Abstract
The latitudinal diversity gradient (LDG) describes the pattern of increasing numbers of species from the poles to the equator. Although recognized for over 200 years, the mechanisms responsible for the largest-scale and longest-known pattern in macroecology are still actively debated. I argue here that any explanation for the LDG must invoke differential rates of speciation, extinction, extirpation, or dispersal. These processes themselves may be governed by numerous abiotic or biotic factors. Hypotheses that claim not to invoke differential rates, such as ‘age and area’ or ‘time for diversification’, eschew focus from rate variation that is assumed by these explanations. There is still significant uncertainty in how rates of speciation, extinction, extirpation, and dispersal have varied regionally over Earth history. However, to better understand the development of LDGs, we need to better constrain this variation. Only then will the drivers of such rate variation – be they abiotic or biotic in nature – become clearer.
Keywords: age and area hypothesis, tropics as older, tropics as stable, climate change, biodiversity gradient
The tropics teem with a diversity of life that dwindles toward the poles. This latitudinal gradient in species richness (LDG) has been studied intensively for over 200 years (1, 2). Despite concerted attention, significant debate still exists regarding the causal mechanisms behind a pattern that transcends clades, ecosystems, and continents (3).
Over 100 hypotheses have been proposed to explain the higher diversity observed at low versus high latitudes (3–11). Although explanations abound, here I contend that rate variation is inherent to all LDG hypotheses. That is, regional differences in species richness must be explained by one or more of the following four processes: speciation, extinction, local extirpation, or dispersal. These processes themselves may be controlled by a suite of abiotic and biotic factors that operate at different spatial and temporal scales (12), including spatiotemporal climate change (13–16), biotic interactions (17–20), and available resources and area (21–25).
Hypotheses that invoke differential rates have been traditionally classified as ‘historical’ hypotheses and are often contrasted with ‘ecological’ hypotheses that invoke constraints on the number of species that can occur together in any given location (8, 26). Differential rates, however, are implicated in both suites of hypotheses. For example, ecological hypotheses suppose that rates of speciation (incipient or not), extinction, extirpation, or dispersal change in association with the number of species in a region (27, 28). Rate variation is therefore inherent to both ecological and historical hypotheses, with the ‘ecological’ designation a reflection of a proposed mechanism that regulates this rate variation.
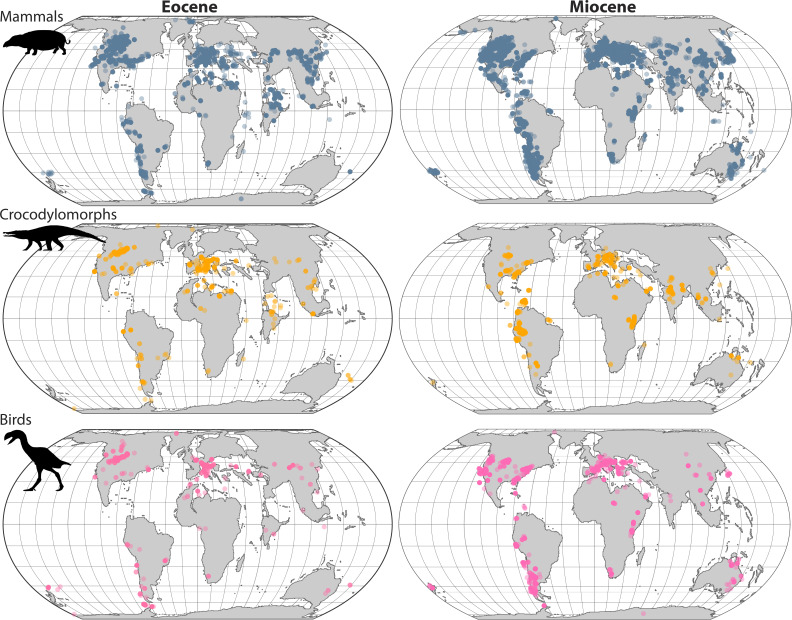
One hypothesis that purports not to invoke differential rates is the ‘time for speciation’ or ‘time for diversification’ hypothesis. Under this formulation, most clades originate at low latitudes and only later disperse to higher latitudes. The greater time that lineages spent equatorially would allow more diversity to accumulate there, even if diversification rates were similar across latitudes once these low-latitude lineages dispersed out of their ancestral home (6, 29–32). Rate variation, however, is still inherent to such an argument, even if somewhat fatuous: Diversification would have been higher at low latitudes simply due to its absence at higher latitudes. Whether the majority of clades have low-latitude origins is an open question that is both supported (33–35) and refuted (36–39) by empirical data. Regardless of where most groups originated, discussions around ‘time for diversification’ often implicitly assume that high-latitude regions were empty of life over much of Earth history. The fossil record, however, suggests these regions were never consistently devoid of life, even if most species were periodically eradicated due to large-scale climate perturbations (see below and Fig. 1).
