Abstract
Introduction
Determining the nature of iris melanocytic tumors based on clinical exam alone remains challenging. Tumor-associated vasculature of iris melanocytic lesions may facilitate the ability to discern between iris nevus and melanoma.
Methods
In a single-institution, retrospective, observational study of 45 patients with pathologically confirmed iris melanoma and 15 patients with iris nevi that were either clinically stable or pathologically confirmed were included. Tumor characteristics and associated vasculature were identified on clinical exam and slit-lamp photographs. Fluorescein angiographic parameters including feeder vessels, intrinsic vessels, leakage, masking, and angiographic silence were assessed.
Results
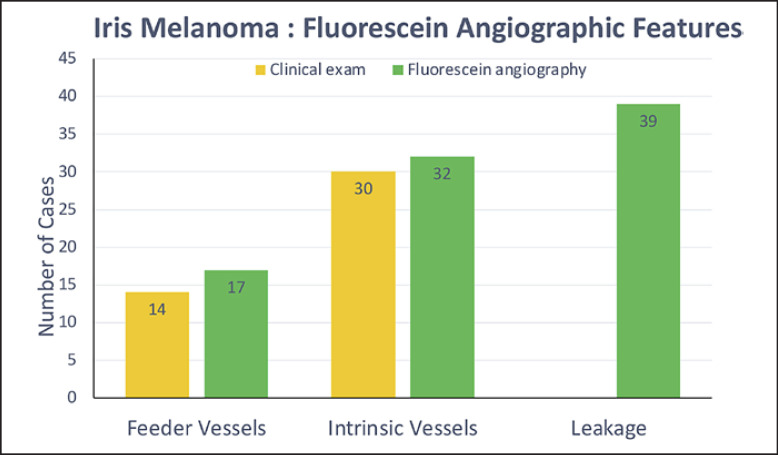
Feeder vessels were present in 17 (43%) melanomas and were absent in the nevus group (p = 0.002). Thirty-three (83%) iris melanomas and 5 (33%) iris nevi were observed to have intrinsic vessels, and a statistically significant association of intrinsic vessels with malignancy (p = 0.001) was noted. Fluorescein leakage was also observed more frequently in iris melanoma 39 (98%) than in nevi 9 (60) with a significant difference (p = 0.001). Angiographic silence occurred in 3 nevi (20%) and was not observed in any melanoma (p = 0.017). Overall, the presence of intrinsic vessels +/− feeder vessels had high sensitivity (0.85) and high positive predictive value (0.87) for diagnosis of iris melanoma.
Conclusions
Anterior segment fluorescein angiography allows for the assessment of tumor-associated vascular patterns and demonstrates utility in differentiating iris nevi from melanoma. Feeder vessels were only observed in iris melanoma and were absent in iris nevi. The intrinsic vessels were present more frequently in melanomas and are thus associated with malignancy. Angiographic silence is indicative of iris nevi.
Keywords: Oncology, Iris nevus, Iris melanoma, Fluorescein angiography, Uveal melanoma, Intrinsic vessels
Introduction
Determining the nature of iris melanocytic tumors based on clinical exam alone remains challenging and may require an extended period of follow-up or biopsy to arrive at an accurate diagnosis [1]. Even though there are overlapping clinical features, distinguishing between iris melanoma and iris nevus is of paramount importance because management and prognosis vary greatly; nevi are observed, while melanomas require intervention such as excision or radiation therapy. Clinical parameters suggestive of iris melanoma include documented growth, a basal diameter greater than 3 mm, infiltrating growth pattern with extension to the anterior chamber angle, sectoral cataract, secondary glaucoma, and abnormal vascularity [2, 3, 4, 5, 6].
Angiogenesis, the growth of new blood vessels, is essential for continued malignant growth. It is thought that small tumors subsist on nutrients and oxygen derived from diffusion alone. However, as tumor size increases and the nutrient supply provided by diffusion is exceeded, an “angiogenic switch” occurs and intrinsic vasculature is developed [7]. The intrinsic vessels are inherently abnormal, disorganized, tortuous, and permeable due to structural defects in endothelial junctions and pericytes [8].
