Abstract
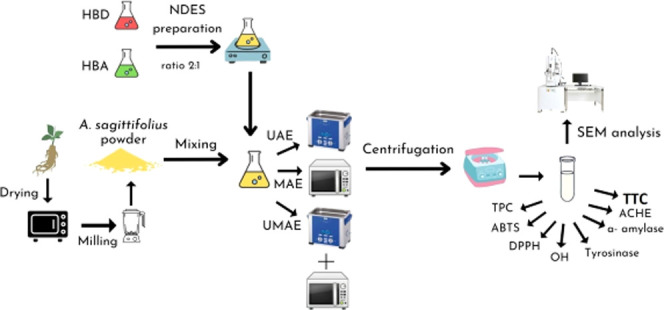
This research extracted phenolics and terpenoids from Abelmoschus sagittifolius (Kurz) Merr roots using natural deep eutectic solvent-based novel extraction techniques. Twelve natural deep eutectic solvents (NADESs) were produced for recovering phenolics and terpenoids. Citric acid/glucose and lactic acid/glucose, with a molar ratio of 2:1, were determined as the most appropriate NADESs for extracting phenolics and terpenoids, respectively. Afterward, the proper conditions for NADES-based ultrasonic-assisted and microwave-assisted extraction were investigated. Then, the time and liquid-to-solid ratios of ultrasonic- and microwave-combined extraction methods and the sequence of ultrasound and microwave treatments were examined. The conditions of ultrasonic-assisted extraction were 40 mL/g liquid-to-solid ratio, 40% water content, 30°C, 5 min, and 600 W ultrasonic power for the highest terpenoid recovery at 69 ± 2 mg UA/g dw, while 150 W ultrasonic power was suitable for phenolic recovery at 9.56 ± 0.17 mg GAE/g dw. The conditions of microwave-assisted extraction were 50 mL/g liquid-to-solid ratio, 20% water content, 400 W microwave power, and 2 min to acquire the highest phenolics and terpenoids at 22.13 ± 0.75 mg GAE/g dw and 90 ± 1 mg UA/g dw, respectively. Under appropriate conditions, the biological activities, phenolic content, and terpenoid content of obtained extracts from four extraction methods, including ultrasonic-assisted, microwave-assisted, ultrasonic–microwave-assisted, and microwave–ultrasonic-assisted extraction, were compared to select the most proper method. The conditions of ultrasonic–microwave-assisted extraction were 40 mL/g liquid-to-solid ratio, 5 min sonication, and 1 min microwave irradiation to obtain the highest phenolic and terpenoid contents (27.07 ± 0.27 mg GAE/g dw and 111 ± 3 mg UA/g dw, respectively). Ultrasonic–microwave-assisted extraction showed the highest phenolic content, terpenoid content, and biological activities among the four extraction techniques. The changes in the surface morphology were determined using scanning electron microscopy. This study demonstrated that ultrasonic–microwave-assisted extraction was an effective and sustainable method in food and pharmaceutical industries for recovering phenolics and terpenoids from Abelmoschus sagittifolius (Kurz) Merr.
1. Introduction
Abelmoschus sagittifolius (Kurz) Merr (A. sagittifolius) is a typical member of the Abelmoschus genus and belongs to the Malvaceae family. A. sagittifolius is widely distributed in China, India, Southeast Asian countries, Africa, and Australia.1A. sagittifolius roots are commonly used to treat various illnesses, such as fever and cough as well as tonic drinking in the traditional medicine of Asian countries. Additionally, A. sagittifolius roots have various macronutrients, including lipids, protein, fatty acids, and bioactive compounds (phenolics and terpenoids), which offer potential health benefits.2 The macronutrients provide materials for human metabolism, while bioactive compounds exhibit various biological activities, including cytotoxic, antioxidant, anti-inflammatory, anti-cancer, and antimicrobial properties.3 Therefore, it is necessary to have a reliable extraction technique for producing enriched phenolic and terpenoid extracts from A. sagittifolius for further application in the pharmaceutical industry
The extraction process refers to using solvents to isolate bioactive compounds in materials. Extraction techniques are divided into conventional and innovative methods.4 Although conventional methods are simple and cost-effective, they have a long operational time, high energy consumption, and limited recovery of bioactive compounds due to the decomposition of thermally sensitive compounds.5 A dispersive micro solid-phase extraction technique is based on sorbent dispersion into samples, leading to the approximation of sorbents and targets. This effect improves the adsorption kinetics of sorbents, increasing the extraction efficiency. The term micro indicates the weight of the employed solid phase in the range of milligrams.6 The properties of sorbents decide the efficiency of this technique. The sorbents with high porosity and surface area showed a high extraction efficiency of targets from samples. This technique is commonly used to extract bisphenol, atrazine, nitrophenol, carbonyl derivatives, hippuric acid, methyl hippuric acid, and sulfonamides from various types of water, such as drinking water, wastewater, and environmental water.6−13 Innovative methods such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) have been developed to address these disadvantages of conventional extraction.5 However, one of the disadvantages of SFE is high-cost devices.5 MAE operates by exposing a polar extraction medium to microwave irradiation, leading to the absorption of microwave energy, converted into a thermal one. The electromagnetic fields of the medium oscillate, causing collisions among dipoles, leading to friction and heat generation. The high temperature evaporates water in cells, causing high pressure and cell wall rupture.14 This rupture creates numerous pores in cell walls, improving the penetration of extractants and, thus, improving the recovery of bioactive compounds. UAE works by inducing ultrasonic dispersion through the molecules of the liquid medium, creating cavitation bubbles through many expansion and compression cycles. The implosion of these bubbles disrupts cell wall structures, increasing mass transfer and facilitating solvent penetration.15 UAE and MAE are considered green technology, as they use little energy, need low solvent consumption, increase extraction yield, and reduce extraction time.5 Therefore, the combination of UAE and MAE is noteworthy as it has proven effectiveness in extracting pectin from jackfruit peels and polysaccharide from Camptotheca acuminata to exhibit antitumor activity.16,17
Natural deep eutectic solvents (NADESs) include hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs), which are cell metabolites.18 NADESs have benefits over organic solvents, such as low production cost, biodegradability, nonvolatile characteristics, and nontoxicity.19 NADESs highly solubilize bioactive compounds due to generating hydrogen bonding networks with these compounds. These hydrogen bonding networks also restrict the mobility of bioactive compounds and preclude bioactive compounds from reacting with oxidative agents. Therefore, NADESs maintain the stability of phenolics and terpenoids during storage.18 The combination of NADESs with UAE and MAE improves the extraction yield of phenolics and terpenoids due to the devastating influence of ultrasound and microwaves on plant cell walls.20 In previous research, Thuc Dinh Ngoc et al. extracted abelsaginol from A. sagittifolius using methanol as a solvent and determined the antioxidant activity of the extract with different free radicals.21 However, there were no studies using NADESs as green solvents for recovering phenolics and terpenoids from A. sagittifolius. Furthermore, the order of ultrasonic and microwave treatments on extraction efficiency was not elucidated.
Therefore, 12 NADESs were designed and prepared in the present study before evaluating their extraction capacity with phenolics and terpenoids from A. sagittifolius. NADESs were prepared by combining three HBDs (acetic acid, citric acid, and lactic acid) and four HBAs (isopropyl alcohol, glycerin, glucose, and choline chloride). The HBDs were selected based on their increasing acidity, while the HBAs were chosen according to the increasing number of hydroxyl groups. Additionally, the HBAs and HBDs have low costs. The research examined the influence of UAE, MAE, ultrasonic–microwave-assisted extraction (UMAE), and microwave–ultrasonic-assisted extraction (MUAE) conditions on the extraction yield of phenolics and terpenoids. The extraction yield and the biological activities with different extraction techniques, including UAE, MAE, UMAE, and MUAE, were compared to select the most effective. The difference in the surface morphology of A. sagittifolius with different extraction techniques was investigated using scanning electron microscopy (SEM).
2. Materials and Methods
2.1. Materials
Dried A. sagittifolius roots were purchased from Tue Lam company, Dong An, Dong Hoi, Quang Binh, Vietnam; then, they were pulverized to acquire dried A. sagittifolius root powder (DAP). Gallic acid monohydrate (purity ≥ 98%), iron (II) sulfate heptahydrate (purity ≥ 99%), absolute ethanol (purity ≥ 99.8%), salicylic acid (purity ≥ 99%), hydrogen peroxide (purity 34.5–36.5%), 1,1-diphenyl-2-picrylhydrazyl (DPPH, purity ≥ 97%), sodium acetate trihydrate (purity ≥ 99%), potassium chloride (purity ≥ 99%), acetylcholine esterase (50 units), 2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, purity ≥ 98%), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, purity 98%), Tris-HCl (purity > 99%), PNPG (purity ≥ 99%), Folin–Ciocalteu reagent (2.1 N), acarbose (purity ≥95%), α-amylase (500000 units), α-glucosidase (750 units), 3,5-dinitrosalicylic acid (DNS, purity 98%), acetylcholine iodine (purity ≥ 98%), tacrine (purity ≥ 99%), 4-nirophenyl-α-d-glucosepiranoside (PNPG), and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB, purity 99%) were obtained from Sigma-Aldrich Chemical Co., Ltd., Singapore, Singapore. The chemicals for NADES production were bought from Xilong Scientific Co., Ltd., Shantou, Guangdong, China, and HiMedia Laboratories, Thane West, Maharashtra, India.
