Abstract
Background
Type 2 diabetes (T2DM) can accelerate the progression of cirrhosis. The potential for oral diabetes medications to counteract the mortality and morbidity of chronic liver diseases is unclear.
Methods
We compared the effectiveness of dual metformin and glucagon-like peptide-1 receptor agonists (GLP1-RA) vs. metformin treatment alone in reducing mortality and hepatic complications in cirrhotic patients with T2DM. We evaluated propensity score-matched cohorts of T2DM and cirrhosis patients treated with metformin or dual metformin and GLP1-RA therapy. Data were obtained from the TriNetX Research Network. Our outcomes were all-cause mortality, composite risk of hepatic decompensation, and hepatocellular carcinoma (HCC).
Results
Compared to patients on metformin alone, dual metformin and GLP1-RA therapy users had a lower risk for both death (hazard ratio [HR] 0.61, 95% confidence interval [CI] 0.42-0.89; P=0.011) and hepatic decompensation (HR 0.65, 95%CI 0.46-0.93; P=0.02) over 5 years. Patients on dual therapy had a lower risk for HCC (HR 0.44, 95%CI 0.26-0.74; P=0.001) compared to mono-metformin therapy patients.
Conclusion
In our multicenter retrospective study, dual therapy was associated with better mortality and morbidity in cirrhosis patients with T2DM compared to those on metformin alone.
Keywords: Cirrhosis, type 2 diabetes mellitus, glucagon-like peptide-1 receptor agonists, hepatocellular carcinoma, nonalcoholic steatohepatitis
Introduction
Type 2 diabetes mellitus (T2DM) is a common comorbidity of nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD) [1,2]. T2DM has been associated with greater cirrhosis disease severity and death [3,4], so using T2DM medications can potentially slow the progression of cirrhosis. Glycemic control and cirrhosis management tend to be synergistic, so improving one often helps with the other [5]. Metformin is the standard first-line oral treatment for T2DM, but some patients require additional therapy, such as glucagon-like peptide-1 receptor agonists (GLP1-RAs), to achieve healthy blood sugar levels [6,7].
GLP1-RAs exhibit various benefits, including decreasing appetite, facilitating weight loss, and enhancing insulin sensitivity. They have been shown to reduce liver inflammation, fibrosis and lipid oxidation [8,9] in preclinical studies, and they have lowered glycemic indices in human studies [10]. Retrospective studies suggest that metformin use in T2DM patients with decompensated cirrhosis is safe and can reduce all-cause mortality [11]. Still, the efficacy of GLP1-RA in T2DM patients with decompensated cirrhosis remains understudied. We sought to determine whether T2DM patients with cirrhosis treated with metformin and a GLP1-RA experienced a reduction in mortality, hepatic decompensation events, and hepatocellular carcinoma (HCC) compared to those patients given metformin alone.
Materials and methods
Study population and design
We used the TriNetX database to build and retrospectively analyze cohorts of T2DM patients with compensated cirrhosis who were either on metformin monotherapy or metformin + GLP1-RA dual therapy. TriNetX, LLC, is a global electronic health records network that provides access to de-identified patient medical records and aggregate summary statistics from 50 healthcare organizations (HCOs) worldwide. We collected patient demographic information, diagnostic and procedural information, and measurements such as labs, vital signs and medications. The platform utilizes standardized coding systems, including International Classifications of Diseases, Tenth Revision (ICD-10), and Current Procedural Terminology (CPT) codes for diagnoses and procedures. RxNorm codes patient medication use and Logistical Observation Identifiers Names and Codes (LOINC) for vital signs and lab values within the TriNetX database. Our study included patients from 1 March 2014 through 2 December 2022.
TriNetX LLC complies with section §164.514(a) of the Health Insurance Portability and Accountability Act Privacy Rule and has received a waiver from the Institutional Review Board (IRB). Since our study only used de-identified patient data for analysis and was not involved in collecting identifiable patient data, it was exempted from IRB approval. TriNetX LLC does not provide protected health information or disclose data on participating HCOs. More details of TriNetX networks have been described previously [12,13].
Data collection and outcomes
We identified patients aged 18 years and above with both T2DM and cirrhosis, regardless of the cause of cirrhosis (e.g., NASH, alcoholic liver disease, viral hepatitis, etc.), using ICD-10 codes. We used RxNorm to determine which of the cirrhotic patients with T2DM were on metformin monotherapy or else on metformin plus a GLP1-RA (i.e., dulaglutide, albiglutide, exenatide, liraglutide, and semaglutide) (Fig. 1). We excluded patients with type 1 diabetes mellitus, patients who had undergone transhepatic intrajugular portosystemic shunt placement, and people who had undergone liver transplantation. We also excluded patients with stage 4-5 chronic kidney disease, since advanced renal disease generally precludes patients from taking metformin, as there is a risk of developing lactic acidosis and worsening kidney injury.
