Learning objectives.
By reading this article, you should be able to:
-
•
Explain the potential benefits of different pacing modes in postoperative cardiac surgical patients.
-
•
List the risk factors for requiring permanent pacemaker implantation after cardiac surgery.
-
•
Describe how to check a temporary pacing system.
-
•
Recognise and manage the most important complications of temporary pacing.
Key points.
-
•
After cardiac surgery, patients often require temporary pacing to optimise their haemodynamic status, prevent atrial fibrillation or protect from sudden bradycardic cardiac death.
-
•
Temporary epicardial pacing carries risk of morbidity and mortality.
-
•
The performance of a temporary pacing system should be checked frequently to reduce the risk of complications.
-
•
The ‘sensing threshold’ is a measured variable. The ‘sensitivity’ is a value that is set based on the sensing threshold.
-
•
Reducing the sensitivity value for a pacing wire allows the pacing box to detect electrical signals of smaller amplitude.
Pathology of the cardiac conduction system poses significant risk to patients after cardiac surgery. As a result, temporary epicardial pacing wires are frequently placed before closing the chest. When combined with a battery-powered pacing box and reusable connecting cables, they form a temporary pacing system. The system allows pacing of the ventricles, atria, or both in order to protect against sudden bradycardic death and other bradycardic dysrhythmias, such as complete heart block. Temporary pacing also allows optimisation of the heart rate to improve cardiac output.
There is no single correct way to pace patients after cardiac surgery. Individual patient factors, the context in which the patient is being paced (i.e. staff experience, the monitoring environment), the complexity and performance of the pacing system (dual or single chamber; sensing thresholds) and how often performance is checked, all influence how the pacemaker system is programmed.
In the clinical case (Box 1), we present a patient who was at low risk of postoperative bradycardic death but nevertheless was placed on an inappropriately complex back-up pacing mode with a system that was functioning poorly. Fortunately, the patient made an uneventful recovery, but the need for chest compressions after cardiac surgery can lead to lasting harm through injury to the right ventricle and coronary grafts, and trauma to the abdominal organs and ribs.1
Box 1.
Definition of terms: sensitivity and sensing threshold
Crucial to the understanding of temporary epicardial pacing is the use of a common language. To ‘increase the sensitivity’ of a pacing system may be understood in conflicting ways by different people. Increasing the sensitivity setting or value renders the pacing system unable to detect signals of smaller amplitude. Increasing the sensitivity of the pacing system, so that signals of smaller amplitude are detected, requires a reduction in the sensitivity setting.
Throughout this article we use the term sensitivity to refer to the set value on the pacing box and the sensing threshold to refer to a measured variable reflective of the amplitude of the signal detected by the pacing system. When a pacing system is able to ‘sense well’, the sensing threshold is high. When the pacing system ‘senses poorly’, the sensing threshold is low or undetectable.
In this article, we provide a practical guide to the management of temporary epicardial pacing after cardiac surgery. We explain the measurement of sensing and capture thresholds, how to set the sensitivity and output given these measured values, and how to choose the best pacing mode in a given setting to ensure optimal safety and efficacy for the patient.
Clinical case: DDD pacing with poor atrial sensing leading to R-on-T
A 50-year-old patient underwent coronary artery bypass grafting following a non-ST elevation myocardial infarction. The operation and postoperative ICU course were uneventful. The patient remained in sinus rhythm at 80-100 beats min-1. The temporary pacemaker system was set in DDD mode at a rate of 60 beats min-1. No temporary pacing was required during the patient’s stay in ICU and no arrhythmias were observed.
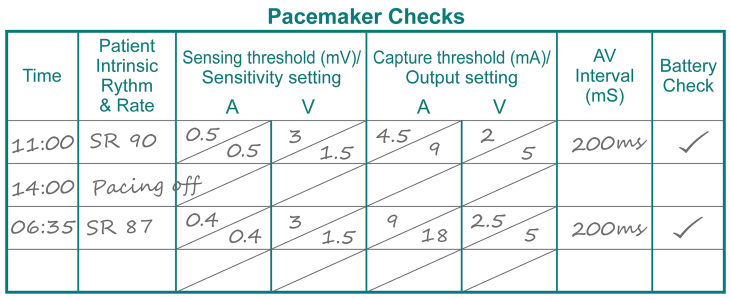
Sensing and capture thresholds were checked by the bedside nurse before discharge from the ICU and were reported as satisfactory (illustrated). A short time later, the patient suffered a cardiac arrest. Return of spontaneous circulation was achieved after three minutes of cardiopulmonary resuscitation and one defibrillation attempt. No further arrhythmia was reported and the patient recovered fully.
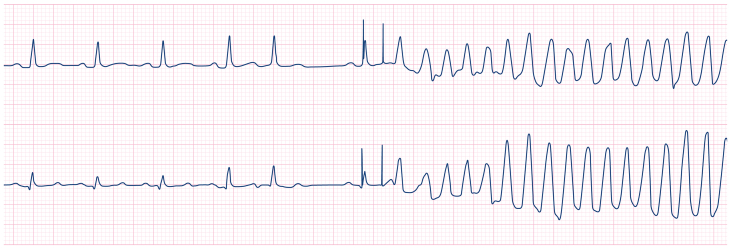
In fact, the atrial sensing threshold was very low. The low atrial sensing threshold resulted in undersensing of an intrinsic P-wave after a post-ectopic pause, which in turn prompted inappropriate atrial then ventricular pacing. Due to ventricular lead blanking during atrial pacing, which occurred simultaneously with the conducted (intrinsic) R-wave, the R-wave was not sensed by the pacing system (see ECG). Failure to sense the intrinsic R-wave led to a ventricular pacing impulse being delivered during the vulnerable period of the T-wave (R-on-T), triggering polymorphic ventricular tachycardia and subsequent ventricular fibrillation.
