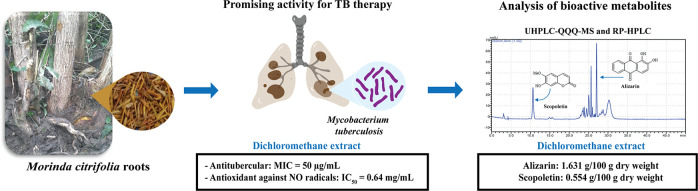
Abstract
Morinda citrifolia is a medicinal plant that has been traditionally used in various therapeutic applications. All parts of M. citrifolia including fruits, leaves, stems, roots, and flowers contain various biologically active phytochemicals. This study aimed to evaluate the antitubercular, antibacterial, and antioxidant activities of M. citrifolia root extracts and spectroscopically analyze the bioactive metabolites. M. citrifolia root extracts were prepared via maceration. The minimum inhibitory concentration (MIC) for antitubercular activity, the inhibition zone for antibacterial activity, and the antioxidant activities in terms of half-maximal inhibitory concentration (IC50) values were determined. 1H-NMR, RP-HPLC, and UHPLC-QQQ-MS analyses were performed to evaluate the secondary metabolites. The results showed that the dichloromethane root extract exhibited relatively good inhibition of M. tuberculosis with an MIC value of 50 μg/mL. All extracts were mostly active against five tested bacterial strains. The ethanolic and dichloromethane root extracts showed the highest antioxidant power against DPPH (IC50 = 0.82 mg/mL) and NO (IC50 = 0.64 mg/mL) radicals, respectively. The 1H-NMR-based screening of the secondary metabolites of all M. citrifolia root extracts confirmed the presence of triterpenes, steroids, phenolics, flavonoids, tannins, and anthraquinones as major bioactive components. Alizarin and scopoletin were detected in the extracts via UHPLC-QQQ-MS, and the alizarin (0.552–3.227 g/100 g dry weight) and scopoletin (0.092–0.554 g/100 g dry weight) contents were quantified via RP-HPLC. The antimicrobial and antioxidant activities of M. citrifolia root extracts and the identification of the main bioactive ingredients are the initial studies that can be beneficial for further in vivo studies and biomedical applications of its bioactive compounds.
Introduction
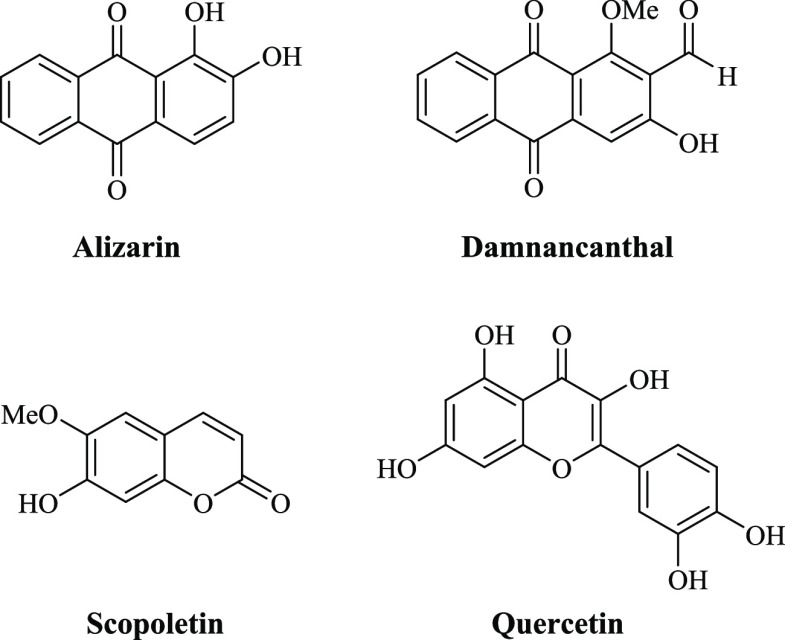
Bioactive compounds extracted and isolated from natural sources including herbal plants, microorganisms, and marine organisms possess various in vitro biological properties such as antibacterial, antifungal, anticancer, and antitubercular activities.1−3 In particular, Morinda citrifolia Linn., also known as noni, is a fruit-bearing plant that belongs to the family of Rubiaceae and is widely found in the Southeast Asia region.4 Many bioactive compounds including anthraquinones, flavonoids, and coumarins found in the crude extracts of M. citrifolia show biological activities including antifungal, antibacterial, antiviral, antioxidant, and anti-inflammatory activities (Figure 1).5,6
Figure 1.