2.2. NADES Production and Screening
In the present study, NADESs were produced by the heating method.22 The mixture of HBAs and HBDs with a proper molar ratio was heated at 85 °C using heating magnetic stirrers (model: C–MAG HS 7, IKA Industrie, Humboldtstraße, Königswinter, Germany). When the mixture of HBAs and HBDs formed a transparent liquid and had no crystals at room temperature, NADESs were successfully produced. The acronym, chemicals, and molar ratios for NADES production are presented in Table 1.
Table 1. NADESs Used in the Present Research.
| no. | HBD | HBA | abbreviation | molar ratio |
|---|---|---|---|---|
| 1 | citric acid | isopropyl alcohol | Ci-Iso | 2:1 |
| 2 | citric acid | choline chloride | Ci-Cho | 2:1 |
| 3 | citric acid | glycerin | Ci-Gly | 2:1 |
| 4 | citric acid | glucose | Ci-Glu | 2:1 |
| 5 | lactic acid | isopropyl alcohol | Lac-Iso | 2:1 |
| 6 | lactic acid | choline chloride | Lac-Cho | 2:1 |
| 7 | lactic acid | glycerin | Lac-Gly | 2:1 |
| 8 | lactic acid | glucose | Lac-Glu | 2:1 |
| 9 | acetic acid | isopropyl alcohol | AA-Iso | 2:1 |
| 10 | acetic acid | choline chloride | AA-Cho | 2:1 |
| 11 | acetic acid | glycerin | AA-Gly | 2:1 |
| 12 | acetic acid | glucose | AA-Glu | 2:1 |
| 13 | ethanol |
DAP was placed into a dark amber glass jar, and NADES (20% water content) was poured into one with the liquid-to-solid ratio at 20 mL/g. The mixture was placed in static conditions for 15 min. Then, the mixture was treated by a centrifugal machine (DM0412, DLAB Scientific Co., Ltd., Shunyi, Beijing, China) at 2200g and 30 °C for 20 min to separate the solid part before phenolics and terpenoids were measured. Ethanol was used as a controlled solvent.
2.3. Ultrasonic-Assisted and Microwave-Assisted Extraction
The quantified amount of DAP was mixed with 25 mL of NADES in the amber glass jar. The mixture was treated by an ultrasonic bath (model: RS22L 40 kHz, Rama Viet Nam Joint Stock Company, District 9, Ho Chi Minh, Vietnam) at distinctive liquid-to-solid ratios (LSRs, the survey ranges from 10 to 50 mL/g), ultrasonic powers (0–750 W with the interval of 150 W), water contents (10–50%, g/g), and temperatures (30–80 °C with a gap of 10 °C) for different extraction times (3, 5, 10, 15, 20, and 25 min).
The specific amount of DAP was blended with 25 mL of NADES in the amber glass flask. The mixture was processed by a microwave oven (model: R-205VN 2450 MHz, Sharp Corporation, Sakai, Osaka, Japan) under different LSRs (10–60 mL/g), different water contents (10–50%, g/g), and different microwave powers (0, 240 W, 400 W, and 800 W) for different retention times (1, 2, 3, and 5 min).
DAP was blended with NADES with different LSRs (20, 30, 40, and 50 mL/g) for different extraction times. Regarding UMAE, the mixture was pretreated by ultrasound for different treatment times (3 and 5 min) before being placed in a microwave oven for extraction times of 1 and 2 min. Regarding MUAE, the mixture was pretreated by microwave irradiation for different treatment times (1 and 2 min), and then the mixture was sonicated for various times (3 and 5 min). Other conditions were kept at the values with the highest extraction efficiency of terpenoids and phenolics in the MAE and UAE processes. After the extraction stages, the solid part of the mixture was removed by a centrifugal machine at 2200g and 30 °C for 20 min before the figure for phenolics and terpenoids in extracts was used to evaluate the extraction efficiency.
2.4. Total Phenolic Contents (TPCs), Total Terpenoid Contents (TTCs), and Antioxidant Activities
TTC was quantified by the Biswajit Biswas method, and TPC was determined using the Folin–Ciocalteu reagent.22,23 TPC and TTC were presented as milligrams of gallic acid equivalent per gram of dried weight (mg GAE/g dw) and milligrams of ursolic acid per gram of dried weight (mg UA/g dw), respectively.
DPPH free radical-quenching ability (DPPH) was measured using an ethanolic DPPH solution.24 ABTS free radical-quenching ability (ABTS) was examined using ABTS+ working solution, and hydroxyl free radical-quenching ability (OH) was determined by the Hongjie Yuan method.24,25 DPPH and ABTS were expressed as micromole Trolox equivalent per gram of dry basis (μM TE/g dw), while OH was expressed as millimole Trolox equivalent per gram of dry basis (mM TE/g dw).
2.5. Enzymatic Inhibitory Activities
The enzymatic inhibitory capacity of DAP extracts was evaluated against tyrosinase, α-amylase, α-glucosidase, and acetylcholine esterase (AChE). The α-amylase inhibition assay was conducted using MingxiaWu with slight modifications.26 Samples (0.1 mL) were mixed with soluble starch (0.1 mL, 1%, m/v) in phosphate buffer (PB, 0.02 M, pH 6). The mixture was immersed in a water bath (SH-WB-6GAN, SH Scientific, Yeondong-myeon, Sejong-si, Republic of Korea) at 37 °C for 10 min. After 0.1 mL of α-amylase (20 U/mL) was blended, the mixture was kept at 37 °C for 10 min. The DNS reagent (0.2 mL) was added to the mixture and then maintained at 100 °C for 5 min. Deionized water (0.9 mL) and PB (2.1 mL) were mixed, and the absorbance was recorded at 540 nm. Acarbose was used as a standard substance, and the results were shown as milligram acarbose equivalent per gram of dry basis (g ACE/g dw).
In tyrosinase inhibition experimentation, samples (0.1 mL) were blended with 1.8 mL of PB (pH 6) and tyrosinase (0.4 mL, 1 U/mL), and then the mixture was placed at ambient temperature for 5 min. Afterward, L-DOPA (1 mL, 2.5 mM in PB) was added to the mixture, which was continuously incubated for 1 min. The absorbance was recorded at 492 nm. Ascorbic acid was employed as a positive control in the tyrosinase inhibitory assay, and the results were expressed as milligram ascorbic acid equivalent per gram of dry basis (mg AE/g dw).27
AChE inhibition capacity was quantified using the Ellman method with slight modifications. 0.2 mL of samples were mixed with 3 mL of Tris-HCl buffer (pH 8.5), DTNB (0.1 mL, 0.9mM), and AChE (0.25 mL, 0.1 U/mL) in Tris-HCl buffer; then the mixture was incubated at ambient temperature in a dark place for 5 min. After incubation, acetylcholine iodine (0.25 mL, 4.5 mM in Tris-HCL) was mixed, and the mixture was maintained at the same conditions for 15 min. The absorbance was determined at 405 nm. Tacrine was used as a standard substance, and the results were shown as milligram tacrine equivalent per gram of dry basis (g TCE/g dw).27
2.6. Determination of Energy Consumption and Recovery Yield
Soxhlet extraction was used to determine DAP′s entire phenolic and terpenoid contents. The Soxhlet extraction procedure was conducted based on the Steven B. Hawthorne method.28 The recovery yield of phenolics and terpenoids from UAE, MAE, UMAE, and MUAE was determined using eq 1, and the energy consumption of the four novel extraction methods was calculated using eq 2.
| 1 |
where Ca is the phenolic or terpenoid content obtained from the four novel extraction methods and Cb is the phenolic or terpenoid content obtained from the Soxhlet extraction method.
| 2 |
where P1 is the ultrasonic power used, t1 is the sonication time, P2 is the microwave power used, and t2 is the microwave irradiation time.
2.7. Surface Morphology of A. sagittifolius Root Powder
The surface morphology of DAP before and after extraction was identified using scanning electron microscopy (SEM, model: Prisma E SEM, Thermo Fisher Scientific, Waltham, Massachusetts). The sample preparation procedure was conducted according to the Patil method.29 Samples were placed on carbon tape (to prevent sample loss) and enclosed on the sample plate. Then, the samples were coated with a gold metal layer. The procedure was carried out under vacuum; the samples were relocated and directly observed under SEM at different magnifications and 5 kV.
