exist on adult HLH.
Methods
In this national, retrospective cohort study, we analysed data from the US National Inpatient Sample database collected between October 1, 2006 and December 31, 2019. Using the International Classification of Diseases (ICD) codes, we identified all adult patients who were admitted non-electively with the diagnosis of HLH. We described demographic characteristics, triggers, and associated conditions. Trends of diagnosis, treatment, and in-hospital mortality were analysed using joinpoint models. In-hospital mortality rates were compared using multivariable logistic regression models that adjusted for demographic characteristics and associated conditions. Finally, we described resource utilization outcomes including cost of hospitalization and length of stay.
Findings
We identified 16,136 non-elective adult HLH admissions. The population pyramid showed a bimodal distribution, with peaks in young adults (16–30 years) and older adults (56–70 years). Joinpoint regression analysis revealed a significant increase in HLH incidence per 100,000 admissions over the study period (Average Annual Percent Change [APC] = 25.3%, p < 0.0001), and no significant change in rates of in-hospital mortality (slope = −0.01; p = 0.95) or administration of in-hospital HLH treatment (slope = 0.46, p = 0.20). The most common associated conditions were malignancy (4953 admissions [30.7%]), infections (3913 admissions [24.3%]), autoimmune conditions (3362 admissions [20.8%]), organ transplant status (639 admissions [4%]), and congenital immunodeficiency syndromes (399 admissions [2.5%]). In-hospital mortality was higher in older adults and males. Furthermore, Congenital immunodeficiency syndromes had the worst in-hospital mortality rate (mortality rate 31.1%, adjusted OR 2.36 [1.56–3.59]), followed by malignancies (mortality rate 28.4%, adjusted OR 1.80 [1.46–2.22]), infections (mortality rate 21.4%, adjusted OR 1.33 [1.10–1.62]), other/no trigger (mortality rate 13.6%, adjusted OR 0.73 [0.58–0.92]), autoimmune (mortality rate 13%, adjusted OR 0.72 [0.57–0.92]), and post-organ transplant status (mortality rate 14.1%, adjusted OR 0.64 [0.43–0.97]). The overall mean length of stay was 14.3 ± 13.9 days, and the mean cost of hospitalization was $54,900 ± 59,800.
Interpretation
We provide insight into the burden of adult HLH in the USA. The incidence has been increasing and the outcomes remain dismal. This signifies the growing need for the development of updated diagnosis and treatment protocols that are specific to adult HLH.
Funding
None.
Keywords: Haemophagocytic lymphohistiocytosis, National Inpatient Sample, Cancer epidemiology
Research in context.
Evidence before this study
Limited data exist on adult Haemophagocytic lymphohistiocytosis (HLH); a search of PubMed from 2000 to 2023 using the terms "hemophagocytic lymphohistiocytosis", "HLH", "adult HLH", and "HLH epidemiology" revealed that most of the available data on HLH pertained to pediatric patients. Most of the evidence on adult HLH was in the form of case reports, series, and small observational studies. Larger reports were limited and often focused on specific aspects of the disease or relied on pooled data from multiple small heterogenous series, which affects the internal validity of the study.
Added value of this study
Using the National Inpatient Sample (NIS) data from 2006 to 2019, our study identified 16,136 non-elective adult HLH admissions. This is the first study that offers a comprehensive analysis of demographic characteristics, diagnosis and treatment trends, outcomes, and resource utilization patterns in a large sample of patients over an extended period.
Implications of all the available evidence
HLH, increasingly acknowledged in adults, exhibits a poor prognosis and notable variations across age, sex, race groups, and underlying causes. This emphasizes the necessity for additional research to comprehend the root mechanisms of HLH and provide explanations for these variations. Furthermore, our results are anticipated to help with the assessment of the effectiveness of emerging therapies that are presently being investigated for the management of HLH.
Introduction
Haemophagocytic lymphohistiocytosis (HLH) is a rare but potentially life-threatening hyperinflammatory syndrome characterized by uncontrolled activation of T cells and macrophages.1 The syndrome is usually characterized by fever, hepatosplenomegaly, cytopenias, and a cytokine storm, which can lead to multiple organ failure and death if not promptly recognized and treated. HLH can occur as a primary genetic disorder or secondary to infections, malignancies, autoimmune diseases, or immunosuppression, among other triggers. Primary HLH is more common in children and young adults, with an estimated incidence of 1.2 per million children per year and mortality rates of 20%–30% within the initial two months of diagnosis.2, 3, 4, 5, 6, 7 Secondary HLH, on the other hand, is the predominant type in older adults, although its exact prevalence is not well established.8 Mortality rates for secondary HLH range from 30% to 40% within the initial two months of diagnosis.9, 10, 11 Due to the wide range of clinical presentations, triggers, and outcomes, HLH remains a diagnostic and therapeutic challenge.12,13
The National Inpatient Sample (NIS) is the largest all-payer inpatient healthcare database in the United States (US), with information on approximately 7 million hospitalizations each year. It has a stratified and weighted sampling process that provides a representative sample of inpatient hospitalizations from community hospitals across 46 states.14 The NIS has been widely used to investigate epidemiological trends, outcomes, and resource utilization of various medical conditions.14, 15, 16
There have been only a small number of studies that have tried to characterize adult HLH in a large sample of patients. A cohort study from England focused on the temporal trends of adult HLH incidence in England between 2003 and 2018.17 Another study pooled various small series from the literature to delineate the triggers, demographic characteristics, and outcomes of adult HLH.13 To date, no large reports used a uniform sample to thoroughly characterize adult HLH, encompassing both clinical and public health-related outcomes.
