Abstract
The perivascular sensory nerve (PvN) Ca2+-sensing receptor (CaR) is implicated in Ca2+-induced relaxation of isolated, phenylephrine (PE)-contracted mesenteric arteries, which involves the vascular endogenous cannabinoid system. We determined the effect of inhibition of diacylglycerol (DAG) lipase (DAGL), phospholipase A2 (PLA2), and cytochrome P-450 (CYP) on Ca2+-induced relaxation of PE-contracted rat mesenteric arteries. Our findings indicate that Ca2+-induced vasorelaxation is not dependent on the endothelium. The DAGL inhibitor RHC 802675 (1 μM) and the CYP and PLA2 inhibitors quinacrine (5 μM) (EC50: RHC 802675 2.8 ± 0.4 mM vs. control 1.4 ± 0.3 mM; quinacrine 4.8 ± 0.4 mM vs. control 2.0 ± 0.3 mM; n = 5) and arachidonyltrifluoromethyl ketone (AACOCF3, 1 μM) reduced Ca2+-induced relaxation of mesenteric arteries. Synthetic 2-arachidonoyl-glycerol (2-AG) and glycerated epoxyeicosatrienoic acids (GEETs) induced concentration-dependent relaxation of isolated arteries. 2-AG relaxations were blocked by iberiotoxin (IBTX) (EC50: control 0.96 ± 0.14 nM, IBTX 1.3 ± 0.5 μM) and miconazole (48 ± 3%), and 11,12-GEET responses were blocked by IBTX (EC50: control 55 ± 9 nM, IBTX 690 ± 96 nM) and SR-141716A. The data suggest that activation of the CaR in the PvN network by Ca2+ leads to synthesis and/or release of metabolites of the CYP epoxygenase pathway and metabolism of DAG to 2-AG and subsequently to GEETs. The findings indicate a role for 2-AG and its metabolites in Ca2+-induced relaxation of resistance arteries; therefore this receptor may be a potential target for the development of new vasodilator compounds for antihypertensive therapy.
Keywords: Ca2+-sensing receptor, arachidonic acid, vasorelaxation
Ionized calcium (Ca2+) is a second messenger involved in a number of cellular functions, such as secretion of hormones, muscle contraction, endocytosis, enzyme control, regulation of gene expression, as well as cell proliferation, differentiation, and apoptosis. The involvement of Ca2+ in coronary and vascular smooth muscle (VSM) reactivity has a bearing on vessel tone and blood pressure control. High Ca2+ intake has been shown to lower blood pressure in animal models of hypertension (31, 32, 40, 50), and diets high in fruits and vegetables, supplemented with predominantly low-fat milk, significantly reduced blood pressure compared with fruit- and vegetable-only diets (1, 3, 4, 57). Current evidence suggests that adequate Ca2+ intake (1,000–1,500 mg/day) is critical to optimal blood pressure regulation, and randomized controlled trials have revealed significant reductions in hypertension risk and blood pressure levels in humans (48, 49). It is now clear that Ca2+ availability to organs that participate in cardiovascular control is a key factor in blood pressure regulation. Although there is abundant clinical evidence to support the blood pressure-lowering effect of dietary Ca2+, mechanisms underlying this phenomenon are not clear, and no plausible explanation as to how Ca2+ lowers blood pressure exists.
In a series of studies, Ca2+-induced relaxation of mesenteric arteries was found to be dependent on an intact perivascular sensory nerve (PvN) network that expresses a Ca2+-sensing receptor (CaR), providing strong evidence that the CaR may serve as the missing link between Ca2+ intake and vascular function (8–10, 36, 51). We have demonstrated that dorsal root ganglion (DRG) expresses a CaR (8, 62, 63) and also provided further evidence that Ca2+-induced relaxation of isolated precontracted mesenteric arteries is mediated in part by a hyperpolarizing endocannabinoid vasodilator transmitter (36). On the basis of this cumulative evidence, we hypothesized that activation of the PvN CaR releases endocannabinoids, 2-arachi-donoylglycerol (2-AG) and anandamide (AEA), that are metabolized by cytochrome P-450 (CYP) and phospholipase A2 (PLA2) to generate hyperpolarizing vasodilator compounds (8, 11, 36). This conclusion was based largely on the finding that the CB1 receptor antagonist SR-141716A and the novel anandamide/abnormal cannabidiol receptor antagonist O-1918 partially inhibited Ca2+-induced relaxation, thus suggesting a role for a hyperpolarizing vasodilator with activity at an endocannabinoid receptor. Furthermore, we demonstrated that iberiotoxin (IBTX), a Ca2+-activated K+ (KCa) channel blocker, reduced Ca2+-induced relaxation of isolated rat mesenteric arteries (36). The involvement of KCa channels is consistent with a role for endothelium-derived factors that modulate myogenic tone. Resistance artery function may be dependent on the balance between localized generation of vasoconstrictor and vasodilator substances (23–25). In the present study, we provide additional evidence to support the linkage between activation of the PvN CaR and vascular relaxation and propose that the receptor mediates the generation of glycerated epoxyeicosatrienoic acids (GEETs), which serve as vasodilators in this system.
MATERIALS AND METHODS
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee. Male Wistar rats, 10–12 wk of age (Harlan Sprague Dawley, Indianapolis, IN), were maintained at constant temperature and humidity with fixed light-dark cycles and provided standard rodent chow (Harlan Tekland, Madison, WI) and water ad libitum.
Drugs.
Acetylcholine (ACh) hydrochloride, phenylephrine (PE), 1,6-bis(cyclohexyloximino-carbonylamino)hexane (RHC 80267), miconazole, quinacrine, IBTX, and SR-141716A were from Sigma (St. Louis, MO); arachidonyltrifluoromethyl ketone (AACOCF3) were from EMD Chemicals (San Diego, CA); and 2-AG and GEET compounds were supplied by Dr. J. R. Falck of the University of Texas Southwestern Medical School.
Vessel isolation.
Mesenteric arteries were dissected from rats that were deeply anesthetized with isoflurane and killed by open-chest cardiac puncture. The small intestine and its feeding vessels were removed in block and placed in physiological salt solution (PSS; mM: 115 NaCl, 4.7 KCl, 1.4 MgSO4·7H2O, 5 NaHCO3, 1.2 KH2PO4, 1.1 Na2HPO4, 1.0 CaCl2, 20 HEPES, and 5 glucose, pH 7.4). Branch I and II arteries were carefully dissected from the surrounding fat and mesenterium, taking care to leave a portion of the omental membrane attached to the vessel. A 40-μm-diameter stainless steel wire was then inserted into the lumen to facilitate easy handling of the vessel segment between solutions and for mounting in the myograph chamber.
Wire myography.
Isometric force generation in isolated arteries was determined by established methods (11). Briefly, 2-mm-long segments of mesenteric branch arteries were mounted in a small-vessel Mulvany-Halpern 510A Auto Dual Wire Myograph (DMT-USA, Marietta, GA) by means of tungsten-free stainless steel wires inserted through the lumen, maintained in PSS with 100 μM ascorbic acid, and gassed with a mixture of 95% air and 5% CO2. The myograph is designed with an automated normalization function controlled from the interface “Normalization” menu, and a standardized procedure was carried out according to the manufacturer’s protocol after 30-min equilibration at 37°C. The following normalization parameters for the mesenteric artery were used: target transmural pressure 13.3 kPa (100 mmHg); time 60 s; IC1/IC100 = 0.9 (IC100 = internal circumference corresponding to target pressure, IC1 = normalized internal circumference); eyepiece calibration 2*Δ (mm/division). The procedure defines the lumen diameter (d100) that the artery would have had in vivo when relaxed and under a transmural pressure of 100 mmHg (2, 30). The arteries were then set to the lumen diameter of d1 = 0.9 × d100, where active force development is maximal. Active force development of ≥10 mN in arteries was considered optimum for the experiment to proceed. The vessels were then challenged with 5 μM phenylephrine (PE) until reproducible contractions were observed. Relaxation was assessed by addition of graded concentrations of Ca2+ and test compounds cumulatively to vessels that were precontracted to maximal tone. When inhibitors were used, tissues were preincubated with the compounds in the bath for 20 min and present during relaxation assays. The inhibitors had no effect on basal tensions. Except for experiments with ACh, where endothelium-replete and endothelium-denuded arteries were used, all studies were carried out with intact mesenteric arteries.
Statistical analysis.
Concentration-response data were analyzed with SigmaStat 3.1 and SigmaPlot 9.0 statistics programs from Systat Software (Point Richmond, CA). Comparisons between groups and within groups were done by analysis of variance (ANOVA), and differences with P < 0.05 were considered significant. EC50 values were determined from fitting data to a four-parameter logistic function in the Pharmacology menu of the SigmaPlot program.