Fig. 1.
Mammal, crocodylomorph, and bird occurrences during the Eocene (56 to 33.9 Ma) and Miocene (23.03 to 5.33 Ma), plotted on a Ypresian and Chattian paleogeography, respectively (40). Using two example time intervals, these maps show that clades were found at high latitudes millions of years ago. The disappearance of some of these groups from high-latitude regions points to broad-scale extinction, extirpation, or movement equatorward. Occurrences were rotated to their paleolatitudes and paleolongitudes in GPlates v.2.2.0 using Scotese’s PALEOMAP PaleoAtlas (41). Fossil distributional data were downloaded from the Paleobiology Database on 22 February 2023 for the class Aves, class Mammalia, and unranked clade Crocodylmorpha. Animal silhouettes are from PhyloPic: Moeritherium from T. Michael Keesey (CC0 1.0), Gastornis giganteus from Scott Hartman (CC BY 3.0), and Duerosuchus piscator from Armin Reindl (CC BY 4.0). Maps were constructed in R v.4.2.1 using the sf package (42) and ggplot2 (43).
The potential origin of many groups in tropical climates, in combination with strongly conserved physiological constraints, is often provided as a mechanism for maintaining LDGs (6, 30, 44–46). Conserved physiology, referred to as phylogenetic niche conservatism (47–49), would prohibit many species from dispersing out of low-latitude regions and would therefore retain higher diversity equatorially. Although physiological tolerances may be conserved within some lineages (50–52) and across some clades (40, 48, 53), fossil data suggest dispersal dynamics were variable, and more species may have dispersed from low to high latitudes than vice versa in many groups (54–58).
The ‘time for speciation’ hypothesis is sister to the ‘age and area’ hypothesis and often discussed concomitantly. The ‘age and area’ hypothesis posits the tropics were larger in extent earlier in the Cenozoic (59–61), which fuelled higher equatorial diversity (30, 61, 62). Like all LDG hypotheses, the ‘age and area’ hypothesis cannot extricate itself from rate variation. The larger historical area of the tropics is thought to have elevated richness by potentially increasing the probability of population isolation and thus speciation, or by decreasing rates of extinction by increasing population size and access to resources (63–65). Environmental heterogeneity may also scale positively with area, further elevating rates of allopatric speciation. Regardless of whether—and why—larger tropical areas scale with richness, the ‘age and area’ hypothesis assumes that tropical-adapted taxa once occupying higher latitudes during warmer intervals have now been driven extinct, extirpated, or forced to migrate equatorward as warmer climates contracted (56, 66, 67). Thus, the historical effect of a once-larger tropical expanse involves differential rates: either through elevated extirpation/extinction at high latitudes, or greater dispersal from high to low latitudes.
