Abstract
In this study, albino Wistar rats that have developed diabetes as a result of the drug streptozotocin (STZ) were treated with camel milk and insulin. For this, 36 rats were divided into six different (n = 6) groups: control, control + camel milk, diabetic control, insulin, camel milk, and combined camel milk + insulin. A 50 mg/kg intraperitoneal injection of STZ was used to induce diabetes. Rats with blood glucose levels exceeding 250 mg/dL after the induction of diabetes were taken into consideration for the study. The diabetic rats were treated with camel milk (50 mL/rat/day), insulin (6 units kg–1 b·wt/day), or their combination daily for 30 days. Throughout the course of the study, the rats’ glucose levels and body weight were checked. In the diabetic control rats, a reduction in body weight and hyperglycemic condition was seen. Improvements in glycemic levels and weight gain were seen in the camel milk, insulin, and combined treatment groups compared to the diabetic control group; however, the combined treated group did not show the same degree of improvement as the alone treated group. Hematological changes in the diabetic control group included reductions in lymphocytes, platelets, total leukocyte count (TLC), and red blood cell (RBC) indices (mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), packed cell volume (PCV), and mean cell hemoglobin concentration (MCHC)). Each group that got insulin and camel milk separately and combined showed improvement in these changes. The liver, kidney, and pancreas in the diabetic control group had worsened morphological alterations. These histopathological alternations were significantly improved in the treatment groups. Hence, this study demonstrates the antidiabetic effects of camel milk in comparison to insulin. These findings highlight the potential of camel milk as an alternative therapy for diabetes, although further research is warranted to fully understand its mechanisms of action and long-term effects.
Introduction
Diabetes mellitus is a multifaceted chronic disorder that causes hyperglycemia (high blood glucose levels) in the body.1 Insufficient insulin synthesis, cellular resistance to insulin binding on its membrane receptors, or a combination of both factors can result in hyperglycemia in the body.1 Over the past three decades, diabetes prevalence has increased. According to the International Diabetes Federation, there were 537 million instances of adult diabetes globally in 2021, making it the ailment that affects more than 10.5% of the adult population worldwide.2 Type-1 diabetes is a chronic metabolic disorder characterized by insufficient endogenous insulin production by the pancreas. Previously referred to as juvenile diabetes or insulin-dependent diabetes, it is distinguished by the diminished or absence of pancreatic insulin secretion (WHO).3 The global prevalence of type 1 diabetes was estimated to be approximately 84 million individuals in 2021. Projections indicate that by the year 2040, this number could potentially increase to 17.4 million individuals.4
Numerous consequences are produced by diabetes mellitus, also known as the “silent killer”, including anomalies of the nervous system, hepatocellular damage, and reproductive malformations.5,6 However, dietary changes, exercise, or medication can help maintain metabolic regulation.7,8 Insulin is a crucial hormone for metabolism. The production of insulin by pancreatic endocrine cells regulates glucose homeostasis by inhibiting the processes of gluconeogenesis and glycogenolysis. Biological systems generate reactive oxygen species (ROS), which serve as mediators in the synthesis of various substances and encompass hydroxyl radicals, nitric oxide radicals, superoxide anions, and peroxyl radicals.9 While the body’s inherent antioxidant defense system is capable of eliminating these free radicals;10 their excessive presence can inflict damage on proteins, lipids, and DNA.11 Notably, free radicals are also generated within the body through the self-oxidation of glucose, resulting in heightened oxidative stress, which is a notable consequence of diabetes. These free radicals circulate through the bloodstream and produce alterations in the morphology and physiology of the hematopoietic system.12 Reduced hemoglobin, MCH, MCHC, PCV, and RBCs are caused by diabetes mellitus, which also affects other hematological indices such as lymphocytes, white blood cells, and platelets.13−16
Insulin therapy is the most widely used treatment for diabetes. Type-I diabetes is also referred to as insulin-dependent diabetes, since exogenous insulin injections can help maintain blood glucose levels and reduce the risk of complications.17,18 According to certain findings, insulinopenic diabetic rats may unintentionally develop insulin resistance when hyperinsulinemia is present.19 The precise determination of insulin dosage is of utmost importance in clinical management for effective diabetes treatment. In clinical scenarios, complete replacement of insulin therapy is unattainable. However, the utilization of conventional drugs is often associated with undesirable side effects, including lipohypertrophy, headaches, abdominal pain, anaphylactic reactions, and hypoglycemia. Consequently, individuals are compelled to seek alternative approaches, such as naturopathic medicines, particularly in many developing nations where approximately three-fourths of the population cannot afford the expenses associated with medical care.20,21 Natural antidiabetic products from plants and animals have gained popularity over the past few decades. Bauhinia forficata, Cecropia obtusifolia (Bertol), Equisetum myriochaetum, and Cucurbita ficifolia (Bouche) are a few of them.22,23 Medicinal plants are frequently utilized as a form of treatment, but their prevalence and variation by region and culture make them challenging to use. Ruminant milk and its products are a source of nutrients and energy and possess positive health effects.24 Camel milk has gained recognition for its distinctive therapeutic and nutritional benefits in recent scientific investigations. Previous studies have demonstrated its effectiveness in treating various conditions such as dropsy, jaundice, tuberculosis, kala-azar, and anemia.25,26 Furthermore, scientific evidence supports the anticancer, antiallergic, and antidiabetic effects of camel milk.27−29 Camel milk is rich in vitamins (particularly A, B2, C—highest concentration, and E) and minerals (including Na, K, Fe, Cu, Zn, and Mg), as well as immunoglobulins (G and A). However, it is not as abundant in sugar, proteins, or cholesterol.29,30 The therapeutic impact of camel milk on Langerhans islet cells, mediated by its immunoglobulins, has led to its use in the treatment of diabetes mellitus in certain regions of Asia, the Middle East, and Africa.31 Its anti-inflammatory effect and high concentration of antioxidants are speculated to contribute to its positive role in diabetes management.32 Additionally, camel milk contains various antioxidant components such as caseins, lactic acid bacteria, bioactive peptides, whey proteins, and lactoferrin. The higher antioxidant activity of camel milk may be attributed to its approximately 6.7 times greater vitamin C content than fresh cow milk.33,34 Camel milk possesses antihyperglycemic properties owing to its higher concentration of insulin-like protein (52 micro units/mL) in comparison to cow milk (16 micro units/mL).29,35,36 Notably, camel milk exhibits the peculiar characteristic of not coagulating in the acidic environment of the stomach, allowing for optimal absorption in the intestine.37 Previous research suggests that camel milk and insulin may serve as effective complementary therapies for individuals with type-I diabetes.38 However, limited scientific investigations have been conducted on the comparative effects of camel milk, insulin, or their combinations. Further research is warranted to explore these aspects comprehensively. This research article investigates and compares the efficacy of insulin and camel milk in the treatment of streptozotocin-induced diabetes in albino rats. The study examines the hematological alterations in the different treatment groups and assesses the histological changes occurring in the liver, kidney, and pancreas.
Materials and Methods
Animal Ethics and Maintenance
Institutional Animal Ethics Committee (IAEC) approved this experimental study. The method used in the care and management of animals was suggested by the Animal Committee for Control and Supervision of Experiments on Animals (CPCSEA).
From the Disease Free Small Animal House, LUVAS, Hisar, Haryana, India, 36 albino Wistar rats (3–4 months old) were purchased. Before the experiment began, the animals were acclimated for a week in polycarbonate cages with a 12:12 h light/dark cycle, and the room temperature was 23 °C. The animals had unrestricted access to a standard diet as well as water.
Chemicals and Reagents Used
From Himedia and Sigma-Aldrich, streptozotocin, citrate monohydrate, and sodium citrate were purchased. This study made use of NPH insulin, namely, Humulin NPH (100 IU/mL). Sterilized bottles of camel milk were purchased from a local farmer daily. Blood was examined using an automated hematological analyzer (Analytical, India). A glucometer (Accu-check) was used to measure the glucose levels throughout the experiment.