2.8. Statistical Analysis
Analysis of variance (ANOVA) was performed by Minitab (v19.1, Minitab Inc, Pennsylvania) with the α value at 5%. The experimental results were conducted three times and shown as mean ± standard deviation (SD). The plots were built by OriginPro (version 2022, OriginLab, Northampton, Massachusetts).
3. Results and Discussion
3.1. Evaluating the Extraction Performance of NADESs
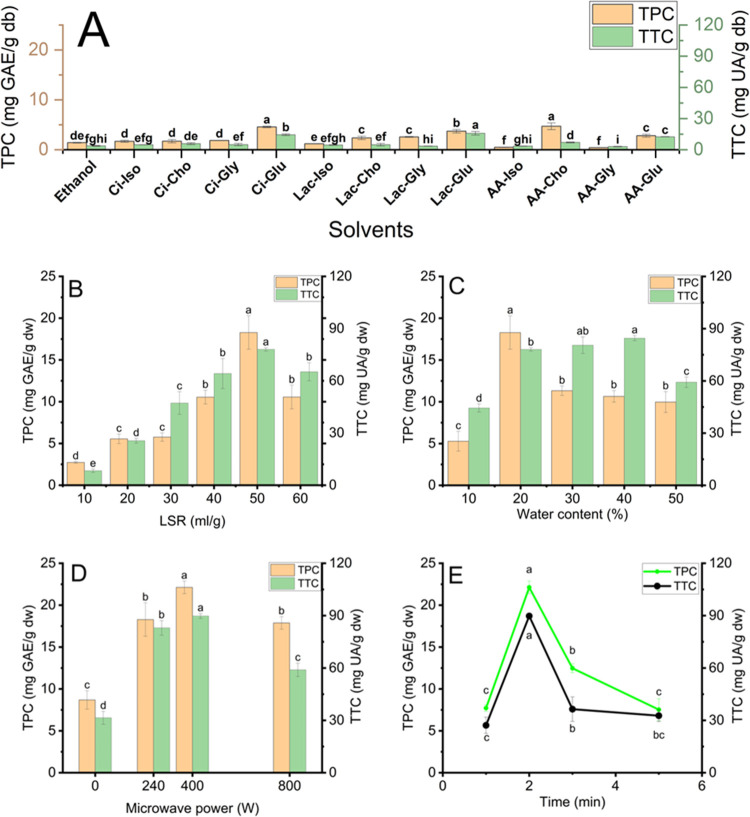
NADES components play an integral role in the extractability of terpenoids and phenolics, since they decide on polarity, viscosity, solvation, and extractability.30 Twelve NADESs were prepared from acetic acid, citric acid, and lactic acid as HBDs and four HBAs (choline chloride, glucose, isopropyl alcohol, and glycerin) with the addition of 20% water. NADES extraction performance with phenolics and terpenoids from DAP was examined and is shown in Figure 1A. The results presented that the extraction efficiency of terpenoids and phenolics from DAP strongly relied on NADES components. Six types of NADESs had the higher extractability of phenolics, while five types of NADESs had that of terpenoids than ethanol (the obtained TPC and TTC are 1.40 ± 0.08 mg GAE/g dw and 3.73 ± 0.41 mg UA/g dw, respectively). Ci-Glu (4.55 ± 0.16 mg GAE/g dw) and AA-Cho (4.70 ± 0.66 mg GAE/g dw) extracted the highest TPC, followed by Lac-Glu (3.70 ± 0.35 mg GAE/g dw). Lac-Glu performed the highest terpenoid extraction (15.79 ± 1.74 mg UA/g dw) among used NADESs. The results indicated that the polarity of phenolics and terpenoids from DAP is high, and phenolics have higher polarity than terpenoids. It can be ascribed to the higher polarity of citric acid than that of lactic acid.31−33 Although Ci-Glu and Lac-Glu have high viscosity, they show the most significant extraction efficiency, probably due to having the highest number of hydroxyl groups. The polarity can significantly influence extracting bioactive compounds from plants more than viscosity because it can directly affect the solubility of target analytes in solvents. The high solubility of target analytes in solvents conformed to the ″like dissolve like″ rule.31 Additionally, the lower extraction performance of phenolics and terpenoids of other NADESs can be attributed to their lower polarity than Ci-Glu and Lac-Glu. The polarity of NADESs is decided by HBA and HBD polarity. Ci-Glu has the highest polarity, followed by Lac-Glu because citric acid had the highest polarity among HBDs, and a similar trend was true in the case of glucose (HBA).31,34−37 Therefore, Ci-Glu was suitable for phenolic recovery and Lac-Glu was proper for terpenoid extraction from DAP to ensure further application in food products.
Figure 1.
Effect of solvents in solid-to-liquid extraction and extracting conditions using Ci-Glu for phenolic recovery and Lac-Glu for terpenoid recovery in the MAE process: (A) solvent types, (B) liquid-to-solid ratios, (C) water content in NADES, (D) microwave power, and (E) extraction time. Different characters (a, b, c, d, e, f, g, h, and i) presented significant statistical differences.
3.2. Effect of Microwave-Assisted Extraction Conditions
The parameters of NADES-based MAE play essential roles in deciding the extraction yield of terpenoids and phenolics from DAP. This present study examined the influence of LSR, water content, microwave power, and extraction time on the extraction yield, and the results are expressed in Figure 1B–E.
3.2.1. Effect of Liquid-to-Solid Ratios in the NADES-Based MAE Process
This research studied the effects of different LSRs (10, 20, 30, 40, 50, and 60 mL/g) under microwave power, water content, and time at 240 W, 20%, and 2 min, respectively. As presented in Figure 1B, TPC, and TTC experienced an increase of 6.7 and 9.6 times, respectively, when LSR increased from 10 to 50 mL/g. The increasing LSR can reduce the overall viscosity of the extraction medium and generate a high gradient concentration. The high LSR can improve the contact area between extractants and solutes and ensure the uniform distribution of materials and exposure to microwave irradiation. These phenomena can facilitate the mass transfer of phenolics and terpenoids from DAP into an extraction medium, increasing extraction yield.6,38 However, a further increase in LSR at 60 mL/g decreased TPC and TTC by 1.7 and 1.2 times, respectively, compared to the LSR at 50 mL/g. It can be attributed to solvents′ insufficient agitation when microwave irradiation is applied to a large volume.39 Therefore, LSR at 50 mL/g was proper for NADES-based MAE to obtain the highest TPC and TTC at 18.28 ± 2.00 mg GAE/g dw and 78.06 ± 0.93 mg UA/g dw from DAP, respectively.
3.2.2. Effect of Water Content in the NADES-Based MAE Process
Figure 1C shows the effect of water content (10–50%) in NADESs on the extraction yield of terpenoids and phenolics under NADES-based MAE conditions at an LSR of 50 mL/g, a microwave power of 240 W, and 30 °C for 2 min. TPC peaked at 18.28 ± 2.00 mg GAE/g dw with 20% water content in Ci-Glu, while TTC reached the highest point at 51.82 ± 4.82 mg UA/g dw with 40% of water content in Lac-Glu. The increase in water content in NADESs can reduce viscosity. The vicious reduction can improve the mobility of polar molecules, accelerating their dipole rotation in the electromagnetic field. Accelerated dipole rotation increases the collision of molecular particles and friction, producing more heat inside DAP. This phenomenon ruptures cell walls, promoting the release of terpenoids and phenolics into Lac-Glu and Ci-Glu.38 Additionally, water addition to Lac-Glu and Ci-Glu can raise the number of hydrogen bonds that improve surface tension. Extensive hydrogen bond networks in solvents can generate a stronger attractive force than external force, increasing mutual interaction on the NADES surface and enhancing surface tension. The high surface tension can favor the mutual interplay between NADESs and DAP, which allow the development of hydrogen bonds among NADESs with terpenoids and phenolics. This phenomenon can promote the dissolvability of terpenoids and phenolics to NADESs, improving extraction yield from DAP.40 However, TPC and TTC decreased to 10.00 ± 1.26 mg GAE/g dw and 45.41 ± 0.74 mg UA/g dw when the water content in Ci-Glu and Lac-Glu increased over 20 and 40%, respectively. Regarding Ci-Glu, the excessive temperature inside DAP can destroy phenolics when the water content is too high.38 Regarding Lac-Glu, the supramolecular structures of NADESs can be devastated at water contents over 40%, which may reduce interaction between solvents and terpenoids in DAP, negatively affecting extraction yield.31 Therefore, the water contents in Ci-Glu at 20% and in Lac-Glu at 40% were suitable for NADES-based UAE to recover the highest TPC and TTC at 18.28 ± 2.00 mg GAE/g dw and 84.52 ± 1.46 mg UA/g dw from DAP, respectively.