Therefore, we conducted a retrospective study using the NIS to examine the baseline characteristics, trends, triggers, associated conditions, outcomes, and resource utilization of adult HLH hospitalizations between 2006 and 2019. Our study aimed to describe the epidemiology and clinical burden of adult HLH in the US and identify potential areas for improvement in diagnosis, management, and surveillance.
Methods
Data source
The Healthcare Cost and Utilization Project (HCUP) develops and maintains the NIS, an all-payer inpatient database available to the public for general research purposes. Every year, HCUP conducts a weighted sampling of 20% of all hospital discharges from US community hospitals, producing more than 7 million entries annually. The NIS includes clinical and resource utilization data obtained from discharge abstracts from participating hospitals. Clinical data, including diagnoses and procedures, is presented using the International Classification of Diseases, 9th and 10th revisions, Clinical Modifications (ICD-CM) coding system. ICD-9-CM was in use until October 2015, and ICD-10-CM is the current version utilized.
As the NIS data is deidentified, the ethics committee at Rochester General Hospital has determined that no specific ethical approval or written consent was required for this research.
Study design and data sampling
HLH-specific code was coined in October 2006. We retrospectively identified patients with HLH between October 1, 2006 and December 31, 2019 using ICD-9-CM code 2884 and ICD-10-CM codes D76x. Additionally, ICD codes were utilized to identify all associated conditions/triggers, confounding factors, procedures, and clinical outcomes. All codes used in the study are included in the Supplementary Material in Supplementary Tables S1 and S2. All codes were chosen by 2 independent authors, and disputes were resolved by joint decision. We excluded paediatric patients (age <18 years) and all elective admissions. Fig. 1 provides a flowchart of the selection process, with the number of discharges at each step.
Fig. 1.
Sampling process, exclusion criteria, and number of hospitalizations in each step. NIS, National Inpatient Sample.
Endpoints
The study aimed to describe the demographic and geographic characteristics of HLH hospitalizations in the US; describe the trends of HLH incidence and outcomes during the study period; describe the associated comorbid conditions and possible secondary causes; compare rates of in-hospital mortality between different demographic groups and associated conditions; and compare inpatient resource utilization between different comorbid groups.
Statistical analysis
Baseline demographics were summarized by presenting counts and percentages for categorical variables and by reporting the mean and standard deviation for continuous variables. The distribution of patients across age groups was further described using a population pyramid. Bimodality was tested by Hartigans’ dip test and LaPlace’s Demon test for multimodality. Hospitals were divided into nine regions based on the U.S. Census Bureau Census Divisions.18 Frequencies and rates of adult HLH admissions per 100,000 admissions were calculated for each region.
A review of the literature identified important triggers and associated conditions.13,17 These conditions were identified using ICD codes (see Supplementary Material for codes, definitions, and assumptions; Supplementary Tables S1 and S2) and described these conditions across the entire sample. To further understand the distribution of these conditions, we examined the frequencies of these conditions in specific subgroups based on age, sex, race, and hospital region. Age displayed a bimodal distribution on initial analysis, reflecting two overlapping populations. To separate the two populations, we fitted a weighted Gaussian mixture model and selected the optimal cutoff as the midpoint between the estimated means of the two subgroups. Specifically, age was divided into two subgroups (<52 and ≥52 years), and the resulting subgroups were used to further analyse the distribution of the associated conditions/triggers.
Trends were analysed using Joinpoint Regression Programme, Version 4.9.1.0.19 The programme uses validated segmented regression models that allow for the identification of significant changes in the slope of a trend line over time.20 We analysed the trends and average Annual Percent Change (APC) for HLH incidence rates per 100,000 admissions, rates of in-hospital mortality, rates of administration of in-hospital HLH treatment, and mean time to start of in-hospital treatment. HLH treatment was defined by the identification of codes for antineoplastic chemo or immunotherapy.