Induction of Diabetes
Animals were given a single dosage of 50 mg/kg of STZ to induce diabetes.39 Animals received an intraperitoneal injection of STZ that had been freshly made in 0.1 M citrate buffer of pH 4.5. A portable glucometer (Accu-check) was used to measure the animals’ fasting blood glucose levels before the injection. Rats under control just received an injection of citrate buffer. To prevent hypoglycemic shock after STZ injection, rats received a 5% glucose solution instead of water for 24 h. After 72 h of STZ injection, the fasting glucose levels of all rats were measured. Rats with fasting glycemia levels over 250 mg/dL were selected for the experimental investigation and divided into various experimental groups.40
Experimental Design (OECD 407)
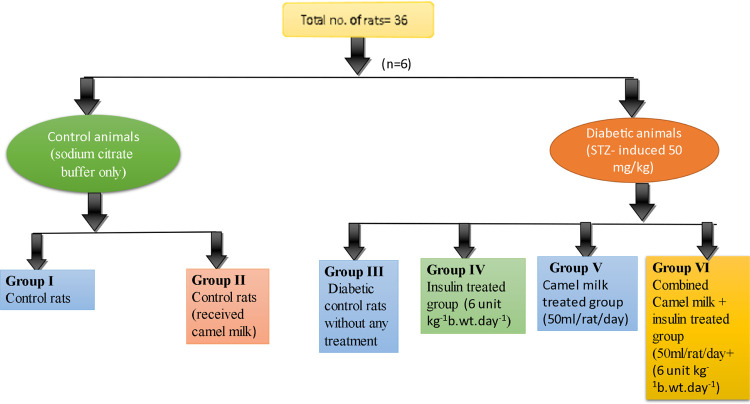
Rats were separated into six groups such as control (group I), control + camel milk (group II), diabetic control (group III), insulin (group IV), camel milk (group V), and combined camel milk + insulin (group VI, Figure 1). STZ injection was given to groups III, IV, V, and VI. Rats were supplied with camel milk 50 mL/rat/day39 and insulin 6 units kg–1b·wt/day–141 after their diabetic state had stabilized for a week. Regular assessments of body weight and fasting glucose levels were observed throughout the experimental investigation. At the end of the study, the animals were sacrificed and blood samples were collected in EDTA-coated vials to assess various hematological parameters, and for histological parameters analysis, the liver, kidney, and pancreas were also removed.
Figure 1.
Representation of different experimental groups.
Parameters Recorded
Body Weight and Glycemic Levels
The body weights of all of the animals were measured daily. Throughout the experiment, the glucose levels were tested every fifth day (0, 5th, 10th, 15th, 20th, 25th, and 30th).
Hematological Parameters
Hematological indices analysis is a common and essential diagnostic procedure for the clinical evaluation of health status. The animals were sacrificed at the end of the experiment (30 days) under carbon dioxide asphyxiation following an overnight fast, and blood was drawn into EDTA-coated vacutainers through a heart puncture following the manufacturer’s instructions. An automated hematology analyzer was used to determine hemoglobin (HB), red blood cells (RBCs), packed cell volume (PCV), total leukocyte count (TLC), lymphocytes, neutrophils, eosinophils, and basophils, as well as mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), and platelets.12,13
Oral Glucose Tolerance Test (OGTT)
OGTT was performed before scarifying the rats (at the end of the experiment). OGTT was evaluated by giving glucose solution (2 g/kg) through the oral route. The glycemic levels were noted before and after the loading of glucose at 0, 30, 60, 90, and 120 min.39
Histological Studies
For the histological study, organs such as the liver, kidney, and pancreas were removed and preserved in 10% formalin. These organs were dehydrated using a graduated alcohol series before being set in paraffin wax. A semiautomated rotator microtome was used to prepare 5 m thick sections. A light microscope with a CCD digital camera attached was used to analyze the sections.42
Statistical Analysis
The obtained data were statistically analyzed by using GraphPad PRISM version 8.0 statistical software and presented as mean ± SD. ANOVA (one-way and two-way) and Tukey’s post hoc test was used to compare the variables of different experimental groups at a significance level of p ≤ 0.05.
Results
Body Weight
The average body weight of each treatment group was evaluated at a fixed time interval of 5 days all through the study duration. The control group presented with a significant (p < 0.05) physiological gain in average body weight from 110.1 ± 4.16 g on day 0 to 136.5 ± 7.66 g on day 30. In comparison, induction of type-1 diabetes resulted in a significant (p < 0.05) decrease in average body weight from 117.8 ± 2.31 g on day 0 to 92.1 ± 2.78 g on day 30 of the study. Administration of insulin to diabetic rats significantly (p < 0.05) ameliorated the body weight loss seen in diabetic rats to a gain of average body weight, as recorded to be 113.1 ± 2.63 g on day 0 to 162.8 ± 6.11 g on day 30. Concurrently, camel milk also resulted in a significant pattern of body weight gain in the diabetic rats from 105 ± 3.34 g on day 0 and 179 ± 5.51 g on day 30. The camel milk-fed control rats presented an average body weight gain almost equivalent to the control group over the time period of the study, which was 103.5 ± 3.61 g on day 0 to 126 ± 1.54 g on day 30. Moreover, combined administration of camel milk and insulin to the diabetic rats also resulted in a significant gain in body weight in comparison to the diabetic group, as recorded to be 112.8 ± 3.25 g on day 0 to 180.6 ± 4.61 g on day 30 (Figure 2).
Figure 2.
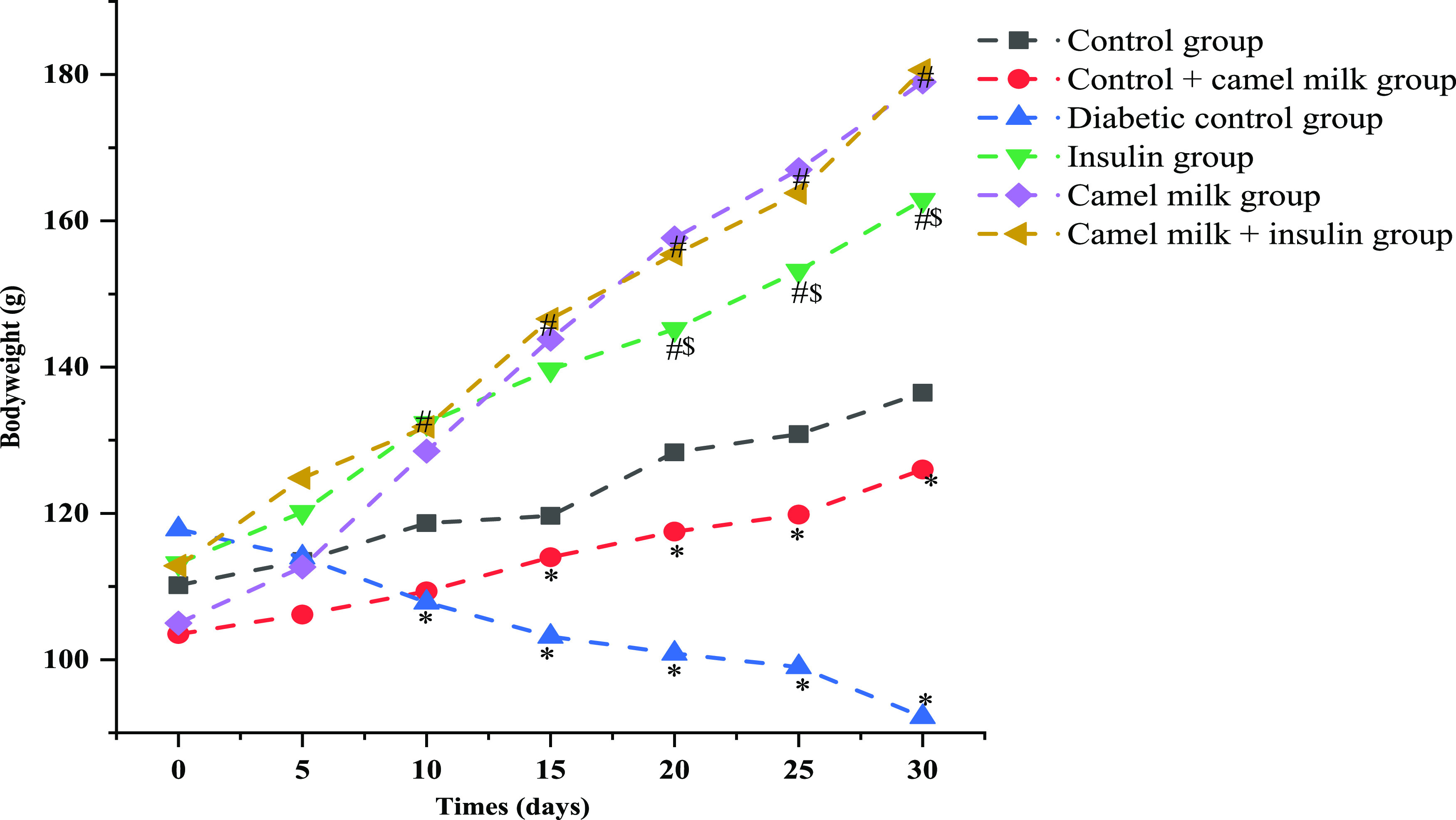
Graphical representation of average body weight pattern of the animals’ post-treatments of camel milk and insulin individually or in combination during the study period. Data are mean ± SD; N = 6; significant at 5% for ANOVA. Here, * represents significance in comparison to control, # represents significance in comparison to diabetic control, and $ represents significance in comparison to the camel milk-treated diabetic group (P value represented with * /#/$ ≤ 0.05).