3.2.3. Effect of Microwave Power in the NADES-Based MAE Process
The effect of microwave power (0, 240, 400, and 800 W) on the extraction yield of phenolics was investigated at 50 mL/g LSR and 20% water content in Ci-Glu for an extraction time of 2 min, while the fixed extracting conditions for terpenoids were 50 mL/g LSR and 40% water content in Lac-Glu for an extraction time of 2 min. As presented in Figure 1D, the extraction yield of phenolics and terpenoids increased when microwave power ranged from 0 to 400 W. An increase in microwave power can increase systematic temperature and pressure, accelerating plant cell wall destruction, vicious decrease, and enhancing solute desorption from the plant matrix, solvent penetration, and solute dissolvability. These phenomena can improve the diffusivity of terpenoids and phenolics into NADESs, promoting extraction yield.38 However, with a continuous increase in microwave power to 800 W, TPC and TTC decreased. The high microwave power can degrade phenolic compounds in DAP, leading to low extraction yield.38 Therefore, microwave power at 400 W was suitable for the NADES-based MAE to obtain the highest TPC and TTC at 22.13 ± 0.75 mg GAE/g dw and 89.78 ± 1.41 mg UA/g dw from DAP, respectively.
3.2.4. Effect of Time in the NADES-Based MAE Process
The effect of microwave irradiation time (1, 2, 3, and 5 min) on the recovery of phenolics was studied at 50 mL/g LSR, 20% water content in Ci-Glu, and 400 W microwave power, while fixed conditions for extracting were 50 mL/g LSR, 400 W microwave power, and 40% water content in Lac-Glu. As illustrated in Figure 1E, as the irradiation time increased to 2 min, TPC and TTC increased to 22.12 ± 0.75 mg GAE/g dw and 46.89 ± 2.67 mg UA/g dw, respectively. Increasing microwave irradiation time increases temperature, enhancing plant cell tissue destruction. Damaged plant cells improve the swelling effect and contact area between solvents and solutes, which improves extraction yield. However, TPC and TTC decreased to 7.52 ± 1.30 mg GAE/g dw and 32.69 ± 3.34 mg UA/g dw, respectively, when the microwave irradiation time was prolonged to 5 min.38 It can be attributed to overheating, which degrades terpenoids and phenolics in DAP. Therefore, microwave irradiation time at 2 min was appropriate for reaching the highest TPC and TTC at 22.13 ± 0.75 mg GAE/g dw and 89.78 ± 1.41 mg UA/g dw from DAP, respectively.
3.3. Effect of Ultrasonic-Assisted Extraction Conditions
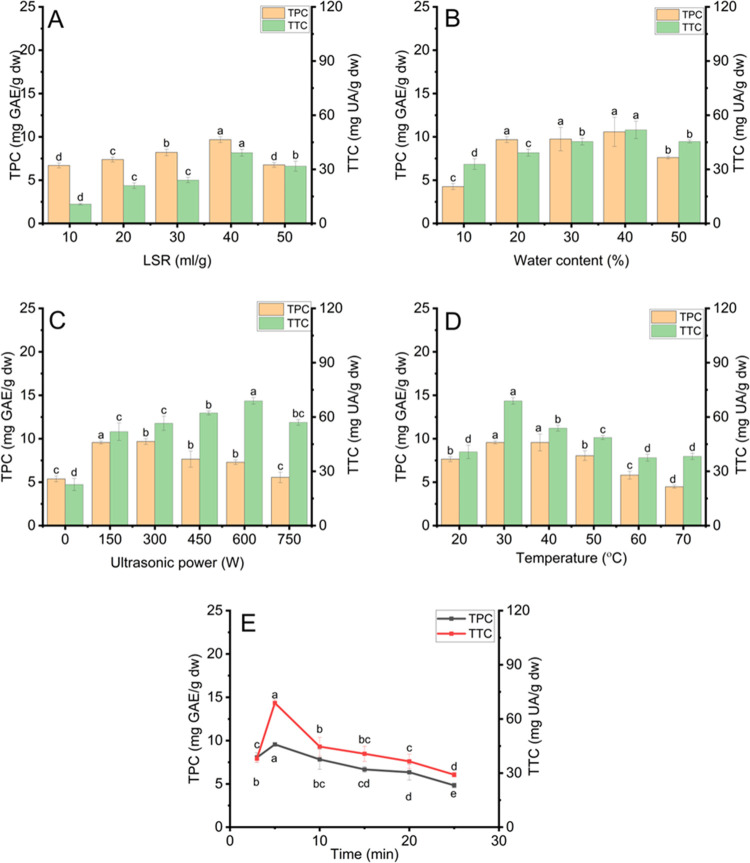
The parameters of NADES-based UAE have vital roles in determining the extraction yield of terpenoids and phenolics from DAP. This work investigated the effect of LSR, water content, ultrasonic power, temperature, and extraction time on the extraction yield, and the results are expressed in Figure 2A–E.
Figure 2.
Effect of extracting conditions using Ci-Glu for phenolic recovery and Lac-Glu for terpenoid recovery in the UAE process: (A) liquid-to-solid ratios, (B) water content in NADES, (C) ultrasonic power, (D) temperature, and (E) extraction time. Different characters (a, b, c, d, and e) presented significant statistical differences.
3.3.1. Effect of Liquid-to-Solid Ratios in the NADES-Based UAE Process
Figure 2A illustrates the effect of different LSRs (10–50 mL/g) on the extraction yield of terpenoids and phenolics under NADES-based UAE conditions at 150 W, 20% water content, and 30 °C for 5 min. The extraction yield of phenolics and terpenoids reached the highest at 9.67 ± 0.33 mg GAE/g dw and 39.23 ± 1.86 mg UA/g dw, respectively, with 40 mL/g LSR, which agrees with previous studies.27 When LSR is appropriately increased, the large solute concentration differences between materials and NADESs facilitate the mass transfer.27 It can be ascribed to decreasing the viscosity of the extraction medium at high LSRs. The reduced viscosity can decrease the cavitation threshold, which is the minimum acoustic pressure to start cavity development during rarefaction cycles. Decreasing the cavitation threshold increases the intensity of cavitation bubbles, which improves the destruction of plant cell walls.29 These phenomena can enhance the driving force for releasing terpenoids and phenolics into Lac-Glu and Ci-Glu, increasing extraction yield. However, when LSR increased to 50 mL/g, TTC and TPC decreased to 6.76 ± 0.27 mg GAE/g dw and 31.76 ± 2.78 mg UA/g dw, respectively. It is shown that if LSR is too high, the excessive NADES can absorb more ultrasonic power. This phenomenon decreases the power absorption of DAP, reducing the destructive effect of cavitation bubbles on cell walls and extraction yield.27 Therefore, the LSR at 40 mL/g was suitable for NADES-based UAE to acquire TPC and TTC at 9.67 ± 0.33 mg GAE/g dw and 39.23 ± 1.86 mg UA/g dw from DAP, respectively, which were lower than those for NADES-based MAE by 1.90 and 2.02 times, respectively.
3.3.2. Effect of Water Content in the NADES-Based UAE Process
Figure 2B demonstrates the effect of water content (10–50%) in Ci-Glu and Lac-Glu on the extraction yield of terpenoids and phenolics under UAE conditions at 40 mL/g, 300W, and 30 °C for 5 min. TPC and TTC reached the highest point at 9.67 ± 0.33 mg GAE/g dw and 51.83 ± 4.82 mg UA/g dw when the water contents were 20 and 40%, respectively. Increasing water content can improve acoustic cavitation intensity due to reducing viscosity. Increasing the cavitation effect can promote cell wall elimination and turbulence, facilitating the extraction of phenolics and terpenoids from DAP.29 However, when water content continuously increased by over 20% in Ci-Glu and 40% in Lac-Glu, the extraction yield of phenolics and terpenoids decreased. The decrease in TPC can be attributed to increasing the polarity of NADESs, which is not favorable for extracting phenolics from DAP. Moreover, the increased water content can break hydrogen bond networks, reducing the interaction between NADESs and terpenoids.25 Therefore, water contents at 20% in Ci-Glu and 40% in Lac-Glu were proper for NADES-based UAE to obtain TPC and TTC at 9.67 ± 0.33 mg GAE/g dw and 51.83 ± 4.82 mg UA/g dw from DAP, respectively, which were lower than those for NADES-based MAE by 1.90 and 1.62 times, respectively.41
3.3.3. Effect of Ultrasonic Power in the NADES-Based UAE Process
The effect of ultrasonic power (0–750 W) on phenolic extraction yield was investigated at 40 mL/g LSR, 30 °C, and 20% water content in Ci-Glu for an extraction time of 5 min. At the same time, the fixed extracting conditions for terpenoids were 40 mL/g LSR, 30 °C, and 40% water content in Lac-Glu for an extraction time of 5 min. As illustrated in Figure 2C, TPC and TTC reached the highest point at 9.56 ± 0.17 mg GAE/g dw and 68.86 ± 1.86 mg UA/g dw when ultrasonic power was at 150 and 600 W, respectively. The increased extraction yield of phenolics and terpenoids can be ascribed to accelerated disruption and sonoporation in plant tissues, resulting from microshearing, localized pressure, and high temperature during ultrasonic treatment, which leads to the promotion of diffusivity and extraction yield.5 However, when ultrasonic power was at 750 W, TPC and TTC decreased to 5.55 ± 0.60 mg GAE/g dw and 57.01 ± 1.54 mg UA/g dw, respectively. The increase of ultrasonic power increases the figure for cavitation bubbles, which results in the deformation, coalescence, and rupture in non-spherical directions of cavitation bubbles. These phenomena reduce the intensity of collapsing bubbles, decreasing the cavitation effect and leading to a drop in extraction yield.5 Therefore, ultrasonic powers at 150 and 600 W were appropriate for the NADES-based UAE to receive TPC and TTC at 9.56 ± 0.16 mg GAE/g dw and 68.86 ± 1.86 mg UA/g dw from DAP, respectively, which were lower than those for NADES-based MAE by 2.28 and 1.30 times, respectively.