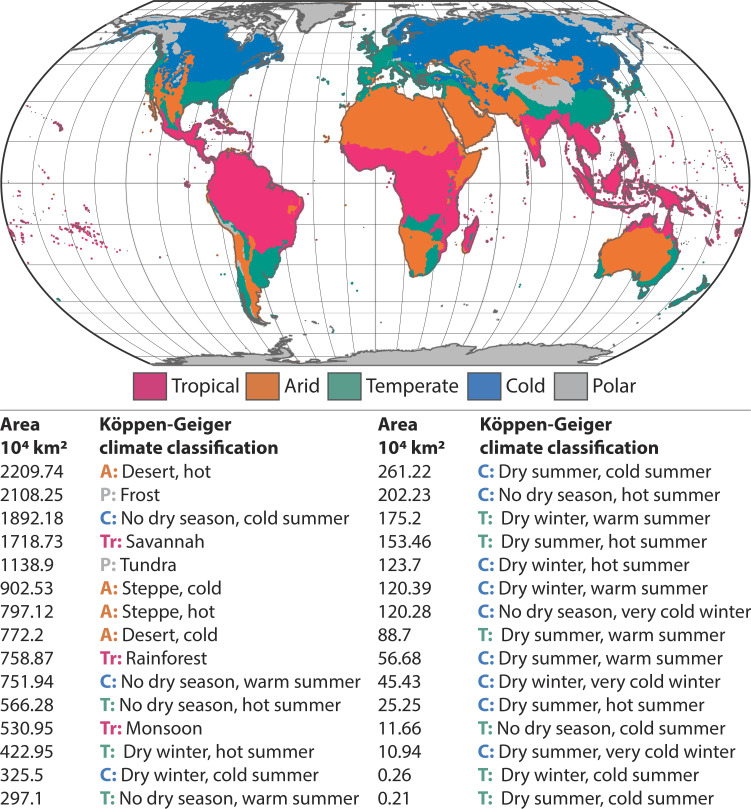
Today, the larger areal extent of the tropics does not provide an explanation for high terrestrial richness at low latitudes, since tropical climate regions on land are no larger than extratropical ones (68–71) (Fig. 2). When examining historical patterns of richness and rate variation, care must be taken to quantify biodiversity dynamics by latitude, and to disentangle these dynamics from the potential drivers of latitudinal patterns, such as spatial climate variation over Earth history. The LDG is a pattern with respect to latitude, not with respect to climate regime. Climate and its variation throughout Earth history is one hypothesized driver of the LDG, but spatial variation in climate does not negate differences in diversification rates across latitudes, which may have occurred as climate changed over time.
Fig. 2.
Present-day Köppen-Geiger climate classifications for tropical, arid, temperate, cold, and polar regions. Tropical climate regimes are not larger in areal extent than extratropical climate regimes. However, individual ‘tropical’ climate classifications span larger areas, on average, than individual ‘temperate’ classifications, listed here. The individual climate categories are ranked from largest to smallest in area (km2) and were calculated using an equal area (Eckert 4) map projection at 10-km resolution from Beck et al. (70). Analyses relied on the R v.4.2.1 computing environment using the sf package (42) and ggplot2 (43).
The original formulation of the ‘age and area’ hypothesis likely dates to Wallace (1), who noted that equatorial regions have suffered less from climate change than temperate regions (6). Wallace’s argument implicitly assumed higher rates of extinction/extirpation at high relative to low latitudes or greater high to low latitude dispersal (7, 71), and provides a corollary to the ‘tropics are stable’ hypothesis that implicates relatively low extinction rates at low latitudes (72).
There is little doubt that high-latitude cooling and glaciation instigated extinction, extirpation, or range shifts at high latitudes over Earth history [often defined as exclusive of 23.26° latitude, though see (68)]. Higher extinction rates at high latitudes have been reported for many clades, including vertebrates (40, 67, 73, 74), invertebrates (74, 75), and plants (76). Comparisons of species’ age distributions based on molecular phylogenies (73, 77) and fossil data (54, 75) additionally suggest a role for high latitude extinction, with greater variance in taxon age at low latitudes suggesting older taxa were preferentially lost at high latitudes (78).
Distributional patterns for groups also reveal a role for high latitude extinction or extirpation. For example, today, beetles with poorer dispersal abilities exhibit a steeper richness gradient across Europe compared to more vagile beetles better able to colonize regions once covered by ice (79). Fossil data show similar evidence: high-latitude regions were richer in the past than they are today for many groups (80–82), particularly during warmer intervals such as the Eocene, around 55 to 45 million years ago (59, 60) (Fig. 1). During this time, the ancestors of bird clades now restricted to low latitudes were found as far north as ~47° paleolatitude, including the Anseranatidae, currently restricted to Australia and southern New Guinea, and the Coliidae, currently restricted to sub-Saharan Africa (40). Greater heat transport from the low to high latitudes in the Eocene warmed polar regions, allowing crocodylomorphs to roam Antarctica and to venture as far north as 73° in the Arctic circle (83). Indeed, many groups whose species are now restricted to lower latitudes today were found at higher latitudes during the Eocene, when temperatures were warmer. Consequently, LDGs may have been shallower during these warm intervals and steepened to their present-day configuration as climate cooled beginning around 34 to 15 million years ago (55, 66, 67, 84, 85).