History and technology
The emergence of pacemaker technology was made necessary by advances in cardiac surgery. By the late 1950 s, an increasing number of operations were being undertaken, largely to correct congenital abnormalities in children. Recovery was often complicated by atrioventricular block (AVB). In the short-term, AVB could often be managed with positive chronotropic drugs. However, a longer-term solution was lacking. Existing methods to externally pace patients via skin electrodes had been unsuccessful, with the systems being bulky, painful and reliant on mains power. The need for a long-term solution to AVB led to the development of pacing wires that could be embedded in the myocardium and exteriorised through the skin. In 1957, a 3-yr-old girl with AVB was the first patient to be successfully treated with a temporary pacing wire and battery-powered pacing box, after repair of a tetralogy of Fallot.2
Modern pacing wires are made of steel with a polytetrafluoroethylene coating to insulate the wire, except at the active ends. Pacing wires may be bipolar, with the cathode and anode housed in the same wire separated by a short insulated section, or unipolar, with two separate wires used to complete a circuit for each paced chamber. Pacing boxes have a variety of settings that can be manipulated to optimise safety and efficacy. There are at least seven manufacturers worldwide that produce pacing boxes of varying complexity.
The need for pacing after cardiac surgery
The normal physiology of cardiac conduction has been recently reviewed in this journal.3 After cardiac surgery, numerous factors may affect the intrinsic pacemakers and conduction system of the heart. These factors include: induced hypothermia; myocardial oedema; myocardial isch-aemia and reperfusion; electrolyte abnormalities; pre-existing conduction abnormality, such as scar or aortic root abscess; surgical handling; suturing near the atrioventricular (AV) node or bundle of His; and use of antiarrhythmic drugs.
Particularly in the early postoperative period, the factors listed above may result in sinus bradycardia, low grade AVB (first degree AVB, Wenckebach), high grade AVB (Mobitz II, complete heart block), or, in extreme cases, ventricular standstill or asystole. Approximately half of all patients undergoing cardiac surgery are affected by some form of dysrhythmia during the postoperative period, the most common being atrial fibrillation (AF).4 The true rate of transient bradyarrhythmia is likely to be underappreciated, attributable in part to the common practice of using back-up (i.e. demand) pacing or pacing to optimise cardiac output.
A large, single-centre study reported that 17.5% of all cardiac surgical patients were paced on arrival to the ICU.5 After coronary artery bypass graft (CABG) surgery, AVB is rare. In 2012, Khorsandi and colleagues reported that AVB complicates only 2.4% of primary CABG procedures.6 AVB is more likely after valve replacement surgery, with rates of 23.5% reported after mitral valve replacement (MVR) and 10.3% after mechanical aortic valve replacement (AVR).7,8
Permanent pacemaker implantation after cardiac surgery
Not all patients with AVB after cardiac surgery require permanent pacemaker (PPM) implantation. A retrospective review of >77,000 operations in the USA found that the rate of PPM implantation during the same admission was 11.3% for AVR plus MVR, 8.6% after MVR and 5.2% after AVR.9 Valve repair and CABG surgery confer a much lower risk. The risk factors for requiring a PPM after cardiac surgery are listed in Table 1.10,11
Table 1.
| Patient factors | Surgical factors |
|---|---|
| Older age | CPB (compared with non-CPB) |
| Increased EuroSCORE | Prolonged CPB time |
| Pulmonary hypertension | Type of surgery (mitral>aortic>CABG) |
| Renal failure | Extent of surgery (2 valves>1 valve>CABG) |
| Diabetes mellitus | Reoperation |
| Reduced LV ejection fraction | |
| Preoperative arrhythmia | |
| Preoperative conduction abnormality on ECG | |
| Aortic root abscess |
Pacing modes and indications
Essentially, two different types of pacing are available on temporary pacing boxes. ‘Fixed’ or ‘asynchronous’ modes do not accommodate the patient's intrinsic rhythm. The advantage of asynchronous modes is that they will continue to pace at a set rate, regardless of electrical interference such as that generated by intraoperative diathermy. However, asynchronous modes may pace over a patient's intrinsic rhythm, with the risk of a pacing impulse being delivered in the vulnerable period of ventricular repolarisation, causing ‘R-on-T’ phenomenon. R-on-T may induce polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF). Therefore, asynchronous modes should not be used postoperatively outside of emergency situations. The second type of pacing is ‘demand’ or ‘synchronous’ pacing. Provided the pacing system is working appropriately, this type of pacing allows (i) the system to be inhibited when normal conduction is present and (ii) triggering of ventricular pacing when intrinsic atrial activity is sensed and conduction to the right ventricle is delayed.
Within these two basic types of pacing, there are several subclassifications. The terminology for pacing modes adheres to the North American Society of Pacing and Electrophysiology (NASPE) and British Pacing and Electrophysiology Group (BPEG) generic pacemaker code and is the same as that used for the programming of permanent devices.12 The coding system is further abbreviated to the NBG (‘NASPE/BPEG Generic’) code. Only the first three letters of the NBG code are relevant to temporary pacing. The first letter refers to the chamber(s) paced, the second to the chamber(s) sensed, and the third to the response of the pacing box to sensing. Responses to sensing include inhibition of pacing (I), triggering of pacing (T), both (D), or asynchronous pacing (O).
Commonly used pacing modes
The commonly used modes of temporary pacing, along with their indications and relative contraindications, are detailed in Table 2.
Table 2.
Pacing modes, indications and contraindications.