Blood Glucose Levels
Throughout the study duration, blood glucose levels were checked every fifth day. All through the study period, the blood glucose levels of the diabetic control group were significantly (p < 0.05) high, i.e., 495.6 ± 91.33 mg/dL on day 0 and 510.3 ± 34.88 mg/dL on day 30 in comparison to healthy control where the blood glucose levels were 135.6 ± 7.47 mg/dL on day 0 while 142.8 ± 9.90 mg/dL on day 30 of the study. Administration of insulin to the diabetic rats significantly (p < 0.05) reduced the glucose levels from 530.3 ± 92.25 mg/dL on day 0 to 175.6 ± 29.15 mg/dL on day 30 of the treatment. Similarly, administration of camel milk to diabetic rats presented a significant (p < 0.05) reduction in blood glucose levels from 565.5 ± 41.44 mg/dL on day 0 to 211.8 ± 19.82 mg/dL on day 30. Additionally, the combined administration of camel milk + insulin to diabetic rats significantly reduced the glucose levels from 524 ± 111.14 mg/dL on day 0 to 236 ± 46.15 mg/dL on day 30 of the treatment (Figure 3).
Figure 3.
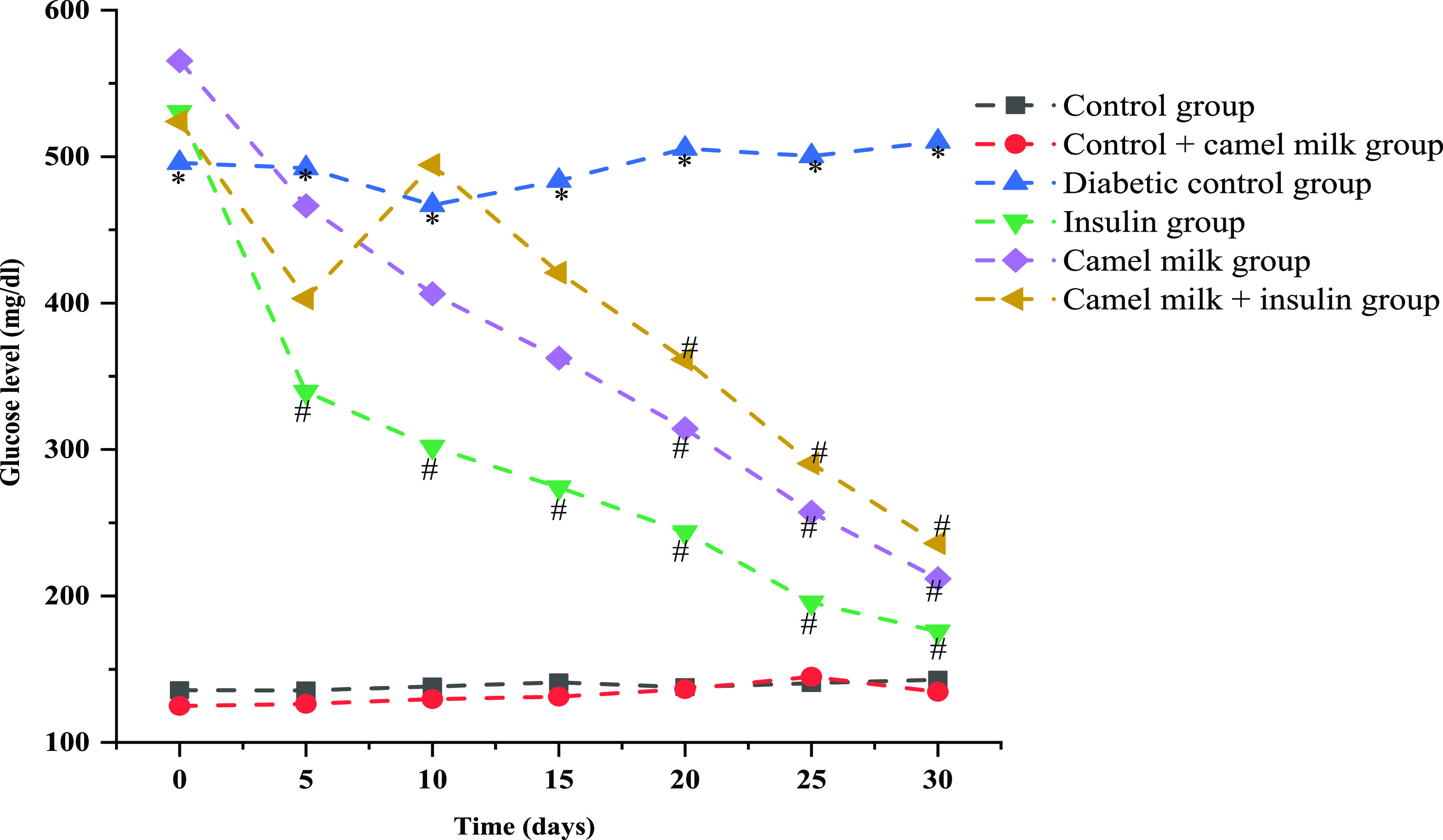
Effect of camel milk and insulin solely and in combination on blood glucose levels (mg/dL) in different experimental groups during the treatment period. Data are mean ± SD; N = 6; significant at 5% for ANOVA. Here, * represents significance in comparison to control, and # represents significance in comparison to diabetic control (P value represented with * /# ≤0.05).
Oral Glucose Tolerance Test (OGTT)
After treatment (30 days) with camel milk and insulin, rats’ responses were different for OGTT. The control group presented a fasting blood glucose level of 123.3 ± 7.33 mg/dL, which increased to 153 ± 8.78 mg/dL in the first 30 min of OGTT. Following that, the blood glucose levels in the control group presented a pattern of a gradual decrease as 153.3 ± 10.80, 133 ± 5.96, and 123.1 ± 7.78 at 60, 90, and 120 min, respectively. In comparison, the diabetic group presented a lower tolerance to oral glucose as the fasting blood glucose levels were observed to be 578 ± 14.73 mg/dL, which increased to 596.5 ± 7.38 mg/dL post 30 min of oral glucose administration. Consequently, the blood glucose levels of diabetic groups were observed to be 591.5 ± 8.44, 571.8 ± 11.79, and 533.3 ± 13.43 mg/dL at 60, 90, and 120 min post oral glucose administration. The insulin-treated diabetic rats presented an enhanced tolerance to oral glucose over the diabetic control rats, as the blood glucose levels were reported to be 178.6 ± 14.34, 201.1 ± 12.7, 186 ± 9.48, 165.5 ± 8.64, and 148.6 ± 6.56 mg/dL at time periods of 30, 60, 90, and 120 min, respectively, post oral glucose administration. Interestingly, the camel milk-treated diabetic rats also showed an enhanced tolerance to oral glucose over the diabetic control rats, as the blood glucose levels were reported to be 211.2 ± 10.30, 244.1 ± 12.41, 245.8 ± 15.6, 238 ± 15.5, and 205.6 ± 10.98 mg/dL at time period 30, 60, 90, and 120 min, respectively, post oral glucose administration. Moreover, the collective administration of insulin and camel milk to diabetic rats also produced a similar level of tolerance to oral glucose. The fasting blood glucose levels were observed to be 196.5 ± 43.16 mg/dL, which increased to 216.6 ± 46.03 mg/dL post 30 min of oral glucose administration. Following, 60, 90, and 120 min post oral glucose administration, the blood glucose levels showed a reducing pattern of 226.1 ± 49.08, 208.1 ± 47.37, and 197.8 ± 48.63 mg/dL, respectively (Figure 4).
Figure 4.
