Abstract
Background
Multidisciplinary team (MDT) meetings aim to optimize patient management. We evaluated the impact of MDT discussions on the management and diagnosis of focal pancreatic lesions in a single tertiary center.
Methods
All patients with an initial diagnosis of solid or cystic pancreatic lesion discussed in our institution’s MDT meeting on pancreatic diseases between January 1, 2020, and December 31, 2021, were included. The impact of MDT discussion on patient management, defined as a modification of the initially proposed therapeutic plan after MDT discussion, as well as the criteria leading to this modification, were the primary outcomes. Impact on diagnosis was the secondary outcome.
Results
A total of 522 patients were included. Of these, 185 (35.4%) and 337 (64.6%) had an initial diagnosis of cystic or solid lesion, respectively. The most common referral query was regarding the management plan (349/522; 66.9%). Endoscopy was the procedure most often proposed before MDT discussion (109/522; 20.9%). Overall, the MDT discussion led to modification of the management plan in 377/522 patients (72.2%), with a statistically significant difference between cystic and solid lesions (63.2% vs. 77.2%; P<0.001). Management modifications were mainly driven by revision of cross-sectional radiological images. MDT discussion led to modification of the diagnosis in 92/522 patients (17.6%), with a significant difference regarding cystic lesions (35.7% vs. 7.7%; P<0.001).
Conclusion
MDT discussion impacts the management of patients with cystic and solid pancreatic lesions, leading to a modification of the initially proposed management in two-thirds of them, mainly through revision of cross-sectional imaging.
Keywords: Multidisciplinary team, pancreatic cancer, cystic lesions, solid lesions
Introduction
The care of cancer patients has become increasingly complex, with most patients now being treated with a combination of therapies, including surgery, chemotherapy, and radiotherapy [1]. As a result, multidisciplinary team (MDT) meetings now play a crucial role in decision making for all cancer patients worldwide [2,3]. MDT meetings allow for multidisciplinary discussion of patient management, focusing on diagnostic and therapeutic aspects [4,5], while improving communication, coordination, and decision-making among healthcare professionals when evaluating treatment options [2,6]. In the field of gastrointestinal cancer, the MDT usually consists of a surgeon, a radiologist, a gastroenterologist, a pathologist, and an oncologist [7,8], although participants may vary depending on the condition being discussed and the goals of the MDT [9]. The inclusion of participants from different clinical areas allows the sharing of viewpoints from multiple specialties [10], so that therapeutic management is both effective and adapted to each patient [11]. For example, the results of several studies have shown that multidisciplinary meetings are associated with changes in the initial diagnosis and staging of tumors [12,13], changes in management with minimization of errors [3], shorter times between treatments [10], and better adherence to existing guidelines [14].
MDT meetings can also be effective in the management of chronic and complex non-cancerous diseases [8]. For pancreatic pathologies of all types, the National Comprehensive Cancer Network (NCCN) guidelines recommend multidisciplinary consultations at high-volume centers for the elaboration of the therapeutic plan [15]. However, there have only been a few studies that have demonstrated the efficacy of MDT management in the context of focal pancreatic pathologies. One study by Haj-Mirzaian et al suggested that MDT management is the best approach for treating patients with pancreatitis of all etiologies [16]. The aim of this study was to evaluate the impact of an MDT approach on the diagnosis and therapeutic management of focal pancreatic lesions at a single tertiary center.
Patients and methods
Study population
All patients whose files were discussed in the weekly MDT meeting for pancreatic diseases between January 2020 and December 2021 were assessed for eligibility. We included all individuals with solid or cystic pancreatic lesions referred to the MDT, while patients referred to MDT with an initial diagnosis of acute, chronic or autoimmune pancreatitis, or any other pathology that could not be classified as a cystic or solid lesion, were excluded from the analysis.
MDT meeting description
The meeting takes place once a week and experts from different disciplines participate, including gastroenterologists, surgeons, radiologists, oncologists, radiation oncologists and pathologists. Patients are referred either by gastroenterologists or by surgeons from the same institution where the MDT takes place, by other physicians from the same institution, or by physicians from other institutions. There is a structured form where the query is clearly described: “diagnosis”, “treatment plan” or both. Each case is presented by the referring physician or by one of the physicians involved in the MDT who has previously reviewed all the relevant information. The patient’s file, cross-sectional radiologic images, endoscopic ultrasound reports and images (if available) and pathology results (whenever applicable) are reviewed and, after discussion, a decision about the proposed therapeutic management or diagnosis is made. This decision is recorded, a complete report is written and included in the patient’s electronic medical file.