In-hospital mortality rates were compared using multivariable logistic regression models. Variables included in the models were age, sex, race, and different associated conditions/triggers. Dummy variables were created for associated conditions with more than two components. Our data met the assumptions of logistic regression including sufficient sample size, absence of significant multicollinearity between the independent variables, linear relationship between “Age” variable and log odds of the outcome, and absence of outliers. Age and sex had less than 1% missing values, which were excluded using listwise deletion. In contrast, race had a higher proportion of missing values (6.6%). To address this, we performed multiple imputation with regression models to create ten datasets, where missing race values were imputed. These imputation models incorporated the same independent variables as the final regression model. The pooled imputed dataset was subsequently utilized for the final analyses. To address the issue of multiplicity, the Bonferroni correction was employed. We divided the α level by the total number of comparisons, resulting in an adjusted α level (0.05/51 = 0.001). Subsequently, we used the adjusted α level to calculate the multiplicity-corrected confidence intervals (CI).
Finally, resource utilization outcomes, including length of stay (LOS) and cost per hospitalization, were described using means and standard deviations.
Sampling, analysis, and reporting were carried out in compliance with The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.21 Analysis was conducted using IBM SPSS version 26, R software package version 4.2 (packages: diptest, version 0.76-0; LaplacesDemon, version 16.1.6; GauPro, version 0.2.11; Forester, version 0.2.0), and Joinpoint Regression Programme, version 4.9.1.0.
Role of the funding source
There was no funding source for this study. Authors AA and AM had access to the dataset and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics
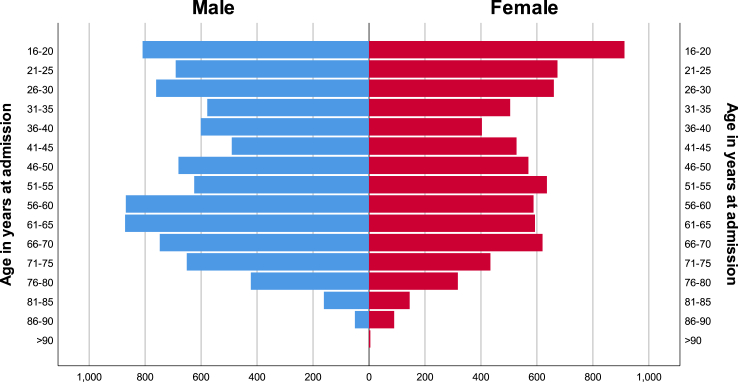
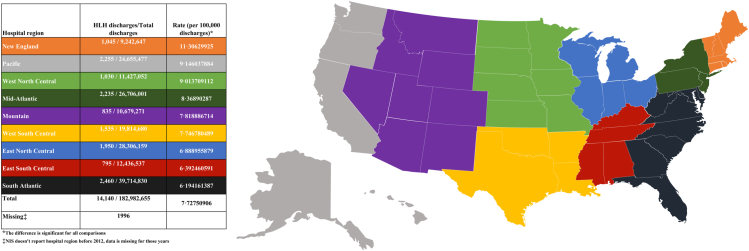
We identified 16,136 weighted non-elective adult HLH admissions between the years 2006–2019. Age, sex, race, and hospital location/teaching status were described in Table 1. Most patients were males and Caucasians. Interestingly, more than 97% of the cases were admitted to urban hospitals, and more than 86% were admitted to teaching hospitals. Age distribution was found to follow a bimodal pattern (p < 0.01), with the highest number of cases falling in age groups 16–30 and 56–70 years old (Fig. 2). Geographically, we found significant differences in rates of adult HLH admissions relative to the total number of admissions in each hospital region (Fig. 3). The New England and South Atlantic regions had the highest and lowest rates, respectively. Supplementary Table S3 further describes the distribution of age, race, sex, and comorbidities in each hospital region.
Table 1.
Demographic characteristics of adult hemophagocytic lymphohistiocytosis hospitalizations in the United States from 2006 to 2019.
| Age–Mean (±SD) | 48.4 (±19) |
| Sex—count (%) | |
| Male | 8754 (54.3) |
| Female | 7382 (45.7) |
| Missing | 01 |
| Race—count (%) | |
| Caucasian | 8243 (51.1) |
| African American | 2865 (17.8) |
| Hispanic | 2228 (13.8) |
| Asian or Pacific Islander | 942 (5.8) |
| Native American | 120 (0.7) |
| Other | 676 (4.2) |
| Missing | 1062 (6.6) |
| Location/teaching status of the hospital—count (%) | |
| Urban teaching | 13,943 (86.4) |
| Urban non-teaching | 1712 (10.6) |
| Rural | 434 (2.7) |
| Missing | 47 (0.3) |
Fig. 2.
Population pyramid showing patient distribution across different age and sex groups.
Fig. 3.
Geographic distribution of adult HLH cases by hospital region.