RESULTS
To determine whether Ca2+-induced relaxation was dependent on the intact endothelium, responses were measured in endothelium-replete and endothelium-denuded arteries. As shown in Fig. 1, removal of the endothelium only reduced Ca2+-induced relaxation by ~20% (Fig. 1A) compared with controls, while ACh-induced responses were reduced by ~80% (Fig. 1B; P < 0.05) at higher concentrations of agonists. A large component of Ca2+-induced relaxation was retained after removal of the endothelium. Figure 2 shows the effect of the diacylglycerol (DAG) lipase (DAGL) inhibitor RHC 80267 on Ca2+-induced relaxation, which was reduced by inhibitor and reversed by washing. The EC50 for Ca2+-induced relaxation was 2.8 ± 0.4 mM in the presence of the inhibitor compared with 1.4 ± 0.3 mM for control, a 50% reduction in Ca2+ sensitivity (P < 0.05). To further explore the possible involvement of DAG and its downstream metabolites in Ca2+-induced relaxation, we examined the effects of the CYP inhibitors miconazole and quinacrine (also an inhibitor of PLA2) as well as AACOCF3 and SR-141716A on Ca2+-induced vascular relaxation. In the presence of 5 μM miconazole, Ca2+-induced relaxation was completely blocked, whereas quinacrine and AACOCF3 partially blocked this response (Figs. 3 and 4). The EC50 in the presence of quinacrine was 4.8 ± 0.4 mM compared with 2.0 ± 0.3 mM for control, about a 2.5-fold reduction in Ca2+ sensitivity (P < 0.05). The CB1 receptor antagonist SR-141716A also blocked Ca2+-induced relaxation of PE-contracted arteries in a concentration-dependent manner as shown in Fig. 5.
Fig. 1.
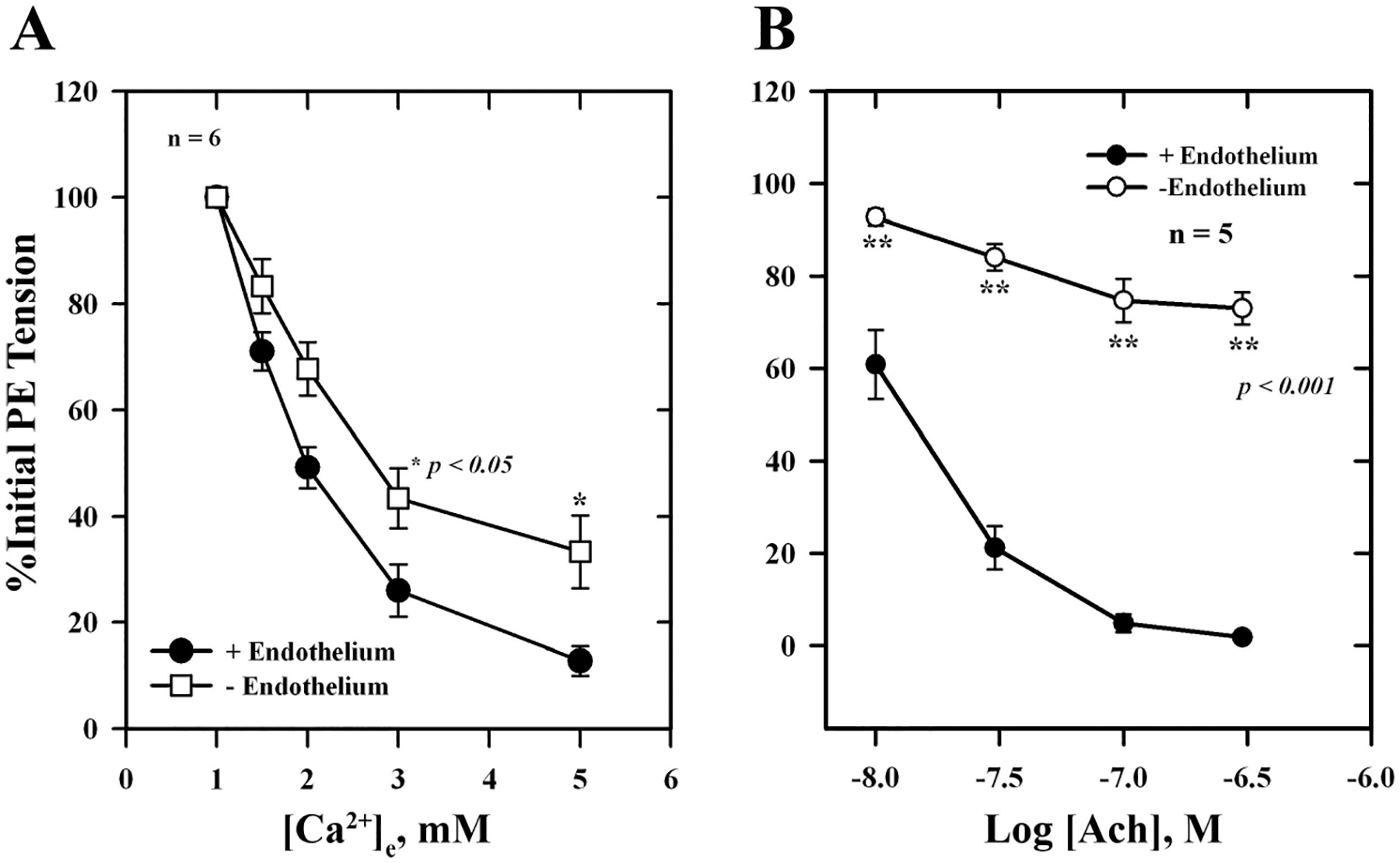
A: extracellular Ca2+ -induced relaxation of isolated, endothelium-replete (■) and endothelium-denuded (□) phenylephrine (PE)-contracted mesenteric arteries. B: acetylcholine (ACh)-induced relaxation of endothelium-replete (●) and endothelium-denuded (○) arteries precontracted with 5 μM PE. Values are means ± SE of 5 experiments. *,**Significantly different from endothelium replete.
Fig. 2.
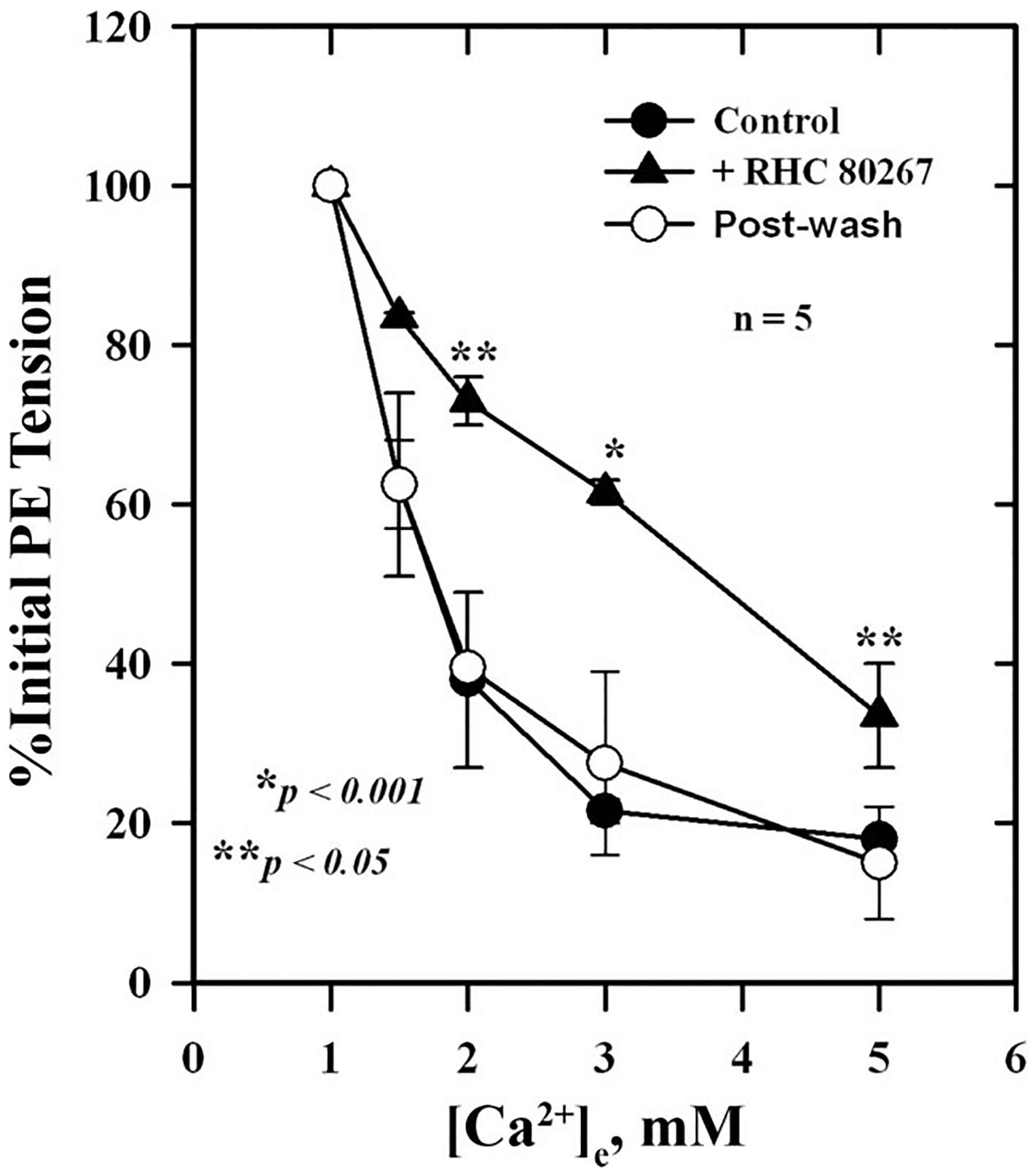
Effect of the diacylglycerol (DAG) lipase (DAGL) inhibitor RHC 80267 on Ca2+-induced relaxation of isolated, PE-contracted mesenteric arteries. Arteries were mounted on a wire myograph in physiological salt solution (PSS) containing 1 mM Ca2+ and equilibrated for 30 min at 37°C with constant aeration. Relaxations were induced by increasing concentrations of and were measured in the presence (▲) and absence (●) of 5 μM RHC 80267 and after washout (○) of the inhibitor. Values are means ± SE of 5 experiments. *,**Significantly different from control.