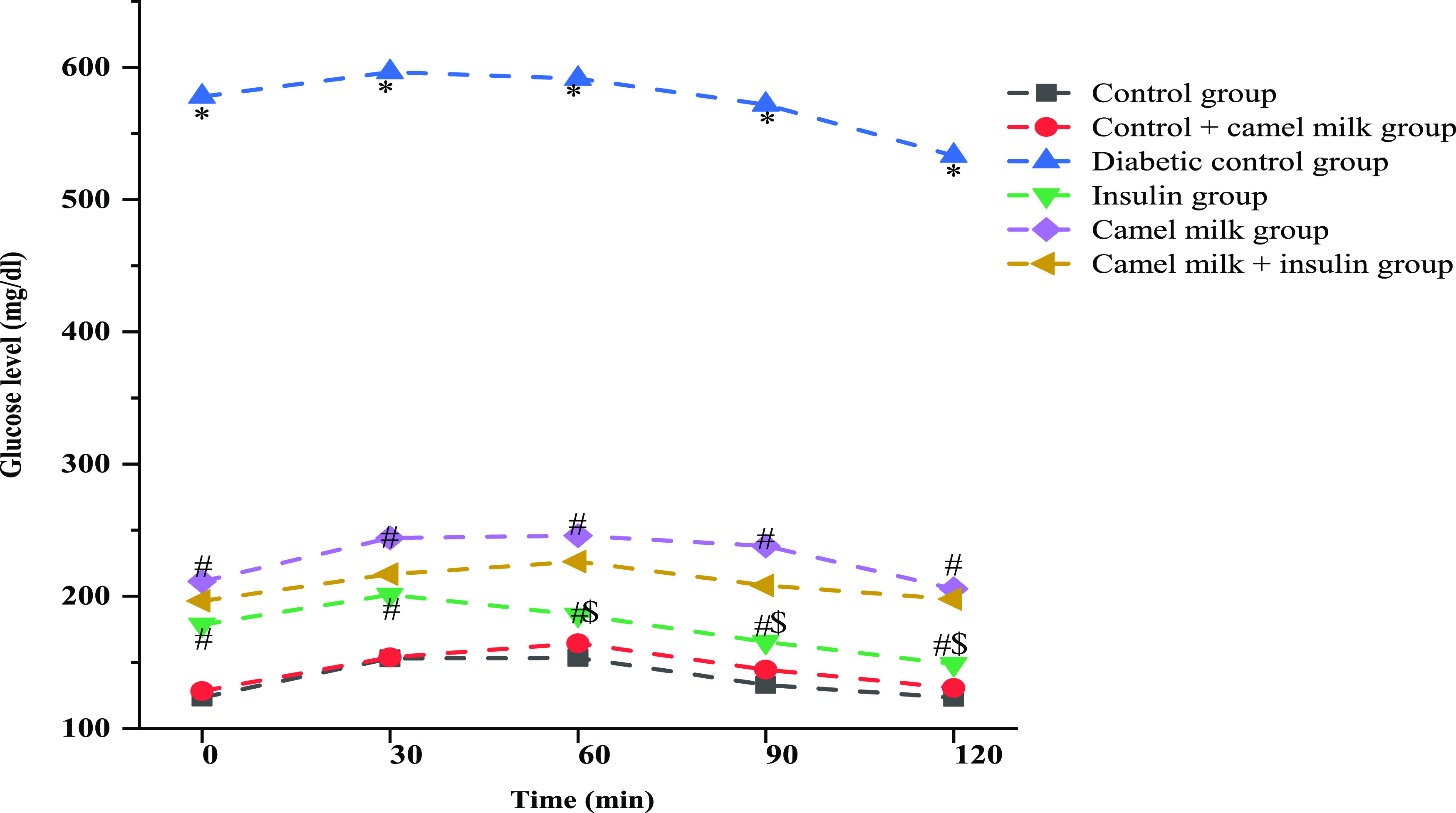
Graphical representation of blood glucose levels observed during OGTT to evaluate the effect of various treatments. Data are mean ± SD; N = 6; significant at 5% for ANOVA. Here, * represents significance in comparison to control, # represents significance in comparison to diabetic control, and $ represents significance in comparison to camel milk (P value represented with * # $ ≤ 0.05).
Hematological Variables in Different Treated Experimental Groups
Hemoglobin, RBCs, MCH, MCV, MCHC, PCV, lymphocytes, platelets, and leukocytes were significantly reduced in the STZ-induced diabetic control group. The insulin and camel milk groups showed a remarkable increase in all these hematological indices, but the increase in the number of platelets was not significant. The camel milk- and insulin-treated group revealed a significant increase in these parameters, especially in platelets, compared to other solely treated groups (Table 1).
Table 1. Different Hematological Indices Observed As Per Treatment Groups during the Studya.
| parameters | control group (I) | control + camel milk group (II) | diabetic control group (III) | insulin group (IV) | camel milk group (V) | camel milk + insulin group (VI) |
|---|---|---|---|---|---|---|
| hemoglobin (Hb) g/dL | 14.9 ± 0.90 | 15.0 ± 0.84 | 10.6 ± 0.58b | 14.4 ± 0.57c | 14.6 ± 0.99c | 14.0 ± 1.19c |
| total leukocyte count (TLC)/cumm | 12850 ± 446.09 | 10566.6 ± 1665.73b | 5550 ± 766.15b | 9766.6 ± 1481.44c | 10016.6 ±1302.94c | 9450 ± 589.06c |
| neutrophil (%) | 22.1 ± 1.47 | 20.3 ± 1.03 | 17.1 ± 2.31b | 26.6 ± 4.80c | 25 ± 0.894c | 24 ± 2.09c |
| lymphocytes (%) | 74.5 ± 5.00 | 74.8 ± 1.72 | 65 ± 1.41b | 71.6 ± 3.82c | 74.1 ± 2.85c | 72.6 ± 3.38c |
| eosinophil (%) | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1.3 ± 0.51 |
| monocyte (%) | 1 ± 0 | 1.3 ± 0.51 | 1.1 ± 0.40 | 1.6 ± 0.51 | 1 ± 0 | 1.8 ± 0.98 |
| basophil (%) | 0 | 0 | 0 | 0 | 0 | 0.1 ± 0.40 |
| RBC count (millions/cmm) | 8.9 ± 0.544 | 8.1 ± 0.96 | 5.5 ± 0.79b | 8.4 ± 0.76c | 8.4 ± 0.66c | 8.1 ± 0.73c |
| PCV (%) | 54.5 ± 1.44 | 50.0 ± 1.65 | 35.8 ± 2.33b | 53.9 ± 3.62c | 53.5 ± 2.58c | 52.1 ± 2.66c |
| MCV (fl.) | 56.9 ± 1.49 | 54.8 ± 3.96 | 42.4 ± 4.85b | 62.5 ± 4.86c | 56.5 ± 1.84c | 52.2 ± 1.54c |
| MCH pictogram | 20.5 ± 1.39 | 18.9 ± 1.38 | 15.5 ± 0.59b | 18.1 ± 0.95c | 18.3 ± 1.23c | 18.3 ± 1.02c |
| MCHC (gm/dL) | 39.3 ± 2.60 | 40.1 ± 4.59 | 23.0 ± 1.55b | 36.5 ± 3.36c | 36.6 ± 3.61c | 35.5 ± 3.16c |
| platelet count (lakh/cmm) | 6.0 ± 2.12 | 5.41 ± 0.77 | 3.1 ± 1.06b | 3.6 ± 0.84 | 3.3 ± 0.99 | 8.7 ± 0.66c,d |
Data are presented as mean ± SD; N = 6. Abbreviations: RBC = red blood cells; PCV = packed cell volume; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; values are significant at 5% for ANOVA.
Represents significance in comparison to control.
Represents significance in comparison to diabetic control.
Represents significance in comparison to camel milk (P value represented with * # $ < 0.05).