3.3.4. Effect of Temperature in the NADES-Based UAE Process
The effect of temperature (30–70°C) on the extraction yield of phenolics was investigated at 40 mL/g LSR, 150 W ultrasonic power, and 20% water content in Ci-Glu for an extraction time of 5 min, while the fixed extracting parameters for terpenoids were 40 mL/g LSR, 600 W ultrasonic power, and 40% water content in Lac-Glu for an extraction time of 5 min. As demonstrated in Figure 2D, TPC was the highest at 30 and 40°C, while TTC peaked at 30 °C. The temperature increase can promote the solubility of phenolics and terpenoids, raising the extraction yield.5 TPC and TTC decreased by 2.1 and 1.8 times, respectively, at 70 °C, compared to those at 30 °C. It can be accounted for the reduction of cavitation bubble sizes and surface tension at elevated temperatures, reducing the intensity of collapsing bubbles. The weakened bubble implosion can decrease the mass transfer of terpenoids and phenolics from DAP to NADESs. Additionally, high temperatures can also decompose these compounds, decreasing the extraction yield.15 Therefore, the proper temperature for NADES-based UAE was 30 °C to obtain TPC and TTC from DAP at 9.56 ± 0.16 mg GAE/g dw and 68.86 ± 1.86 mg UA/g, respectively.
3.3.5. Effect of Time in the NADES-Based UAE Process
The effect of time (3, 5, 10, 15, 20, and 25 min) on TPC was examined at 40 mL/g LSR, 30 °C, 150 W ultrasonic power, and 20% water content in Ci-Glu, while the fixed extracting parameters for terpenoids were 40 mL/g LSR, 600 W ultrasonic power, 30 °C and 40% water content in Lac-Glu. As shown in Figure 2E, the extraction yield of phenolics and terpenoids was the highest at 5 min. It can be ascribed to the higher concentration distinction at the beginning and the cavitation effect imposed on plant cell walls.42 However, TPC and TTC decreased to 4.83 ± 0.27 mg GAE/g dw and 29.11 ± 1.28 mg UA/g dw, respectively, when extraction time was at 25 min. The long time exposure of DAP to ultrasound can cause the destruction of phenolics and terpenoids, decreasing the extraction yield.43 Therefore, extraction time at 5 min was proper for NADES-based UAE to acquire TPC and TTC at 9.56 ± 0.16 mg GAE/g dw and 68.86 ± 1.86 mg UA/g dw from DAP, respectively, which were lower than those for NADES-based MAE by 2.30 and 1.30 times, respectively.
3.4. Effect of NADES-Based UMAE and MUAE
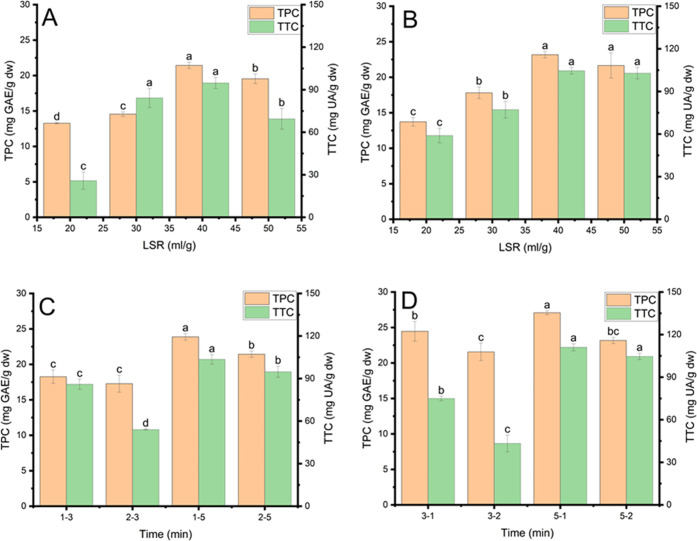
UMAE and MUAE are innovative extraction techniques that combine ultrasound with microwaves. These combinations offer several benefits, such as shortening the extraction time, increasing the extraction yield, and reducing the cost of the extraction process.44 UMAE and MUAE refer to sequential combinations in this study. In UMAE, DAP was first treated by ultrasound before microwave treatment, while the reverse sequence is in MUAE. The effect of NADES-based MUAE and UMAE conditions (LSR and time) on the extraction yield of phenolics and terpenoids and the results are shown in Figure 3A–D.
Figure 3.
Effect of extraction conditions using Ci-Glu for phenolic recovery and Lac-Glu for terpenoid recovery: (A) effect of liquid-to-solid ratios in NADES-based MUAE, (B) effect of liquid-to-solid ratios in NADES-based UMAE, (C) effect of time in NADES-based MUAE, and (D) effect of time in NADES-based UMAE. Different characters (a, b, c, d, and e) presented significant statistical differences.
3.4.1. Effect of LSR in NADES-Based MUAE and UMAE
The effect of LSR (20, 30, 40, and 50 mL/g) on TPC and TTC in NADES-based MUAE and UMAE was investigated. For NADES-based UMAE and MUAE, the ultrasonic conditions for extracting TPC and TTC were fixed at 30 °C for 5 min, 400 W microwave power, and microwave irradiation time at 1 min. Sonication power and water content were set at 150 W and 20%, respectively, in Ci-Glu for phenolic extraction, while these conditions were fixed at 600 W and 40% in Lac-Glu for terpenoid extraction, respectively. TPC and TTC increased with an increase in LSR up to 40 mL/g for MUAE (Figure 3A) and UMAE (Figure 3B). An increase in LSR improves the distribution of ultrasonic waves and microwaves due to a vicious decrease in the extraction medium. This improvement can enhance the acoustic cavitation effect and the heating capacity of microwaves, which promotes fragmentation and the thermal desorption of solute. These phenomena can facilitate solvent penetration and solute diffusion into solvents, improving the extraction yield.5,38 On the other hand, at higher LSRs (>40 mL/g), TPC and TTC decreased. It can be ascribed to the high absorption of ultrasonic and microwave energy of extractants, which deteriorates terpenoids and phenolics. Therefore, 40 mL/g LSR was appropriate for NADES-based MUAE and UMAE to recover phenolics and terpenoids from DAP.
3.4.2. Effect of Time in NADES-Based MUAE and UMAE
The effect of sonication time (3 and 5 min) and microwave irradiation time (1 and 3 min) on TPC and TTC in NADES-based MUAE and MUAE on TPC was studied at 40 mL/g LSR, 30 °C ultrasonic temperature, and 400 W microwave power. Sonication power and water content were set at 150 W and 20%, respectively, in Ci-Glu for phenolic extraction, while these conditions were fixed at 600 W and 40% in Lac-Glu for terpenoid extraction, respectively. The MUAE time was abbreviated 1–3, 2–3, 1–5, and 2–5, corresponding to microwave irradiation and sonication times at 1 and 3, 2 and 3, 1 and 5, and 2 and 5 min, respectively. The acronyms for UMAE were 3–1, 3–2, 5–1, and 5–2, corresponding to sonication and microwave irradiation times at 3 and 1, 3 and 2, 5 and 1, and 5 and 2 min, respectively. As shown in Figure 3C,D, an increase in sonication time promoted the extraction yield of phenolics and terpenoids, while the reverse trend was true in microwave time. It can be explained that microwaves can have a higher thermally destructive effect on phenolics and terpenoids than ultrasound, probably due to the generation of high temperatures during microwave treatment.5,38 Additionally, an increase in sonication time can enhance sonoporation, which promotes NADES penetration into DAP during ultrasonic and microwave treatments, improving the recovery of terpenoids and phenolics.15 Therefore, sonication time (5 min) and microwave irradiation time (1) were proper for NADES-based UMAE and MUAE to extract terpenoids and phenolics from DAP.