| Mode | Meaning | Indications | Relative contraindications |
|---|---|---|---|
| AAI | The atrium is paced, the atrium is sensed and inhibition of pacing occurs in response to sensing | Severe sinus bradycardia Sinus bradycardia with poor cardiac output Prevention of postoperative AF |
High-grade AVB High risk of high grade AVB (DDD/DDI/VVI may be preferred) AF Poor sensing in the atrial wire(s) (Fig. 2) |
| VVI | The ventricle is paced, the ventricle is sensed and inhibition of pacing occurs in response to sensing | Patients at high risk of, or established AVB Ventricular standstill Patients without atrial wires with an inadequate heart rate |
Patients who have better ventricular ejection with physiological depolarisation of the ventricles Poor sensing in the ventricular wire(s) (Fig. 2) |
| DDD | Both the atrium and ventricle are sensed and paced. Either may be inhibited in response to sensing. If the sensed atrial rate is faster than the set pacemaker rate and no ventricular depolarisation occurs within the set AV interval, the ventricular wire will pace the ventricle at the sensed atrial rate (triggering occurs) | High-grade AVB Asystole Sinus bradycardia with high risk of AVB |
Poorly sensing ventricular or atrial wire(s) (Fig. 2) Pacemaker-mediated tachycardia (if not easily remedied by adjusting refractory periods, see section on pacing problems) |
| DDI | Both the atrium and ventricle are sensed and paced. Either may be inhibited in response to sensing. Unlike DDD mode, if the sensed atrial rate is faster than the set pacemaker rate, no ventricular pacing will occur in response to the sensed intrinsic atrial activity provided each depolarisation is conducted to the ventricles (prolongation of the A-V interval is tolerated) | Patients with improved cardiac performance from physiological ventricular depolarisation who need to be protected from bradycardia and high grade AVB Patients with recurrent, paroxysmal AF or atrial flutter with an inadequate ventricular rate Previous episode(s) of pacemaker-mediated tachycardia in DDD mode |
Poorly sensing ventricular or atrial wire(s) (Fig. 2) |
| VOO | The ventricle is paced asynchronously at the set rate. There is no sensing | Emergency use Weaning from bypass with only ventricular wire(s) Pacing dependence when diathermy in use (e.g. for patients returning to theatre) |
Established temporary pacing with appropriate sensing thresholds Not constantly monitored and supervised by a practitioner skilled in rhythm interpretation |
| DOO | Both the atrium and ventricle are paced at the set rate, with a set AV interval between the two There is no sensing | Emergency use Weaning from bypass with both atrial and ventricular wires Pacing dependence when diathermy in use (e.g. for patients returning to theatre) |
Established temporary pacing with appropriate sensing thresholds Not constantly monitored and supervised by a practitioner skilled in rhythm interpretation |
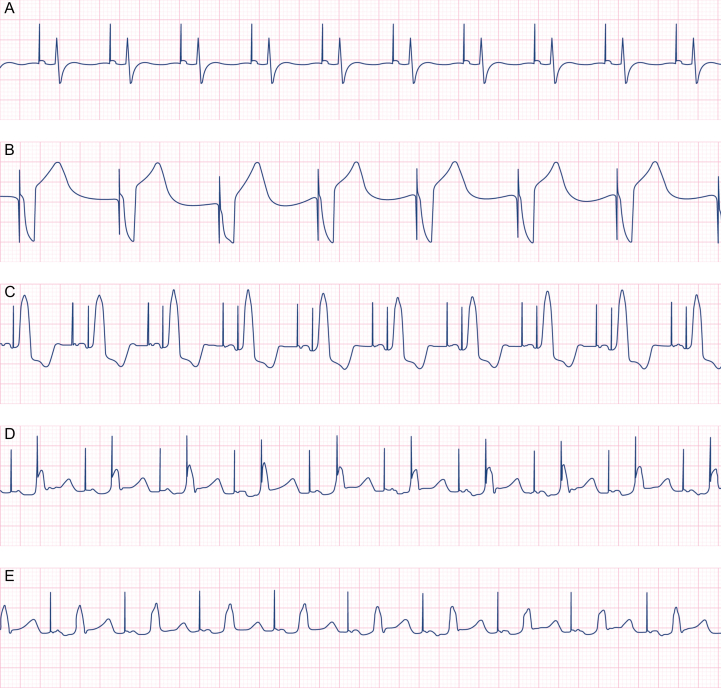
AAI (Fig. 1A)
Fig 1.
Commonly used temporary pacing modes. Pacing detection software is active in all of these examples. (A) Atrial pacing at a rate of 90 beats min−1. No intrinsic atrial activity is seen and so it is impossible to tell from this trace alone whether the pacing system is set in a synchronous (AAI) or asynchronous (AOO) pacing mode. (B) Ventricular pacing at 60 beats min−1. The pacing spike immediately precedes a broad complex QRS. No P-wave is seen. The device may be set in VVI or VOO mode, as no intrinsic ventricular activity is seen. (C–E) DDD pacing at a rate of 90 beats min−1 with a progressive increase in the set atrioventricular interval. (C) The AV interval is set at 150 ms. Both atria and ventricles are extrinsically paced. (D) The AV interval is then increased to 240 ms. This allows sufficient time for the atrial depolarisation to travel down the His-Purkinje system (note the change in R-wave morphology). Because the PR interval is prolonged, the R-wave arrives too late to be sensed by the ventricular lead and so a ventricular pacing spike is seen immediately before the conducted QRS complex. (E) The AV interval is further increased to 270 ms. This allows sufficient time for the conducted QRS complex to be sensed by the ventricular lead and inhibits delivery of a ventricular pacing impulse.
In the presence of normal AV conduction, atrial pacing is beneficial, as the induced depolarisation follows the native conduction pathway, resulting in physiological depolarisation and synchronised contraction of both ventricles, provided that there is no bundle branch block. Synchronised ventricular contraction improves ventricular ejection and atrial systole increases ventricular preload, provided the AV interval is not unduly prolonged.
Atrial pacing in the immediate postoperative period reduces the risk of new AF.13 Rapid atrial pacing may be used in short bursts to treat atrial flutter by terminating the re-entrant flutter wave. For rapid atrial pacing, rates of 260–340 beats min−1 (20–30 beats min−1 faster than the actual atrial flutter rate) are required for up to 20 s in order to ‘capture’ the atria. If effective, cessation of rapid pacing results in the immediate restoration of sinus rhythm.
VVI (Fig. 1B)
In VVI mode, the paced depolarisation stimulates the right ventricular free wall first, meaning the right ventricle is depolarised before the left. (Normally the ventricles depolarise simultaneously in a coordinated way via the left and right bundle branches, which traverse the ventricular septum.) Non-physiological ventricular depolarisation with VVI pacing leads to a broad QRS complex and reduced left ventricular ejection as a result of dyssynchronous septal motion.14 Because only the right ventricle is paced, there is no coordination with atrial systole. As atrial systole contributes up to 25% of ventricular filling, ventricular ejection is generally reduced with VVI mode compared with physiological depolarisation or AV sequential pacing.
DDD and DDI (Fig. 1C–E)
In AV sequential pacing, the atria and the ventricles are paced in sequence, with a predetermined time delay (the AV interval). The AV interval is commonly set between 160 and 200 msec. Both atria and ventricles are paced at a fixed rate, unless intrinsic activity is sensed in either chamber. In DDI mode, if atrial activity is sensed and is faster than the set rate, provided that there is 1:1 conduction to the ventricles, the intrinsic AV interval may exceed the set AV interval without triggering ventricular pacing. The ventricular lead is inhibited. However, in DDD mode, when the sensed atrial rate is faster than the set rate, if the set AV delay is reached without ventricular depolarisation occurring spontaneously, the ventricular lead will pace the ventricle at the sensed atrial rate (i.e. faster than the pacing box's set rate). The ventricular lead is triggered.