With this in mind, studying the tumor-associated vasculature of iris melanocytic lesions may facilitate our ability to discern between iris nevus and melanoma. Anterior segment fluorescein angiography allows for the direct visualization of tumor-associated vasculature in addition to providing an assessment of vessel competency. While overlapping angiographic patterns of iris melanocytic lesions (nevus and melanoma) have included intrinsic vessels, feeder vessels, and leakage [9, 10, 11, 12, 13, 14], the limitation of existing literature lies in the small number of cases in each series that had histopathologic confirmation. Furthermore, histopathologic classification of iris melanocytic lesions has evolved to downgrade spindle A tumors as nevus rather than melanoma [15, 16].
In this study, we explore the utility of fluorescein angiography in differentiating iris nevi from iris melanomas. We analyze the angiographic patterns of biopsy-proven iris melanomas and compare them with corresponding features in clinically stable and/or biopsy-proven iris nevi. Our goal was to identify specific angiographic features that are diagnostic of iris melanoma.
Methods
This research was approved by the Cleveland Clinic Foundation Institutional Review Board (IRB# 21-793). The study adhered to the tenets of the Declaration of Helsinki.
This retrospective, observational clinical study included patients with iris nevi and melanomas that had been imaged with anterior segment fluorescein angiography (AS-FA) at the time of initial presentation and were subsequently managed at the Department of Ophthalmic Oncology, Cole Eye Institute, Cleveland Clinic. Only iris nevi that had demonstrated clinical stability for at least 1 year or had histopathologic confirmation were included. All iris melanomas had pathologic confirmation of the diagnosis.
An extensive chart review was performed to collect patient demographic data, clinical presentation, and tumor characteristics. Digital slit-lamp photography and AS-FA images were also obtained and reviewed. Tumor characteristics were recorded based on clinical examination and digital photographs. Tumor anatomic location (iris or iridociliary), quadrant location, pigmentation, extent of tumor (pupillary, peripheral, pan iris, and central), and visualization of feeder and intrinsic vessels on ophthalmic examination were recorded. Details regarding biopsy modalities including fine needle aspiration biopsy, incisional biopsy, and excisional biopsy with cytological or histopathologic confirmation, respectively, were collected.
AS-FA imaging was performed using the SPECTRALIS® HRA + OCT imaging machine from Heidelberg Engineering (Heidelberg, Germany). The system specifications include a blue light source of a solid-state laser (488 nm) to excite fluorescein and a barrier filter at 500 nm wavelength that separates excitation and fluorescent light. Anterior segment module add-on lens was used to image the anterior segment including iris vasculature. AK-FLUOR® (fluorescein injection, USP) 10% with normal adult dose of 5 mL (500 mg) was injected intravenously over a period of 10 s. Photographs of the iris vasculature were taken starting approximately 15 s after the injection every 2 s for next 30 s. Additional (1-2) photographs were taken at 1 and 5 min after injection. Imaging was performed by experienced ophthalmic photographers of the Cole Eye Institute.
Fluorescein angiographic features for all iris melanocytic tumors were cataloged. The images were not read blind. Analyzed parameters include presence of feeder vessels, intrinsic vessels, and associated leakage. Feeder vessels were identified as vessels that exhibited a combination of earlier hyperfluorescence, greater hyperfluorescence, or larger caliber when compared with normal radial iris vessels of the opposite quadrant. Intrinsic vessels were observed by the presence of visible distinct vascular channels within the tumor boundaries − categorized as fine loops, lacy net, or indeterminate. Fluorescein leakage was defined as ill-defined, patchy hyperfluorescence within the tumor margins with or without extension beyond the margins observed at 1–5-min photographs. Areas of hypofluorescence (obscuration of hyperfluorescence) at the location of the tumor that blocked iris vascular details were recorded as masking of fluorescence.