Figure 1.
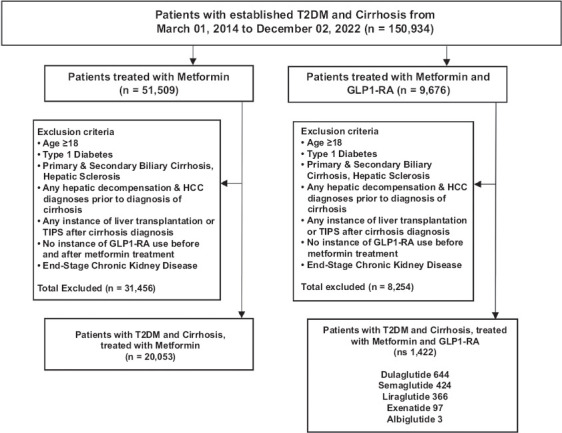
Cohort Construction. Cohort 1 included patients who were initiators of metformin without any instance of GLP1-RA in their electronic medical record (EMR). Cohort 2 inclusion included metformin initiators and additional GLP1-RA therapy. Both cohorts consisted of patients starting on March 01, 2014, to December 02, 2022.
T2DM, type 2 diabetes mellitus; GLP1-RA, glucagon-like peptide-1 receptor agonist; TIPS, transvenous intrahepatic portosystemic shunt; HCC, hepatocellular carcinoma
Since we were interested in determining the relative risk of developing ascites, variceal bleeding, hepatic encephalopathy and HCC between the mono- and dual-therapy groups, we tracked only patients who had compensated cirrhosis at the beginning of our record in March 2014. We therefore excluded patients who already had decompensated cirrhosis at the time that metformin monotherapy or metformin + GLP1-RA therapy was started, and we built our cohorts solely from patients who had compensated cirrhosis with T2DM prior to starting our medications of interest.
After applying the above exclusion criteria, we had 20,053 patients in the monotherapy group and 1422 patients in the dual-therapy group (Fig. 1). We then undertook propensity-score (PS) matching to allow direct comparison between the mono- and dual-therapy cohorts (Table 1, Supplementary Table 1 (1.5MB, pdf) ). We stratified these patients by sex, race, ethnicity and age group (Supplementary Table 2 (1.5MB, pdf) ). In a subset of T2DM patients with NASH cirrhosis, we identified 1841 patients on monotherapy and 317 patients on dual therapy for separate PS-matching (Supplementary Fig. 1 (1.5MB, pdf) , Supplementary Table 3 (1.5MB, pdf) ).
Table 1.
Cohort baseline characteristics
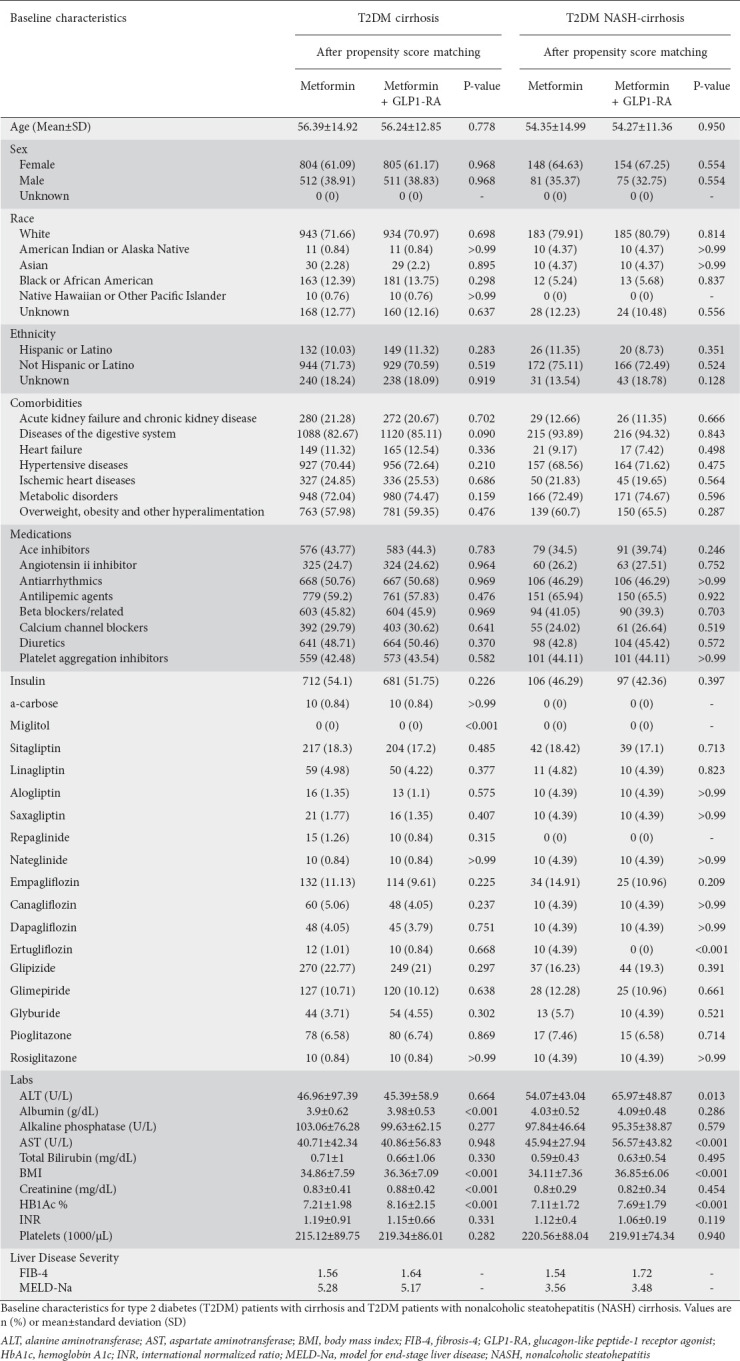
We calculated the Fibrosis-4 (FIB-4) and Model for End-Stage Liver Disease (MELD-Na) scores for each respective cohort from baseline patient data, both before and after PS-matching (Supplementary Tables 4 (1.5MB, pdf) and 5 (1.5MB, pdf) ). Supplementary Table 6 (1.5MB, pdf) presents the study definitions, ICD-10 codes, and variables used to query patients in the TriNetX database.
Our primary outcome was all-cause mortality. Our secondary outcomes were the composite occurrence of hepatic encephalopathy, ascites, and variceal bleeding, as well as the incidence of HCC. All outcomes were recorded up to 5 years after initiation of either monotherapy or metformin-GLP1-RA dual therapy.