Ventricular filling and ejection are usually improved with AV sequential pacing compared with ventricular pacing. However, this is not always the case, as ventricular depolarisation is non-physiological (see VVI above).
It may be difficult to establish whether the ventricles are being captured from above (via the atria) or from below (via the RV free wall epicardial lead). In order to distinguish between the two, the morphology of the QRS and arterial pressure waveform needs to be carefully examined whilst the AV interval is adjusted (Fig. 1 [C–E]).
VOO/DOO
These modes are asynchronous and as such carry the risk of R-on-T phenomenon. To facilitate their use in an emergency, there is a standalone VOO or DOO button on many pacing boxes.
Initiating temporary pacing in the operating room
Temporary pacing is often initiated at the time of weaning from cardiopulmonary bypass (CPB). At this time, the heart's intrinsic pacemakers are recovering from hypothermia and ischaemia and may be too slow for optimal cardiac output. A paced rate of 70–90 beats min−1 is frequently set. Often either AOO or DOO mode is used initially, in order to prevent inappropriate pacemaker inhibition resulting from surgical diathermy. If AV conduction is intact and not unduly prolonged (<200 ms), AOO is generally preferred over DOO, to preserve physiological ventricular depolarisation.
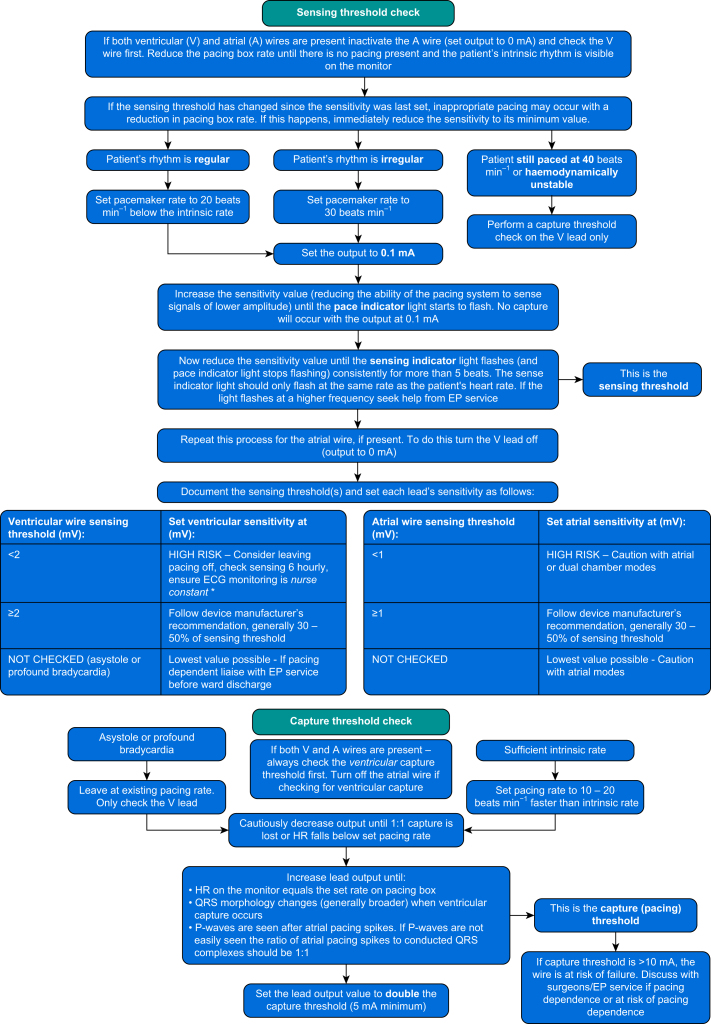
Whilst a full pacemaker check is desirable in the operating room, it is frequently not feasible as the patient is dependent on their paced heart rate for haemodynamic stability. If this is the case, as a minimum, the capture (or pacing) thresholds for both chambers should be measured (Fig. 2) to ensure that pacing function will be maintained during the early postoperative period. Setting the output at just above the capture threshold during chest closure allows dislodgement of the pacing wires to be rapidly detected and rectified. In general, the capture threshold should be <5 mA. A capture threshold of 5–10 mA should prompt a discussion with the surgeon about repositioning or replacing the wires. A capture threshold of >10 mA is unacceptable, as the pacing wires are likely to fail in the early postoperative period. When surgical diathermy is no longer required, the pacemaker should be placed in a demand mode (AAI, VVI, DDD, DDI).
Fig 2.
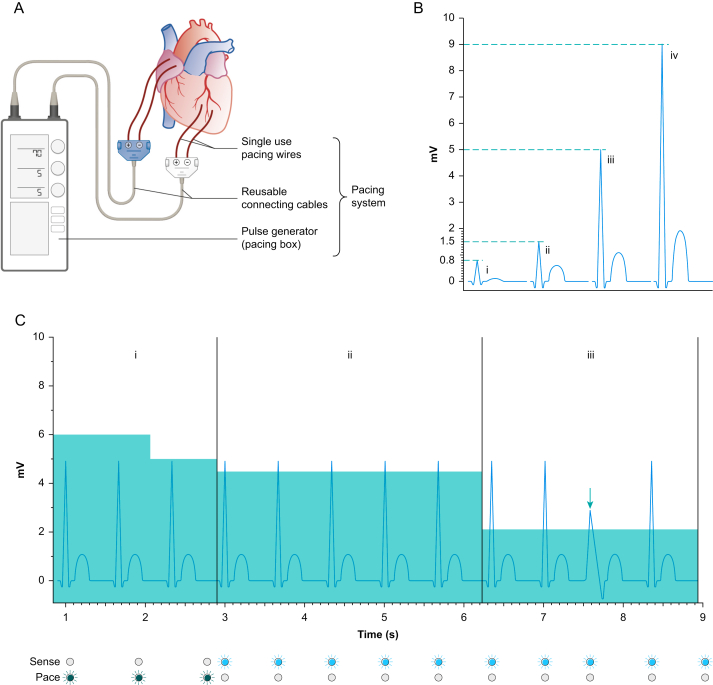
Checking the performance of the pacing system. Ideally the patient is normothermic and normokalaemic before pacing checks. Otherwise, best practice would be to repeat the pacing check when they are. ∗Nurse constant—at all times a nurse is able to see the patient's continuous ECG trace and is able to hear audible alarms from the monitoring system.