Statistical analysis was performed using a two-tailed Fisher test and t test to determine the correlation between AS-FA features and tumor characteristics as well as comparisons between nevi and melanoma. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated.
Results
Demographics
Fifty-five patients were included in this study. Our cohort comprised of 24 males and 31 females. The age of patients ranged from 25 to 79 (mean 57 years). Forty patients with pathologically confirmed iris melanoma (28 excisional biopsies, 10 fine needle aspiration biopsies, and 2 incisional biopsies) and 15 patients with iris nevus that were either clinically stable (10, mean follow-up period of 3 years [range 0.5–9.75 years]) or pathologically confirmed (5) were identified. All patients with iris melanoma had pathologic confirmation (Table 1).
Table 1.
Fluorescein angiographic patterns, Iris nevus and melanoma, and patient and tumor characteristics
| Characteristics | Nevus, n (%) | Melanoma, n (%) |
|---|---|---|
| Age at diagnosis, mean | 58.8 (61, 31–73) | 55.9 (60, 25–79) |
| (median, range), years | ||
| Gender (male/female) | 7 (47); 8 (53) | 17 (43); 23 (57) |
| Laterality (right eye/left eye) | 10 (67); 5 (33) | 15 (37); 25 (63) |
| Pigmentation | ||
| Melanotic | 8 (53) | 11 (27) |
| Amelanotic | 3 (20) | 9 (25) |
| Mixed | 4 (27) | 20 (48) |
| Location/margins | ||
| Pupillary | 9 (60) | 21 (53) |
| Peripheral | 4 (27) | 8 (20) |
| Pan iris | 0 | 7 (17) |
| Central | 2 (13) | 4 (10) |
| Feeder vesselsa | ||
| Visible | 0 | 14 (35) |
| Not visible | 15 (100) | 26 (65) |
| Intrinsic vesselsa | ||
| Visible | 5 (33) | 30 (75) |
| Not visible | 10 (67) | 10 (25) |
| Biopsy approach | ||
| Incisional | 0 | 2 (5) |
| FNAB | 0 | 10 (25) |
| Excision | 5 (33) | 28 (70) |
FNAB, fine needle aspiration biopsy.
By slit-lamp examination and/or photograph.
Iris Nevus (n = 15)
Of the 15 iris nevi, 10 nevi occurred in the right eye and 5 in the left eye. Nine (60%) nevi were located at the pupillary margin, 4 (27%) were peripheral, and 2 (13%) were central. Nevi pigmentation varied with 8 (53%) full melanotic, 3 (20%) amelanotic, and 4 (27%) as partial melanotic (Table 1). All nevi were ≤4.0 mm largest basal diameter (mean 2.1 mm, median 2.0 mm, range 1.0–4.0 mm) and ≤1.0 mm thickness (mean 0.91 mm, median 1.0 mm, range 0.5–1.0 mm) with variable dimension. On review of slit-lamp photos and clinic examination findings, feeder vessels were absent in all cases (100%), but 5 (33%) had intrinsic vessels (Table 1).
Iris Melanoma (n = 40)
Fifteen tumors occurred in the right eye and 25 in the left eye. Of the iris melanomas, 21 (53%) were located at the pupillary margin, 4 (10%) in the central iris, and 7 (17%) extended along the full span of the iris from the pupil to the peripheral iris (pan iris). Tumor pigmentation was variable with 11 (27%) full-melanotic tumors, 9 (25%) amelanotic tumors, and 20 (48%) partial melanotic tumors (Table 1). 36 (90%) melanomas were ≥3.0 mm largest basal diameter (mean 3.4 mm, median 3.4 mm, range 1.3–6.0 mm), and 15 (38%) had thickness of >1.0 mm (mean 1.28 mm, median 1.0 mm, range 0.2–3.0 mm) with variable dimension. On review of slit-lamp photos and clinic examination findings, feeder vessels were present in 14 (35%) tumors, and intrinsic vessels were present in 30 (75%) tumors (Fig. 1).