Data collection
The medical file of each patient was reviewed and demographic data, date of MDT discussion, referring institute, and indication for referral were extracted. Then, based on the written and validated reports of the MDT, we extracted whichever therapeutic option was proposed to the patient after MDT discussion and how this proposition differed from the one suggested by the MDT. The criteria leading to modification of the proposed therapeutic management plan were also recorded. These criteria included cross-sectional imaging review, pathology review, surgical opinion, or other. In addition, we studied whether the first suggested diagnosis was confirmed, or whether another diagnosis was made after MDT discussion; the criteria leading to diagnosis modification were also recorded.
Finally, data were also collected regarding the time from the first cross-sectional imaging to the first presentation of the patient to the MDT, and the time from the last MDT to initiation of the proposed therapeutic management plan.
Outcomes of the study
Primary outcome
The impact of the MDT discussion on patient therapeutic management, defined as a modification of the initially proposed therapeutic plan after all MDT discussions (considering each patient had one or more MDT discussions), as well as the criteria leading to this modification, were the primary outcomes of the study. Modification also included proposing a therapeutic plan when there was none before the MDT. The criteria that influenced management were defined as surgical opinion, cross-sectional imaging review, pathology review, or other.
Secondary outcomes
To identify the impact of MDT discussion on the final diagnosis, defined as a modification of the initially proposed diagnosis, and the criteria that impacted modification. The transition from “unknown” to the proposition of a specific diagnosis was also considered as a modification.
To estimate the time from the first cross-sectional imaging to the first MDT discussion of the patient, and from the last MDT to initiation of patient therapeutic management, based on the MDT decision. This time period did not include subsequent follow-up periods, in case of ex proposed surveillance
Statistical analysis
Statistical analyses were performed using SPSS v.24 software. Categorical and continuous variables are presented as value (%) and as mean ± standard deviation, respectively. Comparisons were performed using a χ2 test or Fisher’s exact test with the Yates’s correction, or non-parametrical tests, as needed. A P-value <0.05 indicated statistical significance.
Ethical considerations
This was a retrospective study that was approved by the ethics committee at the Erasme University Hospital of Brussels in January 2022 (decision number P2021/670).
Results
Demographics
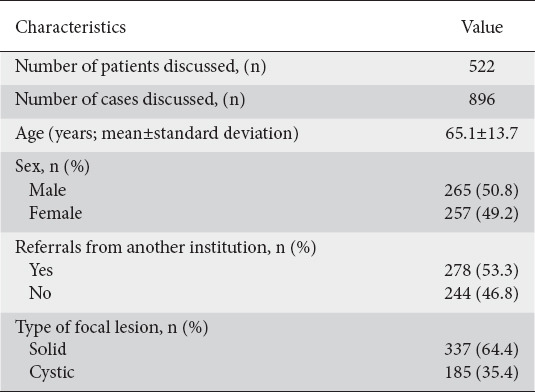
Within a 2-year period, 845 patients were referred to the MDT, with a total of 1329 cases discussed (patients could be discussed more than once). Overall, 522 patients were deemed to meet the inclusion criteria, as shown in Fig. 1. The average age was 65.1±13.6 years, with 257 women (49.2%) and 265 men (50.8%). The initial diagnosis was a solid lesion in 337 patients (64.6%), and a cystic lesion in 185 (35.4%) patients. A total of 278 (53.3%) were referred from another institution. Among all the patients with a solid lesion discussed by the MDT, 62.7% were referred from another institution (209 patients) vs. 37.3% of those with cystic lesions (69 patients) (P<0.001) (Table 1). The mean number of MDT discussions per patient was 1.68±0.9. Patients suffering from cystic lesions were discussed 1.48±0.76 vs. 1.80±0.99 times for those with solid lesions (P=0.04).
Figure 1.
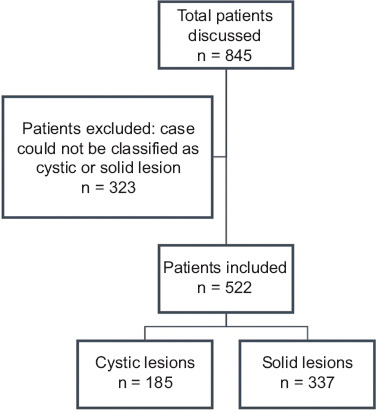
Study flowchart
Table 1.
