Introduction
There are reports of lichenoid eruptions developing after the use of programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors.1 Specifically, lichen planus has been reported secondary to nivolumab, a monoclonal antibody targeting PD-1 receptors.2 PD-1 and PD-L1 are immune checkpoint proteins that play a role in inhibiting T cell activity to maintain self-tolerance, limit tissue damage, and prevent autoimmunity.3 Immune checkpoint inhibitors are associated with immune-related adverse events (iRAE) including vitiligo, and spongiotic, psoriasiform, and lichenoid reactions.4,5
Few treatment options exist for patients who develop corticosteroid-resistant lichen planus from anti-PD-1 agents. Dupilumab, a monoclonal antibody that targets the IL-4 receptor alpha subunit of IL-4 and IL-13 receptors, is approved by the Food and Drug Administration (FDA) for treatment of atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis and prurigo nodularis. A review of the medical literature reveals case reports of patients showing resolution of lichen planus with dupilumab treatment.6,7 We present the case of a 68-year-old female who received treatment for stage III C melanoma with nivolumab, and developed delayed onset lichen planus responsive to dupilumab.
Case report
A 68-year-old female diagnosed with BRAF wild type stage III C melanoma of the right lower leg received adjuvant anti PD-1 treatment after surgical management. The patient received a total of 11 monthly doses of nivolumab. Sixteen months after nivolumab therapy, she presented with progressive symptoms of itching and a skin rash affecting her upper back, gluteal regions, extensor aspects of arms, forearms, thighs and legs (Fig 1). The rash did not respond to treatment with topical corticosteroids.
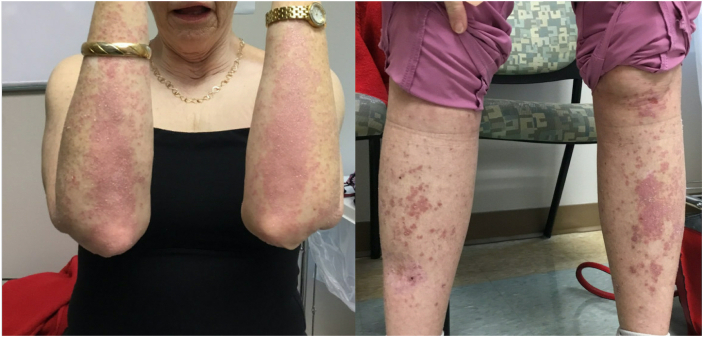
Fig 1.
Multiple flat-topped violaceous papules, some coalescing into plaques, on the forearms and legs.
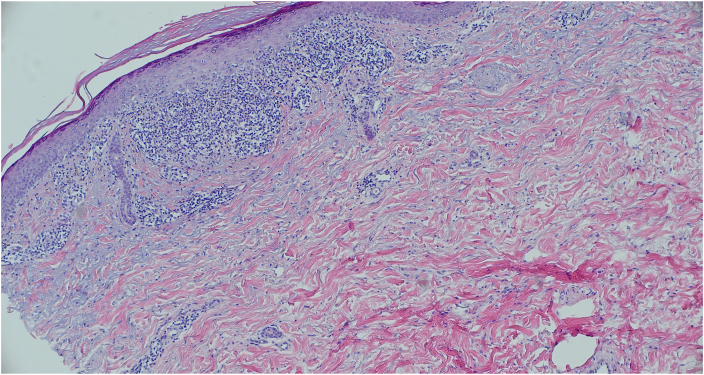
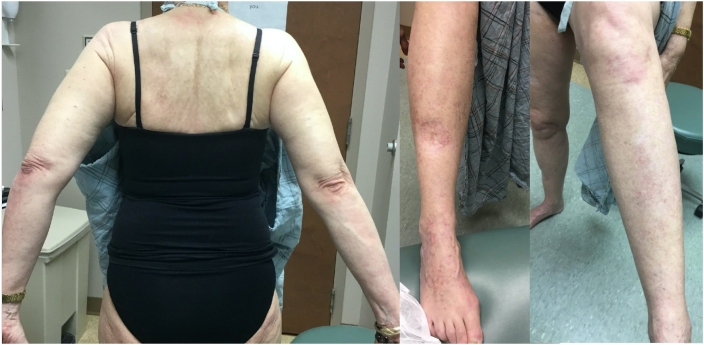
Physical examination revealed multiple violaceous flat topped well-demarcated papules coalescing into plaques of varying sizes and lack of involvement of the nails or mucosal surfaces. There were no bullous lesions or ulcerations. A punch biopsy showed patchy lichenoid lymphocytic infiltrate along the dermal epidermal junction, focal wedge-shaped hypergranulosis, focal cytoid body formation and pigment incontinence (Fig 2). There were no eosinophils seen on histopathology slides. These findings were consistent with lichen planus. The patient had no prior history of lichen planus or other lichenoid eruption. She was treated with a short course of prednisone 20 mg daily and diphenhydramine without relief. She declined higher doses of systemic corticosteroids due to the risk of osteoporosis. Potential treatment with hydroxychloroquine and doxycycline were declined due to her concerns regarding ocular and gastrointestinal side effects. Phototherapy was declined due to her recent history of melanoma. She was started on dupilumab 300 mg every other week after a loading dose of 600 mg. After 10 weeks of treatment with inadequate improvement, the dose of dupilumab was increased to 300 mg every week. After 6 doses of weekly dupilumab treatment, she had almost complete resolution of her symptoms and the rash had improved significantly (Fig 3). Weekly dosing of dupilumab was given for 10 weeks total and then changed back to every 2 weeks dosing. She remained on dupilumab at the time for this report, 11 months after onset of her rash.