Although Cenozoic cooling almost certainly drove high latitude extinction and extirpation, steepening the LDG (56, 67, 76, 79), these processes were likely not the only factors to have influenced the emergence of a modern-style LDG. Growing evidence suggests that low latitude speciation may have contributed to the formation of LDGs today, with perhaps even larger effects on LDG development than extinction dynamics (15, 54, 55, 84). Spatiotemporal climate change may instigate range fragmentation more readily at low latitudes, prompting higher rates of allopatric speciation and therefore piling up species equatorially (13, 14, 86, 87). This mechanism may operate in both the marine and terrestrial realm, with environmental heterogeneity possible across three dimensions (time, space, and depth) in the sea (55).
There is still much to be learned about variation in rates of speciation, extinction, extirpation, and dispersal regionally over Earth history. To better understand the development of LDGs, we need to better constrain this variation – only then will the mechanisms driving rate variation become clearer. This is no easy task. Direct inference from the fossil record can be challenged by gaps in spatial, temporal, and taxonomic coverage. Inferences from molecular phylogenies are similarly fraught: extinction is notoriously difficult to estimate (88, 89), and recent work suggests that any given extant timetree can be explained equally well by a large number of diversification scenarios (90). The latter represents a case of nonidentifiability, meaning that it is difficult to infer the true values of a given model’s underlying parameters. Even approaches that combine fossil and molecular data to estimate evolutionary rates do not necessarily resolve issues of nonidentifiability (91).
Despite these difficulties, deeper insights on regional rate variation are still possible. New phylogenetic methods (92, 93) are providing means to examine whether diversification rate patterns are robust, despite issues of nonidentifiability. Geographic diversification models, such as GeoSSE (94) and ClaSSE (95), may help to elucidate spatial rate variation through time, especially when used in combination with fossil data. These methods should be employed to study new systems of relevance to LDGs, with focus on gathering empirical data for understudied groups, such as invertebrates. Paleontological models are also beginning to estimate rates of diversification regionally (96–98), with more work needed to develop our understanding of how spatial bias may affect rate parameters. Simulation models, such as mechanistic spatial algorithms, provide a new avenue to elucidate rate variation regionally over Earth history (15, 99–101), especially when forced with realistic estimates of how climate, continents, and topography have changed spatially and temporally over time (102). Even without realistic forcers, spatial models may provide null expectations for rate variation in silico (15).
Regional rate variation is best estimated using multiple approaches. This triangulation method echoes recent suggestions by Liow, Uyeda, and Hunt (103) and Meseguer and Condamine (67) to leverage diverse information, including phylogenetic estimates, fossils, developmental biology, and quantitative genetics, to better elucidate macroevolutionary history. For instance, more information on LDG dynamics may be provided when using phylogenetic and fossil data in a total-evidence framework (104) underpinned by several birth–death models that allow for rate variation through time and space. These diversification models can then be coupled to biogeographic analyses that estimate dispersal rates and local extinction rates in low and high latitudinal bands (56).
The richness of the tropics has long intrigued biologists. The pervasiveness of LDGs across ecosystems and clades rightfully deserves attention and begs a mechanistic explanation. Any explanation, however, must invoke differential rates of speciation, extinction, extirpation, or dispersal. Hypotheses that propose ‘more time’ for diversification over rate variation inadvertently eschew focus from the high latitude extinction or dispersal dynamics that are inherent to such arguments. Future work on LDGs should concentrate on better constraining variation in evolutionary rates across space and time using integrative, cross-disciplinary approaches (103, 105). The potential drivers of this rate variation, either biotic or abiotic in nature, will then become clearer.
Acknowledgments
I thank Roger Benson (AMNH), Roger Close (University of Oxford), Alexander Farnsworth (University of Bristol), Daniel Field (University of Cambridge), Phil Mannion (UCL), and Elsa Panciroli (University of Oxford) for helpful discussions that informed this contribution. I am grateful to the contributors of the Paleobiology Database who provided the distributional data for Fig. 1. This research was supported by the Leverhulme Prize and the National Science Research Council grant NE/V011405/1. This is Paleobiology Database publication no. 459.