Demographic characteristics of the study population
The most common referral query was regarding the therapeutic management plan (n=349; 66.9%), followed by queries regarding both diagnosis and therapeutic management (n=99; 19%), and diagnosis alone (n=31; 5.9%). Of the 337 patients with a solid lesion referred to the MDT, 288 were referred for a therapeutic management question (85.5%), 31 for a query regarding both diagnosis and therapeutic management (9.2%), 11 for a follow up (3.3%), and the 7 remaining patients for a question about the diagnosis alone. On the other hand, patients with cystic lesions were most often referred regarding both diagnosis and management (68/185; 36.8%), followed by questions regarding management (61/185; 33%), follow up (32/185; 17.3%), and diagnosis (24/185; 13%).
Primary outcome – impact on proposed therapeutic management plan
For the entire cohort, endoscopy (e.g., biliary drainage by endoscopic retrograde cholangiopancreatography, endoscopic ultrasound with fine needle aspiration/biopsy) was the most commonly proposed therapeutic management before MDT discussion (109/522; 20.9%), followed by chemotherapy (84/522; 16.1%), radiologic follow up (70/522; 13.4%), surgery (49/522; 9.4%), and clinical follow up (33/522; 6.3%), while for 171 patients (32.8%) there was no specific management plan available. Based on the type of lesion, the most common therapeutic management proposal for cystic lesions before MDT was radiologic follow up (53/185; 28.6%) or clinical follow up (26/185; 14.1%), while no treatment was proposed for 81 patients (43.8%). For solid lesions, endoscopy (93/337; 27.6%) and chemotherapy (83/337; 24.6%) were the most common therapeutic management plans, while there was no specific therapeutic management plan for 90 of the 337 (26.7%). There was a significant difference between cystic vs. solid lesion therapeutic management plans before MDT (P<0.001).
As shown in Fig. 2, 377 of 522 (72.2%) patients had a change in their treatment strategy after discussion of their case at the MDT meeting. Changes in treatment strategy were more frequent among patients with solid lesions compared with those with cystic lesions: 260 (77.2%) vs. 117 (63.2%), respectively; P<0.001.
Figure 2.
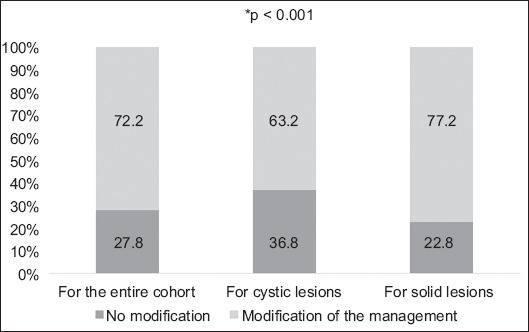
Impact of multidisciplinary team discussion on patient management
After the MDT meeting, chemotherapy was the most commonly proposed treatment (n=193; 37%), followed by radiologic follow up (n=115; 22.0%), clinical follow up (n=65; 12.5%), surgery (n=57; 10.9%), radiotherapy (n=20; 3.8%), endoscopy (n=11; 2.1%), and palliative care (n=11; 2.1%). Among patients with cystic lesions, radiological follow up (n=89; 48.1%), and clinical follow up (n=39; 21.1%) were the most commonly proposed therapeutic management plans, while for solid lesions chemotherapy became the most commonly proposed plan after the MDT meeting (n=181; 53.7%), followed by surgery (n=42; 12.5%), clinical follow up (n=26; 7.7%), and imaging follow up (n=26; 7.7%) (Supplementary Table 1 (188.7KB, pdf) ).
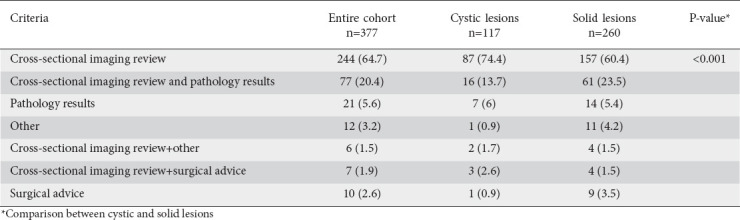
The most common criterion leading to modification of the therapeutic management plan for the entire cohort was revision of the cross-sectional radiological images (computed tomography [CT] or magnetic resonance imaging [MRI]) (n=244; 64.7%), followed by review of both cross-sectional imaging and pathology report (n=77; 20.4%). Among patients with a modification of their initially proposed therapeutic management plan, review of cross-sectional images was the most frequent criterion leading to this change among patients with cystic lesions compared to patients with solid lesions (n=87/74.4% vs. n=157/60.4%, respectively; P=0.01) (Table 2).