Histological Examination
Liver
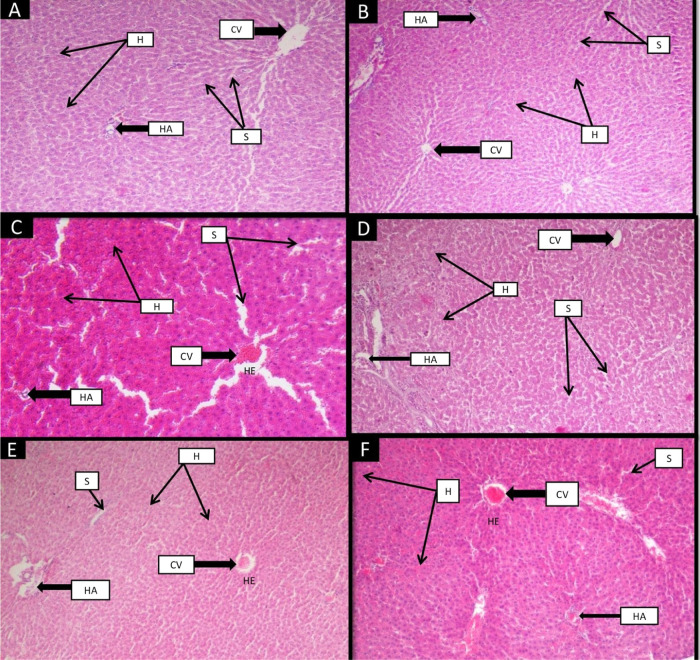
Histological findings of the liver of control rats showed a normal morphological structure. The proper radial arrangement of hepatocytes with a prominent nucleus was observed around the central vein (Figure 5). Hepatic arteries well-spaced with the adequately arranged sinusoidal area were seen in the control liver. Group II (control + camel milk), which had received camel milk, displayed the same pattern as group I (control group). STZ-treated group III (diabetic group) demonstrated damaged hepatocytes with inflammation and hemorrhage in the central vein and the hepatic artery with wide sinusoidal spaces. The pattern of the hepatocyte arrangement and central vein structure improved in group IV (insulin group). As with group IV (insulin), group V (camel milk) considerably improved the change; however, there was a small central hemorrhage. Group VI (camel milk + insulin) attenuates damage to the central vein, hepatocytes, hepatic artery, and sinusoidal dilation. Still, significant hemorrhage was observed in group VI (camel milk + insulin) compared to other treated groups (groups IV and V).
Figure 5.
Effect of camel milk and insulin on the histological structure of the liver of albino Wistar rats (original magnification 10×). (A) Group I (control): H&E-stained liver section showing the normal radial arrangement of hepatocytes around the central vein (CV) along with normal sinusoidal (S) spaces. The arrangement of hepatic plates (H) with the hepatic artery (HA) was visible. (B) Group II (control + camel milk): The liver section of this group shows the normal arrangement of hepatic plates (H) with the central vein (CV). The structure of the hepatic artery (HA) and spaces of the sinusoidal was normal as in the control group. (C) Group III (diabetic control): STZ-induced diabetic group showing radial disarrangement of hepatocytes and hemorrhage (HE) around the central vein (CV). Inflammation and distorted arrangement of hepatic plates (H) and destructed hepatic artery (HA) along with wide sinusoidal (S) spaces were depicted in this group. (D) Group IV (insulin): Showed the improved arrangement of hepatocytes without hemorrhage around the central vein (CV). Inflammation in hepatic plates (H) was reduced, and sinusoidal (S) spaces were also decreased in this group. (E) Group V (camel milk): Showed a slight improvement in hemorrhage (HE) and radial arrangement of hepatocytes around the central vein (CV). A significant reduction in inflammation of hepatic plates (H) and sinusoidal (S) spaces was visible. Inflammation in a hepatic artery (HA) was slightly improved. (F) Group VI (camel milk + insulin): Depicted slightly reduced hemorrhage (HE) in the central vein (CV) and the hepatic artery (HA) compared to the diabetic group. Improved arrangement of hepatic plates (H) and inflammation were also decreased. Sinusoidal (S) spaces were also declining.
Kidney
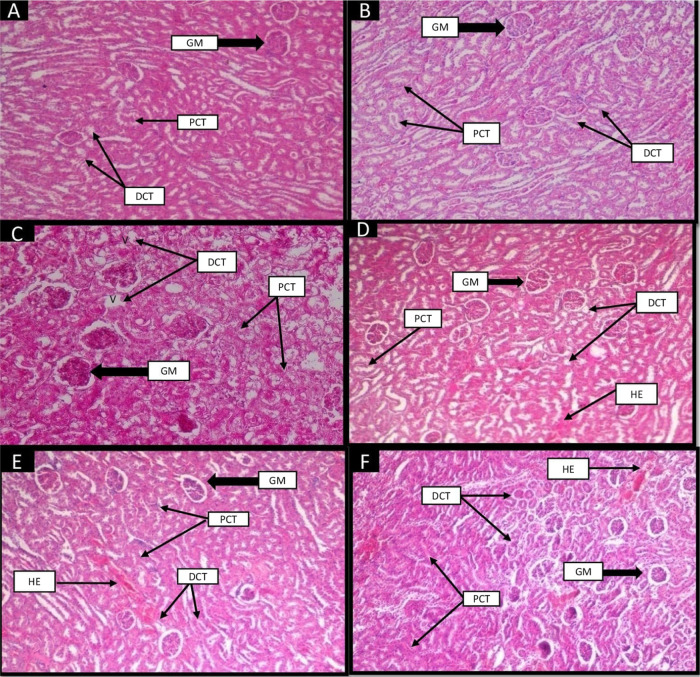
Morphological attributes of the kidney section were normal in group I (control), like prominent glomeruli, proximal convoluted tubules (PCT), and distal convoluted tubules (DCT, Figure 6). Group II (control + camel milk) showed the same normal structure as group I (control group). Group III (diabetic control) disrupted glomeruli, damaged PCTs and DCT, vacuolization, and hemorrhage were observed. Group IV (insulin) reversed the damages shown in diabetic rats with the presence of slight hemorrhage. Group V (camel milk) showed improvisation in the structure of glomeruli, PCT, and DCT with a decrease in hemorrhage. Group VI (camel milk + insulin) showed a slight improvement in the damaged structure compared to the other treated groups.
Figure 6.
Effect of camel milk and insulin on the histological structure of the kidney of albino Wistar rats (original magnification 10×). (A) Group I (control): H&E-stained section of the kidney showed the normal structure of glomerulus (GM), proximal convoluted tubules (PCT), and distal convoluted tubules (DCT). (B) Group II (control + camel milk): showed the normal structure of glomerulus, PCT, and DCT as in the control group. (C) Group III (diabetic control): STZ-induced diabetic group showed tubular (PCT and DCT) degeneration, vacuolization (V), and disruption of the glomerulus (GM). (D) Group IV (insulin): Reverted the damage of glomerulus (GM), improvisation in the structures of PCT and DCT. Vacuolization and tubular degeneration improved in this group. Slightly hemorrhage appeared in tubules. (E) Group V (camel milk): Depicted improvement in structures of the glomerulus, PCT, and DCT. Hemorrhage and slight vacuolization appeared in this group. (F) Group VI (camel milk + insulin): Showed slightly reverted structure of glomerulus, PCT, and DCT. Hemorrhages were present in tubules.
Pancreas
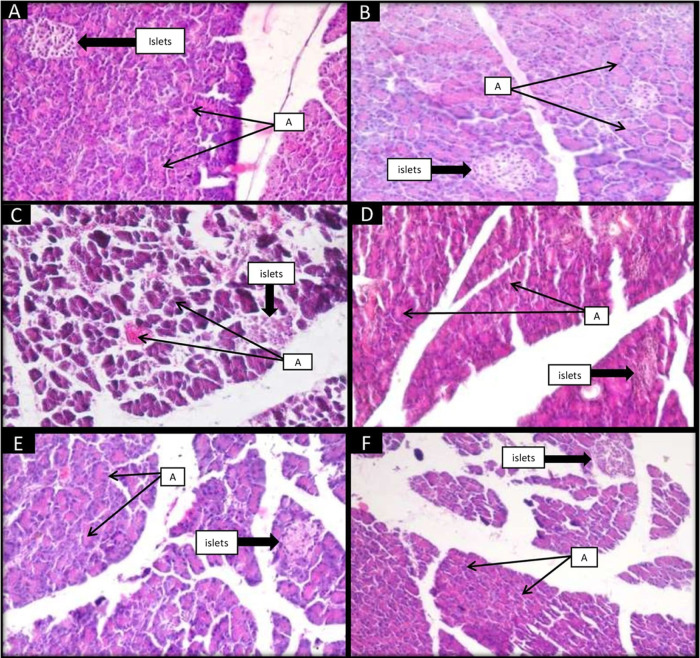
Group I (control) exhibited normal structures of the pancreas with intact islets and acini (Figure 7). Group II (control + camel milk) showed normal morphology as observed in group I (control). Group III (diabetic control) showed destructed islets with damaged acini. Congestion and hemorrhage were present in acini. All these damages reverted in treated group IV (insulin) and group V (camel milk). In group VI (camel milk+ insulin), these changes were also improved significantly as in the separately treated group.