Fig. 3.
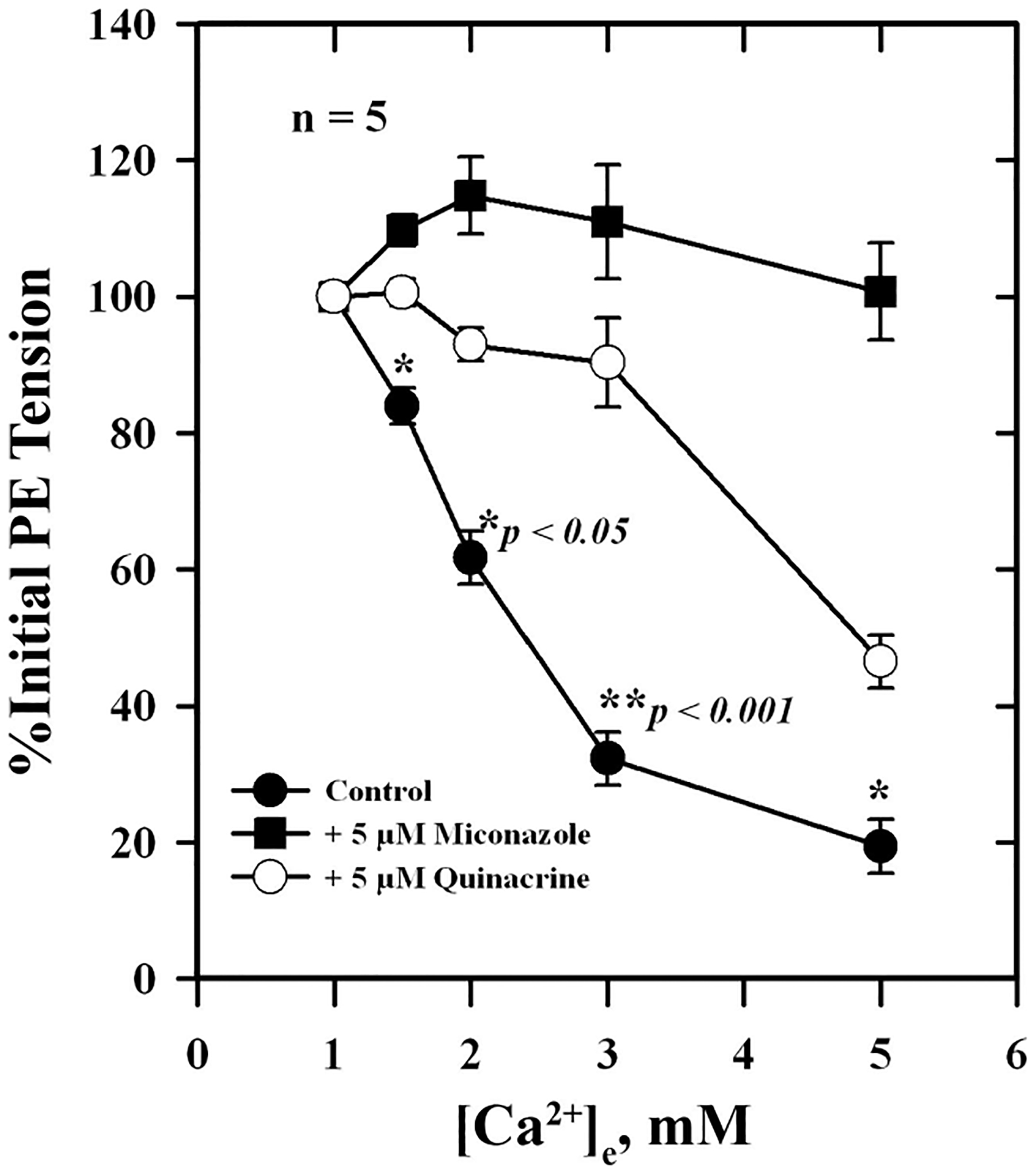
Effects of miconazole and quinacrine on Ca2+-induced relaxation of isolated, PE-contracted mesenteric arteries. Arteries were mounted on a wire myograph in PSS containing 1 mM Ca2+ and equilibrated for 30 min at 37°C with constant aeration. Relaxations to increasing concentrations of were measured in the absence (●) or presence of 5 μM miconazole (■) or 5 μM quinacrine (○). Values are means ± SE of 5 experiments. *,**Significantly different from quinacrine and miconazole.
Fig. 4.
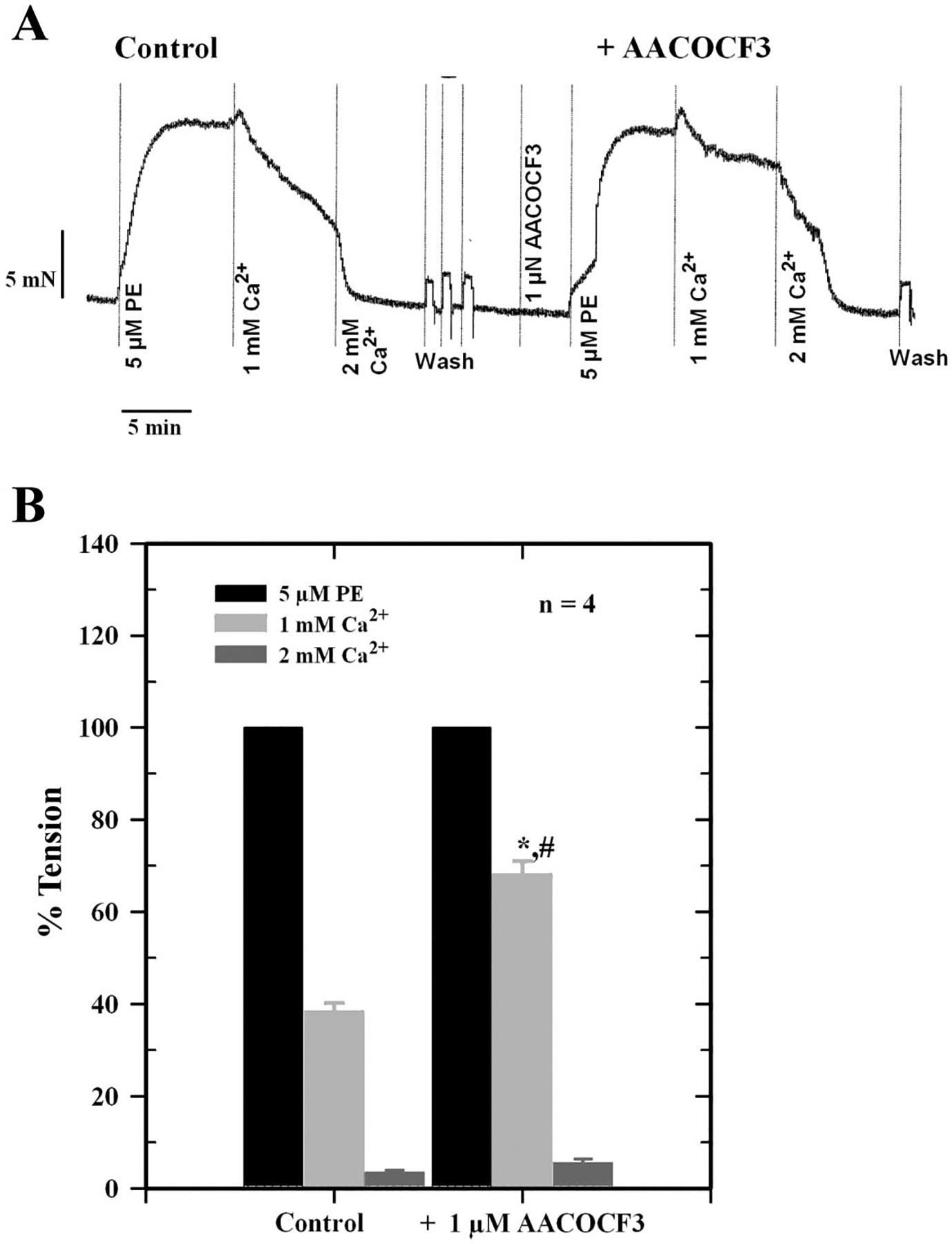
Effect of the specific phospholipase A2 (PLA2) inhibitor arachidonyltrifluoromethyl ketone (AACOCF3) on Ca2+-induced relaxation of PE-contracted mesenteric artery. A: force tracings showing typical relaxation of vessel in the absence (Control) or presence of 1 μM AACOCF3. B: histogram showing inhibition of Ca2+-induced relaxation of mesenteric arteries by AACOCF3. Data are means ± SE of 4 separate experiments. *Significantly different from PE tension; #significantly different from control (P < 0.05).