Performing a pacemaker check
In order to be safe, a pacing system needs to be able to reliably sense the patient's intrinsic rhythm (Fig. 3) so as to avoid an R-on-T phenomenon. In order to be effective, the system needs to be able to reliably capture the chamber(s) at a reasonable output current.
Fig 4.
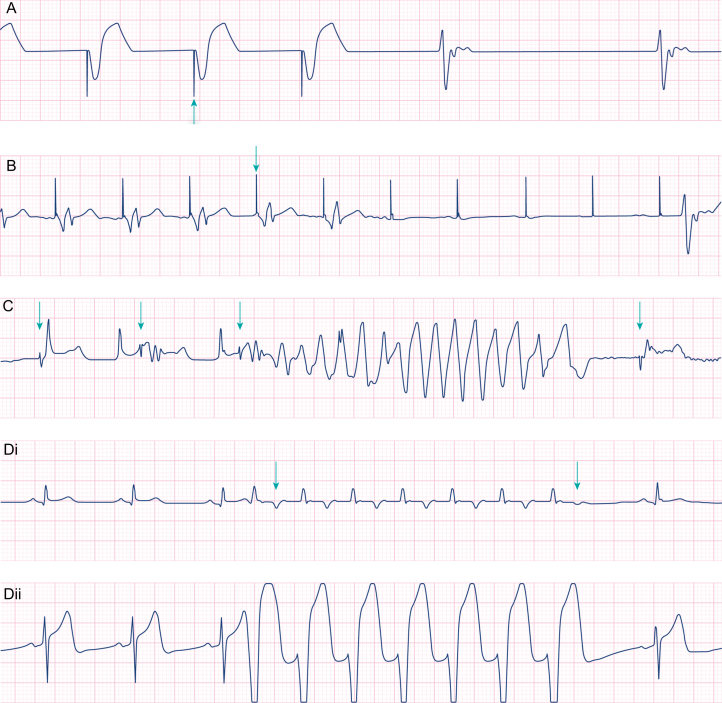
Pacing problems. (A) Failure to pace. With pacing detect activated on the monitor, pacing spikes (arrow) are seen to precede broad ventricular complexes at 56 beats min−1. After three paced beats there is a failure of the pacing system to emit an impulse. A slow intrinsic escape rhythm is seen. (B) Failure to capture. Pacing spikes (arrow) induce ventricular complexes for five beats whereafter the spikes fail to induce ventricular activity. There is a 3.5-s pause before an intrinsic escape beat occurs. (C) R-on-T as a result of ventricular lead undersensing. Pacing detect is deactivated in this example but regular pacing impulses can still be identified (arrows) at a rate of ∼60 beats min−1. The pacing system does not detect the patient's R-waves and inappropriate pacing impulses are delivered. The third pacing impulse is delivered during the T-wave (R-on-T) and non-sustained polymorphic ventricular tachycardia is induced. Inappropriate pacing continues after the dysrhythmia. (D) Pacemaker-mediated tachycardia. Two leads of an ECG are shown (lead II and V). Pacemaker detect is off. The pacemaker is set in DDD mode at a rate of 50 beats min−1 and AV interval of 200 ms. A spontaneous ventricular ectopic (first arrow) is conducted in a retrograde direction up the His-Purkinje system to the atria. This retrograde P-wave is sensed by the atrial lead as intrinsic atrial activity and prompts the pacing system to emit a ventricular impulse (triggering occurs) 200 ms after the P-wave is detected. The paced ventricular depolarisation is again conducted up the His-Purkinje system to the atria. A re-entrant loop is created, producing a broad complex monomorphic tachycardia of 120 beats min−1. In this example, the retrograde conduction up the His-Purkinje system fails, giving rise to the different T-wave morphology seen (second arrow). The re-entry is stopped, and normal sinus rhythm recurs. Alternatively, pausing the pacing system will also break this re-entry and lead to restoration of normal sinus rhythm.
Fig 3.
The pacing system, the sensitivity setting and the sensing threshold. (A) A temporary pacing system comprises single use pacing wires, reusable connecting cables and the pacing box or pulse generator. (B) Unlike in a standard 12-lead ECG, the amplitude of the electrical signal generated by cardiac chamber depolarisation, delivered to the pacing box, may vary significantly. The type of pacing wires used, the method of fixation to the epicardium, the integrity of the connecting cable (which may have been autoclaved numerous times) and the location chosen on the heart all have an effect on this signal. In some patients the R-wave amplitude may be so small (<0.8 mV) that the R-wave cannot be discriminated from background electrical ‘noise’ (i). In others, the R-wave amplitude may be just detectable (ii), readily detectable (iii), or easily detectable (iv) against background electrical noise. (C) The sensitivity is a set value on the pacing box (represented in this figure by the teal shading). The sensitivity defines how large the amplitude of an electrical signal has to be in order to be sensed by the pacing system. This patient has an R-wave amplitude of approximately 5 mV. With the sensitivity set at 6 mV the pacing system is unable to detect the intrinsic R-waves and pacing impulses are delivered at the set rate with synchronous flashing of the pacing indicator light (dark green in this example). The pacing impulses and the R-waves are dissociated. In this example the set rate is 70 beats min−1, 20 beats min−1 slower than the patient's intrinsic rate (i). Reduction of the sensitivity to 5 mV fails to allow sensing as the R-wave signal is at or just below 5 mV amplitude. Reduction of the sensitivity to 4.5 mV allows regular sensing of the R-wave and the sensing indicator light (blue in this example) flashes in synchrony with the patient's R wave (ii). The sensitivity is then set at 2 mV, as this allows detection of normal sinus activity, and ventricular ectopic beats (arrow) with smaller R-wave amplitude (iii).
The procedure for performing a pacing check varies depending on the presence of an underlying intrinsic rhythm that can sustain the patient's blood pressure. Ideally the patient should have a normal temperature (>36 °C) and normal serum potassium before the first routine pacing check. If a more urgent pacing check is indicated, best practice is to repeat the check once the patient's temperature and potassium have returned to normal. If the patient is being paced, the ‘pause’ button can be used to see if there is an intrinsic rhythm. Unplugging the pacing leads is not recommended. If no intrinsic rhythm is seen whilst the system is paused, a gradual reduction in the paced rate, over some minutes, may be necessary to look for development of an intrinsic escape rhythm. Whilst checking for the intrinsic rhythm, the patient's ECG and arterial blood pressure should be continuously monitored.