Statistical analysis
We identified covariates such as age, sex, race, ethnicity, labs, medications, surgical procedures and comorbidities. Lab values were obtained from the same date as the baseline characteristics. We then used the TriNetX platform to perform PS-matching between the 2 cohorts and constructed mono- and dual-therapy groups of 1316 patients each. We repeated the matching process for all T2DM patients with cirrhosis on monotherapy vs. dual therapy by sex (e.g., men on metformin vs. men on dual therapy), race (e.g., Non-Whites on metformin vs. Non-Whites on metformin and GLP1-RA), ethnicity (Non-Hispanics on metformin vs. Non-Hispanics on metformin and GLP1-RA), and age groups (patients aged 60-85 on metformin vs. patients aged 60-85 on metformin and GLP1-RA). Using similarly identified covariates, PS-matching was performed in a separate analysis for mono- and dual-therapy cohorts in T2DM patients with NASH cirrhosis. Supplementary Tables 1 (1.5MB, pdf) and 3 (1.5MB, pdf) identify all covariates that were used for PS-matching. TriNetX provides real-time live analytics on its platform. Continuous variables were normally distributed and are represented as mean ± standard deviation and 95% confidence interval (CI), while categorical variables are reported as counts and percentages. A normal z-test was performed for binary and categorical variables and a t-test for continuous variables in summary statistics. The platform analytics balances each patient from the smaller cohorts by choosing matches from the larger cohort through the 1:1 greedy-nearest-neighbor approach, using logistic regression from the scikit-learn package in Python version 3.7 with Scipy 1.5.2. This approach used a caliper of 0.1 pooled standard deviations and randomization of the order of records with fixed seeding to increase the reproducibility of matching. We used Kaplan-Meier analysis to estimate the probability of our outcomes occurring and compared the distribution of the event-free curve with log-rank tests using the R survival package v3.2-3. With the same R package, we estimated hazard ratios and 95%CIs using Cox proportional hazards models. Patients documented to have had our outcomes of interest before the inception window of receiving mono or dual oral therapy were excluded from the analysis. As of this writing, the TriNetX platform does not perform chi-square or Fisher’s exact testing. Statistical significance was considered with a 2-sided P value of 0.05 or less.