Bioactive chemicals isolated from M. citrifolia.
However, reports on the antitubercular activity of M. citrifolia extracts are comparatively scarce.7−9 Tuberculosis (TB) is an infectious disease caused by bacillus Mycobacterium tuberculosis, which continuously spreads around tropical regions. TB was ranked 13th in the list of common causes of death due to microbial infections in 2021.10 In addition, TB multidrug resistance is a public health threat affecting first- and second-line antitubercular drugs.11,12 Therefore, the discovery of bioactive compounds with antitubercular activity is essential.
In this context, diterpene and steroid derivatives showing inhibition of the M. tuberculosis H37Rv strain with minimum inhibitory concentration (MIC) values between 2.0 and 128.0 μg/mL were isolated from the hexane fraction of an ethanolic leaf extract of M. citrifolia using vacuum liquid chromatography, column chromatography, and high-performance liquid chromatography (HPLC).7 Additionally, the ethanolic extract of the M. citrifolia fruit exhibited antitubercular activity against the M. tuberculosis H37Rv strain.8
Furthermore, some studies evaluated the antibacterial activity of M. citrifolia extracts.5,13,14 The fresh fruit juice was investigated for antimicrobial activity, and inhibitory activity was found against both Gram-positive (e.g., Staphylococcus aureus and Staphylococcus epidermidis) and Gram-negative (e.g., Pseudomonas aeruginosa, Escherichia coli, and Proteus vulgaris) bacteria with at least 10 mm of inhibition zone.13 The antibacterial activity of the petroleum ether, chloroform, ethyl acetate, n-butanol, and aqueous extracts of M. citrifolia leaves was evaluated using the disk diffusion method,14 and it was found that the n-butanol leaf extract was the most active against Bacillus subtilis, S. aureus, E. coli, and P. vulgaris with inhibition zones of 12–15 mm.
Numerous bioactive compounds derived from M. citrifolia are important for pharmaceuticals, nutraceuticals, and foods. More than 200 biologically active compounds have been identified and are promising as therapeutic agents.5 Alizarin as a bioactive anthraquinone was highly abundant in roots of M. citrifolia as compared to its leaves and fruits.15 This anthraquinone has a wide range of multi-pharmacological effects including anti-pathogenicity of microorganisms.16,17 In addition, alizarin possessed the inhibition of biofilm produced by S. aureus and S. epidermidis at a concentration of 10 μg/mL, and it also possessed anti-hemolytic activity.18 Scopoletin is regarded as one of bioactive coumarins derived from M. citrifolia, and it exhibited various pharmacological actions including antiproliferative, anti-inflammatory, and antioxidant activities.16,19
In traditional medicine, M. citrifolia has been used for treating TB in a variety of cultures including Hawaii, Southeast Asia, the Pacific Islands, the Philippines, and Bangladesh.20,21 The M. citrifolia roots were also documented for treating bacterial infection.22
In this study, we evaluated the antitubercular, antibacterial, and antioxidant activities of M. citrifolia root extracts, aiming particularly at gaining more insight into the scarcely reported antitubercular activity of M. citrifolia extracts. Moreover, we investigated the phytochemicals in M. citrifolia extracts using phytochemical screening and proton nuclear magnetic resonance (1H-NMR) spectroscopy. In addition, the identification and quantification of alizarin and scopoletin were conducted by reverse-phase HPLC (RP-HPLC) and ultra HPLC coupled with triple quadrupole tandem mass spectrometry (UHPLC-QQQ-MS) techniques.