If an adequate intrinsic rhythm is present, the sensing threshold should be measured before the capture threshold. If no intrinsic rhythm can be found then the sensing threshold(s) of the pacing system cannot be established. Only the capture threshold can be determined, by gradually reducing the pacing box output until capture of the chamber is lost. As loss of capture may result in abrupt decrease in cardiac output and arterial pressure, the operator should be prepared to immediately increase the output of the pacing box.
A suggested protocol for checking the sensing and capture threshold of a temporary pacing system is shown in Figure 2. Sensitivity and output settings should be based on the thresholds for sensing and capture, respectively. The thresholds for sensing and capture may vary substantially, particularly in the first 48 h after surgery. The pacing system should be checked at least daily. Pacing checks should be done with the patient in bed or a reclinable chair, where effective CPR can be immediately administered. A defibrillator should be close by.
Pacing problems
Table 3 summarises the problems that can occur with temporary pacing, along with the common causes and their management. Further explanation is provided below for some of the causes.
Table 3.
Pacing problems, causes and management. In all patients, pacing detect should be activated on the monitor. Electrolyte derangements should be normalised and the patient should be warmed to >36°C. If the capture threshold rises too close to the output setting, respiratory variation may lead to intermittent failure to capture (see below). The patient's intrinsic R–R interval and to a lesser extent, the R-wave amplitude and the capture threshold, often vary with the respiratory cycle. If the set pacing rate is close to the intrinsic rate, this may result in regular, appropriate inhibition of pacing. This is a common cause of intermittent pacing. If the sensing threshold (R-wave amplitude) falls toward the sensitivity setting, respiratory variation may lead to intermittent undersensing. CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; EP, electrophysiology; PVARP, postventricular atrial refractory period.
| Problem | Explanation | Causes | Management |
|---|---|---|---|
| Failure to pace (Fig. 4A) |
The pacing box is programmed to deliver a pacing spike but none occurs. No pacing spikes are seen and the patient's monitored heart rate is less than the set pacing rate. | Wire migration OR Improper wire connection |
Conduct a systematic check (from patient to pacing box): - Check position of wires at skin looking for evidence of migration - Check connection of wires to connecting cables - Check connection of connecting cables to pacing box - Consider replacing the connecting cable - Check pacing box switched on, battery indicator and pacing mode - If haemodynamic compromise, consider transcutaneous pacing and arrange insertion of temporary transvenous wire |
| Oversensing | - Perform a sensing check - Consider increasing sensitivity value (reducing sensing capability of pacing box) to prevent inappropriate inhibition from background electrical noise - Assess for shivering and actively warm if hypothermic - Consider use of neuromuscular blocking agents if significant pacing inhibition is occurring - If diathermy interference (e.g. in operating room) change to asynchronous mode |
||
| Cross-talk | - Seek advice from EP team - Consider deactivating PPM in immediate perioperative period if sensing and pacing capability of the temporary system is adequate - If adequate sensing and capture of permanent system, and appropriate rate, turn off temporary system |
||
| Failure to capture (Fig. 4B) | The pacing box delivers pacing spikes at the appropriate frequency but fails to capture the paced chamber. The patient's monitored heart rate is less than the set pacing rate | Wire migration OR Improper wire connection |
- With atrial pacing consider—has the patient developed atrial fibrillation? Conduct a systematic check (from patient to pacing box): - Check position of wires at skin looking for evidence of migration - Check connection of wires to connecting cables - Check connection of connecting cables to pacing box - Consider replacing the connecting cable - Check capture threshold(s) and set output(s) appropriately - Consider echocardiography to assess for pericardial effusion as a cause of failure to capture/change in capture threshold - If haemodynamic compromise, consider transcutaneous pacing and arrange insertion of new epicardial pacing wires OR a temporary transvenous pacing wire |
| Inappropriate output setting(s) | - Perform a full pacing check. Adjust output setting(s) as necessary based on capture threshold(s) as per Fig. 2 | ||
| Intermittent pacing, irregular pacing, or both | Pacing spikes appear irregular, only intermittently capture the intended chamber, or both | Inappropriate output setting(s) | - Perform a full pacing check. Adjust output setting(s) as necessary based on capture threshold(s) as per Fig. 2 |
| Native heart rate is similar to paced rate | - Assess underlying native rate, by decreasing set pacing rate - Assess ECG for respiratory variation in native R–R interval - Increase paced rate to >10 beats min−1 above, or reduce rate to 20 beats min−1 or more below the native rate |
||
| Undersensing | - Check sensing threshold for ventricular (and atrial if present) wires - Set sensitivity appropriately as per Figure 2 - Consider turning pacing box off if native rhythm is adequate and consult with EP team - Do not discharge to ward with a poorly sensing pacing system turned on |
||
| Oversensing | - Manage as per oversensing above | ||
| Pacing system tracking atrial arrhythmia (e.g. AF or atrial flutter) in DDD mode | - Consider switch to VVI or DDI mode - Consider electrical cardioversion - Consider rapid atrial pacing for atrial flutter (see text AAI for details) - Treat atrial arrhythmia pharmacologically as per institutional protocol |
||
| R-on-T induced VT or VF (Fig. 4C) | The pacing system delivers a pacing spike over the vulnerable part of the T-wave. Polymorphic VT/VF is provoked | Undersensing of atrial or ventricular leads Frequent ectopy |
Immediate management: - Pause pacemaker - External defibrillation - CPR as dictated by institutional protocol for cardiac surgical life support When ROSC attained: - Reduce output setting to minimum (0.1 mA) - Immediate sensing threshold check and adjustment of sensitivity as appropriate - Consider leaving pacing box switched off if system is sensing poorly - Assess telemetry for R-on-T and seek expert advice |
| Pacemaker-mediated tachycardia (Fig. 4D) | See text for explanation | Ventricular ectopy AND First degree AV block or an Accessory pathway AND a relatively short PVARP setting |
Immediate management: - Pause pacing box and assess rhythm. If true pacemaker-mediated tachycardia, the broad complex tachycardia will be terminated - If no change to monitored rhythm, then treat as broad complex tachycardia using standard advanced life support (ALS) protocol. Specific management: - Cautiously increase PVARP setting (this will be limited by the set pacemaker rate and the AV interval) - Consider switching to DDI or VVI mode |
Undersensing
With undersensing, the system fails to sense the intrinsic depolarisation of the cardiac chamber, allowing the delivery of an inappropriate pacing impulse. Undersensing can occur after inappropriate setting of the sensitivity (i.e. the sensitivity setting is too high), or secondary to variation in the sensing threshold, as a result of any of the factors that also affect the capture threshold (see Failure to capture, Table 3).