Table 2.
Criteria leading to a modification of the management plan, n (%)
Secondary outcomes
i. Impact on initial diagnosis
Regarding the initial diagnosis, adenocarcinoma was the most frequently suggested diagnosis before the MDT discussion (n=250; 47.9%), followed by intraductal papillary mucinous neoplasm (IPMN) (n=108; 20.7%), neuroendocrine tumor (NET) (n=42; 8%), and serous cystadenoma (n=23; 4.4%). For cystic lesions, the most frequent diagnosis before MDT discussion was IPMN (n=102; 55.1%), followed by serous cystadenoma (n=21;11.4%), and mucinous cystadenoma (n=17; 9.2%). On the other hand, adenocarcinoma was the most frequent initial diagnosis among patients with solid lesions (n=250; 74.2%), followed by NET (n=39; 11.6%). The diagnosis was unknown for 28 of 185 patients with cystic lesions (15.1%), and for 4 patients with solid lesions (1.2%) before MDT discussion.
Overall, MDT discussion led to modification of the diagnosis in 92 of 522 patients (17.6%). Following the MDT discussion, a modification was made in 66 patients (35.7%) with a cystic lesion compared to 26 patients (7.7%) with a solid lesion (P<0.001) (Fig. 3).
Figure 3.
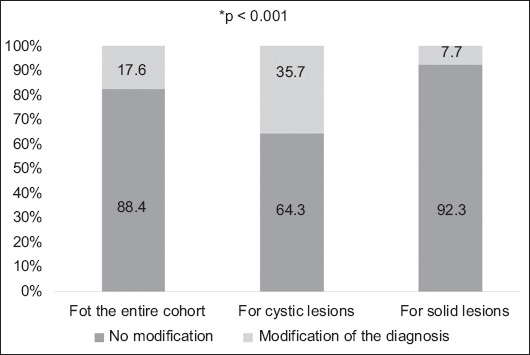
Impact of multidisciplinary team discussion on patient diagnosis
For the entire cohort, the most common diagnosis after MDT discussion remained adenocarcinoma (n=266; 51%). Among patients with cystic lesions, the most frequent diagnosis after MDT discussion was IMPN (n=93; 50.3%), followed by serous cystadenoma (n=30; 16.2%), adenocarcinoma (n=14; 7.6%), and mucinous cystadenoma (n=12; 6.5%). Adenocarcinoma remained the most frequent diagnosis for solid lesions (n=252; 74.8%), followed by NET (n=34; 10.1%) (Supplementary Table 2 (188.7KB, pdf) ).
The most common criterion leading to modification of the initial diagnosis was revision of the cross-sectional radiological images (n=50; 54.3%), followed by review of the pathology results (n=37; 40.2%). Modification of diagnosis for cystic lesions was mainly due to a review of the cross-sectional imaging (n=40; 60.6%) and review of the pathology results (n=22; 33.3%), while for solid lesions, the main criteria were review of the pathology results (n=15; 57.7%) followed by revision of the cross-sectional imaging (n=10; 38.5%).
ii. Time from diagnosis to MDT and from MDT to therapeutic management
Overall, the median time between the first cross-sectional imaging and the first time the patient was discussed at the MDT meeting was 43±41 days, and the delay between the last MDT meeting and the initiation of therapeutic management was 21±24 days. There was a significant difference between solid and cystic lesions. In fact, solid lesions were managed more promptly compared to cystic lesions (time from MDT meeting to management initiation 17 vs. 28 days, respectively; P<0.001).
Discussion
The findings of our study suggest that MDT discussion significantly impacted the therapeutic management of patients with focal pancreatic lesions. Our data showed that the initially proposed therapeutic management plan was altered after MDT discussion in 72.2% of the patients and led to a change in the proposed diagnosis in 17.6%. This rate is higher than those previously reported, where the percentage of patients who experienced changes in treatment plans after the MDT meeting ranged from 4.5% to 52% [10,13,17]. However, the populations in those studies consisted of patients with kidney, breast or digestive cancers [10,13,17], and may not represent the specific population of patients with pancreatic lesions. Another study performed by Chingkoe et al on 252 patients with pancreatic pathologies, including pancreatitis, malignant pathologies and cystic lesions, reported a 38.5% rate of change in management plan after MDT discussion [18]. Recently, Quero et al evaluated the impact of a multidisciplinary tumor board on reviews of 148 patients with pancreatic adenocarcinoma, and reported a change in diagnosis and management for 44 (29.7%) and 27 (18.2%) cases, respectively [19].