Triggers and associated conditions
In our sample, malignancy was the most frequently associated condition (30.7%), followed by infections (24.3%), autoimmune conditions (20.8%), organ transplant status (4%), and congenital immunodeficiency syndromes (2.5%), respectively. Conditions included in congenital immunodeficiency syndromes are listed in Supplementary Tables S1 and S2. We did not identify a trigger or an associated condition in 5782 hospitalizations (35.8%). Non-Hodgkin lymphomas (NHL) were the most common malignancies, with T-cell lymphomas slightly more frequent than B-cell lymphomas (7.1 vs. 5.8, p < 0.01). Amongst infections, viral infections were the most common (21.6%) and Ebstein-Barr virus (EBV) was the most common viral infection (5.5%). None of the patients in our sample had COVID-19 infection. The rates of other triggers and associated conditions are illustrated in detail in Fig. 4.
Fig. 4.
List of triggers and associated diseases. 1. Chronic myeloid leukemia (CML) is counted in myeloid leukemias. 2. The list of bacterial pathogens is not inclusive; many pathogens (e.g, gram positive bacteria, E. coli, pseudomonas, opportunistic fungi) were not included either due to lack of specific coding, clinical irrelevance, or concern that they could be nosocomially acquired. 3. Include Coccidioides, Paracoccidioides, and Blastomyces. Created with BioRender.com.
The distribution of these conditions also varied between different subgroups (Supplementary Table S4). In older adults (≥52 year), the most frequently associated conditions were malignancies (38.3%), infections (19.8%), and autoimmune conditions (14.4%), respectively. While in younger adults (age <52 year), infections were the most common (28.2%), followed by autoimmune (26.6%) and malignancies (24%). Furthermore, males had more malignancies (37.4% vs. 22.7%) and infections (26.8% vs. 21.3%) and lower autoimmune conditions (29.8% vs. 13.3%) than females. Significant variability was also observed in different racial groups.
Trends of diagnosis, outcomes, and treatment practices
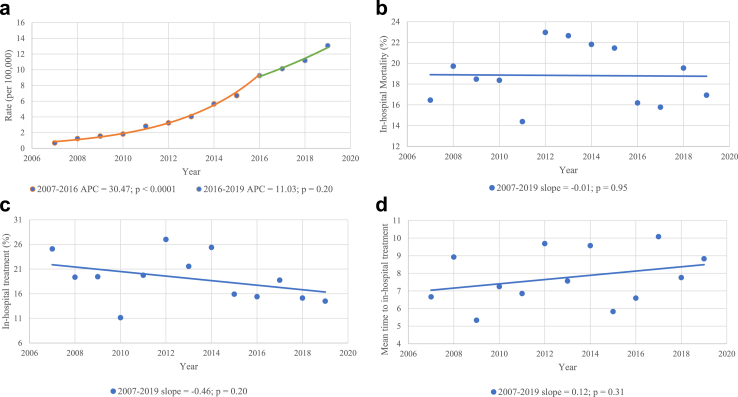
Fig. 5a demonstrates the change in HLH rates per 100,000 admissions per year. Supplementary Table S5 and Supplementary Figure S1 show the absolute number of adult HLH admissions per year. We found a significant increase in the rate of HLH over the study period (APC 25.3%, CI 20.2–30.7%, p < 0.0001). Most of this increase occurred between 2007 and 2016 (APC 30.5%, CI 26.4–34.7%, p < 0.0001). While the change between 2016 and 2019 was not statistically significant (APC 11.0%, CI −6.6 to 32%, p = 0.20). Fig. 5b–d demonstrate the annual change in the rates of in-hospital mortality, administration of HLH-specific treatment, and mean time from admission to the administration of HLH-specific treatment, respectively. There was no significant change in these measures between 2007 and 2019. Subgroup analysis of mortality rates revealed different trends across various triggers; however, none of these subgroups reached statistical significance (Supplementary Figures S2–S5).
Fig. 5.
Trends and annual percentage changes. (a) Adult HLH rate per 100,000 admissions; (b) Rate of in-hospital mortality; (c) Rate of in-hospital HLH treatment; (d) Mean time to in-hospital treatment. APC, Annual Percent Change.