Fig. 5.
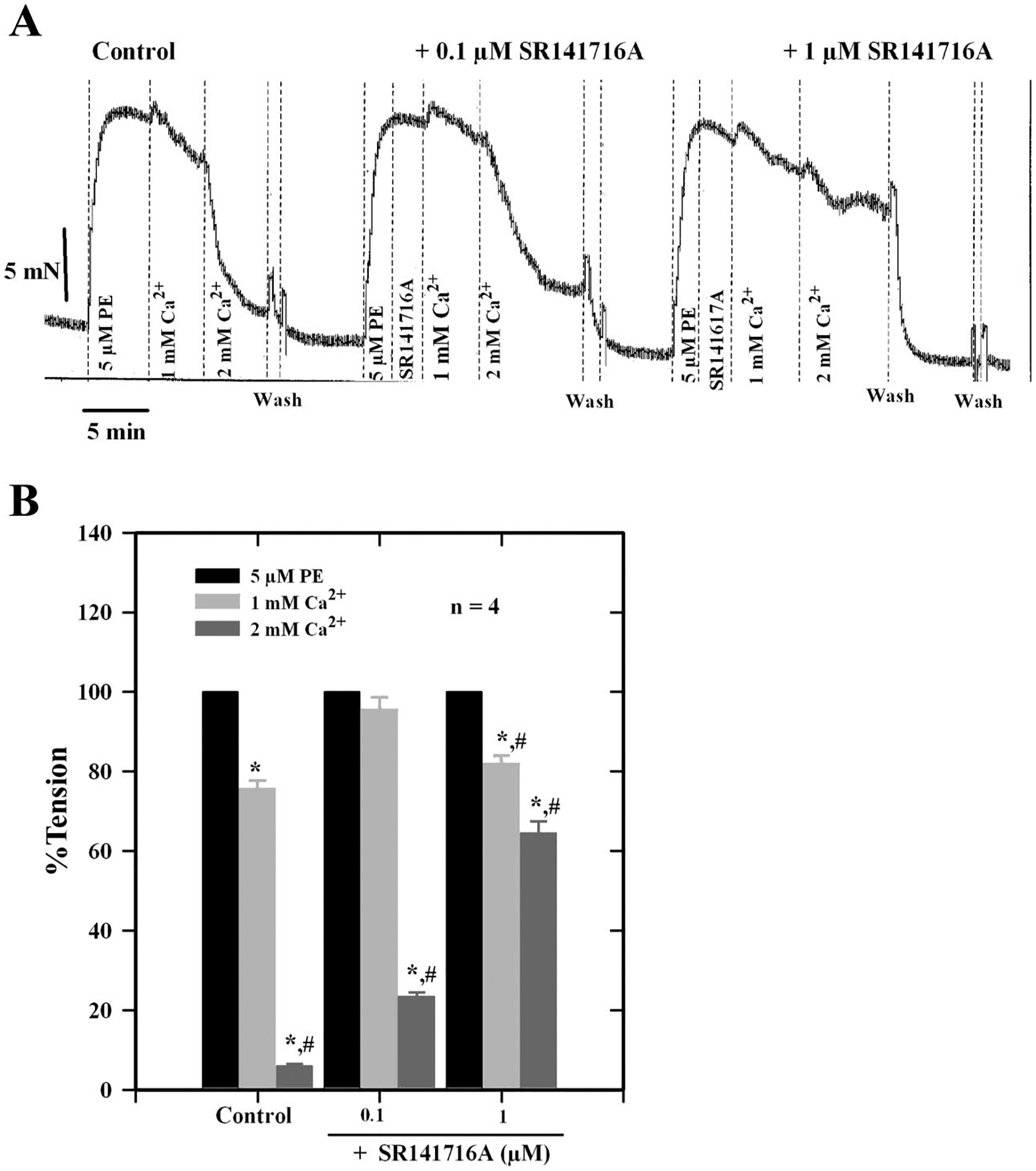
A: force tracings showing the effect of graded concentrations of SR-141716A on Ca2+-induced relaxation of a PE-contracted mesenteric artery. B: histogram showing the concentration-dependent inhibition of Ca2+-induced relaxation of mesenteric arteries by SR-141716A, a CB1 receptor antagonist. Data are means ± SE of 4 separate experiments. *Significantly different from PE tension; #significantly different from controls (P < 0.05).
To determine whether 2-AG and its CYP metabolites are able to relax PE-contracted mesenteric arteries, we tested synthetic 2-AG and GEET compounds (5,6-GEET, 8,9-GEET, 11,12-GEET, and 14,15-GEET) derived from CYP metabolism of 2-AG as well as the effect of CB1 and KCa channel blockade on responses. Figure 6A shows relaxation of PE-contracted mesenteric artery segments following graded additions of Ca2+ or 2-AG. 2-AG induced concentration-dependent relaxation with an EC50 value of 0.96 ± 0.14 nM. The curve was shifted to the right in the presence of 100 nM IBTX (EC50 = 1.3 ± 0.5 μM) (Fig. 6B). Miconazole (1 μM) also blocked 2-AG relaxation by ~48 ± 3% (Fig. 6C). Similar concentration-response curves were obtained for the GEET compounds (Fig. 7A). The 5,6-, 11,12-, and 14,15-GEET regioisomers relaxed precontracted arteries with EC50 values ranging from 18 to 55 nM, and the EC50 for 8,9-GEET was 450 nM. The data show that isomers of GEET induced relaxation of PE-contracted arteries, which was blocked by IBTX, a KCa channel blocker (Table 1, Fig. 7B, and Fig. 8). The concentration-response curve was shifted to the right by the inhibitor. The EC50 for the 11,12-GEET response in the presence of 100 nM IBTX was ~690 ± 96 nM compared with 55 ± 4 nM for control (P < 0.001), about a 12-fold reduction in sensitivity. The 14,15-GEET response was also attenuated by 1 μM SR-141716A, a CB1 antagonist with an EC50 of 20 ± 3 nM, but a large component of this response (≈40% at the highest concentration) was unaffected (Fig. 8).
Fig. 6.