Various factors could explain this discrepancy. First, the impact of MDT meetings varies depending on the type of cancer and the stage of disease [10], and pancreatic pathologies are known to be very complex, with the choice of treatment being sometimes very challenging [20]. Second, in our study, “benign” pancreatic diseases, e.g., acute and chronic pancreatitis and autoimmune pancreatitis, were not included, a fact that could affect the percentage of patients with an altered management plan, since benign pancreatic diseases often require only simple clinical and/or radiological follow up. Third, the fact that our study included cases referred from smaller centers, just after diagnosis and before any therapeutic plan had been set, could have affected the rate of modification of the initial plan detected in our study compared to previous results reported in the literature [18,19].
In the current study, a change in the therapeutic management plan was proposed for three quarters of patients with solid lesions, mainly patients with adenocarcinoma (Fig. 4, 5). The number of patients who were to receive chemotherapy increased from 83 to 181; this increase may have been due to correction of the staging of adenocarcinoma by cross-sectional imaging review. Indeed, revision of cross-sectional radiological images alone was the sole indication for a modified treatment plan for more than half of these patients. In the results published by Chingkoe et al [18], reinterpretation of the images in the context of pancreatic pathologies was beneficial in 143 of the 252 cases (56.7%). These data highlight the importance of high-quality imaging and expert review, since these may allow for better tumor staging and lead to higher rates of detection of metastatic disease or tumoral invasion around the major abdominal vessels [1,20,21], placing chemotherapy as the most suitable first treatment option.
Figure 4.
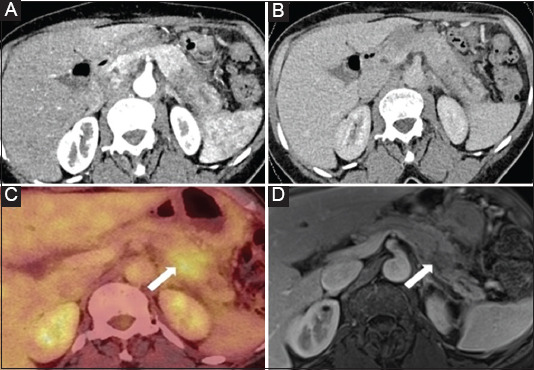
A 70-year-old woman with idiopathic acute pancreatitis. A and B: CT with iodinate contrast medium in the arterial (A) and venous (B) phase show swelling of the pancreatic tail with heterogeneous enhancement and mid ductal dilation, associated with mild inflammatory infiltration of peripancreatic fat. The features were presented as focal autoimmune pancreatitis, but careful analysis of the images detected a focal hypovascular tissular abnormality in the proximal portion of the pancreatic tail and a concomitant lack of visualization of the main pancreatic duct: the 2 features together (mass and stricture) supported the idea of a neoplasm. (C) FDG PET shows focal hypermetabolism in the pancreatic tail. (D) MR image using T1 post-gadolinium injection in the portal venous phase, performed 2 months after the CT, shows regression of inflammatory signs in the pancreatic and peripancreatic caudal region, and delineation of a hypoenhancing 2-cm focal lesion with persistent upstream dilatation of the main pancreatic duct, suggesting the diagnosis of pancreatic ductal adenocarcinoma causing inaugural pancreatitis. EUS-FNB confirmed the diagnosis.
CT, computed tomography; FDG PET, 18-F-fluorodeoxyglucose positron emission tomography; MR, magnetic resonance; EUS-FNB, endoscopic ultrasound fine-needle biopsy
Figure 5.
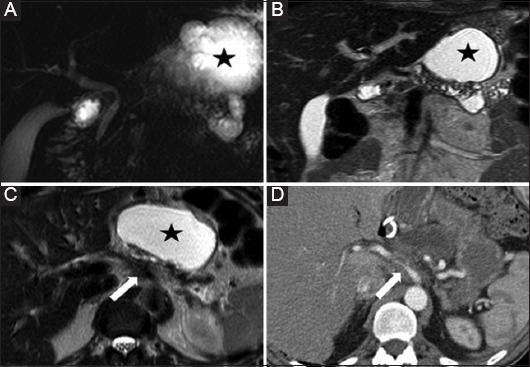
A 55-year-old man with acute pancreatitis of unknown origin. MRCP (A) MR T2 w images in coronal (B) and axial (C) planes show features of chronic pancreatitis with main pancreatic duct irregularity in the body and tail, and parenchymal atrophy. There is a pseudocyst (star) in the lesser sac. No mass was detected. Careful analysis of the images revealed the presence of tissue arising from the pancreatic body at the level of the ductal stricture encasing the celiac artery (arrow), suspicious for vascular invasion from ductal adenocarcinoma. The finding was conformed at CT after iodine contrast medium injection (D) and subsequent tissue sampling.