In-hospital mortality
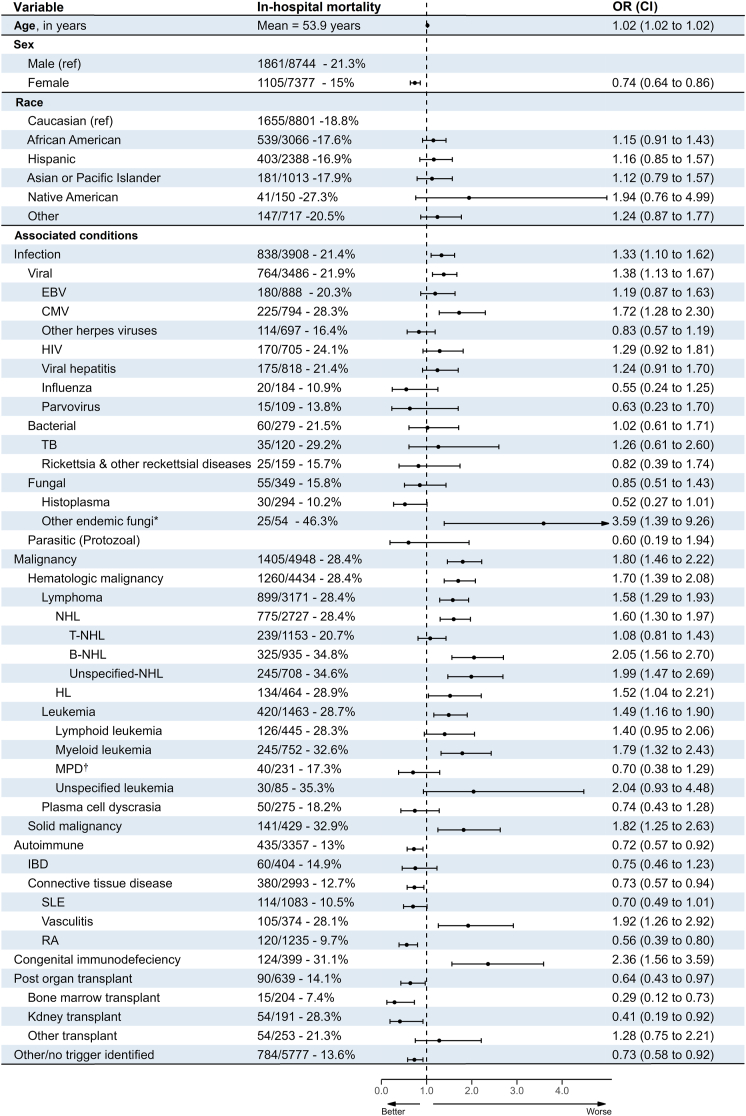
A total of 2966 (18.4%) patients died during the hospitalization. Multivariate logistic regression analysis showed that females had lower in-hospital mortality than males (15% vs. 21.3% adjusted OR 0.74 [0.64–0.86]). Meanwhile, non-Caucasians had higher odds of mortality than Caucasians but they were not statistically significant. In-hospital mortality rates were also different per different associated conditions. Congenital immunodeficiency syndromes had the worst outcomes (mortality rate 31.1%, adjusted OR 2.36 [1.56–3.59]), followed by malignancies (mortality rate 28.4%, adjusted OR 1.80 [1.46–2.22]), infections (mortality rate 21.4%, adjusted OR 1.33 [1.10–1.62]), other/no trigger (mortality rate 13.6%, adjusted OR 0.73 [0.58–0.92]), autoimmune (mortality rate 13%, adjusted OR 0.72 [0.57–0.92]), and post-organ transplant status (mortality rate 14.1%, adjusted OR 0.64 [0.43–0.97]). Fig. 6 compares in-hospital mortality rates for different demographic and comorbidity groups.
Fig. 6.
In-hospital mortality rates and adjusted odds for different demographic groups and associated conditions. ∗Includes coccidioides, Paracoccidioides, and blastomycosis. †Includes polycythemia vera, essential thrombocythemia, and myelofibrosis. Chronic myeloid leukemia was counted in leukemias. OR, odds ratio; CI, confidence interval; EBV, Epstein–Barr virus; CMV, Cytomegalovirus; HIV, human immunodeficiency virus; TB, tuberculosis; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MPD, Myeloproliferative diseases; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis.
Resource utilization
The mean LOS was 14.3 ± 13.9 days. HLH associated with infections had the longest hospital stays (mean LOS 18.1 ± 16.5 days), while HLH with no identified trigger/associated conditions had the shortest stays (mean LOS 11.4 ± 11.5).
On the other hand, the mean cost of individual HLH hospitalizations was $54,900 ± 59,800. Congenital immunodeficiency syndromes were associated with the highest costs (mean $80,100 ± 101,400), and those with no identified trigger/associated condition were associated with the lowest costs (mean $41,100 ± 46,100). Table 2 illustrates LOS and cost in dollars of HLH hospitalizations divided by associated conditions.
Table 2.
Resource utilization.
| Associated condition | LOS, in days–mean ± SD | Cost, in dollars–mean ± SDa |
|---|---|---|
| All patients | 14.3 ± 13.9 | $54,900 ± 59,800 |
| Infections | 18.1 ± 16.5 | $70,900 ± 70,300 |
| Malignancy | 17.2 ± 15.5 | $69,900 ± 61,700 |
| Autoimmune condition | 14 ± 14.8 | $54,700 ± 73,100 |
| Congenital immunodeficiency | 15.6 ± 12.7 | $80,100 ± 101,400 |
| No trigger | 11.4 ± 11.5 | $41,100 ± 46,100 |
Rounded to the nearest 100.