When pacing the atrium, inappropriate pacing during the atrial refractory period can cause AF. When pacing the ventricle, inappropriate pacing during the ventricular refractory period (i.e. R-on-T phenomenon) can produce polymorphic VT or VF. In patients paced in AV sequential modes (e.g. DDD), poor sensing in the atrial lead can also produce inappropriate ventricular pacing and R-on-T. This is because whenever an atrial pacing impulse is delivered, the pacing system's ventricular sensing is transiently disabled (blanking), to prevent that impulse being misinterpreted by the system as an intrinsic R-wave. If an intrinsic P-wave goes unsensed, an inappropriate atrial pacing impulse delivered on the conducted R-wave may lead to the pacing system missing the conducted R-wave. The pacing box may then deliver a ventricular pacing impulse (at the set AV interval) on the T-wave (see clinical vignette).
If undersensing is undetected or left unresolved, an R-on-T phenomenon may generate VT or VF. There are numerous case reports of cardiac arrest being caused by this mechanism.15, 16, 17, 18 As such, patients with pacing systems that sense poorly should not be discharged to the ward on back-up pacing. Undersensing may be resolved or improved by turning the sensitivity setting down. Ultimately, if the sensing threshold is too low (Fig. 2), the risk of R-on-T cannot be fully mitigated and the only solution is to turn the pacing box off or—if the patient is fully pacemaker dependant—to keep the patient in a highly monitored environment with immediate access to a defibrillator.
Oversensing
With oversensing, the pacing box misinterprets another electrical signal as cardiac chamber depolarisation and pacing is inappropriately inhibited. The other electrical signal may be from another cardiac chamber, a T-wave or skeletal muscle activity. In the ICU this phenomenon is particularly relevant in the shivering patient. Oversensing commonly occurs in the operating room when diathermy is in use. Oversensing can be resolved by increasing the sensitivity setting, causing low amplitude electrical signals to be ignored.
Pacemaker-mediated tachycardia
Pacemaker-mediated tachycardia occurs when a ventricular ectopic depolarisation (either native or produced by an inappropriate pacing impulse) is conducted in a retrograde fashion up the bundle of His or an accessory pathway, producing a retrograde P-wave in the atria. To prevent a retrograde P-wave from being misinterpreted as intrinsic atrial activity, many pacing boxes have a postventricular atrial refractory period (PVARP) setting. However, if the P-wave is sufficiently delayed and arrives after the PVARP, it will be sensed by the atrial pacing circuit and trigger the pacing box to produce a ventricular pacing spike after the programmed AV interval (ventricular triggering occurs). This creates a re-entry loop, producing a broad complex tachycardia at a rate that is usually just lower than the upper ventricular rate limit of the pacing system. Turning the pacing box off usually resolves the problem.
New atrial tachyarrhythmias (AF or atrial flutter) may be ‘tracked’ in DDD mode, with the pacing box rapidly stimulating the ventricles in response to the sensed atrial events. To prevent this, most pacing boxes have a set upper limit on the atrial rate that will lead to inhibition of the ventricular pacing impulse. If the problem persists, the mode should be changed to DDI or VVI.
Pacemaker crosstalk
If a temporary pacing system is used postoperatively in a patient with a PPM, it is possible for one system to fail to capture the patient's cardiac chambers but succeed in inhibiting the other system. PPM leads may be displaced or damaged during cardiac surgery. If a PPM is to be used postoperatively, the system should be checked and appropriately set before transferring the patient to the ICU. Crosstalk is managed by turning off one of the pacing systems.
Removal of pacing wires
Institutional guidelines should inform the timing of wire removal, with the postoperative day, risk of arrhythmia, coagulation status and the monitoring environment all being considered. In the absence of pacing dependence or a significant ongoing risk of bradyarrhythmia, wires are usually removed sometime after the second postoperative day, but at least 24 h before hospital discharge. In our institution, wires are only removed if heparin prophylaxis has been withheld, the platelet count >100×109 L−1, and the international normalised ratio <3.0. Pacing wires should be removed before commencing direct acting oral anticoagulants (e.g. dabigatran).
Generally, pacing wires are removed by an experienced bedside nurse. After cleaning the skin around the exit site and removing any retaining sutures, constant gentle downward traction is applied to release each wire in turn. If there is undue resistance, the attempt should be aborted and the surgeon informed. Occasionally, it may be safest to cut a retained wire flush to the skin, but this carries a small risk of localised abscess formation and migration of the retained wire.19 MRI incompatibility is also a theoretical risk. As such, long-term surgical follow-up of patients with retained wires is recommended.
Cardiac tamponade is a well-recognised complication after removal of pacing wires. The incidence of tamponade requiring chest reopening in this setting is 9–18 per 10,000 surgeries.20, 21, 22 Given this low incidence and the background risk of tamponade after cardiac surgery, risk factors specific to wire removal are not well described. All patients should be observed closely in the 24 h after removal. Any sign of new-onset hypotension or shock should prompt an urgent echocardiogram to exclude tamponade.
Discharge and referral to the electrophysiology service
A close relationship with the electrophysiology (EP) service is essential for safe and effective care of patients with temporary pacing systems. Any patient who is pacing dependent requires continuous monitoring and review by the EP service before ICU discharge. EP review should also be considered for those patients with ongoing risk of AVB, poor sensing and capture thresholds, or recurrent tachyarrhythmia.
Conclusions
Patients often require temporary pacing after cardiac surgery. However, no pacing strategy is uniformly safe. The pacing thresholds, settings and mode should be reviewed frequently to ensure pacing is as safe and effective as possible. Asynchronous modes (DOO, VOO) should not be used except in an emergency or in the operating room when the patient's chest is open. The capture threshold should be checked in the operating room before chest closure, and if the threshold is high (>10 mA) the pacing wires should be replaced. When the patient is in the ICU, both the capture threshold and sensing threshold should be checked when the patient is normothermic and normokalaemic. Accurately determining the sensing threshold and setting the sensitivity accordingly are essential to prevent R-on-T phenomena. If the sensing threshold is too low (Fig. 2), pacing carries a risk of R-on-T and should not be used in an unmonitored environment.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CMEditor comment: Please provide expansion for ROSCE/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Acknowledgements
The authors would like to thank Dr Andrew Martin for his thoughtful review of the manuscript and Roger Hulley for his skilled assistance in producing figures for this article.