Results
We identified patients with T2DM and cirrhosis confirmed by ICD-10 codes, after applying exclusion criteria, including 20,053 on monotherapy and 1422 on dual therapy. Patients on metformin and GLP1-RA comprised 644 patients on dulaglutide, 424 on semaglutide, 366 on liraglutide, 97 on exenatide, and 3 on albiglutide. After PS-matching, the monotherapy cohort (n=1316) included 804 (61.09%) women, 943 Whites (71.66%), and 132 (10.03%) Hispanic/Latino patients. The dual-therapy cohort (n=1316) included 805 (61.17%) women, 934 Whites (70.97%) and 149 (11.32%) Hispanic/Latino patients (Table 1). Supplementary Table 7 (1.5MB, pdf) outlines the preceding patient diagnoses associated with the cirrhosis burden.
In the subset of T2DM patients with NASH cirrhosis on mono- and dual therapy, cohorts were overall similar concerning age, sex and race/ethnicity breakdown. However, levels of aspartate aminotransferase, body mass index (BMI), and hemoglobin A1c (HbA1c) were slightly higher in the dual-therapy group even after PS-matching (Table 1).
Primary outcome: mortality
After PS-matching, we found that the dual metformin and GLP1-RA therapy group had decreased 5-year mortality risk (hazard ratio [HR] 0.61, 95%CI 0.42-0.89; P=0.011) compared to the monotherapy group (Table 2, Fig. 2A). Men on dual therapy were about half as likely to die within 5 years (HR 0.55, 95%CI 0.30-0.97; P=0.046) compared to men on monotherapy. Women on dual therapy likewise had half the mortality risk (HR 0.50, 95%CI 0.29-0.87; P=0.01) compared to women on monotherapy (Table 2). White patients on metformin and GLP1-RA had a lower risk of death (HR 0.55, 95%CI 0.33-0.89; P=0.014) relative to White patients on metformin. We found no statistically significant differences in mortality risk for Non-White patients or based on ethnicity and age groups. Looking at patients with NASH cirrhosis specifically, those on dual therapy had a lower 5-year mortality risk (HR 0.13, 95%CI 0.03-0.58; P=0.002) than those on metformin monotherapy (Table 2 and Supplementary Figure 2 (1.5MB, pdf) ).
Table 2.
Five-year Kaplan-Meier estimates for mortality
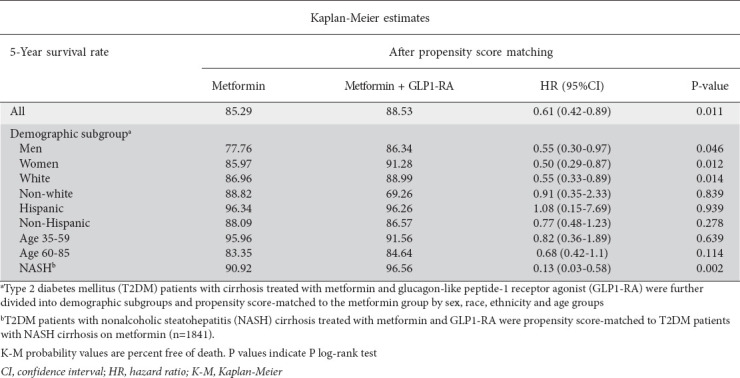
Figure 2.
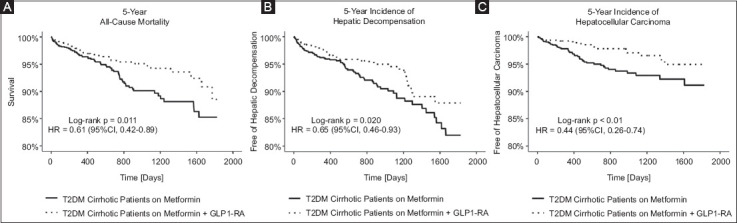
Five-year all-cause mortality, composite hepatic decompensation and hepatocellular carcinoma for all T2DM cirrhosis patients. Kaplan-Meier probability values are percent free of death (A), composite hepatic decompensation (B) and hepatocellular carcinoma (C). P values indicate P log-rank test
T2DM, type 2 diabetes mellitus; GLP1-RA, glucagon-like peptide-1 receptor; HR, hazard ratio; CI, confidence interval
Composite hepatic decompensation
After PS-matching, we found that the composite risk of developing decompensated cirrhosis over 5 years was lower in the dual-therapy group (HR 0.65, 95%CI 0.46-0.93; P=0.02) (Table 3, Fig. 2B). We found no statistically significant differences in the composite risk for hepatic decompensation (HR 0.75 95%CI 0.36-1.56; P=0.44) for men. Conversely, women on dual therapy were at lower risk for decompensation (HR 0.54, 95%CI 0.31-0.92; P=0.02) compared to women on metformin therapy alone. White and Non-Hispanic patients on dual treatment were half as likely to have a decompensation event as were White and Non-Hispanic patients on monotherapy.