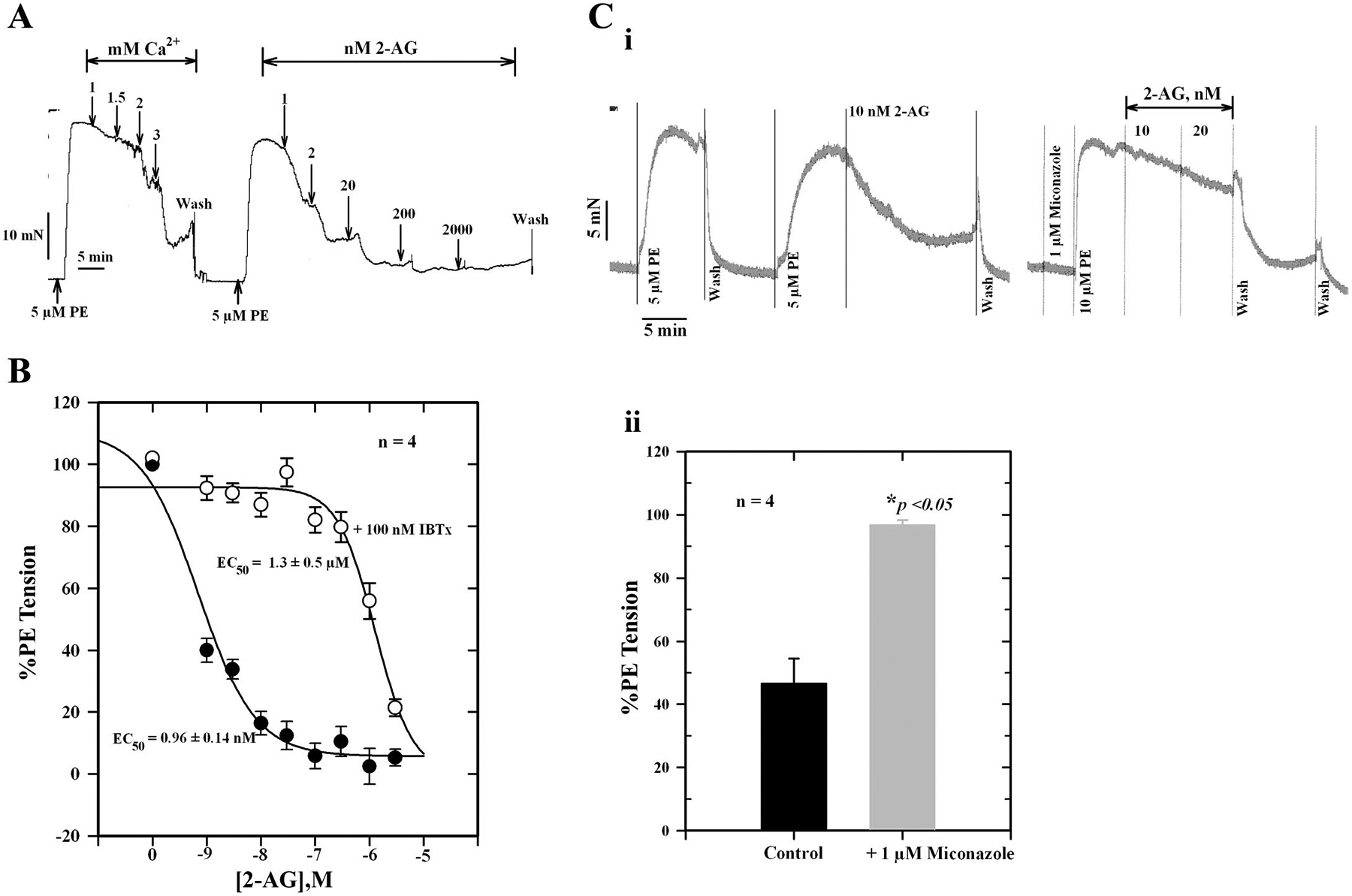
A: force tracings showing relaxation of an isolated, PE-contracted mesenteric artery segment to graded concentrations of Ca2+ and 2-arachidonoylglycerol (2-AG). B: effect of 100 nM iberiotoxin (IBTX) on 2-AG-induced relaxation of isolated, PE-precontracted mesenteric arteries. IBTX shifted the 2-AG concentration-effect curve to the right. C: i: force tracings showing the effect of 1 μM miconazole on 2-AG-induced relaxation. ii: Histogram showing the inhibition of relaxation by miconazole. Values are means ± SE of 4 separate experiments. *Significantly different from control (P < 0.05).
Fig. 7.
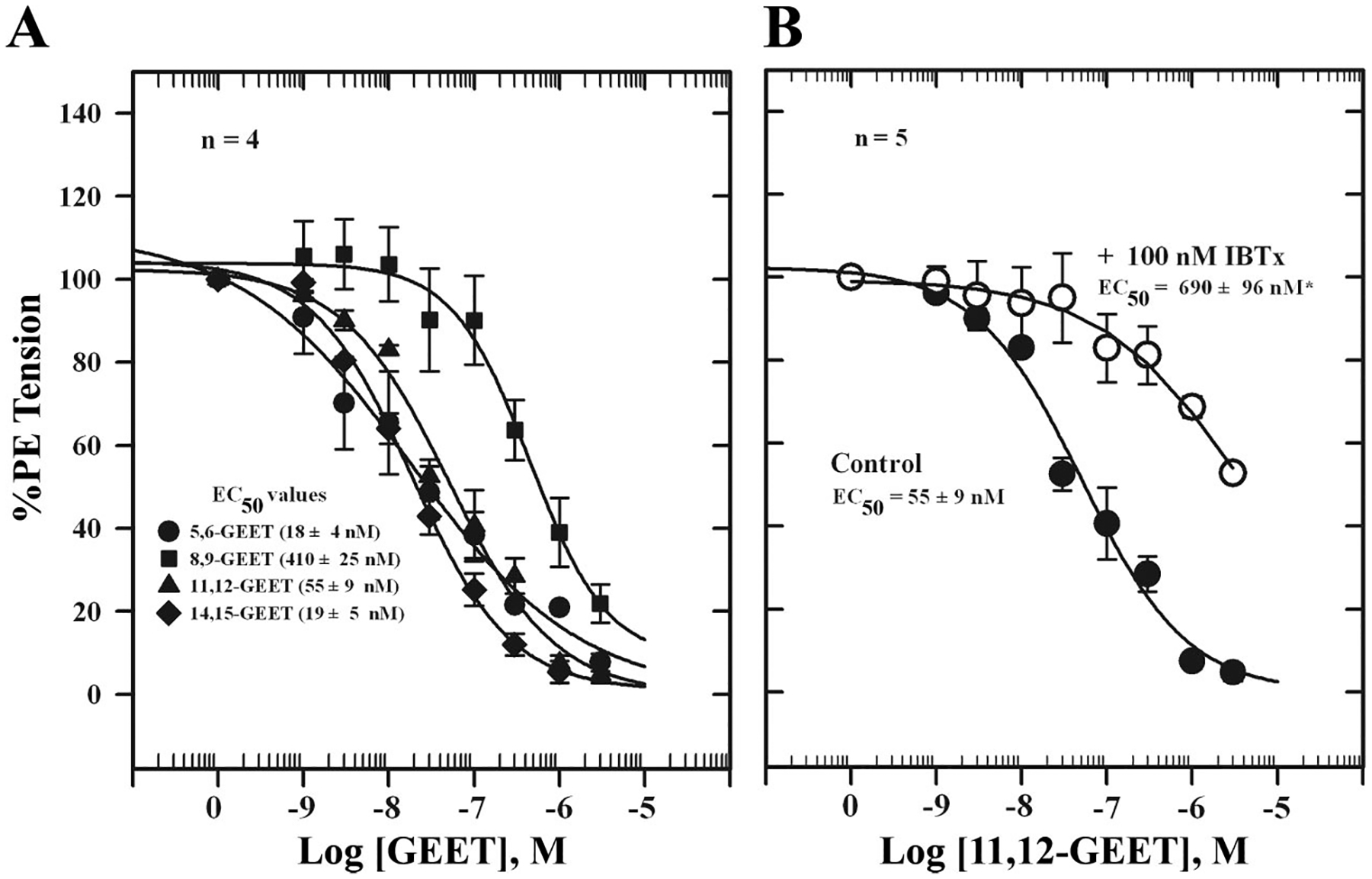
A: glycerated epoxyeicosatrienoic acid (GEET)-induced relaxation of isolated, PE-contracted mesenteric arteries mounted in a wire myograph in PSS containing 1 mM Ca2+ and equilibrated for 30 min at 37°C with constant aeration and responses to GEET isomers determined. B: effect of the Ca2+-activated K+ (KCa) channel inhibitor IBTX on 11,12-GEET-induced relaxation of isolated, PE-contracted mesenteric arteries incubated with 100 nM IBTX for 10 min before cumulative additions of 11,12-GEET. Values are means ± SE of 5 experiments. *Significantly different from control (P < 0.05).
Table 1.
EC50 values for GEET-induced relaxation of isolated, PE-contracted rat mesenteric arteries in presence and absence of 100 nM IBTX
| PE Tension, mN | EC50, nM | |||
|---|---|---|---|---|
| GEET Isomer | Control | + 100 nM IBTX | Control | +100 nM IBTX |
| 5,6-GEET | 12.6±1.5 | 11.3±1.2 | 18±4 | 260±60 (P < 0.007) |
| 8,9-GEET | 11.8±1.1 | N.D. | 410±25 | N.D. |
| 11,12-GEET | 10.8±1.6 | 8.7±2.0 | 55±9 | 690±96 (P < 0.001) |
| 14,15-GEET | 13.2±0.9 | 11.7±1.2 | 19±5 | 520±80 (P < 0.001) |
Values are means ± SE for 4 experiments. GEET, glycerated epoxyeicosatrienoic acid; PE, phenylephrine; IBTX, iberiotoxin; N.D, not determined.
Fig. 8.