Biographies
Michael Gillham BSc FANZCA FCICM is a consultant cardiovascular anaesthetist and intensivist. He is the deputy clinical director of the Cardiac and Vascular Intensive Care Unit at Auckland City Hospital. His major interests include patient safety, quality improvement and electrophysiology. He has published research in perioperative arrhythmia and pacing and has coedited a textbook in cardiothoracic critical care.
Thomas Barr MBBCh BSc is a specialty trainee in anaesthesia and intensive care at Auckland City Hospital. He has an interest in the critically unwell patient with cardiac disease and the teaching of basic sciences.
Matrix codes: 1A03, 1B04, 2C07, 3C00, 3G00
References
- 1.Miller A.C., Rosati S.F., Suffredini A.F., Schrump D.S. A systematic review and pooled analysis of CPR-associated cardiovascular and thoracic injuries. Resuscitation. 2014;85:724–731. doi: 10.1016/j.resuscitation.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol. 2006;8:17–81. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C.J., Lever N., Cooper J.O. Antiarrhythmic drugs and anaesthesia: part 1. mechanisms of cardiac arrhythmias. BJA Educ. 2023;23:8–16. doi: 10.1016/j.bjae.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peretto G., Durante A., Limite L.R., Cianflone D. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract. 2014;2014 doi: 10.1155/2014/615987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cote C.L., Baghaffar A., Tremblay P., Herman C.R. Prediction of temporary epicardial pacing wire use in cardiac surgery. J Card Surg. 2020;35:1933–1940. doi: 10.1111/jocs.14870. [DOI] [PubMed] [Google Scholar]
- 6.Khorsandi M., Muhammad I., Shaikhrezai K., Pessotto R. Is it worth placing ventricular pacing wires in all patients post-coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. 2012;15:489–493. doi: 10.1093/icvts/ivs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berdajs D., Schurr U.P., Wagner A., Seifert B., Turina M.I., Genoni M. Incidence and pathophysiology of atrioventricular block following mitral valve replacement and ring annuloplasty. Eur J Cardiothorac Surg. 2008;34:55–61. doi: 10.1016/j.ejcts.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Hwang Y.M., Kim J., Lee J.H., et al. Conduction disturbance after isolated surgical aortic valve replacement in degenerative aortic stenosis. J Thorac Cardiovasc Surg. 2017;154:1556–1565. doi: 10.1016/j.jtcvs.2017.05.101. [DOI] [PubMed] [Google Scholar]
- 9.Moskowitz G., Hong K.N., Giustino G., et al. Incidence and risk factors for permanent pacemaker implantation following mitral or aortic valve surgery. J Am Coll Cardiol. 2019;74:2607–2620. doi: 10.1016/j.jacc.2019.08.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T.K.M., Arroyo D., Martin A., McGeorge A., Gillham M. Permanent pacemaker implantation after cardiac surgery: rates, predictors and a novel risk score. N Z Med J. 2018;131:88–91. [PubMed] [Google Scholar]
- 11.Al-Ghamdi B., Mallawi Y., Shafquat A., et al. Predictors of permanent pacemaker implantation after coronary artery bypass grafting and valve surgery in adult patients in current surgical era. Cardiol Res. 2016;7:123–129. doi: 10.14740/cr480w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein A.D., Daubert J.C., Fletcher R.D., et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol. 2002;25:260–264. doi: 10.1046/j.1460-9592.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 13.Arsenault K.A., Yusuf A.M., Crystal E., et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;1:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwak J.G., Kim S.J., Song J.Y., et al. Permanent epicardial pacing in pediatric patients: 12-year experience at a single center. Ann Thorac Surg. 2012;93:634–639. doi: 10.1016/j.athoracsur.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 15.Chen M.Y., Mundangepfupfu T. Sustained ventricular tachycardia secondary to R-on-T phenomenon caused by temporary ventricular epicardial pacemaker undersensing after cardiac surgery. Anesthesiology. 2020;132:374. doi: 10.1097/ALN.0000000000002990. [DOI] [PubMed] [Google Scholar]
- 16.Nakamori Y., Maeda T., Ohnishi Y. Reiterative ventricular fibrillation caused by R-on-T during temporary epicardial pacing: a case report. JA Clin Rep. 2016;2:3. doi: 10.1186/s40981-016-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodoropoulos K.C., Liakopoulou A., Tsagkaropoulos S., Kassimis G., Antonitsis P., Anastasiadis K. Under-sensing by a temporary pacemaker after cardiac surgery and ventricular fibrillation. Lancet. 2022;399:677. doi: 10.1016/S0140-6736(21)02443-0. [DOI] [PubMed] [Google Scholar]
- 18.Chemello D., Subramanian A., Nanthakumar K. Cardiac arrest caused by undersensing of a temporary epicardial pacemaker. Can J Cardiol. 2010;26:13–14. doi: 10.1016/s0828-282x(10)70334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaikhrezai K., Khorsandi M., Patronis M., Prasad S. Is it safe to cut pacing wires flush with the skin instead of removing them? Interact Cardiovasc Thorac Surg. 2012;15:1047–1051. doi: 10.1093/icvts/ivs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahon L., Bena J.F., Morrison S.M., Albert N.M. Cardiac tamponade after removal of temporary pacer wires. Am J Crit Care. 2012;21:432–440. doi: 10.4037/ajcc2012585. [DOI] [PubMed] [Google Scholar]
- 21.Bougioukas I., Jebran A.F., Grossmann M., et al. Is there a correlation between late re-exploration after cardiac surgery and removal of epicardial pacemaker wires? J Cardiothorac Surg. 2017;12:3. doi: 10.1186/s13019-017-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote C.L., Baghaffar A., Tremblay P., Herman C. Incidence of tamponade following temporary epicardial pacing wire removal. J Card Surg. 2020;35:1247–1252. doi: 10.1111/jocs.14564. [DOI] [PubMed] [Google Scholar]