MRCP, magnetic resonance cholangiopancreatography; MR, magnetic resonance; CT, computed tomography
Proper patient selection for operative resection is also not an easy task. The NCCN classification of loco-regional pancreatic adenocarcinoma divides pancreatic adenocarcinoma into three categories—resectable, borderline resectable, or locally advanced—based on the tumor contact(s) with the major abdominal vessels such as the superior mesenteric artery or the portal vein, thus justifying the participation of an experienced radiologist in the MDT meeting [15,22]. With resectable pancreatic adenocarcinoma, upfront surgical resection is recommended, but the guidelines for borderline resectable tumors often involve neoadjuvant chemotherapy. Neoadjuvant chemotherapy seems to improve the chances of performing R0 surgery for this type of tumor, improving the average survival, while non-resectable tumors are considered first for chemotherapy as upfront curative oncological surgery is unfeasible [15,21,22]. Furthermore, the role of radiotherapy—and in particular stereotactic body radiation therapy—is promising, but still requires further validation in randomized controlled trials (e.g., the STEREOPAC trial [NCT05083247]) [15,23-25].
Endoscopy was the most common therapeutic plan before MDT for patients with solid lesions, referring either to biliary drainage by endoscopic retrograde cholangiopancreatography, or to endoscopic ultrasound with fine needle aspiration/biopsy. Patients who are candidates for chemotherapy require both confirmation of malignancy through tissue acquisition and resolution of jaundice through biliary drainage [26,27]. The above endoscopic modalities had been performed before the last MDT discussion; therefore, endoscopy represented only a minor therapeutic management option at the end.
According to the results of this study, therapeutic management of pancreatic cystic lesions was also significantly impacted by MDT discussion, with 63.2% of patients experiencing a modification in the proposed plan. After MDT discussion, clinical or imaging follow up was the most common proposition. The therapeutic management of cystic lesions varies according to their nature. If the risk of neoplastic evolution is nil or exceptional (such as in serous cystadenomas), there is a priori no indication for surgery, except in cases of compression of a structure such as the main bile duct. Follow up by imaging or clinical examination is therefore usually sufficient [28].
With regard to the role of MDT discussion in the definition of the diagnosis of pancreatic lesions, we documented a change in 17.6%, with a statistically significant difference between solid and cystic lesions (7.7% vs. 35.7%, respectively). Other studies, particularly those focused on malignant solid lesions such as adenocarcinoma, have reported higher numbers that ranged from 23-42% of cases for modification of diagnosis [1,12,20,29]. The difference between our percentage and those found in the literature can be explained by the fact that, in the studies mentioned above, a change in staging was considered as a change in diagnosis, while in our study, such changes were not recorded in that way. In the results published by Chingkoe et al, cystic lesions underwent a change of diagnosis in 39% of cases, comparable to the percentage found in our study (35.7%) [18]. The challenge with cystic lesions is to provide accurate diagnosis and determine their malignant potential before deciding between surgery, surveillance or discharging the patient [30]. CT and MRI imaging accuracy rates range from 73-97% for differentiating benign and malignant cystic lesions, making image review by an experienced radiologist at an MDT meeting essential [30]. In our study, providing a diagnosis after MDT in patients with an unspecified cystic lesion was also considered a modification, as it may determine further therapeutic management. One of the important things to note about our study is that 14 adenocarcinomas were discovered among the cystic lesions after review of the case in the MDT meeting, leading to an adaptation in management. In these cases, the cystic lesion was a consequence of ductal obstruction due to an adenocarcinoma (e.g., a retention cyst or a dilated duct) and not a true cystic lesion, making the final diagnosis more challenging.
Concerning the time until the MDT meeting and time from MDT decision to therapeutic management initiation, we observed a difference between cystic lesions and solid lesions. Indeed, solid lesions were managed more rapidly, and this difference can be explained by the fact that most solid lesions are suspicious for malignancy, calling for prompt management. However, the lack of a comparative group of patients without MDT does not allow us to know whether the delays were affected in a favorable or unfavorable way by the MDT discussion. Further studies should be conducted to evaluate the impact of MDT discussion on the time to treatment initiation, as well as the impact of any delay on the patient’s prognosis and outcome.