Table 3.
Five-year Kaplan-Meier estimates for composite hepatic decompensation
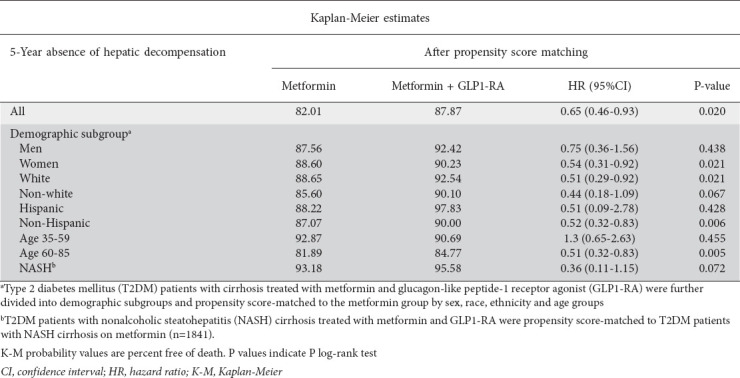
While there were no differences in hepatic decompensation risk for patients aged 35-59 years, we found that patients aged 60-85 years on metformin-GLP1-RA therapy had half the risk of hepatic decompensation (HR 0.51, 95%CI 0.32-0.83; P=0.006) over 5 years compared to those aged 60-85 years on monotherapy. We found no statistically significant differences in incidence of hepatic decompensation between the cohorts when looking specifically at Non-White patients, Hispanics, and those with NASH cirrhosis (Table 3).
HCC
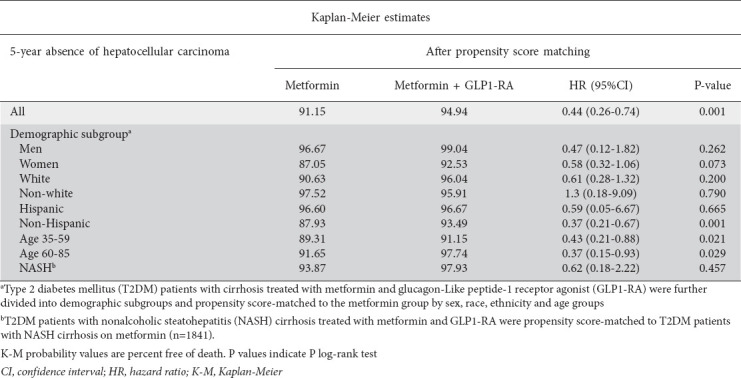
Upon PS-matching, the metformin-GLP1-RA group had less than half the risk for HCC over 5 years (HR 0.44, 95%CI 0.26-0.74; P=0.001) compared with the monotherapy group (Table 4, Fig. 2C). While there was no significant difference in 5-year risk and occurrence of HCC between Hispanic patients in the T2DM therapy cohorts, Non-Hispanic patients on dual therapy were at lower risk for HCC (HR 0.37, 95%CI 0.21-0.67; P=0.001) compared to Non-Hispanic individuals on dual therapy. Across both age groups, patients on dual therapy were at lower risk and less likely to develop HCC over 5 years (Table 4). We found no statistically significant differences in the risk or occurrence of HCC between the cohorts based on sex, race, or confirmed NASH diagnosis (Table 4).
Table 4.
Five-year Kaplan-Meier estimates for hepatocellular carcinoma
Discussion
Our study highlights the association between dual metformin and GLP1-RA therapy use in cirrhotic patients with T2DM and the significantly lower rates of mortality, hepatic decompensation events and HCC compared to patients on metformin monotherapy in PS-matched analyses (Supplementary Tables 8 (1.5MB, pdf) -10 (1.5MB, pdf) ). The benefit of dual metformin and GLP1-RA therapy in our study can be attributed to the multiple benefits of GLP1-RA, improving glycemic indices, improving comorbidities associated with T2DM, and potentially reducing liver inflammation and disease progression. In addition to the glucose-regulating properties of metformin [6], GLP1-RA treatment can benefit patients by improving insulin resistance, inhibiting gastric emptying, improving postprandial hyperglycemia, and reducing body weight [17].