Author contributions
E.E.S. designed research; performed research; analyzed data; and wrote the paper.
Competing interests
The author declares no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the main text.
References
- 1.Wallace A. R., Tropical Nature and Other Essays (Macmillan, New York, 1878). [Google Scholar]
- 2.Forster J. R., “Observations made during a voyage round the world, on physical geography, natural history, and ethic philosophy” in Foundations of Biogeography, Lomolino M. V., Sax D. F., Brown J. H., Eds. (University of Chicago Press, Chicago, IL, 1778), pp. 19–27. [Google Scholar]
- 3.Willig M. R., Kaufman D. M., Stevens R. D., Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (2003). [Google Scholar]
- 4.Fine P. V., Ecological and evolutionary drivers of geographic variation in species diversity. Annu. Rev. Ecol. Evol. Syst. 46, 369–392 (2015). [Google Scholar]
- 5.Hillebrand H., On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Mittelbach G. G., et al. , Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Pianka E. R., Latitudinal gradients in species diversity: A review of concepts. Am. Nat. 100, 33–46 (1966). [Google Scholar]
- 8.Rohde K., Latitudinal gradients in species diversity: The search for the primary cause. Oikos 65, 514–527 (1992). [Google Scholar]
- 9.Field R., et al. , Spatial species-richness gradients across scales: A meta-analysis. J. Biogeogr. 36, 132–147 (2009). [Google Scholar]
- 10.Nils Chr S., The tropics: Cradle or museum? Oikos 43, 417–420 (1984). [Google Scholar]
- 11.Brodie J. F., Mannion P. D., The hierarchy of factors predicting the latitudinal diversity gradient. Trends Ecol. Evol. 38, 15–23 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Whittaker R. J., Willis K. J., Field R., Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 28, 453–470 (2001). [Google Scholar]
- 13.Dynesius M., Jansson R., Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl. Acad. Sci. U.S.A. 97, 9115–9120 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haffer J., Prance G. T., Climatic forcing of evolution in Amazonia during the Cenozoic: On the refuge theory of biotic differentiation. Amazoniana 16, 579–607 (2001). [Google Scholar]
- 15.Saupe E. E., et al. , Spatio-temporal climate change contributes to latitudinal diversity gradients. Nat. Ecol. Evol. 3, 1419–1429 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Klopfer P. H., Environmental determinants of faunal diversity. Am. Nat. 93, 337–342 (1959). [Google Scholar]
- 17.Dobzhansky T., Evolution in the tropics. Am. Sci. 38, 209–221 (1950). [Google Scholar]
- 18.Paine R. T., Food web complexity and species diversity. Am. Nat. 100, 65–75 (1966). [Google Scholar]
- 19.Schemske D. W., Mittelbach G. G., Cornell H. V., Sobel J. M., Roy K., Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (2009). [Google Scholar]
- 20.Williams C. B., Patterns in the Balance of Nature (Academic Press, New York, 1964). [Google Scholar]
- 21.Connell J. H., Orias E., The ecological regulation of species diversity. Am. Nat. 98, 399–414 (1964). [Google Scholar]
- 22.Currie D. J., Energy and large-scale patterns of animal and plant species richness. Am. Nat. 137, 27–49 (1991). [Google Scholar]
- 23.Rosenzweig M. L., Species Diversity in Space and Time (Cambridge University Press, Cambridge, 1995). [Google Scholar]
- 24.Pigot A. L., Tobias J. A., Jetz W., Energetic constraints on species coexistence in birds. PLoS Biol. 14, e1002407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabosky D. L., Hurlbert A. H., Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Allen A. P., Gillooly J. F., Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol. Lett. 9, 947–954 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Rabosky D. L., Ecological limits on clade diversification in higher taxa. Am. Nat. 173, 662–674 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Hurlbert A. H., Stegen J. C., On the processes generating latitudinal richness gradients: Identifying diagnostic patterns and predictions. Front. Genet. 5, 420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens P. R., Wiens J. J., Explaining species richness from continents to communities: The time-for-speciation effect in emydid turtles. Am. Nat. 161, 112–128 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Wiens J. J., Donoghue M. J., Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Marin J., Hedges S. B., Time best explains global variation in species richness of amphibians, birds and mammals. J. Biogeogr. 43, 1069–1079 (2016). [Google Scholar]