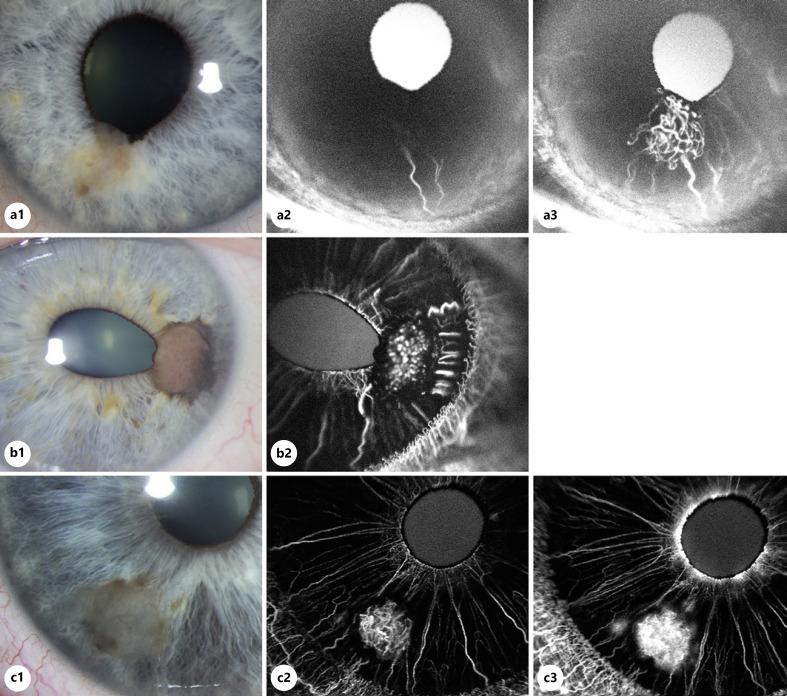
Fig. 1.
Angiographic features of iris melanoma. Comparison of clinical findings and anterior segment fluorescein angiography.
Angiographic Features
AS-FA characteristics including the presence of feeder vessels, intrinsic vessels, and leakage were identified in melanomas and contrasted with nevi.
Feeder Vessels
AS-FA demonstrated the presence of feeder vessels in 17 (43%) melanomas and was not observed in the nevus group, a statistically significant finding (p = 0.002) (Table 2). In the majority of tumors, feeder vessels approached the tumor from the periphery in 15 (88%) rather than from the pupillary margin (n = 1, 6%) (Fig. 2a). Tumor location (pupillary margin n = 13 (33%) and non-pupillary location (n = 4, 10%), p = 0.505) and tumor size (largest basal diameter and height) did not correlate with the presence of feeder vessels (p = 0.248, p = 0.108).
Table 2.
Iris nevus and melanoma and fluorescein angiographic features
| Characteristics | Nevus, n (%) | Melanoma, n (%) | p value |
|---|---|---|---|
| Feeder vessels | 0.002 | ||
| Present | 0 (0) | 17 (43) | |
| Central | 0 (0) | 1 (6) | |
| Peripheral | 0 (0) | 15 (88) | |
| Both | 0 (0) | 1 (6) | |
| Intrinsic vessels | 0.001 | ||
| Present | 5 (33) | 33 (83) | |
| Loops | 0 (0) | 12 (36) | |
| Lacy | 5 (100) | 21 (64) | |
| Fluorescein leakage present | 9 (60) | 39 (98) | 0.001 |
| Silent pattern | 3 (20) | 0 (0) | 0.0173 |
| Intrinsic vessels +/− feeder vessels | |||
| Sensitivity | 0.85 | ||
| Specificity | 0.67 | ||
| PPV | 0.87 | ||
| NPV | 0.62 |
Fig. 2.