Discussion
HLH is a rare and life-threatening disease, often requiring prompt diagnosis and treatment. In recent years, there has been a growing interest in understanding the epidemiology and outcomes of this condition. Our study aimed to provide a comprehensive analysis of HLH admissions in the US between 2006 and 2019. We identified a total of 16,136 non-elective adult HLH admissions, we found that most patients were males and Caucasians, and the age displayed a bimodal distribution, with the highest number of cases falling in age groups 16–30 and 56–70 years old. These findings are similar to those reported in other studies.13,17 The bimodality suggests distinct underlying triggers and associated conditions, which we confirmed in our subgroup analysis. The initial peak was linked to the higher incidence of infections and autoimmune conditions, whereas the second peak was associated with higher incidence of malignancies. Furthermore, we found that more than 97% of the cases were admitted to urban hospitals, and more than 86% were admitted to teaching hospitals. This may reflect the fact that teaching hospitals often have more resources and expertise to manage complex cases such as HLH. Interestingly, we also found significant geographic differences in rates of adult HLH admissions. Hospitals in the New England region, for example, had approximately double the rate of the South Atlantic region. This might reflect a higher academic interest in these states, which leads to increased testing for rare conditions like HLH. Furthermore, while these differences might also be due to actual differences in underlying demographics and associated conditions, our data did not show any meaningful differences in these variables that could explain the variability in incidence rate.
Our trends analysis showed a significant increase in the rate of HLH over the study period (APC of 25.3%). This is consistent with previous studies, which have reported an increasing incidence of HLH in various populations.17 Whether this trend is related to increased awareness of the disease or an actual increase in its occurrence is unknown. The recent development and validation of the H-Score for diagnosing secondary HLH may have also contributed to enhanced detection of this condition in adults.22,23 On the other hand, We found that this was not accompanied by a significant improvement in the overall in-hospital mortality rates. This highlights the need for new therapeutic approaches and novel treatments. Presently, clinical practice in adult HLH is mostly based on paediatric HLH-94 and HLH-2004 protocols, which use etoposide, glucocorticoids, and calcineurin inhibitors as the mainstay of treatment.1,12,24 Promising new agents have shown benefit in observational studies and small clinical trials. These include anakinra (an IL-1 blocker), ruxolitinib (JAK1/2 inhibitor), alemtuzumab (CD52 monoclonal antibody), and emapalumab (anti–IFN-γ monoclonal antibody).25, 26, 27, 28, 29, 30, 31 However, larger randomized clinical trials on these agents are lacking which limits their widespread use. We also found no significant change in the rate or time of administration of in-hospital antineoplastic chemotherapy or immunotherapy. This could be partially attributed to the fact that many physicians may initially prioritize treatments aimed at addressing the underlying condition, particularly when patients are clinically stable. For example, HLH secondary to autoimmune conditions can sometimes be treated with steroids alone, obviating the need for antineoplastic therapy. However, clinicians must exercise a cautious approach and maintain a low threshold to initiate treatment without waiting for confirmatory testing, as delaying HLH-specific therapy can adversely affect patient outcomes.32
The most frequently associated condition with HLH in our sample was malignancy (30.7%), followed by infections (24.3%), autoimmune conditions (20.8%), organ transplant status (4%), and congenital immunodeficiency syndromes (2.5%). Interestingly, our results were very similar to those reported by West et al. who analysed data from hospital admissions and death certificates of adult patients with HLH in England from 2003 to 2018 and found associated malignancies in 32.9% of the cases and rheumatological diseases in 19.3%.17 In contrast, a review of the literature by Ramos-Casals et al. showed higher rates of associated infections (50%) and neoplasms (48%); and lower rates of autoimmune diseases (13%).13 Many of the studies reported in this review were cause-specific, which could have influenced the overall rates of these conditions in the pooled cohort. Thus, we believe that our results represent a closer look at reality, while also acknowledging the inherent limitations stemming from the coding system.
In terms of outcomes, our study found in-hospital mortality rates varied depending on associated conditions, with congenital immunodeficiency syndromes having the worst in-hospital mortality rates, followed by malignancies, infections, other/no trigger, autoimmune, and post-organ transplant status, respectively. These results are consistent with previous studies cited in the literature.33, 34, 35, 36, 37 However, with regard to the impact of sex, two different systematic reviews reported conflicting results.35,37 The large sample size in our study provides strong evidence that the male sex is associated with worse outcomes. The variability in outcomes highlights the importance of identifying the underlying trigger and associated conditions of HLH to optimize management and improve outcomes.