Preclinical NASH models have shown that GLP1-RA treatment reduced levels of liver inflammation, hepatic steatosis, and plasma alanine aminotransferase and triglycerides [8,9]. Other preclinical models have demonstrated the benefit of GLP1-RAs in reducing liver disease by acting on hepatic stellate cells and improving microvascular function, reducing intrahepatic systemic resistance and attenuating liver fibrosis [18]. Moreover, GLP1-RA treatment has been shown to suppress transforming growth factor alpha and hepatocyte growth factor, both of which are signaling proteins instrumental in the migration of HCC cells [19]. Randomized clinical trials have likewise shown that GLP1-RA treatment in diabetic and non-cirrhotic NASH patients improved liver fibrosis markers, decreased fibrosis-4 indices, and improved diabetes status [10].
Patients with decompensated cirrhosis have markedly poorer survival compared to those with compensated cirrhosis [24]. Preclinical studies show that GLP1-RAs can reduce liver fibrosis [18], implicated in the development of portal hypertension [25], a critical hemodynamic complication that accelerates the progression to a decompensated cirrhosis state. Retrospective studies support the hypothesis that GLP1-RAs can also provide hepatic decompensation benefit [26]. In conjunction with those studies, our findings suggest that T2DM patients with cirrhosis on dual metformin and GLP1-RA therapy have lower risks and the occurrence of developing decompensation over 5 years. Other evidence suggests that certain GLP1-RAs, such as liraglutide, can increase heart rate and the risk for variceal bleeding in patients simultaneously taking β-blockers [27]. Thus, prospective confirmatory studies are needed to evaluate the safety of GLP1-RAs in diabetes patients with cirrhosis.
To our knowledge, there are no ongoing clinical trials using GLP1-RAs in diabetic patients with decompensated cirrhosis. Few studies have examined the implications of GLP1-RA treatment on the natural progression of cirrhosis, particularly cirrhosis due to NASH. Our retrospective study encompassed a large population in a multicenter setting. Metformin is a standard therapeutic in T2DM treatment, in conjunction with lifestyle modifications. Using additional antidiabetics, such as a GLP1-RA, represents a clinical decision that may also be influenced by metabolic parameters, as evidenced by higher baseline BMI and HbA1c in the dual-therapy group at baseline. Other researchers have demonstrated that GLP1-RAs are associated with a similar reduction in HbA1c, lipid profiles and adverse cardiovascular outcomes in T2DM patients across baseline BMI categories compared to metformin [14,15]. Furthermore, semaglutide is now approved for obesity management and has been shown to reduce body weight in patients without diabetes [16]. These findings collectively underscore the role of GLP1-RA in patients with risk factors for NASH, and cirrhosis patients regardless of HbA1c status.
We acknowledge that any mortality benefit from GLP1-RAs cannot be attributed entirely to glucose and weight control. Previous cardiovascular outcomes trials have underscored that GLP1-RA provides cardiovascular benefits by preventing atherosclerotic events and cardiomyopathy, which could be attributed to decreased M2 macrophage polarization and decreased monocyte-endothelium adhesion, as shown by preclinical studies [20]. In a similar vein, renal injury is a common complication of cirrhosis and T2DM that can eventually require patients to undergo hemodialysis [21]. Others have noted that end-stage renal disease (ESRD) increased 3-year mortality twofold in cirrhosis patients, particularly those with hepatic decompensation [22]. While GLP1-RAs can slow the progression of the nephropathy [23], our study excluded patients with ESRD, so we could not corroborate such findings.
Liver malignancy can accelerate the course of liver complications and is known to be an independent risk factor for increased mortality in decompensated cirrhotic patients [28,29]. We found not only a lower risk and occurrence of HCC over 5 years, but also benefits seen at earlier time points (Supplementary Table 11 (1.5MB, pdf) ), suggesting protective effects of T2DM therapy, given the significant impact of HCC on both compensated and decompensated cirrhosis patient mortality. Our study demonstrates a potential therapeutic strategy in reducing disease progression from compensated to decompensated cirrhosis and developing a primary malignancy, which are significant risk factors for increased morbidity and mortality.