Fig 2.
Punch biopsy showing patchy lichenoid lymphocytic infiltrate along the dermal epidermal junction, focal wedge-shaped hypergranulosis, focal cytoid body formation and pigment incontinence.
Fig 3.
At 7 months follow-up, there is significant improvement after treatment with dupilumab.
Discussion
Cutaneous toxicities are one of the most common adverse effects of PD-1 agents. There are reports of cutaneous iRAEs being associated with longer survival in patients with melanoma who are treated with nivolumab.5 Although cutaneous toxicities in immune checkpoint treatment may be an indicator of good treatment response, such toxicities, if severe, can result in premature termination of treatment. Based on the 2018 American Society of Clinical Oncology (ASCO) management guidelines for iRAE, Grade I & II lichenoid eruptions can be managed with topical, oral, or intralesional corticosteroids with additional use of antihistamines in hypertrophic cases.5 Grade III eruptions require suspension of anti-PD-1 therapy and treatment with oral corticosteroids, acitretin, cyclosporine, or promethazine and topical calcineurin inhibitors. Grade IV eruptions should prompt cessation of anti-PD-1 treatments and patients should be treated with IV corticosteroids with close monitoring.5 The use of nivolumab 3 mg/kg every 2 weeks has been associated with low risk of cutaneous iRAEs.1 Alternatively, the use of phototherapy in conjunction with topical corticosteroids can help prevent lichenoid eruptions in patients on anti-PD-1 immunotherapy.1 A review of our patient’s medications at the time of her clinical presentation revealed that nivolumab was the most likely culprit medication.
While the exact pathogenesis of lichen planus is not known, it has largely been attributed to cell-mediated cytotoxicity. Interferon-gamma is elevated in lesions of lichen planus and is involved in CD8+ T-cell mediated keratinocyte death.8 Although dupilumab has been shown to successfully treat lichen planus,6,7 it has also been reported to cause lichenoid eruptions when used for the treatment of atopic dermatitis likely due to a Th1 predominant response from Th2 suppression by dupilumab.9 There are few reports detailing possible mechanisms by which lichen planus can respond to dupilumab. One mechanism looks at IL-6, a proinflammatory factor stimulated by TNF alpha in the Th1 pathway, which is found in increased levels in patients with lichen planus.7 One of the functions of IL-6 is to promote IL-4 activity and potentially increase Th2 response.7 It is possible that dupilumab-induced inhibition of the Th2 response is responsible for the clinical improvement seen in patients with lichen planus; however, further study of its mechanism of action is warranted to provide clarity.
Although the majority of autoimmune side effects occur during anti-PD-1 therapy or within weeks after discontinuation of therapy, delayed-onset autoimmune side effects are increasingly reported in the literature. Delayed onset of autoimmune side effects can result from persistence of PD-1 inhibition and anti-tissue T cell activity evoked by new introduction of tissue antigenic determinants.10 Clinicians should be aware of delayed presentation of iRAEs to prevent delays in diagnosis and effective treatment.10
As the check point inhibitory agents are widely used to treat advanced cancer, there is increased likelihood of encountering moderate to severe cutaneous iRAEs compromising effective treatment outcomes in patients. Since cutaneous iRAEs can be associated with good treatment response, there is a need for more effective management options for these cutaneous reactions to enable patients to stay on check point inhibitory agents for their expected treatment duration. This case demonstrates successful treatment of nivolumab-induced lichen planus with dupilumab despite resistance to topical and low dose systemic corticosteroid treatment.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Statement of patient consent: All patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Bhardwaj M., Chiu M.N., Pilkhwal Sah S. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocul Toxicol. 2022;41(1):73–90. doi: 10.1080/15569527.2022.2034842. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz M., Mese S.G., Celik U. Nivolumab-induced lichen planus. J Oncol Pharm Pract. 2020;26(3):758–760. doi: 10.1177/1078155219866248. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh C., Luong G., Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021;12(9):2735–2746. doi: 10.7150/jca.57334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villadolid J., Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazemi S., Murphrey M., Hawkes J.E. Rapid resolution of widespread cutaneous lichen planus and generalized pruritus in an elderly patient following treatment with dupilumab. JAAD Case Rep. 2022;30:108–110. doi: 10.1016/j.jdcr.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pousti B.T., Jin A., Sklovar L., et al. Dupilumab for the treatment of lichen planus. Cutis. 2021;107(4):E8–E10. doi: 10.12788/cutis.0232. [DOI] [PubMed] [Google Scholar]
- 8.Shao S., Tsoi L.C., Sarkar M.K., et al. IFN-gamma enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11(511) doi: 10.1126/scitranslmed.aav7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim T.E., Shin M.K. De novo case of lichenoid eruption following dupilumab treatment. JAAD Case Rep. 2021;13:71–72. doi: 10.1016/j.jdcr.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parakh S., Cebon J., Klein O. Delayed autoimmune toxicity occurring several months after cessation of anti-PD-1 therapy. Oncol. 2018;23(7):849–851. doi: 10.1634/theoncologist.2017-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]