Angiographic patterns of iris melanoma. A well-circumscribed, partially pigmented, transparent-appearing melanoma with pupil involvement and ectropion uveae is seen at 6:30 on slit-lamp photography (3.0 × 3.0 × 1.0 mm). Tumor-associated feeder and intrinsic vessels are visible (a1). Anterior segment fluorescein angiogram at 28 s demonstrates early filling of linear peripheral feeder vessels prior to normal iris vessels (a2). Angiogram taken 38 s after dye injection shows greater hyperfluorescence and larger caliber of feeder vessels when compared to adjacent normal iris vasculature (3). Intrinsic vessels are also seen within the tumor (a3). Small incision iridectomy with pupilloplasty was performed with histopathologic confirmation of iris melanoma (spindle cell type). A nodular melanoma centered at 3:00 with corectopia and intrinsic and feeder vessels visible on slit-lamp photo (3.0 × 2.5 × 2.1 mm) (b1). Fluorescein angiogram highlights the “fine loop” morphology of intrinsic vessels and presence of peripheral feeder vessels (b2). Iridocyclectomy was performed due to angle involvement, and histopathology was consistent with iris melanoma (epithelioid cell type). Iris melanoma at 7:30 with a translucent center and rim of pigment (2.5 × 2.2 × 0.8 mm) with visible intrinsic vessels seen on slit-lamp photo (c1). Anterior segment fluorescein angiogram at 35 s shows “lacy” intrinsic vessels (c2) which demonstrate diffuse leakage within the margins of the lesion at 1 min, and a rim of masking corresponding to pigmentation at the margin of the lesion is also observed (c3). Small incision iridectomy was performed, and histopathology was consistent with iris melanoma (mixed spindle and epithelioid cell type).
Intrinsic Vessels
Thirty-three (83%) iris melanomas were observed to have intrinsic vessels on AS-FA. The morphology of intrinsic vessels could be described as loops in 12 (36%), lacy in 21 (64%), and lacking any discernible pattern in 7 (17%) tumors (Table 2) (Fig. 2b). Although lacy pattern of intrinsic vessels could be detected in nevi, it was a less frequent finding in 5 (33%), with a statistically significant association of intrinsic vessels with malignancy (p = 0.001). Identification of intrinsic vessels on AS-FA did not correlate with melanoma pigmentation, as they were observed in 24 (60%) melanomas with complete or partial pigmentation and 9 (23%) amelanotic tumors (p = 0.175). The presence of intrinsic vessels was also not reflective of tumor size (largest basal diameter and height) (p = 0.927, p = 0.423). Overall, the presence of intrinsic vessels +/− feeder vessels had the sensitivity of 0.85 and specificity of 0.67 with PPV of 0.87 and NPV 0.62 for diagnosis of iris melanoma (Table 2).
Leakage
Leakage of fluorescein within the tumor with or without extension beyond the margins was the most common angiographic feature of iris melanomas, occurring in 39 (98%) melanomas in comparison to 9 (60%) nevi (p = 0.001) (Table 2) (Fig. 2c). Varying amount of masking of fluorescein leakage was observed both in melanomas (30, 75%) and nevi (9, 60%) (p = 0.326). All such tumors were either completely or partially melanotic, and none were amelanotic melanomas (Fig. 2c).
Silent Pattern
A silent angiogram was defined by normal appearance of iris vessels with absence of feeder vessels, intrinsic vessels, or leakage (Fig. 3). This pattern was present in some nevi (n = 3, 20%) but was not observed in any melanomas (0%), a statistically significant angiographic finding (p = 0.017) (Table 2).
Fig. 3.
Angiographic patterns of iris nevus. Slit-lamp photo shows a 2 × 2 mm, flat, uniformly pigmented iris lesion without intrinsic or feeder vessels at 4:00, without angle involvement on gonioscopy (a). The nevus has been stable for 10 years (photographic documentation). Anterior segment fluorescein angiogram images at 1 min (not shown) and 5 min (b) show normal radial iris vessel pattern with normal caliber and intensity of fluorescence similar to surrounding vessels. Angiogram is considered silent due to absence of tumor-associated vasculature leakage and masking at the location of the nevus.