- 32.Miller E. C., Román-Palacios C., Evolutionary time best explains the latitudinal diversity gradient of living freshwater fish diversity. Glob. Ecol. Biogeogr. 30, 749–763 (2021). [Google Scholar]
- 33.Farrell B. D., Mitter C., Futuyma D. J., Diversification at the insect-plant interface. Bioscience 42, 34–42 (1992). [Google Scholar]
- 34.Latham R. E., Ricklefs R. E., “Continental comparisons of temperate-zone tree species diversity” in Species Diversity in Ecological Communities: Historial and Geographical Perspectives, Ricklefs R. E., Schluter D., Eds. (University of Chicago Press, Chicago, IL, 1993), pp. 294–314. [Google Scholar]
- 35.Jansson R., Rodríguez-Castañeda G., Harding L. E., What can multiple phylogenies say about the latitudinal diversity gradient? A new look at the tropical conservatism, out of the tropics and diversification rate hypotheses. Evolution 67, 1741–1755 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Rabosky D. L., et al. , An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Soria-Carrasco V., Castresana J., Diversification rates and the latitudinal gradient of diversity in mammals. Proc. R. Soc. B Biol. Sci. 279, 4148–4155 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raja N. B., Kiessling W., Out of the extratropics: The evolution of the latitudinal diversity gradient of Cenozoic marine plankton. Proc. R. Soc. B Biol. Sci. 288, 20210545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales-Barbero J., Gouveia S. F., Martinez P. A., Historical climatic instability predicts the inverse latitudinal pattern in speciation rate of modern mammalian biota. J. Evol. Biol. 34, 339–351 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Saupe E. E., et al. , Climatic shifts drove major contractions in avian latitudinal distributions throughout the Cenozoic. Proc. Natl. Acad. Sci. U.S.A. 116, 12895–12900 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scotese C. R., PALEOMAP PaleoAtlas for GPlates and the PaleoData plotter program, PALEOMAP Project (2016).
- 42.Pebesma E., Simple features for R: Standardized support for spatial vector data. R J. 10, 439–446 (2018). [Google Scholar]
- 43.Wickham H., ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, New York, 2016). [Google Scholar]
- 44.Romdal T., Araújo M. B., Rahbek C., Life on a tropical planet: Niche conservatism explains the global diversity gradient. Glob. Ecol. Biogeogr. 22, 344–350 (2013). [Google Scholar]
- 45.Buckley L. B., et al. , Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B Biol. Sci. 277, 2131–2138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etienne R. S., et al. , A minimal model for the latitudinal diversity gradient suggests a dominant role for ecological limits. Am. Nat. 194, E122–E133 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Wiens J. J., Speciation and ecology revisited: Phylogenetic niche conservatism and the origin of species. Evolution 58, 193–197 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Peterson A., Soberón J., Sánchez-Cordero V., Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Ricklefs R. E., Latham R. E., Intercontinental correlation of geographical ranges suggests stasis in ecological traits of relict genera of temperate perennial herbs. Am. Nat. 139, 1305–1321 (1992). [Google Scholar]
- 50.Saupe E. E., et al. , Macroevolutionary consequences of profound climate change on niche evolution in marine molluscs over the past three million years. Proc. R. Soc. Lond. B Biol. Sci. 281, 20141995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antell G. S., Fenton I. S., Valdes P. J., Saupe E. E., Thermal niches of planktonic foraminifera are static throughout glacial–interglacial climate change. Proc. Natl. Acad. Sci. U.S.A. 118, e2017105118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martínez-Meyer E., Townsend Peterson A., Hargrove W. W., Ecological niches as stable distributional constraints on mammal species, with implications for Pleistocene extinctions and climate change projections for biodiversity. Glob. Ecol. Biogeogr. 13, 305–314 (2004). [Google Scholar]
- 53.Khaliq I., et al. , Global variation in thermal physiology of birds and mammals: Evidence for phylogenetic niche conservatism only in the tropics. J. Biogeogr. 42, 2187–2196 (2015). [Google Scholar]