Figure 7.
Effect of camel milk and insulin on histology of (H&E-stained) pancreas of albino Wistar rats (original magnification 20×). (A) Group I (control): Showed the normal architecture of islets. Normal acini (A) surrounded by a nucleus, which contains zymogen granules. (B) Group II (control + camel milk): showed the intactness of islets as in the control group with the normal acini. (C) Group III (diabetic control): STZ-induced diabetic group showed many degenerative changes like disrupted islets and acini, cytoplasmic vacuolations, hemorrhage in acini, and extravasation. (D) Group IV (insulin): showed an improved structure of islets, decreased cytoplasmic vacuolations, slight congestion of blood vessels, and reverted damage of acini. (E) Group V (camel milk): depicted that the intactness of islets was improved, cytoplasmic vacuolations were reduced, and acini were improved with slight hemorrhage. (F) Group VI (camel milk + insulin): showed a slight improvement in the intactness of islets, restoration of the normal architecture of acini with the nucleus, and reduction in cytoplasmic vacuolation.
Discussion
This study compares the efficacy of camel milk and insulin when used individually or in combination to treat STZ-induced diabetic rats. Diabetic rats had considerably lower body weight and higher glycemic levels. However, the insulin and camel milk groups both exhibited more favorable effects on these parameters individually than they did together in the combination group (camel milk + insulin). Camel milk and insulin improved the morphological features of the liver, kidney, and pancreas, which were well confirmed by previous studies.43,44 Body weight was monitored in this study to infer the rat’s health state because it is one of the most widely studied indicators to study the development and growth of laboratory rats.45 During one week of the experimental study, the body weight of the diabetic rats decreased because of a persistent hyperglycemia condition that was observed in multiple prior studies.39,44,46 Loss of insulin function, which controls the anabolic process, is the second most common reason for weight loss in diabetic control rats.47,48 Through activating GLUT-2, insulin promotes the absorption of fatty acids and amino acids as well as glucose uptake by the liver and muscles. The glucose uptake mechanism is diminished due to the interruption of insulin secretion in tissues.49 Long-term low-energy generation in tissues promotes proteolysis and lipolysis, depletes triglyceride reserves, and results in weight loss.50 In this study, camel milk and insulin were administered individually or in combination to treat STZ-induced diabetic rats. The control diabetic rats showed a considerably average body weight loss of 21% compared to the control healthy rats that gained 23% average body weight over the study period (Figure 2). Camel milk contains an insulin-like protein that resists stomach acid and upholds glucose homeostasis (58.67 IU/L).30,51 Camel milk has a lot of zinc, encouraging the pancreas to secrete insulin.36 According to previous findings, diabetic rats treated with camel milk showed improvement in their body weight.39,43 As we saw in other treatment groups, the combined group also maintained body weight. According to previous studies by Zafar et al.52 and Etuk EU,53 the pancreatic β cells are directly destroyed by STZ, which causes insufficient insulin secretion and higher glucose levels in STZ-induced diabetic rats. In this investigation, the diabetic control group showed signs of hyperglycemia compared to the control group. On the other hand, it has been noted that hyperglycemia can also cause pancreatic β-cell malfunction, decreased β-cell mass, and insulin shortage.54 But since the findings indicated that STZ had partially damaged the pancreas, it was anticipated that the body would not be able to produce enough endogenous insulin to control the high glucose levels that had been noticed. It has been demonstrated that exogenous insulin is more effective at lowering blood glucose levels in severely diabetic rats (glucose > 400 mg/dL).55 In the present study, the administration of insulin to diabetic rats not only reduced the high blood glucose concentration by 3 times over the study period (Figure 3) but also resulted in net body weight gain by a factor of 43% (Figure 2). More of interest, administration of camel milk to diabetic rats showed a 2.6-fold reduction in blood glucose concentration (Figure 3) while a net gain of 70% average body weight (Figure 2). Moreover, the collective administration of insulin and camel milk to the diabetic rats resulted in a 60% average body weight gain while a 2.2-fold reduction in blood glucose concentration. In diabetic mice, camel milk statistically reduced blood glucose and HbA1c levels.56 Several previous findings revealed the antidiabetic property of camel milk due to the presence of an insulin-like protein.30,39,43,44,51 According to prior studies, the majority of diabetic complications are triggered by oxidative stress.57,58 Hematological indices affected by oxidative stress include hemoglobin glycosylation, a decrease in RBC count, and a rise in TLC count.12 Due to the RBC membrane’s glycosylation in diabetic control rats, which is a direct result of hyperglycemia, the RBC count fell.59 In the diabetic control group, several RBC indices such as MCV, PCV, MCH, MCHC, and HB decreased. Camel milk with an exogenous dosage of insulin reduces oxidative stress and elevates hematological markers. Vitamin C and zinc, two antioxidants, in camel milk inhibit diabetes-induced hemolysis by triggering the body’s natural antioxidant defenses.30,60 According to earlier investigations, the total leukocyte count was considerably lower in the diabetic group than that in the control group.15 Supplemental insulin boosted lymphocyte and TLC counts to levels that are close to the insulin group. Leukocyte and lymphocyte counts were significantly restored in the camel milk group. This effect of camel milk may be produced by various immune-boosting substances like zinc, vitamin C, and vitamin E.43 Due to internal and exterior blood loss caused by high blood glucose levels, the platelet count dropped in diabetic rats.13,16 Insulin improves platelet count and controls glucose homeostasis in the insulin group. Due to the insulin-like protein in camel milk, the platelet count also increases. Compared to other treated groups, the combined group demonstrated a considerable increase in platelet count. In the present study, histological changes observed in the liver, kidney, and pancreas in STZ-induced diabetic rats were similar to previous findings of Usman et al.43 and Ahmed et al.61 Apoptosis in hepatic cells, hemorrhage in the central vein, congested sinusoidal spaces, and congestion in the central vein were seen in the liver segment of the diabetic group. The insulin treated and camel milk treated groups showed improvements in all these changes. These structural alterations were also improved by the combined group, although the hemorrhage in the central vein and hepatic plates did not considerably improve. The kidneys of the diabetic group of rats exhibited severe histological alterations, including renal glomeruli degeneration, mesangial hyperplasia, glomerular sclerosis and desquamation of the tubular epithelium, and vacuolar degeneration in addition to significant inflammatory cell infiltration.62 Likewise, in the present investigation, the histological results of the diabetic kidney’s sections showed morphological alterations to the glomerulus, PCT, and DCT structures. Treatment with camel milk and its exosomes alone or in combination (CM + EXO) resulted in a significant improvement in the histology of the kidney, with the absence of glomerular sclerosis.62 The present histological study of the insulin-treated group also showed remarkable improvement in the structure of the kidney. In the camel milk group also, these abnormalities were markedly reversed. These structures were also improved by the combined group, but not as significantly as in the separately treated groups. The diabetic group showed obvious degenerative alterations and a reduction in the size of islets. One prominent aspect was the loss of many islets of Langerhans cells. Marked cytoplasmic vacuolations were observed in the study of Faried et al.63 Similarly, in the present experiment, pancreatic islets and other types of acini were completely lost and severely damaged in the diabetic group. In insulin, camel milk, and the combined group, all of these morphological alterations were reversed.
Conclusions
The present study aimed to investigate and compare the efficacy of camel milk and insulin in streptozotocin-induced diabetic albino rats, with the objective of assessing whether camel milk could be a viable alternative or complementary therapy for diabetes management. The current observations are consistent with the established role of insulin as the primary treatment for diabetes due to its direct regulation of glucose metabolism. Furthermore, the study revealed a positive impact of camel milk on overall metabolic function and nutrient utilization in diabetic animals. Particularly, camel milk exhibited a positive recovery effect on pancreatic tissue in diabetic subjects, potentially surpassing the restorative capacity of insulin. The findings of this study have provided valuable insights into the potential benefits of camel milk and its role in glycemic control. In conclusion, while insulin remains the established gold standard for diabetes management, camel milk holds potential as an adjunct therapy or alternative treatment option for individuals with diabetes. Further investigation is warranted to elucidate the precise mechanisms of action and identify the bioactive components responsible for the observed beneficial effects of camel milk. Additionally, clinical trials are necessary to evaluate the safety, efficacy, and long-term effects of camel milk in human subjects with diabetes.