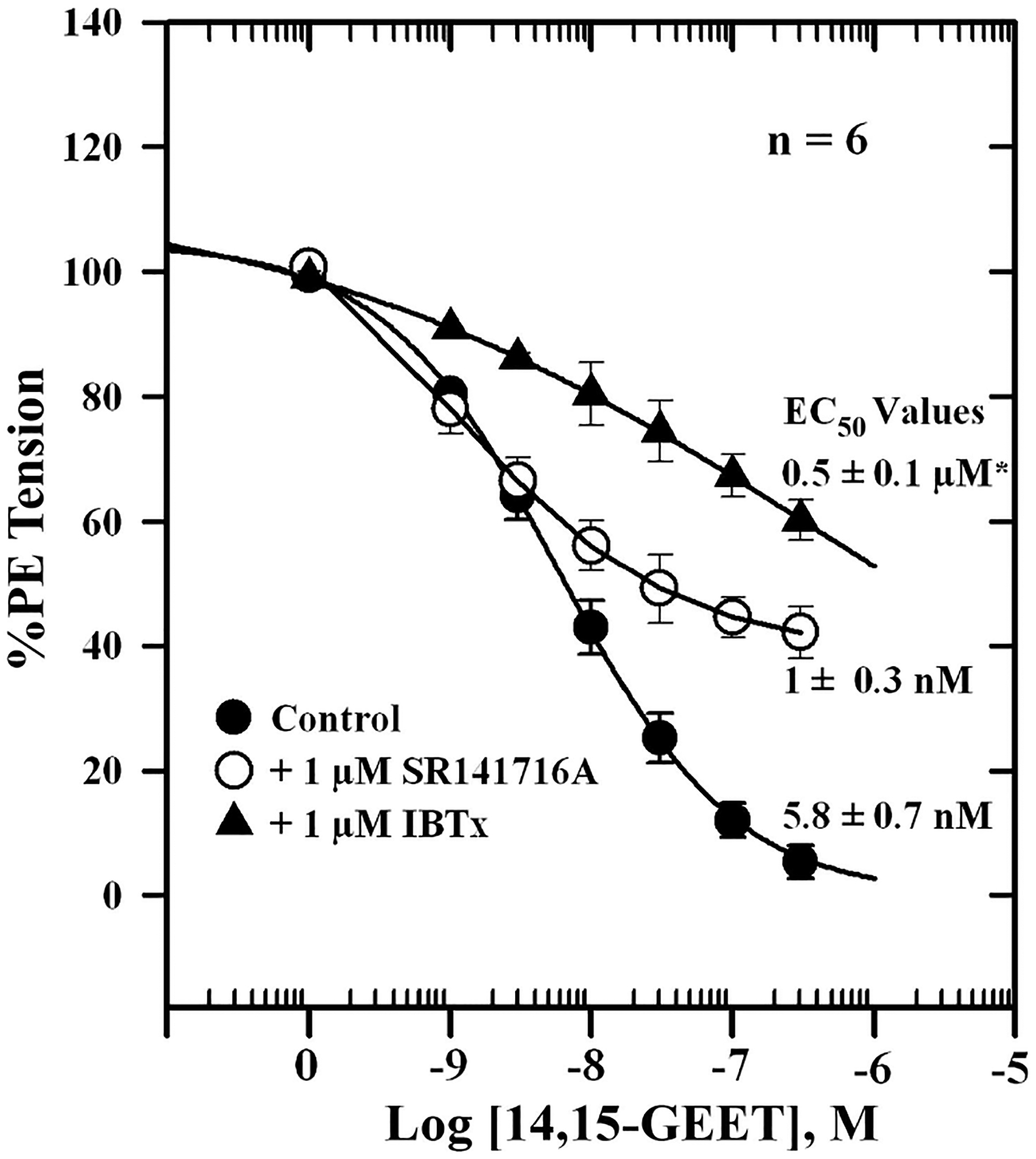
Effects of the CB1 cannabinoid receptor antagonist SR-141716A and IBTX on 14,15-GEET-induced relaxation of isolated, PE-contracted mesenteric arteries. Arteries were mounted in a wire myograph in PSS containing 1 mM Ca2+ and equilibrated for 30 min at 37°C with constant aeration. Responses to 14,15-GEET (●) or 14,15-GEET in the presence of 1 μM SR-141716A (○) or 1 μM IBTX (▲) were determined. Values are means ± SE of 6 experiments. *Significantly different from control (P < 0.05).
DISCUSSION
Earlier studies from our laboratory demonstrate that Ca2+-induced relaxation of PE-contracted mesenteric arteries is reduced by two different KCa channel blockers, IBTX (36) and O-1918 (11), and a CB1 antagonist. These findings have led to the hypothesis that extracellular Ca2+ evokes the release of a hyperpolarizing vasodilator with activity at an endocannabinoid receptor. The results of the present study confirm that Ca2+-induced relaxation of isolated, PE-contracted rat mesenteric arteries is largely independent of a functional endothelium, indicating a mechanism that involves vasodilators apart from endothelium-derived factors such as nitric oxide. A major component of the relaxation is due to endothelium-independent factors, consistent with a role for GEET, and possibly CYP epoxygenase metabolites of arachidonic acid (AA). Thus agonist activation of the perivascular CaR leads to the generation of hyperpolarizing factors from 2-AG, and possibly AA, that activate KCa channels, resulting in the relaxation of smooth muscles.
The CaR signals through the Gαq-phospholipase C pathway (7); thus downstream DAG production is involved. DAG is a strong activator of protein kinase C (PKC) and is metabolized by enzymes such as DAGL to 2-AG (27, 61). In neuronal cells, activation of the metabotropic glutamate receptor, a C family G protein-coupled receptor (GPCR) similar to the CaR, leads to the breakdown of DAG to 2-AG and subsequently to AA (20, 21, 38). The reduction in relaxation observed in the presence of RHC 80267, a selective inhibitor of DAGL and a tool for investigating the role of DAG and AA in physiological processes, suggests that Ca2+-induced relaxation involves the conversion of DAG to 2-AG, a known vasodilator, and its downstream metabolites, AA by PLA2 and GEET by CYP epoxygenase. RHC 80267 has been shown to potentiate ACh-induced relaxation of contracted mesenteric arteries via inhibition of acetylcholinesterase in the vascular wall (28), an effect independent of inhibition of nitric oxide synthase, PKC, or PLA2. Inhibition of CYP by miconazole and CYP/PLA2 with quinacrine as well as blockade of Ca2+-induced relaxation by the specific PLA2 inhibitor AACOCF3 in the present study suggest that Ca2+-induced relaxation is mediated by CYP metabolites of 2-AG and/or its PLA2 metabolites that may have been converted to vasodilators downstream. Synthetic GEET metabolites of 2-AG relaxed PE-contracted mesenteric arteries, which was blocked by IBTX, and the 2-AG effect was blocked by IBTX and miconazole, indicating that the process involves hyperpolarization. The CB1 receptor antagonist SR-141716A also partially blocked Ca2+-induced and GEET-induced relaxation, suggesting involvement of the endocannabinoid system. Therefore, these results support the notion that Ca2+-induced relaxation of isolated mesenteric arteries is mediated by DAG metabolites. Thus activation of the sensory nerve CaR leads to release of 2-AG and its metabolism to the CYP metabolite GEET that contribute to Ca2+-induced relaxation. The higher EC50 for 8,9-GEET was unexpected and suggests isomerization, decomposition, or a lower potency for this isomer. The GEET regioisomers are labile compounds and degrade over time.
CYP metabolites of the AA monoxygenase pathway are major players in the bioactivation and physiological actions of AA (15–17). CYP hydroxylase-derived 20-hydroxyeicosatetraenoic acid (20-HETE) is a potent vasoconstrictor and CYP epoxygenase-derived EET a natriuretic vasodilator in the kidney (24, 25). In rat mesenteric artery, involvement of ACh-induced CYP-derived metabolites of AA in hyperpolarization is minimal (26), and chronic hypoxia was shown to enhance CYP2C9 expression in association with increased 11,12-EET production leading to KCa-dependent hyperpolarization of VSM and attenuated reactivity (22). Furthermore, CYP metabolites of AA may be important mediators of angiotensin II-induced constriction of rat mesenteric arteries in vivo (18). In the peripheral vasculature, AA is metabolized via CYP-dependent pathways to EET or 20-HETE, the latter being metabolized further to prostaglandins (56). EETs (5,6-, 8,9-, 11,12-, and 14,15-EET) are vasodilators that show no predictable stereoselectivity in their dilator responses (64, 65). Apart from their properties as powerful vasodilators, these compounds have also been shown to be powerful and selective angiogenic lipids, suggesting a physiological role for them in angiogenesis and de novo vascularization (53). The inhibition of Ca2+-induced relaxation of PE-contracted mesenteric arteries by quinacrine and AACOCF3 suggests that a pathway through PLA2 is also involved. Thus it appears that stimulation of the perivascular nerve CaR leads to PLA2 activation and release of AA from membrane phospholipids and metabolism by CYP to vasodilatory compounds. The CYP epoxygenases metabolize endogenous pools of AA to EETs, and several isoforms of CYP have been identified as predominant stereoselective epoxygenases in human, rat, and mouse tissues (19, 20, 56, 58). There is abundant evidence to indicate that EETs are vasodilators produced from AA in the endothelium by CYP epoxygenase and hyperpolarize VSM cells by opening KCa channels (5, 12–14, 22). Several studies have also shown that CYP metabolites regulate cellular Ca2+ responses by exerting their effects on store-operated calcium entry and voltage-gated Ca2+ entry channels; however, the nature of some of these Ca2+ entry pathways has not been fully determined. The mechanism by which CYP metabolites of AA activate intracellular second messenger systems is not clear, although specific GPCRs may be involved. Specific CYPs have been shown to localize in VSM and endothelium and contribute to the regulation of vascular tone and homeostasis. However, nerve-derived hyperpolarizing factors may also play a role. Studies on astrocytes, which express glutamatergic and γ-aminobutyric acid receptors, have shown the involvement of increased Ca2+ and EET production in the regulation of cerebrovascular blood flow (29, 35, 39). Furthermore, increases in intracellular Ca2+ concentration in astrocytic processes precede vasodilation of arterioles within cortical slices (59). It is therefore reasonable to assume from the present studies that CaR-mediated generation of EETs in perivascular nerves may also play a role in Ca2+-induced relaxation. This mechanism may therefore be important in counteracting the effects of vasoconstrictor CYP metabolites that are associated with increased vascular tone as in hypertension. A recent study by Ohanian et al. (52) demonstrated a functional role for the CaR in regulating myogenic tone in rat subcutaneous small arteries, which was dependent on PKC.