Discussion
Tumor-associated vasculature of melanocytic iris lesions is routinely assessed on clinical exam. A multicenter trial by Khan et al. [17] which included 124 biopsy-proven iris melanomas, demonstrated intrinsic vessels in 56% of tumors and feeder vessels in 7%. Conway and colleagues identified prominent tumor vessels as a feature associated with the epithelioid subtype of iris melanoma [18]. While vasculature can be identified on clinical exam or with newer technology such as optical coherence angiography without injection of the dye, tumor pigmentation limits the detection of vessels and study of vascular architecture in many cases [19]. Our study highlights that tumor-associated vasculature is readily identified on fluorescein angiography in cases where it is not evident on slit-lamp photography. In the melanoma group, the presence of intrinsic and feeder vessels was noted in 3 additional cases on fluorescein angiography when compared to slit-lamp photos where vessels were not visible. Furthermore, the innate qualities of the vessels and breakdown of the blood-iris barrier (leakage) cannot be determined with slit-lamp exam, photography, or optical coherence angiography.
Anterior segment fluorescein angiography of iris melanocytic lesions provides a dynamic assessment of tumor-associated vasculature. Our study revealed three key angiographic findings: (1) feeder vessels are only observed in iris melanoma and are absent in iris nevi; (2) while intrinsic vessels (and fluorescein leakage) are found in both iris nevi and iris melanomas, they are more frequently observed in iris melanomas and are significantly associated with malignancy; and (3) angiographic silence is only demonstrated by iris nevi and does not occur in iris melanomas. Overall, the presence of intrinsic vessels +/− feeder vessels had high sensitivity (0.85) and high PPV (0.87) for diagnosis of iris melanoma (Table 2).
Prior studies have also described the differential patterns of tumor-associated vasculature in melanocytic iris lesions. An aggregate of published studies reveals that feeder vessels were present in 6% of iris nevi (n = 13/201, range 0–77%) [11, 13, 14] and feeder vessels in 19% (n = 13/67, range 0–71%) of biopsy-proven iris melanoma [9, 10, 11, 13, 14, 20, 21]. Our findings are consistent with those reported by Brovkina et al. [13] who noted that feeder vessels exclusively occur in iris melanoma. Among published studies, intrinsic vessels were found in 24–100% of iris melanomas and 0–33% of iris nevi [9, 10, 11, 13, 14, 20, 21]. Our study identified a significant association of intrinsic vessels (and their leakage) with malignancy. It may be that the presence of intrinsic vessels is a function of size, since iris melanomas, in general, have greater basal dimension and thickness.
Similar to our observations, angiographic leakage has been shown in both iris nevi and melanoma [9, 10, 11, 13, 14, 20, 21]. Fluorescein leakage, appears to be a secondary finding that reflects incompetence of intrinsic vessels, the extent of which is influenced by tumor pigmentation (masking). Moreover, the area and intensity of leakage increase with time since injection and may be difficult to interpret. Hence, we did not include leakage as an independent variable in predictive value calculations.
With respect to angiographic silence, Brovkina et al. [13] and Kottow et al. [20] highlight an association with iris nevi rather than iris melanoma. We observed angiographic silence exclusively in iris nevi. Overall, the presence of intrinsic vessels +/− feeder vessels had high sensitivity (0.85) and high PPV (0.87) for diagnosis of iris melanoma, although specificity (0.67) and NPV (0.62) were lower implying that presence of these angiographic feature supported the diagnosis of iris melanoma and absence of these findings could not exclude melanoma. Sensitivity, specificity, and PPV/NPV could not be calculated from published studies due to lack of patient-level data.