Our study has notable limitations. TriNetX does not provide imaging or biopsy data, so we relied on ICD-10 code diagnoses to build our cohorts, particularly our NASH patients. In doing so, we may have undercounted the number of patients with cirrhosis and NASH. The current literature [30] cites ICD-10 codes K76.0 and K75.8 for patients diagnosed with NASH, but these are not necessarily consistently used amongst the varied institutions that contribute patients to the TriNetX database. Though we did not conduct sensitivity analyses, previous studies have demonstrated that ICD-10 codes have high positive predictive values for patients with cirrhosis and its related complications [31,32].
Another shortcoming of our study was that we did not have access to patient-level data. Thus, the MELD-Na score, which predicts mortality, and the FIB-4 score, which predicts scarring of liver tissue, were calculated based on averages of aggregate patient lab values across cohorts of interest. Furthermore, we could not look at patient-specific causes of mortality on an individualized basis, given the privacy regulations of TriNetX. Therefore, we used all-cause mortality as the primary outcome of our study. After PS-matching, we found that in mono- and dual-therapy cohorts, over 70% of patients had a high prevalence of cardiovascular and metabolic diseases, suggesting the influence of comorbidities on patient mortality. We found no hepatic decompensation benefits for patients with NASH cirrhosis on dual therapy, but this may have been due to the relatively small number of confirmed NASH patients (Supplementary Table 12 (1.5MB, pdf) ). There was a similar challenge for our analyses of Non-White and Hispanic patients.
In conclusion, our study is the first to compare metformin monotherapy with metformin + GLP1-RA dual therapy in T2DM patients with cirrhosis in a multicenter fashion. We have highlighted a possible mortality and morbidity benefit, even when looking across demographic groups. Further prospective studies can help elucidate these effects and their potential role for GLP1-RA in patients with cirrhosis and T2DM.
Summary Box.
What is already known:
Glucagon-like peptide-1 receptor agonists (GLP1-RAs) improve glycemic indices in type 2 diabetes mellitus (T2DM) patients
Chronic hyperglycemia has been linked to the progression of liver disease
Little is known about the impact of GLP1-RAs on T2DM patients with cirrhosis
Randomized clinical trials for GLP1-RAs in T2DM patients with liver disease have excluded cirrhotic patients
What the new findings are:
Dual metformin and GLP1-RA therapy reduced mortality in T2DM patients with cirrhosis
Lower mortality was seen in men, women, Whites, and the subset of T2DM patients with cirrhosis due to nonalcoholic steatohepatitis
Patients on dual metformin and GLP1-RA therapy had a lower risk of hepatic decompensation and hepatocellular carcinoma than those on metformin alone
Biography
Renaissance School of Medicine at Stony Brook University, Stony Brook, NY; Morehouse School of Medicine, Atlanta, GA; Stony Brook Medicine, Stony Brook, NY, USA
Footnotes
Conflict of Interest: None
References
- 1.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 3.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120:829–834. doi: 10.1016/j.amjmed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Labenz C, Nagel M, Kremer WM, et al. Association between diabetes mellitus and hepatic encephalopathy in patients with cirrhosis. Aliment Pharmacol Ther. 2020;52:527–536. doi: 10.1111/apt.15915. [DOI] [PubMed] [Google Scholar]
- 5.Boursier J, Anty R, Carette C, et al. AFEF and SFD. Management of diabetes mellitus in patients with cirrhosis:An overview and joint statement. Diabetes Metab. 2021;47:101272. doi: 10.1016/j.diabet.2021.101272. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment:standards of medical care in diabetes-2021. Diabetes Care. 2021;44((Suppl 1)):S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 7.Brunton SA, Wysham CH. GLP-1 receptor agonists in the treatment of type 2 diabetes:role and clinical experience to date. Postgrad Med. 2020;132:3–14. doi: 10.1080/00325481.2020.1798099. [DOI] [PubMed] [Google Scholar]
- 8.Somm E, Montandon SA, Loizides-Mangold U, et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res. 2021;227:75–88. doi: 10.1016/j.trsl.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Trevaskis JL, Griffin PS, Wittmer C, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G762–G772. doi: 10.1152/ajpgi.00476.2011. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:an updated meta-analysis of randomized controlled trials. Metabolites. 2021;11:73. doi: 10.3390/metabo11020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan DE, Serper M, John BV, et al. Veterans Outcomes and Cost Associated with Liver disease Study Group. Effects of metformin exposure on survival in a large national cohort of patients with diabetes and cirrhosis. Clin Gastroenterol Hepatol. 2021;19:2148–2160. doi: 10.1016/j.cgh.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Stacey J, Mehta MD. Using EHR data extraction to streamline the clinical trial process. [[Accessed 19 June 2023]];Clin Researcher. 2017 3:2–7. Available from: https://trinetx.com/wp-content/uploads/2017/03/ACRP-APRIL17-TRINETX.pdf . [Google Scholar]