Endocannabinoids such as AEA are membrane-derived signaling molecules released “on demand” from nerves (54), blood cells, and endothelial cells. AEA is synthesized from its precursor N-arachidonoylphosphatidylethanolamine from membrane phospholipids by hydrolysis through multiple pathways and plays a role in the regulation of numerous physiological and pathological processes (42–44). These findings indicate that biosynthetic and degrading enzymes are key regulators of lipid signaling in the vasculature (41, 47). Therefore, signaling pathways that lead to the activation of these regulatory enzymes will be important in maintaining normal vascular tone, and this process may be altered under disease conditions, such as in hypertension, where vascular reactivity is increased. Our earlier finding (36) that Ca2+-induced relaxation is reduced by the CB1 antagonist SR-141716A and the present data indicating attenuation of the 14,15-GEET-induced response in the presence of SR-141716A support the involvement of a CB1 receptor pathway in the GEET response. A large component of the response, however, was unaffected by the CB1 antagonist, suggesting the involvement of an alternate pathway. If AEA is produced after Ca2+ addition to precontracted arteries it could activate AEA receptors (11) or be metabolized by PLA2 to AA and then by CYP to vasodilatory compounds.
A number of studies have established associations between genetically controlled alterations in blood pressure and the activity and/or transcriptional regulation of CYP enzymes that are linked to the pathophysiology of hypertension (45, 46), a leading cause of cardiovascular, cerebral, and renal morbidity/mortality. Zhao and Imig (66) have shown that EETs and 20-HETE levels in the kidney are altered in diabetes, pregnancy, and animal models of hypertension, and blockade of EET formation is associated with salt-sensitive hypertension (34, 37). Thus abnormalities in the CYP system may contribute to the pathogenesis of cardiovascular diseases such as hypertension. A preponderance of CYP hydroxylase-derived AA metabolites in the vasculature will likely contribute to hypertension (60), while CYP epoxygenase-derived AA metabolites will reduce it. Desensitization of the CaR occurs with repeated stimulation (6), further suggesting that an understanding of the regulation of the signaling pathways for this receptor may shed light on altered vasodilator release in hypertension.
In conclusion, the present study demonstrates that Ca2+-induced relaxation of both intact and endothelium-denuded mesenteric arteries is mediated by a mechanism involving the synthesis of CYP metabolites of 2-AG and AA, GEETs and EETs, respectively, and that GEET-induced relaxation operates through activation of KCa channels. Furthermore, the results indicate that hyperpolarizing compounds of the endocannabinoid pathway may also play a role. On the basis of these findings, we propose that agonist activation of the perivascular nerve CaR stimulates Ca2+-dependent PLA2 and N-acetyl transferase to release AEA/AA from membrane phospholipids as well as 2-AG from DAG as substrates for CYP-mediated synthesis of nerve-derived vasodilators, which then diffuse into the underlying smooth muscle cells to induce relaxation (Fig. 9). We showed in an earlier study (6) that activation of the DRG CaR, stably expressed in HEK293 cells, leads to the mobilization of . The DRG houses cell bodies of sensory nerves that send efferent processes to tissues such as the perivascular adventitia. Therefore activation of the CaR at the perivascular nerve terminal can lead to the release of hyperpolarizing vasodilator compounds, such as CYP metabolites of AA and 2-AG, that can then diffuse into and relax adjacent smooth muscle cells (8, 9, 11, 36).
Fig. 9.
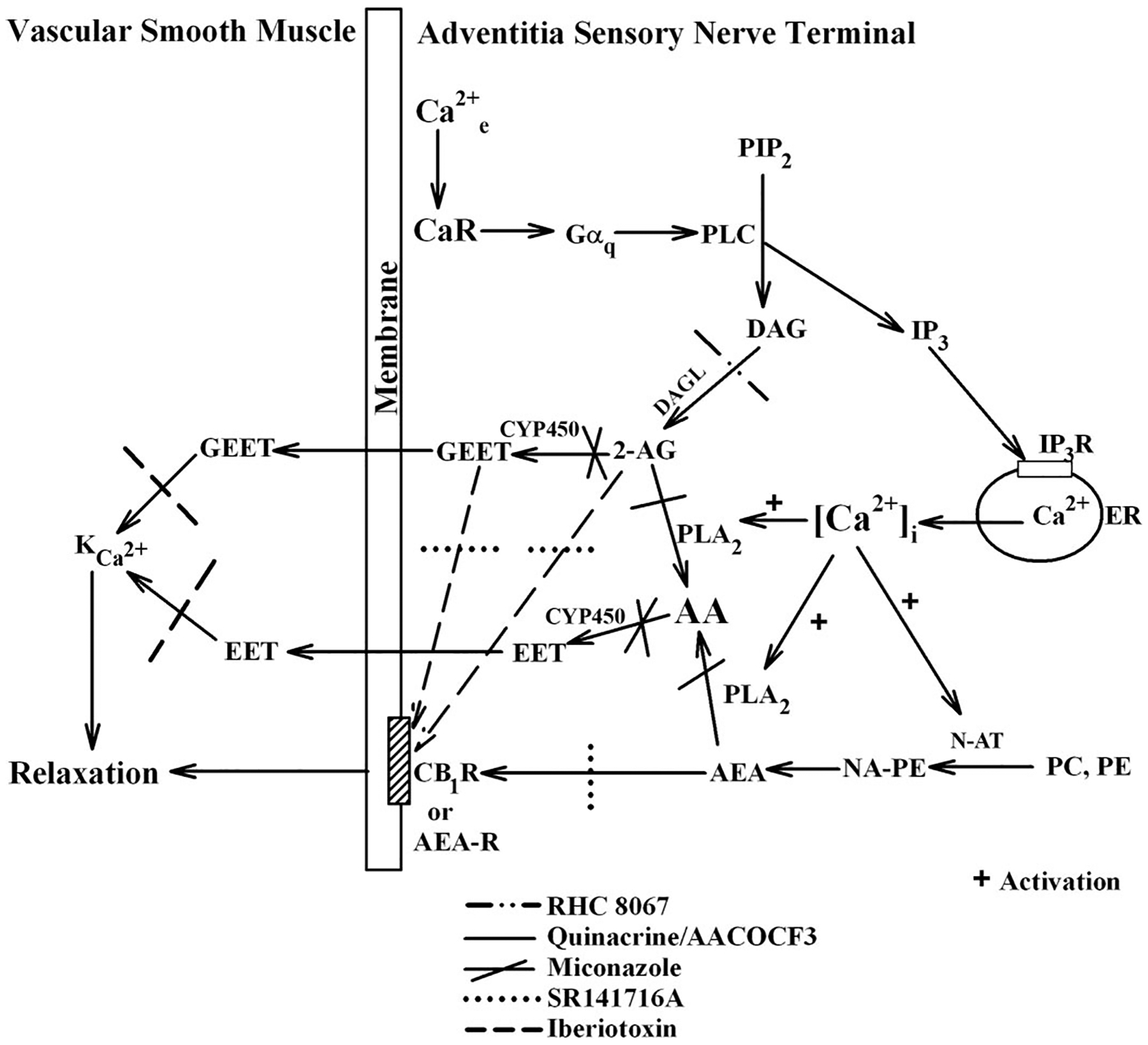
Proposed model for perivascular nerve Ca2+-sensing receptor (CaR)-mediated vasodilator release. Agonist activation of the CaR at the nerve terminal leads to receptor coupling to Gαq and activation of phospholipase C (PLC) and hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 then binds to its receptor (IP3R) in the endoplasmic reticulum (ER) membrane to release Ca2+ into the cytoplasm. Released Ca2+ then activates 1) Ca2+-sensitive N-acetyl transferase (N-AT) to generate anandamide (AEA) from phosphatidylcholine (PC) and phosphatidylethanolmine (PE) through N-arachidonoylphosphatidylethanolamine (NAPE) and 2) Ca2+-sensitive PLA2 to generate arachidonic acid (AA), a substrate for cytochrome P-450 (CYP), from AEA. DAG, on the other hand is metabolized by DAGL to give 2-AG, which in turn is metabolized by PLA2 to give AA and/or by CYP to give GEET. The AA produced is metabolized by CYP to give EET. Both EET and GEET then cross the plasma membrane to the adjacent smooth muscle layer to activate KCa channels, resulting in hyperpolarization of the muscle cells and vascular relaxation. AEA can also bind to the CB1 receptor (CB1R) or an AEA receptor in the plasma membrane to activate the cannabinoid pathway and cause relaxation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-064761, UH1-HL-059868, and R25-HL-059868.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akita S, Sacks FM, Svetkey LP, Conlin PR, Kimura G. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressurenatriuresis relationship. Hypertension 42: 8–13, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Angus JA, Wright CE. Techniques to study the pharmacodynamics of isolated large and small blood vessels. J Pharmacol Toxicol Methods 44: 395–407, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336: 1117–1124, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension. A scientific statement from the American Heart Association. Hypertension 47: 296–308, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation 107: 769–776, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Awumey EM, Howlett AC, Putney JW Jr, Diz DI, Bukoski RD. Ca2+ mobilization through dorsal root ganglion Ca2+-sensing receptor stably expressed in HEK293 cells. Am J Physiol Cell Physiol 292: C1895–C1905, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bukoski RD. The perivascular sensory nerve Ca2+ receptor and blood pressure regulation: a hypothesis. Am J Hypertens 11: 1117–1123, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Bukoski RD, Bian K, Wang Y, Mupanomunda M. Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension 30: 1431–1439, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Bukoski RD. Dietary Ca2+ and blood pressure: evidence that Ca2+-sensing receptor activated, sensory nerve dilator activity couples changes in interstitial Ca2+ with vascular tone. Nephrol Dial Transplant 16: 218–221, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Bukoski RD, Batkai S, Jarai Z, Wang Y, Offertaler L, Jackson WF, Kunos G. CB1 receptor antagonist SR141716A inhibits Ca2+-induced relaxation in CB1 receptor-deficient mice. Hypertension 39: 251–260, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84: 484–488, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Campbell WB, Holmes BB, Falck JR, Capdevila JH, Gauthier KM. Regulation of potassium channels in coronary smooth muscle by adenoviral expression of cytochrome P-450 epoxygenase. Am J Physiol Heart Circ Physiol 290: H64–H71, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxygenase. J Lipid Res 41: 163–181, 2000. [PubMed] [Google Scholar]
- 16.Capdevila JH, Falck JR. The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochem Biophys Res Commun 285: 571–576, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72: 683–689, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Chu ZM, Croft KD, Kingsbury DA, Falck JR, Reddy KM, Beilin LJ. Cytochrome P450 metabolites of arachidonic acid may be important mediators in angiotensin II-induced vasoconstriction in the rat mesentery in vivo. Clin Sci (Lond) 98: 277–282, 2000. [PubMed] [Google Scholar]