Our study has some limitations. First, its retrospective design; the problem of missing data with this type of study is well known. However, in our case, we obtained the data based on written and validated recording of the patient’s MDT, which is associated with a low probability of inadequate or missing data. Moreover, the lack of a long-term follow-up analysis did not allow for an assessment of the potential benefits of MDT discussion in terms of patients’ prognosis. Finally, combining patients with cystic and solid lesions in the analysis could lead to a heterogenous group, with benign and malignant pathologies, and therefore interfere with the results and their comparison with previous studies. The strengths of our study are the number of patients reviewed in a reference center, and the fact that, to the best of our knowledge, this study is the first to compare the impact of MDT discussion on the management of pancreatic solid and cystic lesions.
Overall, our study highlights the fact that discussing cystic lesions in a MDT meeting is of the utmost important for providing accurate diagnosis. Moreover, MDT discussion has an important impact on the therapeutic management of patients with solid pancreatic lesions.
Summary Box.
What is already known:
Multidisciplinary team (MDT) meetings play a crucial role in decision making for all cancer patients, allowing better communication, coordination and decision-making among healthcare professionals when evaluating treatment options
Several studies have shown that multidisciplinary meetings are associated with changes in the initial diagnosis and staging of tumors, minimization of errors, and better adherence to existing guidelines
The impact of MDT discussion on the management of patients with focal pancreatic lesions has not been investigated
What the new findings are:
MDT discussion significantly impacts the management of patients with both cystic and solid pancreatic lesions
MDT discussion leads to a modification of the initially proposed management in two-thirds of patients, mainly through revision of cross-sectional imaging
The impact of MDT on lesion diagnosis was more significant for cystic lesions than for solid lesions
Biography
Erasme University Hospital, HUB; Institute Jules Bordet, HUB, Brussels, Belgium
Footnotes
Conflict of Interest: None
Funding: CB disclosed receipt of the following financial support: doctoral grants from the “Association Jules Bordet” [grants number: 2019-31 and 2021-03] and by the “Fonds de la Recherche Scientifique – FNRS” [Grant number FC 33593].
References
- 1.Pawlik TM, Laheru D, Hruban RH, et al. Johns Hopkins Multidisciplinary Pancreas Clinic Team. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams:a systematic review. Ann Surg Oncol. 2011;18:2116–2125. doi: 10.1245/s10434-011-1675-6. [DOI] [PubMed] [Google Scholar]
- 3.Back MF, Ang EL, Ng WH, et al. Improvements in quality of care resulting from a formal multidisciplinary tumour clinic in the management of high-grade glioma. Ann Acad Med Singap. 2007;36:347–351. [PubMed] [Google Scholar]
- 4.Wright FC, De Vito C, Langer B, Hunter A. Expert Panel on Multidisciplinary Cancer Conference Standards. Multidisciplinary cancer conferences:a systematic review and development of practice standards. Eur J Cancer. 2007;43:1002–1010. doi: 10.1016/j.ejca.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Devitt B, Philip J, McLachlan SA. Team dynamics, decision making, and attitudes toward multidisciplinary cancer meetings:health professionals'perspectives. J Oncol Pract. 2010;6:e17–e20. doi: 10.1200/JOP.2010.000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39:838–841. doi: 10.1111/j.1445-5994.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 7.Sidhom MA, Poulsen M. Group decisions in oncology:doctors'perceptions of the legal responsibilities arising from multidisciplinary meetings. J Med Imaging Radiat Oncol. 2008;52:287–292. doi: 10.1111/j.1440-1673.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 8.Bekkali NLH, Murray S, Winter L, et al. The role of multidisciplinary meetings for benign pancreatobiliary diseases:a tertiary centre experience. Frontline Gastroenterol. 2017;8:210–213. doi: 10.1136/flgastro-2016-100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruhstaller T, Roe H, Thürlimann B, Nicoll JJ. The multidisciplinary meeting:An indispensable aid to communication between different specialities. Eur J Cancer. 2006;42:2459–2462. doi: 10.1016/j.ejca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings:A systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. doi: 10.1016/j.ctrv.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Jerusalem G, Coucke P. Apport de la consultation oncologique multidisciplinaire dans le choix des options thérapeutiques [The role of multidisciplinary tumor board discussions in treatment decisions. Revue Med Liège. 2011;66:311–314. French. [PubMed] [Google Scholar]