- 54.Jablonski D., Roy K., Valentine J. W., Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Fenton I., Aze T., Farnsworth A., Valdes P., Saupe E. E., Origination of the modern-style diversity gradient 15 million years ago. Nature 614, 708–712 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Quintero I., Landis M. J., Jetz W., Morlon H., The build-up of the present-day tropical diversity of tetrapods. Proc. Natl. Acad. Sci. U.S.A. 120, e2220672120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rolland J., Condamine F. L., Beeravolu C. R., Jiguet F., Morlon H., Dispersal is a major driver of the latitudinal diversity gradient of Carnivora. Glob. Ecol. Biogeogr. 24, 1059–1071 (2015). [Google Scholar]
- 58.Condamine F. L., Sperling F. A. H., Wahlberg N., Rasplus J.-Y., Kergoat G. J., What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 15, 267–277 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Westerhold T., et al. , An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369, 1383–1387 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Zachos J., Pagani M., Sloan L., Thomas E., Billups K., Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Fine P. V. A., Ree R. H., Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat. 168, 796–804 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Jetz W., Fine P. V., Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Losos J. B., Schluter D., Analysis of an evolutionary species–area relationship. Nature 408, 847–850 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Kisel Y., Barraclough T. G., Speciation has a spatial scale that depends on levels of gene flow. Am. Nat. 175, 316–334 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Chown S. L., Gaston K. J., Areas, cradles and museums: The latitudinal gradient in species richness. Trends Ecol. Evol. 15, 311–315 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Mannion P. D., Upchurch P., Benson R. B. J., Goswami A., The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 29, 42–50 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Meseguer A. S., Condamine F. L., Ancient tropical extinctions at high latitudes contributed to the latitudinal diversity gradient. Evolution 74, 1966–1987 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Feeley K. J., Stroud J. T., Where on earth are the “tropics”? Front. Biogeogra. 10, e38649 (2018). [Google Scholar]
- 69.Rohde K., The larger area of the tropics does not explain latitudinal diversity gradients in species diversity. Oikos 79, 169–172 (1997). [Google Scholar]
- 70.Beck H. E., et al. , Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 5, 180214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schluter D., Speciation, ecological opportunity, and latitude. Am. Nat. 187, 1–18 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Fischer A. G., Latitudinal variations in organic diversity. Evolution 14, 64–81 (1960). [Google Scholar]
- 73.Weir J. T., Schluter D., The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315, 1574–1576 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Hawkins B. A., Diniz-Filho J. A. F., Jaramillo C. A., Soeller S. A., Post-Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. J. Biogeogr. 33, 770–780 (2006). [Google Scholar]
- 75.Stehli F. G., Douglas R. G., Newell N. D., Generation and maintenance of gradients in taxonomic diversity. Science 164, 947–949 (1969). [DOI] [PubMed] [Google Scholar]
- 76.Eiserhardt W. L., Borchsenius F., Plum C. M., Ordonez A., Svenning J. C., Climate driven extinctions shape the phylogenetic structure of temperate tree floras. Ecol. Lett. 18, 263–272 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Stevens R. D., Historical processes enhance patterns of diversity along latitudinal gradients. Proc. R. Soc. Lond. B 273, 2283–2289 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller E. C., Wiens J. J., Extinction and time help drive the marine-terrestrial biodiversity gradient: Is the ocean a deathtrap? Ecol. Lett. 20, 911–921 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Baselga A., Lobo J. M., Svenning J. C., Aragón P., Araújo M. B., Dispersal ability modulates the strength of the latitudinal richness gradient in European beetles. Glob. Ecol. Biogeogr. 21, 1106–1113 (2012). [Google Scholar]
- 80.Blois J. L., Hadly E. A., Mammalian response to cenozoic climatic change. Annu. Rev. Earth Planet. Sci. 37, 181–208 (2009). [Google Scholar]
- 81.Smith R. Y., Basinger J. F., Greenwood D. R., Early eocene plant diversity and dynamics in the falkland flora, Okanagan highlands, british columbia, Canada. Palaeobiodiversity Palaeoenvironments 92, 309–328 (2012). [Google Scholar]