When compared to existing literature, our study population demonstrates a few unique features. To begin with, we describe the angiographic findings in histopathologically proven iris melanomas, which represents the largest such reported series [9, 10, 11, 12, 13, 14, 20, 21, 22]. Some of the cases in prior studies diagnosed as melanoma may in fact have been iris nevi as the classification system has evolved by downgrading spindle A melanoma to nevi [15, 23, 24]. Furthermore, our cohort of iris nevi represents an enriched population of iris nevi suspicious to be melanoma. These nevi clinically resembled iris melanoma and were referred to a tertiary care oncology center for evaluation and underwent excision in select cases. It is therefore very likely that our study population of nevi represents a higher grade of iris nevi, and the angiographic pattern of nevi observed by us may not be applicable to garden variety iris nevus and may be the underlying reason for observed differences from the published studies. On the other hand, the angiographic patterns observed herein are more relevant for differentiating nevus from melanoma. As the AS-FA images were not read blindly, the interpretation of angiographic features may be subject to potential bias.
In conclusion, anterior segment fluorescein angiography appears to be useful in discerning iris melanoma from iris nevi based on characteristic vascular features. Our observations need to be validated on larger but similarly well-characterized study population.
Statement of Ethics
The study protocol (IRB# 21-793) was approved by the Cleveland Clinic Institutional Review Board. Patient consent was not required in accordance with local or national guidelines. The research complied with the guidelines for human studies and that the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
Arun D. Singh is the Editor-in-Chief of Ocular Oncology and Pathology and has reported having relevant financial activities outside the submitted work: Aura Biosciences (stock options, consultancy), IsoAid LLC (consultancy), Immunocore (consultancy), and Isoaid (consultancy). The rest of the authors have no relevant financial activities.
Funding Sources
This work was supported by a Research to Prevent Blindness Challenge Grant, Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine.
Author Contributions
Arun D. Singh and Janani Singaravelu both contributed with the writing of the manuscript. Jaquelyn Wrenn assisted with data collection. Alexander Melendez-Moreno performed the statistical analysis.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Mark Harrod, CRA, OCT-C (Cole Eye Institute; Cleveland Clinic, Cleveland, OH) ophthalmic photographer who imaged most of these cases.
Funding Statement
This work was supported by a Research to Prevent Blindness Challenge Grant, Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine.
References
- 1.Damato B, Singh AD, Ohio Library and Information Network . Clinical ophthalmic oncology: uveal tumors. Cham, Switzerland: Springer; 2019. [Google Scholar]
- 2.Harbour JW, Augsburger JJ, Eagle RC., Jr Initial management and follow-up of melanocytic iris tumors. Ophthalmology. 1995 Dec;102((12)):1987–93. doi: 10.1016/s0161-6420(95)30765-8. [DOI] [PubMed] [Google Scholar]
- 3.Arentsen JJ, Green WR. Melanoma of the iris: report of 72 cases treated surgically. Ophthalmic Surg. 1975;6((2)):23–37. [PubMed] [Google Scholar]
- 4.Shields JA, Sanborn GE, Augsburger JJ. The differential diagnosis of malignant melanoma of the iris. A clinical study of 200 patients. Ophthalmology. 1983 Jun;90((6)):716–720. doi: 10.1016/s0161-6420(83)34500-0. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman LE. Clinical Pathology of Iris tumors. Am J Ophthalmol. 1963 Aug;56:183–195. doi: 10.1016/0002-9394(63)91848-8. [DOI] [PubMed] [Google Scholar]