Acknowledgments
The authors are highly thankful to the Department of Zoology for providing facilities to carry out this research work.
The authors declare no competing financial interest.
Notes
The protocols were approved by the Institutional Animal Ethical Committee (IAEC) of M.D.U., Rohtak.
References
- Johar D.; Maher A.; Aboelmagd O.; Hammad A.; Morsi M.; Warda H.-F.; Awad H.-I.; Mohamed T.-A.; Zaky S. Whole-food phytochemicals antioxidative potential in alloxan-diabetic rats. Toxicol. Rep. 2018, 5, 240–250. 10.1016/j.toxrep.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Saeedi P.; Karuranga S.; Pinkepank M.; Ogurtsova K.; Duncan B.-B.; Magliano D. -J.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109–119. 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Diabetes; World Health Organization, 2023. [Google Scholar]
- Gregory G. -A.; Robinson T. -I.; Linklater S. -E.; Wang F.; Colagiuri S.; de Beaufort C.; Ogle G. -D.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 2022, 10, 741–760. 10.1016/S2213-8587(22)00218-2. [DOI] [PubMed] [Google Scholar]
- Papaspyros N.-S.The History of Diabetes. In The History of Diabetes Mellitus; Verlag G. T., Ed.; Thieme: Stuttgart, G, 1964; p 4. [Google Scholar]
- Hajam Y. A.; Rai S.; Ghosh H.; Basheer M. Combined administration of exogenous melatonin and insulin ameliorates streptozotocin induced toxic alteration on hematological parameters in diabetic male Wistar rats. Toxicol. Rep. 2020, 7, 353–359. 10.1016/j.toxrep.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade K.; Wu K.-D.; Hwu C.-M.; Ting C.-T.; Pei D.; Pesich R.; Hebert J.; Chen Y.-D.-I.; Pratt R.; Olshen R.; Masaki K. Genetic variation in the human urea transporter-2 is associated with variation in blood pressure. Hum. Mol. Genet. 2001, 10, 2157–2164. 10.1093/hmg/10.19.2157. [DOI] [PubMed] [Google Scholar]
- Lindstrom J.; Ilanne-Parikka P.; Peltonen M.; Aunola S.; Eriksson J.-G.; Hemio K.; Ham ainen H.; arkonen P.; Keinanen Kiukaanniemi S.; Laakso M.; Louheranta A.; Mannelin M.; Paturi M.; Sundvall J.; Valle T. T.; Uusitupa M.; Tuomilehto J. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- Yang H.; Villani R.-M.; Wang H.; Simpson M.-J.; Roberts M.-S.; Tang M. The Role of Cellular Reactive Oxygen Species in Cancer Chemotherapy. J. Exp. Clin. Cancer. Res. 2018, 37, 1 10.1186/s13046-017-0664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieger K.; Schiavone S.; Miller F.; Krause K.-H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, 13659. 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- Kurutas E. B. The Importance of Antioxidants Which Play the Role in Cellular Response Against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam Y.-A.; Rai S.; Ghosh H.; Basheer M. Combined administration of exogenous melatonin and insulin ameliorates streptozotocin induced toxic alteration on hematological parameters in diabetic male Wistar rats. Toxicol. Rep. 2020, 7, 353–359. 10.1016/j.toxrep.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyedemi S. O.; Yakubu M.-T.; Afolayan A.-J. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin induced diabetic rats. J. Med. Plant Res. 2011, 5, 119–125. [Google Scholar]
- Stookey J.-D.; Burg M.; Sellmeyer D.-E.; Greenleaf J.-E.; Arieff A.; Van Hove L.; Gardner C.; King J.-C. A proposed method for assessing plasma hypertonicity in vivo. Eur. J. Clin. Nutr. 2007, 61, 143–146. 10.1038/sj.ejcn.1602481. [DOI] [PubMed] [Google Scholar]
- Hillson R. Herbs and diabetes. Pract. Diabetes. 2019, 36, 159–160. 10.1002/pdi.2236. [DOI] [Google Scholar]
- Jarald E.; Joshi S. B.; Jain D. Diabetes and herbal medicines. Iran. J. Pharmacol. Ther. 2008, 20 (7), 97–106. [Google Scholar]
- Mortensen H.-B.; Hougaard P. Hvidøre Study Group on Childhood Diabetes. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. Diabetes Care 1997, 20, 714–720. 10.2337/diacare.20.5.714. [DOI] [PubMed] [Google Scholar]
- Holl R.-W.; Swift P.-G.; Mortensen H.-B.; Lynggaard H.; Hougaard P.; Aanstoot H. J.; Chiarelli F.; Daneman D.; Danne T.; Dorchy H.; Garandeau P.; et al. Insulin injection regimens and metabolic control in an international survey of adolescents with type 1 diabetes over 3 years: results from the Hvidore study group. Eur. J. Pediatr. 2003, 162, 22–29. 10.1007/s00431-002-1037-2. [DOI] [PubMed] [Google Scholar]
- Okamoto M.-M.; Anhê G.-F.; Sabino-Silva R.; Ferreira Marques M.-F.-D.-S.; Freitas H.-S.; Mori R.-C.-T.; Melo K.-F.-S.; Machado U. F. Intensive insulin treatment induces insulin resistance in diabetic rats by impairing glucose metabolism-related mechanisms in muscle and liver. J. Endocrinol. 2011, 211, 55. 10.1530/JOE-11-0105. [DOI] [PubMed] [Google Scholar]
- Hays N.-P.; Galassetti P.-R.; Coker R.-H. Prevention and treatment of type 2 diabetes: current role of lifestyle, natural product, and pharmacological interventions. Pharmacol. Ther. 2008, 118, 181–191. 10.1016/j.pharmthera.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malseed R.-T.Springhouse Nurses Drug Guide, 6th ed.; Lippincott Williams: Philadelphia, 2005. [Google Scholar]
- Yeung A.-W.-K.; Tzvetkov N. T.; Durazzo A.; Lucarini M.; Souto E. B.; Santini A.; Gan R.-Y.; Jozwik A.; Grzybek W.; Horbańczuk J.-O.; Mocan A.; et al. Natural products in diabetes research: Quantitative literature analysis. Nat. Prod. Res. 2021, 35, 5813–5827. 10.1080/14786419.2020.1821019. [DOI] [PubMed] [Google Scholar]
- Gori M.; Campbell R.-K. Natural products and diabetes treatment. Diabetes Educ. 1998, 24, 201–208. 10.1177/014572179802400210. [DOI] [PubMed] [Google Scholar]
- Sharma R.; Rajput Y.-S. Therapeutic potential of milk and milk products. Ind. Dairy. 2006, 58, 70. [Google Scholar]
- Ali A. -A.; Al-Attar S. -A. -A. The activity of camel milk to treated immunity changes that induced by Giardia lamblia in male rats. Anna. Trop. Med. Health. 2020, 23, 120–124. 10.36295/ASRO.2020.23415. [DOI] [Google Scholar]
- Abdurahman A.; Food and nutritional sciences . Haemoglobin Concentration among Camel Milk and Cow Milk Consuming Pastoralist Communities of Somali Region, Eastern Ethiopia. MS Thesis; Addis Ababa University: Ethiopia, 2018. [Google Scholar]
- Yang J.; Dou Z.; Peng X.; Wang H.; Shen T.; Liu J.; Li J.; Gao Y. Transcriptomics and proteomics analyses of anti-cancer mechanisms of TR35–An active fraction from Xinjiang Bactrian camel milk in esophageal carcinoma cell. Clin. Nut. 2019, 38, 2349–2359. 10.1016/j.clnu.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Gizachew A.; Teha J.; Birhanu T.; Nekemte E. Review on medicinal and nutritional values of camel milk. Nat. Sci. 2014, 12, 35–41. [Google Scholar]
- Hammam A. R. A. Compositional and therapeutic properties of camel milk: A review. Emir. J. Food. Agri. 2019, 31, 148–152. 10.9755/ejfa.2019.v31.i3.1919. [DOI] [Google Scholar]
- Mullaicharam A.-R. A review on medicinal properties of camel milk. World. J. Pharm. Sci. 2014, 237–242. [Google Scholar]