- 19.DeLozier TC, Tsao CC, Coulter SJ, Foley J, Bradbury JA, Zeldin DC, Goldstein JA. CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products. J Pharmacol Exp Ther 310: 845–854, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 99: 10819–10824, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol 66: 1260–1264, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Earley S, Pastszyn A, Walker BR. Cytochrome P-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol 285: H127–H136, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Fleming I Cytochrome P450 2C is an EDHF synthase in coronary arteries. Trends Cardiovasc Med 10: 166–170, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Fleming I Cytochrome P450 enzymes in vascular homeostasis. Circ Res 89: 753–762, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Fleming I Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol Res 49: 525–533, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Evidence against a role of cytochrome P450-derived arachidonic acid metabolites in endothelium-dependent hyperpolarization by acetylcholine in rat isolated mesenteric artery. Br J Pharmacol 120: 439–446, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauthier KM, Baewer DV, Hittner S, Hillard CJ, Nithipatikom K, Reddy DS, Falck JR, Campbell WB. Endothelium-derived 2-arachidonylglycerol: an intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am J Physiol Heart Circ Physiol 288: H1344–H1351, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Ghisdal P, Vandenberg G, Hamaide M, Wibo M, Morel N. The diacylglycerol lipase inhibitor RHC-80267 potentiates the relaxation to acetylcholine in rat mesenteric artery by anticholinesterase action. Eur J Pharmacol 517: 92–102, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia 55: 1214–1221, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Halpern W, Mulvany MJ, Warshaw DM. Mechanical properties of smooth muscle cells in the walls of arterial resistance vessels. J Physiol 275: 85–101, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatton DC, Scrogin KE, Metz JA, McCarron DA. Dietary calcium alters blood pressure reactivity in spontaneously hypertensive rats. Hypertension 13: 622–629, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Hatton DC, McCarron DA. Dietary calcium and blood pressure in experimental models of hypertension. A review. Hypertension 23: 513–530, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int 71: 1105–1115, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest 104: 751–760, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliff JJ, Close LN, Selden NR, Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol 92: 653–658. [DOI] [PubMed] [Google Scholar]
- 36.Ishioka N, Bukoski RD. A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J Pharmacol Exp Ther 289: 245–250, 1999. [PubMed] [Google Scholar]
- 37.Iwai N, Inagami T. Isolation of preferentially expressed genes in the kidneys of hypertensive rats. Hypertension 17: 161–169, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-α in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol 72: 612–621, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol 100: 307–317, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotchen TA, Ott CE, Whitescarver SA, Resnick LM, Gertner JM, Blehschmidt NG. Calcium and calcium regulating hormones in the “prehypertensive” Dahl salt sensitive rat (calcium and salt sensitive hypertension). Am J Hypertens 2: 747–753, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 45: 4720–4726, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Kunos G. Activation of anandamide synthesis in mouse macrophages and its role in endotoxin-induced hypotension. J Biol Chem 278: 45034–45039, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA 103: 13345–13350, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RJ, Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 54: 1–7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevilla JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2412–2420, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makita K, Falck HR, Capdevilla JH. Cytochrome P450, the arachidonic acid cascade, and hypertension: new vistas for an old enzyme system. FASEB J 10: 1456–1463, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302: 84–88, 2003. [DOI] [PubMed] [Google Scholar]
- 48.McCarron DA. Calcium metabolism and hypertension. Kidney Int 35: 717–736, 1989. [DOI] [PubMed] [Google Scholar]
- 49.McCarron DA, Reusser ME. Finding consensus in the dietary calciumblood pressure debate. J Am Coll Nutr 18: 398S–405S, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Muntzel MS, Hatton DC, Metz JA, McCarron DA. Dietary calcium alters blood pressure in neonatal spontaneously hypertensive rats. Am J Hypertens 2: 158–162, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Mupanomunda M, Wang Y, Bukoski RD. Effect of chronic sensory denervation on Ca2+-induced relaxation of isolated mesenteric resistance arteries. Am J Physiol Heart Circ Physiol 274: H1655–H1661, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Ohanian J, Gatfield KM, Ward DT, Ohanian V. Evidence for a functional calcium-sensing receptor that modulates myogenic tone in rat subcutaneous small arteries. Am J Physiol Heart Circ Physiol 288: H1756–H1762, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem 280: 27138–27146, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Ralevic V Cannabinoid modulation of peripheral autonomic and sensory neurotransmission. Eur J Pharmacol 472: 1–21, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Reusser ME, McCarron DA. Reducing hypertensive cardiovascular disease risk of African Americans with diet: focus on the facts. J Nutr 136: 1099–1102, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Sacks FM, Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol 22: III6–III10, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, Mugge A, Lindpaintner K, Huesing A, Maisch B, Zeldin DC, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation 110: 2132–2136, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol 128: 659–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su P, Kaushal KM, Kroetz DL. Inhibition of renal AA ω-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol Regul Integr Comp Physiol 275: R426–R428, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Tang X, Edwards EM, Holmes BB, Falck JR, Campbell WB. Role of phospholipase C and diacylglyceride lipase pathway in arachidonic acid release and acetylcholine-induced vascular relaxation in rabbit aorta. Am J Physiol Heart Circ Physiol 290: H37–H45, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Bukoski RD. Distribution of the perivascular nerve Ca2+ receptor in rat arteries. Br J Pharmacol 125: 1397–1404, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Bukoski RD. Use of acute phenolic denervation to show the neuronal dependence of Ca2+-induced relaxation of isolated arteries. Life Sci 64: 887–894, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Wang MH, Guan H, Nguyen X, Zand BA, Nasjletti A, Laniado-Schwartzman M. Contribution of cytochrome P-450 4A1 and 4A2 to vascular 20-hydroxyeicosatraenoic acid synthesis in rat kidneys. Am J Physiol Renal Physiol 276: F246–F253, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BKCa channels. Am J Physiol Heart Circ Physiol 280: H2430–H2440, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Zhao X, Imig JD. Kidney CYP450 enzymes: biological actions beyond drug metabolism. Curr Drug Metab 4: 73–84, 2003. [DOI] [PubMed] [Google Scholar]