- 6.Kersten RC, Tse DT, Anderson R. Iris melanoma. Nevus or malignancy? Surv Ophthalmol. 1985 May-Jun;29((6)):423–433. doi: 10.1016/0039-6257(85)90207-3. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339((6219)):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 8.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020 May;77((9)):1745–70. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dart JK, Marsh RJ, Garner A, Cooling RJ. Fluorescein angiography of anterior uveal melanocytic tumours. Br J Ophthalmol. 1988 May;72((5)):326–337. doi: 10.1136/bjo.72.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodes BL, Gildenhar M, Choromokos E. Fluorescein angiography in pigmented iris tumors. Arch Ophthalmol. 1979 Jun;97((6)):1086–8. doi: 10.1001/archopht.1979.01020010540005. [DOI] [PubMed] [Google Scholar]
- 11.Jakobiec FA, Depot MJ, Henkind P, Spencer WH. Fluorescein angiographic patterns of iris melanocytic tumors. Arch Ophthalmol. 1982 Aug;100((8)):1288–99. doi: 10.1001/archopht.1982.01030040266014. [DOI] [PubMed] [Google Scholar]
- 12.Demeler U. Fluorescence angiographical studies in the diagnosis and follow-up of tumors of the iris and ciliary body. Adv Ophthalmol. 1981;42:1–17. [PubMed] [Google Scholar]
- 13.Brovkina AF, Chichua AG. Value of fluorescein iridography in diagnosis of tumours of the iridociliary zone. Br J Ophthalmol. 1979 Mar;63((3)):157–160. doi: 10.1136/bjo.63.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandello F, Brancato R, Lattanzio R, Carnevalini A, Rossi A, Coscas G. Biomicroscopy and fluorescein angiography of pigmented iris tumors. A retrospective study on 44 cases. Int Ophthalmol. 1994;18((2)):61–70. doi: 10.1007/BF00919241. [DOI] [PubMed] [Google Scholar]
- 15.Jakobiec FA, Silbert G. Are most iris “melanomas” really nevi? A clinicopathologic study of 189 lesions. Arch Ophthalmol. 1981 Dec;99((12)):2117–32. doi: 10.1001/archopht.1981.03930020993002. [DOI] [PubMed] [Google Scholar]
- 16.Jakobiec FA, Coleman DJ, Chattock A, Smith M. Ultrasonically guided needle biopsy and cytologic diagnosis of solid intraocular tumors. Ophthalmology. 1979 Sep;86((9)):1662–81. doi: 10.1016/s0161-6420(79)35349-0. [DOI] [PubMed] [Google Scholar]
- 17.Khan S, Finger PT, Yu GP, Razzaq L, Jager MJ, de Keizer RJ, et al. Clinical and pathologic characteristics of biopsy-proven iris melanoma: a multicenter international study. Arch Ophthalmol. 2012 Jan;130((1)):57–64. doi: 10.1001/archophthalmol.2011.286. [DOI] [PubMed] [Google Scholar]
- 18.Conway RM, Chua WC, Qureshi C, Billson FA. Primary iris melanoma: diagnostic features and outcome of conservative surgical treatment. Br J Ophthalmol. 2001 Jul;85((7)):848–854. doi: 10.1136/bjo.85.7.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skalet AH, Li Y, Lu CD, Jia Y, Lee B, Husvogt L, et al. Optical coherence tomography angiography characteristics of Iris melanocytic tumors. Ophthalmology. 2017 Feb;124((2)):197–204. doi: 10.1016/j.ophtha.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kottow M. Fluorescein angiographic behaviour of iris masses. Ophthalmologica. 1977;174((4)):217–223. doi: 10.1159/000308605. [DOI] [PubMed] [Google Scholar]
- 21.Geisse LJ, Robertson DM. Iris melanomas. Am J Ophthalmol. 1985 Jun;99((6)):638–648. doi: 10.1016/s0002-9394(14)76028-3. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Bron AJ, Easty D. A study of iris masses by fluorescein angiography. Trans Ophthalmol Soc U K. 1971;91:199–205. [PubMed] [Google Scholar]
- 23.McLean IW, Zimmerman LE, Evans RM. Reappraisal of callender's spindle a type of malignant melanoma of choroid and ciliary body. Am J Ophthalmol. 1978 Oct;86((4)):557–564. doi: 10.1016/0002-9394(78)90307-0. [DOI] [PubMed] [Google Scholar]
- 24.McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of callender's classification of uveal melanoma at the armed forces institute of pathology. Am J Ophthalmol. 2018 Nov;195:lvi–lx. doi: 10.1016/j.ajo.2018.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.