The resource utilization analysis confirmed that adult HLH admissions were associated with high costs and lengthy stays. The mean cost was $54,800, which is approximately 5 times greater than the mean cost of all medical stays in the U.S. in 2019 ($11,100). The mean LOS was 14.3 days, which is about 3 times longer than the mean LOS of all medical stays in the U.S. in 2019.38
Our study has several strengths; we used a nationally representative database to sample the largest descriptive cohort on adult HLH in the literature, spanning 13 years of practice. This allowed us to produce accurate national estimates and trends which would be difficult to produce otherwise given the rarity of the condition. On the other hand, there are also several limitations to consider. First, the database relied on administrative data, which may be subject to coding errors leading to potential loss or misclassification of clinically relevant information. Second, we used ICD-10 codes to identify cases of adult HLH which introduces a certain degree of diagnostic uncertainty due to the absence of universally recognized diagnostic criteria. Third, we were unable to confirm the causal relationship between HLH and the associated conditions. Fourth, we were limited by the data available to us by the NIS, which doesn’t report laboratory values, or specific treatments administered. Fifth, the NIS captures data on individual admissions rather than the entire disease process; therefore, it is impossible to estimate the overall mortality. Instead, we focused on in-hospital mortality which could potentially underestimate the severity of this disease. Finally, the results of the study might not accurately reflect outpatient HLH cases as the database is limited to inpatient settings.
In conclusion, our study provides valuable insights into the epidemiology, triggers, and outcomes of adult HLH in the US. Our findings highlight the need for continued efforts to improve the diagnosis and management of this rare and complex condition.
Contributors
A.A. and A.M. accessed and verified the underlying data; A.A. and A.M. were involved in the conception and design of the project; O.A. and A.O. collected and verified the required ICD codes; A.A. and O.A. analysed the data; A.A. and A.H. wrote the manuscript and created the graphs; S.J. supervised the conception and design and critically revised the manuscript for scientific content. All writers approved the final manuscript.
Data sharing statement
Data is publicly available upon request from Healthcare Cost and Utilization Project website: https://hcup-us.ahrq.gov/.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
During the preparation of this work, the authors used ChatGPT (https://chat.openai.com; openAI, San Francisco, CA, USA) in order to improve the readability of the work. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102143.
Appendix A. Supplementary data
References
- 1.Henter J.I., Horne A., Aricó M., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 2.Henter J.I., Elinder G., Söder O., Ost A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80(4):428–435. doi: 10.1111/j.1651-2227.1991.tb11878.x. [DOI] [PubMed] [Google Scholar]
- 3.Meeths M., Horne A., Sabel M., Bryceson Y.T., Henter J.I. Incidence and clinical presentation of primary hemophagocytic lymphohistiocytosis in Sweden. Pediatr Blood Cancer. 2015;62(2):346–352. doi: 10.1002/pbc.25308. [DOI] [PubMed] [Google Scholar]
- 4.Ishii E., Ohga S., Imashuku S., et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58–65. doi: 10.1532/IJH97.07012. [DOI] [PubMed] [Google Scholar]
- 5.Luo Z.B., Chen Y.Y., Xu X.J., Zhao N., Tang Y.M. Prognostic factors of early death in children with hemophagocytic lymphohistiocytosis. Cytokine. 2017;97:80–85. doi: 10.1016/j.cyto.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Dao A.T., Luong V.T., Nguyen T.T., et al. Risk factors for early fatal outcomes among children with hemophagocytic lymphohistiocytosis (HLH): a single-institution case-series in Vietnam. Pediatr Hematol Oncol. 2014;31(3):271–281. doi: 10.3109/08880018.2013.858198. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y.H., Han X.R., Xia F.Q., Poonit N.D., Liu L. Clinical features and prognostic factors of early outcome in paediatric hemophagocytic lymphohistiocytosis: a retrospective analysis of 227 cases. J Pediatr Hematol Oncol. 2022;44(1):e217–e222. doi: 10.1097/MPH.0000000000002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George M.R. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J Blood Med. 2014;5:69–86. doi: 10.2147/JBM.S46255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R., Cui T., He L., et al. A study on early death prognosis model in adult patients with secondary hemophagocytic lymphohistiocytosis. J Healthc Eng. 2022;2022 doi: 10.1155/2022/6704859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito-Zerón P., Kostov B., Moral-Moral P., et al. Prognostic factors of death in 151 adults with hemophagocytic syndrome: etiopathogenically driven analysis. Mayo Clin Proc Innov Qual Outcomes. 2018;2(3):267–276. doi: 10.1016/j.mayocpiqo.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jumic S., Nand S. Hemophagocytic lymphohistiocytosis in adults: associated diagnoses and outcomes, a ten-year experience at a single institution. J Hematol. 2019;8(4):149–154. doi: 10.14740/jh592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan M.B., Allen C.E., Weitzman S., Filipovich A.H., McClain K.L. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos-Casals M., Brito-Zerón P., López-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 14.Healthcare cost and utilization project (HCUP). Introduction to the HCUP national inpatient sample (NIS) 2019. 2019. https://www.hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2019.pdf [Google Scholar]