3.5. Comparison of UAE, MAE, UMAE, and MUAE Techniques
TTC, TPC, and biological activities obtained from DAP by NADES-based UMAE, MAE, UAE, and MUAE at proper conditions are illustrated in Table 2. The results indicated that TPC and TTC from NADES-based UMAE and MUAE were higher than those recovered using NADES-based UAE and MAE. This result can be accounted for the synergistic effects of microwaves and ultrasound on the plant matrix, which improves the diffusion and solubilization of terpenoids and phenolics. Moreover, TPC and TTC recovered by NADES-based UMAE were higher than those by MUAE. For NADES-based UMAE, the cavitation effect generates numerous microchannels on a material surface when ultrasound is first applied to DAP. These channels can accelerate mass and heat transfer when microwaves are used. Additionally, the elevated temperature generated by microwaves can decrease viscosity and cause cell rupture, improving the release of terpenoids and phenolics. On the other hand, NADES-based MUAE can form extensive hydrogen bond networks with high-molecular-weight polysaccharides in DAP, increasing the overall viscosity of extraction media. After microwave treatment, DAP is sonicated at ambient temperature; thus, the viscosity of extraction media can return. The high viscosity can increase the cavitation threshold and decrease the intensity of cavitation bubbles, impairing the release of terpenoids and phenolics.29 It can be concluded that NADES-based UMAE had a higher extraction yield than NADES-based MUAE.
Table 2. Comparing TPC, TTC, Biological Activities, Recovery Yield, and Energy Consumption in Four Different Extraction Techniquesa.
| criteria/extraction techniques | NADES-based UMAE | NADES-based MAE | NADES-based UAE | NADES-based MUAE |
|---|---|---|---|---|
| Ci-Glu | ||||
| conditions | ||||
| LSR (mL/g) | 40 | 50 | 40 | 40 |
| water content (%) | 20 | 20 | 20 | 20 |
| ultrasonic power (W) | 150 | 150 | 150 | |
| temperature (°C) | 30 | 30 | 30 | |
| sonication time (min) | 5 | 5 | 5 | |
| microwave power (W) | 400 | 400 | 400 | |
| microwave irradiation time (min) | 1 | 2 | 1 | |
| TPC (mg GAE/g dw) | 27.07 ± 0.27a | 22.13 ± 0.75c | 9.56 ± 0.17d | 23.88 ± 0.46b |
| recovery yield (%) | 77.52 ± 0.78a | 63.37 ± 2.15c | 27.38 ± 0.49d | 68.38 ± 1.32b |
| ABTS (μM TE/g dw) | 755 ± 50a | 606 ± 21b | 529 ± 45c | 637 ± 11b |
| DPPH (μM TE/g dw) | 123 ± 3a | 91 ± 4c | 87 ± 2c | 115 ± 4b |
| OH (mM TE/g dw) | 104 ± 0.31a | 100 ± 0.67b | 87 ± 0.8c | 104 ± 0.29a |
| tyrosinase inhibition (mg AE/g dw) | 648 ± 29a | 379 ± 33c | 259 ± 43d | 566 ± 43b |
| α-amylase inhibition (g ACE/g dw) | 10.39 ± 0.06a | 9.1 ± 0.37c | 8.59 ± 0.1d | 9.71 ± 0.08b |
| AChE inhibition (g TCE/g dw) | 3.83 ± 0.12a | 3.31 ± 0.08c | 2.94 ± 0.08d | 3.59 ± 0.04b |
| energy consumption (Wh) | 19.2 | 13.3 | 12.5 | 19.2 |
| Lac-Glu | ||||
| conditions | ||||
| LSR (mL/g) | 40 | 50 | 40 | 40 |
| water content (%) | 40 | 40 | 40 | 40 |
| ultrasonic power (W) | 600 | 600 | 600 | |
| temperature (°C) | 30 | 30 | 30 | |
| sonication time (min) | 5 | 5 | 5 | |
| microwave power (W) | 400 | 400 | 400 | |
| microwave irradiation time (min) | 1 | 2 | 1 | |
| TTC (mg UA/g dw) | 111 ± 3a | 90 ± 1c | 69 ± 2d | 104 ± 3b |
| recovery yield (%) | 82.71 ± 2.23a | 67.06 ± 0.75c | 51.42 ± 1.49d | 77.50 ± 2.21b |
| ABTS (μM TE/g dw) | 900 ± 17a | 722 ± 23c | 663 ± 17d | 842 ± 21b |
| DPPH (μM TE/g dw) | 120 ± 1a | 87 ± 1c | 87 ± 3c | 110 ± 3b |
| OH (mM TE/g dw) | 52 ± 0.33a | 44 ± 0.15c | 36 ± 0.89d | 48 ± 0.31b |
| tyrosinase inhibition (mg AE/g dw) | 309 ± 17a | 232 ± 14b | 121 ± 31c | 285 ± 25b |
| α-amylase inhibition (g ACE/g dw) | 15.78 ± 0.10a | 9.5 ± 0.38c | 8.03 ± 0.05d | 14.86 ± 0.14b |
| AChE inhibition (g TCE/g dw) | 4.36 ± 0.09a | 4.21 ± 0.11a | 3.36 ± 0.07b | 4.25 ± 0.04a |
| energy consumption (Wh) | 56.67 | 13.3 | 50 | 56.67 |
Different characters (a, b, c, and d) showed significant statistical differences.
In terms of antioxidant activity, NADES-based UMAE had the highest ABTS, DPPH, and OH at 755 ± 50 μM TE/g dw, 123 ± 3 μM TE/g dw, and 104 ± 0.31 mM TE/g dw, respectively, followed by NADES-based MUAE, MAE, and UAE when using Ci-Glu as a solvent. A similar trend was true in the case of using Lac-Glu. The combination of ultrasound and microwaves can facilitate the degradation of glycosidic bonds between bioactive compounds and macromolecules (proteins and polysaccharides) more than merely ultrasonic or microwave treatment, producing more free bioactive compounds. The presence of free bioactive compounds can enhance the quenching of ABTS, DPPH, and OH radicals.45 Additionally, extracts attained by UMAE had higher antioxidant activity than MUAE. The initial application of ultrasound can cause cell wall degradation, fragmentation, and particle size reduction, improving the contact between microwaves and materials. This contact can increase heat distribution during microwave treatment, which enhances the mass transfer and release of bioactive compounds, increasing antioxidant activity. On the other hand, microwave treatment is first applied, causing a decrease in particle size, which can reduce the effect of ultrasound on the material surface. This phenomenon can decrease in extraction yield of bioactive compounds, decreasing antioxidant activity.15,46
Regarding enzyme inhibitory activity, Ci-Glu-based and Lac-Glu-based UMAE showed the highest tyrosinase, α-amylase, and AChE inhibitory capacity, followed by NADES-based MUAE, MAE, and UAE. UMAE treatment can break the glycoside bonds between polysaccharides and bioactive compounds, which generate their mono and oligo forms. These forms are more flexible than polymeric ones, which can easily bind with the catalytic triad in enzyme active sites. This binding can prevent the accessibility of substrates to enzyme active sites, improving the inhibitory capacity of extracts obtained by UMAE.47 Additionally, the high inhibitory capacity of extracts from UMAE can be attributed to the difference in the phenolic and terpenoid profiles. Alu′datt et al. 2017 demonstrated that extractable and non-extractable phenolic recovered from lemon juice increased α-amylase activity in lieu of inhibition.48 Lemon had differences in the phenolic profile relying on species and part of a fruit, and the profile can directly influence α-amylase inhibitory capacity.49 Ioanna Stamogiannou et al. 2021 demonstrated that quercetin and kaempferol had a cellulase inhibitory effect, while the reverse trend was true in the case of sinapic acid and vanillic acid. OCH3 groups can enhance enzyme activity, modify cellulose structure, and improve enzyme attachment. The aromatic rings can bind with the enzyme active site, reducing the contact between the enzyme and the substrate, which can increase inhibitory capacity.47
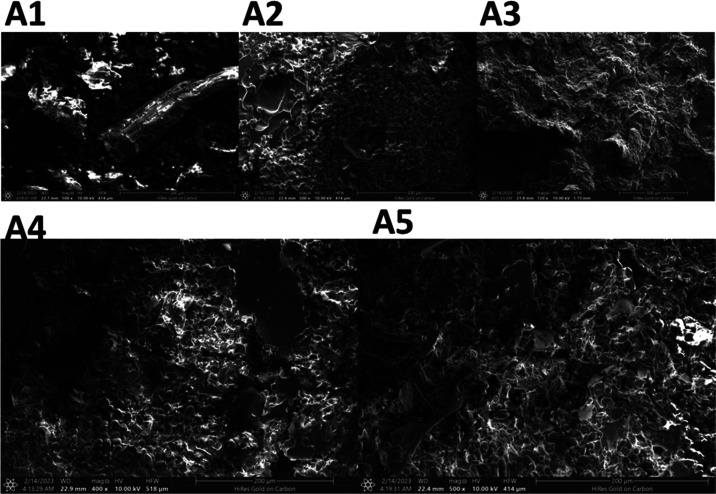
3.6. Surface Morphology Variation of DAP
The destruction of plant cells can promote the diffusion of phenolics and terpenoids from plant materials, improving the extraction yield.25 Therefore, the DAP surface morphology variations after treatment with different extraction techniques were examined using SEM. As shown in Figure 4A1–A5, the surface morphology of DAP was smooth before treatment (Figure 4A1), while that of treated DAP was rough and had numerous pores. The destructive level of the DAP surface was in the following order: A5 > A4 > A2 > A3 > A1. For NADES-based UMAE and MUAE, the cell wall could be destroyed more severely than that for NADES-based UAE and MAE, probably due to the synergistic effect of ultrasound and microwaves.5,38 SEM results also agreed with the explanation (Section 3.5) for the higher extraction yield and antioxidant activity of NADES-based UMAE than NADES-based MUAE, MAE, and UAE.