- 82.Wilf P., Labandeira C. C., Johnson K. R., Cúneo N. R., Richness of plant–insect associations in Eocene Patagonia: A legacy for South American biodiversity. Proc. Natl. Acad. Sci. U.S.A. 102, 8944–8948 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mannion P. D., Chiarenza A. A., Godoy P. L., Cheah Y. N., Spatiotemporal sampling patterns in the 230 million year fossil record of terrestrial crocodylomorphs and their impact on diversity. Palaeontology 62, 615–637 (2019). [Google Scholar]
- 84.Crame J. A., Early Cenozoic evolution of the latitudinal diversity gradient. Earth Sci. Rev. 202, 103090 (2020). [Google Scholar]
- 85.Marcot J. D., Fox D. L., Niebuhr S. R., Late Cenozoic onset of the latitudinal diversity gradient of North American mammals. Proc. Natl. Acad. Sci. U.S.A. 113, 7189–7194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson S. T., Overpeck J. T., Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology 26, 194–220 (2000). [Google Scholar]
- 87.Rangel T. F. L., Diniz-Filho J. A. F., Colwell R. K., Species richness and evolutionary niche dynamics: A spatial pattern–oriented simulation experiment. Am. Nat. 170, 602–616 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Moore B. R., Höhna S., May M. R., Rannala B., Huelsenbeck J. P., Critically evaluating the theory and performance of Bayesian analysis of macroevolutionary mixtures. Proc. Natl. Acad. Sci. U.S.A. 113, 9569–9574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rabosky D. L., Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816–1824 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Louca S., Pennell M. W., Extant timetrees are consistent with a myriad of diversification histories. Nature 580, 502–505 (2020). [DOI] [PubMed] [Google Scholar]
- 91.Louca S., McLaughlin A., MacPherson A., Joy J. B., Pennell M. W., Fundamental identifiability limits in molecular epidemiology. Mol. Biol. Evol. 38, 4010–4024 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kopperud B. T., Magee A. F., Höhna S., Rapidly changing speciation and extinction rates can be inferred in spite of nonidentifiability. Proc. Natl. Acad. Sci. U.S.A. 120, e2208851120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Höhna S., Kopperud B. T., Magee A. F., CRABS: Congruent rate analyses in birth–death scenarios. Methods Ecol. Evol. 13, 2709–2718 (2022). [Google Scholar]
- 94.Rolland J., Condamine F. L., Jiguet F., Morlon H., Faster speciation and reduced extinction in the tropics contribute to the mammalian latitudinal diversity gradient. PLoS Biol. 12, e1001775 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldberg E. E., Igić B., Tempo and mode in plant breeding system evolution. Evolution 66, 3701–3709 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Allen B., et al. , Estimating spatial variation in origination and extinction in deep time: A case study using the Permian-Triassic marine invertebrate fossil record. Paleobiology, 1–18, 10.1017/pab.2023.1 (2023). [DOI]
- 97.Flannery-Sutherland J. T., Silvestro D., Benton M. J., Global diversity dynamics in the fossil record are regionally heterogeneous. Nat. Commun. 13, 2751 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liow L. H., et al. , Higher origination and extinction rates in larger mammals. Proc. Natl. Acad. Sci. U.S.A. 105, 6097–6102 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pontarp M., et al. , The latitudinal diversity gradient: Novel understanding through mechanistic eco-evolutionary models. Trends Ecol. Evol. 34, 211–223 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Rangel T. F., et al. , Modeling the ecology and evolution of biodiversity: Biogeographical cradles, museums, and graves. Science 361, eaar5452 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Hagen O., Skeels A., Onstein R. E., Jetz W., Pellissier L., Earth history events shaped the evolution of uneven biodiversity across tropical moist forests. Proc. Natl. Acad. Sci. U.S.A. 118, e2026347118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haywood A. M., et al. , What can palaeoclimate modelling do for you? Earth Syst. Environ. 3, 1–18 (2019). [Google Scholar]
- 103.Liow L. H., Uyeda J., Hunt G., Cross-disciplinary information for understanding macroevolution. Trends Ecol. Evol. 38, 250–260 (2023). [DOI] [PubMed] [Google Scholar]
- 104.Brée B., Condamine F. L., Guinot G., Combining palaeontological and neontological data shows a delayed diversification burst of carcharhiniform sharks likely mediated by environmental change. Sci. Rep. 12, 21906 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pennell M., Alternate histories in macroevolution. Proc. Natl. Acad. Sci. U.S.A. 120, e2300967120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the main text.