- 12.Kirkegård J, Aahlin EK, Al-Saiddi M, et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br J Surg. 2019;106:756–764. doi: 10.1002/bjs.11093. [DOI] [PubMed] [Google Scholar]
- 13.Chang JH, Vines E, Bertsch H, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management:the University of Pennsylvania experience. Cancer. 2001;91:1231–1237. doi: 10.1002/1097-0142(20010401)91:7<1231::aid-cncr1123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Devitt B, Philip J, Singh M, McLachlan SA. Understanding patients'attitudes toward cancer multidisciplinary meetings:a mixed methods study. JCO Oncol Pract. 2020;16:e175–e182. doi: 10.1200/JOP.19.00274. [DOI] [PubMed] [Google Scholar]
- 15.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 16.Haj-Mirzaian A, Patel BN, Fishman EK, Zaheer A. Value of multidisciplinary collaboration in acute and chronic pancreatitis. Abdom Radiol (NY) 2020;45:1458–1467. doi: 10.1007/s00261-019-02320-9. [DOI] [PubMed] [Google Scholar]
- 17.Van Hagen P, Spaander MC, van der Gaast A, et al. Rotterdam Oesophageal Tumour Study Group. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making:a prospective cohort study. Int J Clin Oncol. 2013;18:214–219. doi: 10.1007/s10147-011-0362-8. [DOI] [PubMed] [Google Scholar]
- 18.Chingkoe CM, Brook A, Moser AJ, Mortele KJ. Subspecialized radiology review at multidisciplinary pancreas conference:impact on patient management. Abdom Radiol (NY) 2018;43:2783–2789. doi: 10.1007/s00261-018-1549-5. [DOI] [PubMed] [Google Scholar]
- 19.Quero G, Salvatore L, Fiorillo C, et al. The impact of the multidisciplinary tumor board (MDTB) on the management of pancreatic diseases in a tertiary referral center. ESMO Open. 2021;6:100010. doi: 10.1016/j.esmoop.2020.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Herman JM, Wolfgang CL, Zheng L. Multidisciplinary management of pancreatic cancer. Surg Oncol Clin N Am. 2013;22:265–287. doi: 10.1016/j.soc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni NM, Soloff EV, Tolat PP, et al. White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology's disease-focused panel for pancreatic ductal adenocarcinoma:Part I, AJCC staging system, NCCN guidelines, and borderline resectable disease. Abdom Radiol (NY) 2020;45:716–728. doi: 10.1007/s00261-019-02289-5. [DOI] [PubMed] [Google Scholar]
- 22.Fogel EL, Shahda S, Sandrasegaran K, et al. A multidisciplinary approach to pancreas cancer in 2016:a review. Am J Gastroenterol. 2017;112:537–554. doi: 10.1038/ajg.2016.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchart C, Navez J, Closset J, et al. Novel strategies using modern radiotherapy to improve pancreatic cancer outcomes:toward a new standard?Ther Adv Med Oncol . 2020;12:1758835920936093. doi: 10.1177/1758835920936093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchart C, Engelholm JL, Closset J, et al. Isotoxic high-dose stereotactic body radiotherapy integrated in a total multimodal neoadjuvant strategy for the treatment of localized pancreatic ductal adenocarcinoma. Ther Adv Med Oncol. 2021;13:17588359211045860. doi: 10.1177/17588359211045860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preoperative mFOLFIRINOX (or Gem-Nab-P) +/- Isotoxic High-dose SBRT for Borderline Resectable Pancreatic Adenocarcinoma (STEREOPAC) [[Accessed 6 July 2023]]. Available from: https://www.clinicaltrials.gov/study/NCT05083247 . [DOI] [PMC free article] [PubMed]
- 26.Pouw RE, Barret M, Biermann K, et al. Endoscopic tissue sampling - Part 1:Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:1174–1188. doi: 10.1055/a-1611-5091. [DOI] [PubMed] [Google Scholar]
- 27.Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting:indications, choice of stents, and results:European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910–930. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S, Wang WT, Wu WC, Lou WH, Zeng MS, Rao SX. Magnetic resonance morphologic features predicts progression of incidental cystic lesions during follow-up. Diagn Interv Radiol. 2020;26:396–402. doi: 10.5152/dir.2020.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen MFC, Storkholm JH, Hansen CP. The results of pancreatic operations after the implementation of multidisciplinary team conference (MDT):A quality improvement study. Int J Surg. 2020;77:105–110. doi: 10.1016/j.ijsu.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Farrell JJ. Pancreatic cysts and guidelines. Dig Dis Sci. 2017;62:1827–1839. doi: 10.1007/s10620-017-4571-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