Figure 4.
Variations of DAP surface morphology before and after treatment; (A1): non-treatment (500 magnification, resolution: 1536 × 1094); (A2) ultrasonic treatment (500 magnification, resolution: 1536 × 1094); (A3): microwave treatment (120 magnification, resolution: 1536 × 1094); (A4): microwave–ultrasonic treatment (500 magnification, resolution: 1536 × 1094); (A5): ultrasonic–microwave treatment (500 magnification, resolution: 1536 × 1094).
3.7. Choice of Extraction Methods
The choice of the extraction method is one of the most crucial steps in the recovery of phenolics and terpenoids from DAP. Table 2 also shows the energy consumption and recovery yield of the four extraction methods. Regarding the extraction of phenolics, although the energy consumption of UAE was the lowest at 12.5 Wh, the recovery yield of phenolics was the lowest at 27.38 ± 0.49%. Regarding the recovery of terpenoids, the energy expenditure of the UAE was quite high; however, the recovery yield was the lowest. MAE showed high energy efficiency in the extraction of phenolics and terpenoids. The UMAE and MUAE showed the same energy expenditure; however, the UMAE expressed a higher recovery yield of phenolics and terpenoids than MUAE. In addition to energy efficiency, other criteria, such as biological activities, the amount of solvent used, and recovery yield, are considered important factors in selecting the extraction method for phenolics and terpenoids from DAP. Although MAE showed the highest energy efficiency, the above criteria were lower than those for UMAE. Therefore, UMAE was suitable for attaining phenolics and terpenoids from DAP.
4. Conclusions
In this research, TPC obtained using Ci-Glu at (4.55 ± 0.16 mg GAE/g dw) and TTC acquired using Lac-Glu (15.79 ± 1.74 mg UA/g dw) from DAP were highly efficient compared to those using ethanol (TPC at 1.40 ± 0.08 mg GAE/g dw and TTC at 3.73 ± 0.41 mg UA/g dw). Regarding NADES-based MAE, the appropriate conditions for phenolic extraction were 50 mL/g LSR, 20% water content in Ci-Glu, and 400 W microwave power, while those of terpenoids were 50 mL/g LSR, 400 W microwave power, and 40% water content in Lac-Glu for 2 min. In terms of NADES-based UAE, the appropriate conditions for phenolic extraction were 40 mL/g LSR, 30 °C, 150 W ultrasonic power, and 20% water content in Ci-Glu, while those of terpenoids were 40 mL/g LSR, 600 W ultrasonic power, 30 and 40% water content in Lac-Glu for 5 min. Regarding NADES-based UMAE and MUAE, LSR at 40 mL/g, sonication time at 5 min, and microwave time at 1 min were suitable for extracting phenolics and terpenoids from DAP. Additionally, NADES-based UMAE extracts had the highest biological activities, TPC, and TTC. SEM images showed that NADES-based UMAE had the most destructive effect on the DAP surface among the four extraction techniques. NADES-based UMAE was the combination of green solvents, UAE, and MAE, which presented a higher extraction yield of phenolics and flavonoids than that using a single method. The sequence of applying ultrasound and microwave irradiation is also determined that has not been elucidated in previous studies. The advantages of NADES-based UAE result from the material surface destruction of ultrasound and NADESs and the mass transfer improvement of microwave-based heating. These effects significantly improve extraction yield via mass transfer, reduce solvent consumption, and shorten extraction time. Therefore, NADES-based UMAE could be considered a green, sustainable, and effective method for recovering phenolics and terpenoids from DAP. DAP extracts can be a potential source of bioactive compounds for food products.
Acknowledgments
We acknowledge Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for supporting this study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article
Author Contributions
T.P.V.: conceptualization, methodology, investigation, software, visualization, formal analysis, data curation, and writing—original draft. T.V.P.: investigation and formal analysis. T.N.H.T.: investigation and visualization. L.T.V.V.: formal analysis and investigation. T.T.V.: visualization and investigation. N.D.P.: visualization. D.Q.N.: visualization, supervision, and writing—review & editing
The authors declare no competing financial interest.
Notes
There is a declaration that the authors have no known personal relationships or competing monetary interests that could have impacted the work reported in this paper
References
- Dinh Ngoc T.; Ha M. V. T.; Nguyen Le T.; Nguyen T. V. A.; Mechler A.; Hoa N. T.; Vo Q. V. Antioxidant Activity of Natural Samwirin A: Theoretical and Experimental Insights. ACS Omega 2021, 6, 27546. 10.1021/acsomega.1c04569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo M.-H.; Nguyen H.; Nguyen T.-B.; Le T.-D.; Le Q.-H. Experimental Study of the Effect of Ultrasound on the Freezing Process of Bo Chinh Ginseng. Appl. Sci. 2023, 13, 408 10.3390/app13010408. [DOI] [Google Scholar]
- Ludwiczuk A.; Skalicka-Woźniak K.; Georgiev M.. Terpenoids. In Pharmacognosy; Elsevier, 2017; pp 233–266. [Google Scholar]
- Chemat F.; Abert-Vian M.; Fabiano-Tixier A. S.; Strube J.; Uhlenbrock L.; Gunjevic V.; Cravotto G. Green extraction of natural products. Origins, current status, and future challenges.. TrAC, Trends Anal. Chem. 2019, 118, 248–263. 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- Rao M. V.; Sengar A. S.; C K S.; Rawson A. Ultrasonication - A green technology extraction technique for spices: A review. Trends Food Sci. Technol. 2021, 116, 975–991. 10.1016/j.tifs.2021.09.006. [DOI] [Google Scholar]
- Behbahani M.; Rabiee G.; Bagheri S.; Amini M. M. Ultrasonic-assisted d-μ-SPE based on amine-functionalized KCC-1 for trace detection of lead and cadmium ion by GFAAS. Microchem. J. 2022, 183, 107951 10.1016/j.microc.2022.107951. [DOI] [Google Scholar]
- Behbahani M.; Sobhi H. R.; Esrafili A. Combination of ultrasonic-assisted dispersive liquid phase micro-extraction with magnetic dispersive solid-phase extraction for the pre-concentration of trace amounts of atrazine in various water samples. Int. J. Environ. Anal. Chem. 2021, 101, 609–620. 10.1080/03067319.2019.1670170. [DOI] [Google Scholar]
- Behbahani M.; Bagheri S.; Omidi F.; Amini M. M. An amino-functionalized mesoporous silica (KIT-6) as a sorbent for dispersive and ultrasonication-assisted micro solid phase extraction of hippuric acid and methylhippuric acid, two biomarkers for toluene and xylene exposure. Microchim. Acta 2018, 185, 505 10.1007/s00604-018-3038-5. [DOI] [PubMed] [Google Scholar]
- Izanloo M.; Esrafili A.; Behbahani M.; Ghambarian M.; Reza Sobhi H. Trace quantification of selected sulfonamides in aqueous media by implementation of a new dispersive solid-phase extraction method using a nanomagnetic titanium dioxide graphene-based sorbent and HPLC-UV. J. Sep. Sci. 2018, 41, 910–917. 10.1002/jssc.201700814. [DOI] [PubMed] [Google Scholar]
- Salimi M.; Behbahani M.; Sobhi H. R.; Ghambarian M.; Esrafili A. Dispersive solid-phase extraction of selected nitrophenols from environmental water samples using a zirconium-based amino-tagged metal–organic framework nanosorbent. J. Sep. Sci. 2018, 41, 4159–4166. 10.1002/jssc.201800764. [DOI] [PubMed] [Google Scholar]
- Sobhi H. R.; Behbahani M.; Ghambarian M.; Salimi M.; Esrafili A. Extraction of carbonyl derivatives from ozonated wastewater samples using hollow fiber liquid phase microextraction followed by gas chromatography-electron capture detection. Microchem. J. 2019, 148, 331–337. 10.1016/j.microc.2019.05.020. [DOI] [Google Scholar]