- Agrawal R. -P.; Saran S.; Sharma P.; Gupta R. -P.; Kochar D. -K.; Sahani M. S. Effect of camel milk on residual β-cell function in recent onset type 1 diabetes. Diabetes Res. Clin. Pract. 2007, 77, 494–495. 10.1016/j.diabres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Limon A.; Gallegos-Perez J. -L.; Reyes-Ruiz J.-M.; Aljohi M. -A.; Alshanqeeti A. -S.; Miledi R. The endogenous GABA bioactivity of camel, bovine, goat and human milks. Food. Chem. 2014, 145, 481–487. 10.1016/j.foodchem.2013.08.058. [DOI] [PubMed] [Google Scholar]
- Faye B.; Konuspayeva G.; Bengoumi M. Vitamins of Camel Milk: A Comprehensive Review. J. Camelid Sci. 2019, 12, 17–32. [Google Scholar]
- Khan M.-Z.; Xiao J.; Ma Y.; Ma J.; Liu S.; Khan A.; et al. Research Development on Anti-Microbial and Antioxidant Properties of Camel Milk and Its Role as an Anti-Cancer and Anti-Hepatitis Agent. Antioxid. 2021, 10, 788. 10.3390/antiox10050788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran P.; Ejtahed H.-S.; Angoorani P.; Eslami F.; Azizi F. Camel milk has beneficial effects on diabetes mellitus: A systematic review. Int. J. Endocrinol. Metab. 2017, In press, 15. 10.5812/ijem.42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaia M.-A.; Hablas M.-A.; Abdel-Rahman K.-M.; El-Mougy S.-A. Milk composition of majaheim, wadah and hamra camels in Saudi Arabia. Food chem. 1995, 52, 115–122. 10.1016/0308-8146(94)P4189-M. [DOI] [Google Scholar]
- Agrawal R.-P.; Tantia P.; Jain S.; Agrawal R.; Agrawal V. Camel milk: a possible boon for type 1 diabetic patients. Cell. Mol. Biol. 2013, 59, 99–107. [PubMed] [Google Scholar]
- El-Sayed M.-K.; Al-Shoeibi Z.-Y.; Abd El-Ghany A. A.; Atef Z.-A. Effects of camel’s milk as a vehicle for insulin on glycaemic control and lipid profile in type 1 diabetics. Am. J. Biochem. Biotechnol. 2011, 7, 179–e89. 10.3844/ajbbsp.2011.179.189. [DOI] [Google Scholar]
- Meena S.; Rajput Y. S.; Pandey A.-K.; Sharma R.; Singh R. Camel milk ameliorates hyperglycaemia and oxidative damage in type-1 diabetic experimental rats. J. Dairy Res. 2016, 83, 412–419. 10.1017/S002202991600042X. [DOI] [PubMed] [Google Scholar]
- Deeds M.-C.; Anderson J.-M.; Armstrong A.-S.; Gastineau D.-A.; Hiddinga H.-J.; Jahangir A.; Eberhardt N.-L.; Kudva Y.-C. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab. Anim. 2011, 45, 131–140. 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A.; Alzohairy M. A.; Mohieldein A.-H. Antidiabetic effects of camel milk in streptozotocin-induced diabetic rats. Am. J. Biochem. Mol. Biol. 2012, 3, 151–158. 10.3923/ajbmb.2013.151.158. [DOI] [Google Scholar]
- Bancroft J. D.; Layton C.; Suvarna S.-K.. Bancroft’s Theory and Practice of Histological Techniques; Churchill Livingstone Elsevier, 2013. [Google Scholar]
- Usman M.; Ali M.-Z.; Qureshi A.-S.; Ateeq M.-K.; Nisa F. U. Short term effect of dose dependent camel milk in Alloxan induced diabetes in female albino rats. J. Anim. Plant Sci. 2018, 28. [Google Scholar]
- Korish A.-A.; Gader A.-G.-M.-A.; Alhaider A.-A. Comparison of the hypoglycemic and antithrombotic (anticoagulant) actions of whole bovine and camel milk in streptozotocin-induced diabetes mellitus in rats. J. Dairy Sci. 2020, 103, 30–41. 10.3168/jds.2019-16606. [DOI] [PubMed] [Google Scholar]
- Sengupta P. A scientific review of age determination for a laboratory rat: how old is it in comparison with human age. Biomed. Int. 2011, 2, 81–89. [Google Scholar]
- Ng A.-X.-H.; Ton S.-H.; Kadir K.-A. Low-dose insulin treatment ameliorates glucose metabolism in type 1 diabetic rats. J. Diabetes Metab. 2016, 7, 635 10.4172/2155-6156.1000635. [DOI] [Google Scholar]
- King A.-J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova L.; Smirnova I.-V.; Rawal S.; Dotson A.-L.; Benedict S.-H.; Stehno-Bittel L. Variations in rodent models of type 1 diabetes: islet morphology. J. Diabetes Res. 2013, 2013, 1–13. 10.1155/2013/965832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn P.-F.; Dice J.-F. Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22, 830–844. 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Eiselein L.; Schwartz H.-J.; Rutledge J.-C. The challenge of type 1 diabetes mellitus. ILAR journal 2004, 45, 231–236. 10.1093/ilar.45.3.231. [DOI] [PubMed] [Google Scholar]
- Agrawal R.-P.; Beniwal R.; Sharma S.; Kochar D.-K.; Tuteja F.-C.; Ghorui S.-K.; Sahani M.-S. Effect of raw camel milk in type 1 diabetic patients: 1-year randomised study. J. Camel Pract. 2005, 12, 27. [DOI] [PubMed] [Google Scholar]
- Zafar M.; Naeem-ul-Hassan Naqvi S. Effects of STZ-Induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: a comparative study. Int. J. Morphol. 2010, 28, 135–142. 10.4067/S0717-95022010000100019. [DOI] [Google Scholar]
- Etuk E.-U. Animal models for studying diabetes mellitus. Agric. Biol. J. N. Am. 2010, 1, 130–134. [Google Scholar]
- Nichols C.-G.; Remedi M.-S. The diabetic beta-cell: hyperstimulated vs hyperexcited. Diabetes Obes. Metab. 2012, 14 Suppl 3, 129–135. 10.1111/j.1463-1326.2012.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qinna N. -A.; Badwan A. -A. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des., Dev. Ther. 2015, 9, 2515. 10.2147/DDDT.S79885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain H.; Wattoo F. -H.; Wattoo M. -H. S.; Gulfraz M.; Masud T.; Shah I.; Ali S.; Alavi S. -E. Camel milk as an alternative treatment regimen for diabetes therapy. Food Sci. Nut. 2021, 9, 1347–1356. 10.1002/fsn3.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovics B.; Kotorman M.; Varga I.-S.; Hai D.-Q.; Varga C. Oxidative stress in experimental diabetes induced by streptozotocin. Acta. Physiol. Hung. 1997, 85, 29–38. [PubMed] [Google Scholar]
- Kavalalı G.; Tuncel H.; Goksel S.; Hatemi H.-H. Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J. Ethnopharmacol. 2003, 84, 241–245. 10.1016/S0378-8741(02)00315-X. [DOI] [PubMed] [Google Scholar]
- Abbas M.; Siddiqi M.-H.; Khan K.; Zahra K.; Naqvi A. u. N. Haematological evaluation of sodium fluoride toxicity in oryctolagus cunniculus. Toxicol. Rep. 2017, 4, 450–454. 10.1016/j.toxrep.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parivash R.; Najmeh K.; Sedigheh A.; Mahbubeh S. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2655–2661. 10.5897/AJPP11.480. [DOI] [Google Scholar]
- Ahmed D.; Kumar V.; Verma A.; Shukla G. -S.; Sharma M. Antidiabetic, antioxidant, antihyperlipidemic effect of extract of Euryale ferox salisb. with enhanced histopathology of pancreas, liver and kidney in streptozotocin induced diabetic rats. Springerplus 2015, 4, 315 10.1186/s40064-015-1059-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shaban A.-M.; Raslan M.; Qahl S.-H.; Elsayed K.; Abdelhameed M.-S.; Oyouni A.-A.-A.; Al-Amer O.-M.; Hammouda O.; El-Magd M.-A. Ameliorative Effects of Camel Milk and Its Exosomes on Diabetic Nephropathy in Rats. Membranes 2022, 12, 1060. 10.3390/membranes12111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faried M. -A.; El-Mehi A. -E. -S. Aqueous anise extract alleviated the pancreatic changes in streptozotocin-induced diabetic rat model via modulation of hyperglycaemia, oxidative stress, apoptosis and autophagy: a biochemical, histological and immunohistochemical study. Folia Morphol. 2020, 79, 489–502. 10.5603/FM.a2019.0117. [DOI] [PubMed] [Google Scholar]