- 13.Stapff M. Use of electronic health data in clinical development. Pharm Ind. 2017;79:204–210. [Google Scholar]
- 14.Chitnis AS, Ganz ML, Benjamin N, Langer J, Hammer M. Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the United States:a retrospective cohort study. Adv Ther. 2014;31:986–999. doi: 10.1007/s12325-014-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S, McGuire DK, Bain SC, et al. Effects of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes:results of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2020;22:2487–2492. doi: 10.1111/dom.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilding JPH, Batterham RL, Calanna S, et al. STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Wang M, Wen Z, et al. GLP-1 receptor agonists:beyond their pancreatic effects. Front Endocrinol (Lausanne) 2021;12:721135. doi: 10.3389/fendo.2021.721135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Mesquita FC, Guixé-Muntet S, Fernández-Iglesias A, et al. Liraglutide improves liver microvascular dysfunction in cirrhosis:Evidence from translational studies. Sci Rep. 2017;7:3255. doi: 10.1038/s41598-017-02866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada N, Matsushima-Nishiwaki R, Kobayashi K, Tachi J, Kozawa O. GLP-1 reduces the migration of hepatocellular carcinoma cells via suppression of the stress-activated protein kinase/c-Jun N-terminal kinase pathway. Arch Biochem Biophys. 2021;703:108851. doi: 10.1016/j.abb.2021.108851. [DOI] [PubMed] [Google Scholar]
- 20.Galatou E, Mourelatou E, Hatziantoniou S, Vizirianakis IS. Nonalcoholic steatohepatitis (NASH) and atherosclerosis:explaining their pathophysiology, association and the role of incretin-based drugs. Antioxidants (Basel) 2022;11:1060. doi: 10.3390/antiox11061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheen YJ, Kung PT, Sheu WH, Kuo WY, Tsai WC. Impact of liver cirrhosis on incidence of dialysis among patients with type 2 diabetes. Diabetes Ther. 2020;11:2611–2628. doi: 10.1007/s13300-020-00919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung TH, Tsai CC, Tseng KC, et al. High mortality of cirrhotic patients with end-stage renal disease. Medicine (Baltimore) 2016;95:e3057. doi: 10.1097/MD.0000000000003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Górriz JL, Soler MJ, Navarro-González JF, et al. GLP-1 receptor agonists and diabetic kidney disease:a call of attention to nephrologists. J Clin Med. 2020;9:947. doi: 10.3390/jcm9040947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407–1414. doi: 10.1111/j.1478-3231.2012.02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis:Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75(Suppl 1):S49–S66. doi: 10.1016/j.jhep.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon TG, Patorno E, Schneeweiss S. Glucagon-like peptide-1 receptor agonists and hepatic decompensation events in patients with cirrhosis and diabetes. Clin Gastroenterol Hepatol. 2022;20:1382–1393. doi: 10.1016/j.cgh.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vukotic R, Raimondi F, Brodosi L, et al. The effect of liraglutide on β-blockade for preventing variceal bleeding:a case series. Ann Intern Med. 2020;173:404–405. doi: 10.7326/L20-0041. [DOI] [PubMed] [Google Scholar]
- 28.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma:a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis:a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Labenz C, Huber Y, Michel M, et al. Impact of NAFLD on the incidence of cardiovascular diseases in a primary care population in Germany. Dig Dis Sci. 2020;65:2112–2119. doi: 10.1007/s10620-019-05986-9. [DOI] [PubMed] [Google Scholar]
- 31.Tapper EB, Korovaichuk S, Baki J, et al. Identifying patients with hepatic encephalopathy using administrative data in the ICD-10 era. Clin Gastroenterol Hepatol. 2021;19:604–606. doi: 10.1016/j.cgh.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkholder DA, Moran IJ, DiBattista JV, Lok AS, Parikh ND, Chen VL. Accuracy of International Classification of Diseases-10 codes for cirrhosis and portal hypertensive complications. Dig Dis Sci. 2022;67:3623–3631. doi: 10.1007/s10620-021-07282-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