- 15.Badheka A., Bangalore Prakash P., Allareddy V., Allareddy V. Retrospective study of haemophagocytic syndrome hospitalisations in children in the USA. BMJ Paediatr Open. 2018;2(1) doi: 10.1136/bmjpo-2018-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel A.R., Desai P.V., Banskota S.U., Edigin E., Manadan A.M. Hemophagocytic lymphohistiocytosis hospitalizations in adults and its association with rheumatologic diseases: data from nationwide inpatient sample. J Clin Rheumatol. 2022;28(1):e171–e174. doi: 10.1097/RHU.0000000000001670. [DOI] [PubMed] [Google Scholar]
- 17.West J., Stilwell P., Liu H., et al. Temporal trends in the incidence of hemophagocytic lymphohistiocytosis: a nationwide cohort study from England 2003-2018. Hemasphere. 2022;6(11) doi: 10.1097/HS9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Census Bureau Census Bureau regions and divisions with state FIPS codes. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- 19.Statistical Methodology and Applications Branch SRP. National Cancer Institute . 2022. Joinpoint regression program. Version 4.9.1. [Google Scholar]
- 20.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Fardet L., Galicier L., Lambotte O., et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 23.Debaugnies F., Mahadeb B., Ferster A., et al. Performances of the H-score for diagnosis of hemophagocytic lymphohistiocytosis in adult and paediatric patients. Am J Clin Pathol. 2016;145(6):862–870. doi: 10.1093/ajcp/aqw076. [DOI] [PubMed] [Google Scholar]
- 24.Trottestam H., Horne A., Aricò M., et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–4584. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baverez C., Grall M., Gerfaud-Valentin M., et al. Anakinra for the treatment of hemophagocytic lymphohistiocytosis: 21 cases. J Clin Med. 2022;11(19):5799. doi: 10.3390/jcm11195799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakoory B., Carcillo J.A., Chatham W.W., et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44(2):275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar B., Aleem S., Saleh H., Petts J., Ballas Z.K. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37(7):638–643. doi: 10.1007/s10875-017-0439-x. [DOI] [PubMed] [Google Scholar]
- 28.Wohlfarth P., Agis H., Gualdoni G.A., et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2019;34(9):723–731. doi: 10.1177/0885066617711386. [DOI] [PubMed] [Google Scholar]
- 29.Zhou D., Huang X., Li X., et al. P1243: ruxolitinib combined with dexamethasone in adult patients with newly diagnosed hemophagocytic lymphohistiocytosis: interim analysis of a prospective, single-center, single-arm, phase 2 clinical trial. Hemasphere. 2022;6(Suppl):1128–1129. [Google Scholar]
- 30.Marsh R.A., Allen C.E., McClain K.L., et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60(1):101–109. doi: 10.1002/pbc.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallurupalli M., Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019;134(21):1783–1786. doi: 10.1182/blood.2019002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imashuku S., Kuriyama K., Teramura T., et al. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol. 2001;19(10):2665–2673. doi: 10.1200/JCO.2001.19.10.2665. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M., Li L., Zhang Q., et al. Clinical features and outcomes in secondary adult hemophagocytic lymphohistiocytosis. QJM. 2018;111(1):23–31. doi: 10.1093/qjmed/hcx183. [DOI] [PubMed] [Google Scholar]
- 34.Otrock Z.K., Eby C.S. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220–224. doi: 10.1002/ajh.23911. [DOI] [PubMed] [Google Scholar]
- 35.Hayden A., Park S., Giustini D., Lee A.Y., Chen L.Y. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: a systematic scoping review. Blood Rev. 2016;30(6):411–420. doi: 10.1016/j.blre.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Knaak C., Nyvlt P., Schuster F.S., et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. doi: 10.1186/s13054-020-02941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knaak C., Schuster F.S., Nyvlt P., et al. Treatment and mortality of hemophagocytic lymphohistiocytosis in adult critically ill patients: a systematic review with pooled analysis. Crit Care Med. 2020;48(11):e1137–e1146. doi: 10.1097/CCM.0000000000004581. [DOI] [PubMed] [Google Scholar]
- 38.Owens P.L., Liang L., Barrett M.L., Fingar K.R. 2022. Comorbidities associated with adult inpatient stays, 2019.https://hcup-us.ahrq.gov/reports/statbriefs/sb303-Comorbidities-Adult-Hospitalizations-2019.pdf [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.