- Sobhi H. R.; Ghambarian M.; Behbahani M.; Esrafili A. Application of dispersive solid phase extraction based on a surfactant-coated titanium-based nanomagnetic sorbent for preconcentration of bisphenol A in water samples. J. Chromatogr. A 2017, 1518, 25–33. 10.1016/j.chroma.2017.08.064. [DOI] [PubMed] [Google Scholar]
- Sobhi H. R.; Mohammadzadeh F.; Behbahani M.; Yeganeh M.; Esrafili A. Application of a modified MWCNT-based d-μSPE procedure for determination of bisphenols in soft drinks. Food Chem. 2022, 385, 132644 10.1016/j.foodchem.2022.132644. [DOI] [PubMed] [Google Scholar]
- Cunha S. C.; Fernandes J. O. Extraction techniques with deep eutectic solvents. TrAC, Trends Anal. Chem. 2018, 105, 225–239. 10.1016/j.trac.2018.05.001. [DOI] [Google Scholar]
- Kumar K.; Srivastav S.; Sharanagat V. S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.-Y.; Liu J.-P.; Huang X.; Du L.-P.; Shi F.-L.; Dong R.; Huang X.-T.; Zheng K.; Liu Y.; Cheong K.-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT 2018, 90, 577–582. 10.1016/j.lwt.2018.01.007. [DOI] [Google Scholar]
- Sun H.; Li C.; Ni Y.; Yao L.; Jiang H.; Ren X.; Fu Y.; Zhao C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019, 206, 557–564. 10.1016/j.carbpol.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Bener M.; Şen F. B.; Önem A. N.; Bekdeşer B.; Çelik S. E.; Lalikoglu M.; Aşçı Y. S.; Capanoglu E.; Apak R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633 10.1016/j.foodchem.2022.132633. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- More P. R.; Jambrak A. R.; Arya S. S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. 10.1016/j.tifs.2022.08.016. [DOI] [Google Scholar]
- Ngoc T. D.; Thi Ha M. V.; Le T. N.; Thi H. V.; Anh Nguyen T. V.; Mechler A.; Hoa N. T.; Vo Q. V. A Potent Antioxidant Sesquiterpene, Abelsaginol, from Abelmoschus sagittifolius: Experimental and Theoretical Insights. ACS Omega 2022, 7, 24004–24011. 10.1021/acsomega.2c02974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Li L.; Chen S.; Wang L.; Lin X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014 10.1016/j.seppur.2020.117014. [DOI] [Google Scholar]
- Biswas B.; Golder M.; Abid M. A.; Mazumder K.; Sadhu S. K. Terpenoids enriched ethanol extracts of aerial roots of Ceriops decandra (Griff.) and Ceriops tagal (Perr.) promote diuresis in mice. Heliyon 2021, 7, e07580 10.1016/j.heliyon.2021.e07580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L.; Fröhlich K.; Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. 10.1016/j.foodchem.2011.04.045. [DOI] [Google Scholar]
- Gao Y.; Dong Q.; Zhao S.; Zhao Y.; Zhang Y.; Wang H.; Wang Y.; Wang W.; Wang L.; Wang H. Efficient ultrasound-assisted enzymatic method for extraction of immunostimulant QS-21 from Quillaja saponaria Molina. Ind. Crops Prod. 2022, 189, 115807 10.1016/j.indcrop.2022.115807. [DOI] [Google Scholar]
- Wu M.; Yang Q.; Wu Y.; Ouyang J. Inhibitory effects of acorn (Quercus variabilis Blume) kernel-derived polyphenols on the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase IV. Food Biosci. 2021, 43, 101224 10.1016/j.fbio.2021.101224. [DOI] [Google Scholar]
- Babotă M.; Frumuzachi O.; Gâvan A.; Iacoviţă C.; Pinela J.; Barros L.; Ferreira I. C. F. R.; Zhang L.; Lucini L.; Rocchetti G.; et al. Optimized ultrasound-assisted extraction of phenolic compounds from Thymus comosus Heuff. ex Griseb. et Schenk (wild thyme) and their bioactive potential. Ultrason. Sonochem. 2022, 84, 105954 10.1016/j.ultsonch.2022.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne S. B.; Grabanski C. B.; Martin E.; Miller D. J. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. 10.1016/S0021-9673(00)00091-1. [DOI] [PubMed] [Google Scholar]
- Patil S. S.; Pathak A.; Rathod V. K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021, 70, 105267 10.1016/j.ultsonch.2020.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; van Spronsen J.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Xu M.; Ran L.; Chen N.; Fan X.; Ren D.; Yi L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970 10.1016/j.foodchem.2019.124970. [DOI] [PubMed] [Google Scholar]
- National Library of Medicine . Citric Acid. https://pubchem.ncbi.nlm.nih.gov/compound/311 (accessed October 26, 2022).
- National Library of Medicine . Lactic acid. https://pubchem.ncbi.nlm.nih.gov/compound/612#section=MeSH-Entry-Terms (accessed October 26, 2022).
- National Library of Medicine . Isopropyl Alcohol. https://pubchem.ncbi.nlm.nih.gov/compound/3776#section=Computed-Properties (accessed June 30, 2023).
- National Library of Medicine . Glycerin. https://pubchem.ncbi.nlm.nih.gov/compound/753 (accessed June 30, 2023).
- National Library of Medicine . Acetic acid. https://pubchem.ncbi.nlm.nih.gov/compound/176#section=Chemical-and-Physical-Properties (accessed October 26, 2022).
- National Library of Medicine . Glucose. https://pubchem.ncbi.nlm.nih.gov/compound/5793#section=Chemical-and-Physical-Properties (accessed June 30, 2023).
- Chemat F.; Cravotto G.. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Springer Science & Business Media, 2012. [Google Scholar]
- Chen Y.; Xie M.-Y.; Gong X.-F. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. 10.1016/j.jfoodeng.2006.10.018. [DOI] [Google Scholar]
- Huang H.; Zhu Y.; Fu X.; Zou Y.; Li Q.; Luo Z. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from gardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. 2022, 380, 132216 10.1016/j.foodchem.2022.132216. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Mena A.; Ochoa-Martínez L. A.; Herrera S. M. G.; Rutiaga-Quiñones O. M.; González-Laredo R. F.; Olmedilla-Alonso B.; Vega-Maturino S. Coloring potential of anthocyanins from purple sweet potato paste: Ultrasound-assisted extraction, enzymatic activity, color and its application in ice pops. Food Chem. Adv. 2023, 100358 10.1016/j.focha.2023.100358. [DOI] [Google Scholar]
- Komal V. M.; Virendra K. R. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. 2020, 149, 107841 10.1016/j.cep.2020.107841. [DOI] [Google Scholar]
- Iftikhar M.; Zhang H.; Iftikhar A.; Raza A.; Begum N.; Tahamina A.; Syed H.; Khan M.; Wang J. Study on optimization of ultrasonic assisted extraction of phenolic compounds from rye bran. LWT 2020, 134, 110243 10.1016/j.lwt.2020.110243. [DOI] [Google Scholar]
- Jha A. K.; Sit N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. 10.1016/j.tifs.2021.11.019. [DOI] [Google Scholar]
- Dibanda R. F.; Akdowa E. P.; P A. R.; Tongwa Q. M.; F C. M. M. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020, 302, 125308 10.1016/j.foodchem.2019.125308. [DOI] [PubMed] [Google Scholar]
- de Almeida Pontes P. V.; Ayumi Shiwaku I.; Maximo G. J.; Caldas Batista E. A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346 10.1016/j.foodchem.2021.129346. [DOI] [PubMed] [Google Scholar]
- Stamogiannou I.; Van Camp J.; Smagghe G.; Van de Walle D.; Dewettinck K.; Raes K. Impact of phenolic compound as activators or inhibitors on the enzymatic hydrolysis of cellulose. Int. J. Biol. Macromol. 2021, 186, 174–180. 10.1016/j.ijbiomac.2021.07.052. [DOI] [PubMed] [Google Scholar]
- Alu’datt M. H.; Rababah T.; Alhamad M. N.; Al-Mahasneh M. A.; Ereifej K.; Al-Karaki G.; Al-Duais M.; Andrade J. E.; Tranchant C. C.; Kubow S.; et al. Profiles of free and bound phenolics extracted from Citrus fruits and their roles in biological systems: content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food Funct. 2017, 8, 3187–3197. 10.1039/C7FO00212B. [DOI] [PubMed] [Google Scholar]; Research.
- Durmus N.; Kilic-Akyilmaz M. Bioactivity of non-extractable phenolics from lemon peel obtained by enzyme and ultrasound assisted extractions. Food Biosci. 2023, 53, 102571 10.1016/j.fbio.2023.102571. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article