Materials and Methods
Plant Materials and Chemicals
M. citrifolia roots were collected in Kamphaeng Phet, Thailand, in 2017 and authenticated by the botanist at the Queen Sirikit Botanic Garden Herbarium in Chiang Mai, Thailand (Voucher No. 105894).23,24 All commercially available solvents and chemicals/reagents were purchased from commercial companies and used without further purification including scopoletin (≥99% purity; Sigma Aldrich, Germany), alizarin (>95% HPLC; Tokyo Chemical Industry, Japan), rifampin (≥97% purity; Sigma Aldrich, Germany), tetracycline hydrochloride (≥95% purity; Sigma Aldrich, Germany), chloramphenicol (≥98% purity; Sigma Aldrich, Germany), quercetin hydrate (≥95% purity; Sigma Aldrich, Germany), gallic acid (≥97.5% purity; Sigma Aldrich, Germany), hexane [analytical reagent (AR) grade; RCI Labscan, Thailand], dichloromethane (AR grade; RCI Labscan, Thailand), ethyl acetate (AR grade; RCI Labscan, Thailand), ethanol (AR grade; RCI Labscan, Thailand), methanol (AR grade; RCI Labscan, Thailand), dimethylsulfoxide (AR grade; RCI Labscan, Thailand), acetonitrile (HPLC grade; Quality Reagent Chemical, Thailand), sodium nitroprusside (SNP) dihydrate (≥99% purity; Sigma Aldrich, Germany), sulfanilamide (>98% purity, Sigma Aldrich, Germany), and N-(1-naphthyl)ethylenediamine dihydrochloride (>98% purity, Sigma Aldrich, Germany).
Preparation of M. citrifolia Extracts
According to our previous studies, air-dried finely ground powders of M. citrifolia roots were individually extracted with ethanol and water using maceration.23 Additionally, dried M. citrifolia root powders were sequentially extracted via gradient maceration with extracting solvents of increasing polarity, i.e., hexane, dichloromethane, ethyl acetate, and ethanol.24 All crude extracts were evaluated for their antitubercular and antibacterial activities and analyzed for the identification and quantification of the main bioactive metabolites.
Antitubercular Activity
All M. citrifolia root extracts were evaluated for the inhibition of the M. tuberculosis H37Rv strain using a microplate Alamar Blue assay to determine the MIC values.25 Each root extract was individually dissolved in dimethylsulfoxide for preparing stock solutions with a concentration of 10,000 μg/mL. The crude extract solutions (100 μL) in Middlebrook 7H9GC broth (400 μg/mL) were two-fold diluted in 96-well microplates containing Middlebrook 7H9GC broth (100 μL), followed by a serial dilution in the 96-well microplates. A suspension (100 μL) of M. tuberculosis (prepared at approximately 105–106 CFU/mL) was added to each well to achieve concentrations ranging from 100 to 0.1 μg/mL. The prepared microplates were incubated at 37 °C for 7 days. Solutions of 20% Tween 80 (12.5 μL) and Alamar Blue (20 μL) were subsequently added to each well of the microplates, which were then further incubated at 37 °C for 16–24 h. Determination of the mycobacterial growth was observed according to a color change from blue to pink. The absence of a color change indicated the occurrence of inhibition, and the MIC value was recorded as the lowest concentration for each extract. The M. tuberculosis H37Ra strain used in this study was obtained from a stock culture of Ramathibodi Hospital in Bangkok, Thailand. Rifampin, a stock solution in dimethylsulfoxide with a concentration of 32 μg/mL, was used as a standard drug for a positive control.
Antibacterial Activity
All crude root extracts were evaluated for the antibacterial activity using a disk diffusion method.26 All selected bacterial strains (Bacillus cereus, S. aureus, S. epidermidis, E. coli, and P. aeruginosa) from glycerol stocks were streaked on Mueller–Hinton agar (MHA) plates and incubated at 37 °C overnight. A single colony of each bacterial cell was picked up and dissolved in a tube containing 0.85% saline solution (10 mL). Each bacterial suspension was prepared to adjust the turbidity to 0.5 McFarland standard. The prepared bacterial suspensions were spread and covered on a surface of nutrient agar plates. The extracts and tetracycline hydrochloride and chloramphenicol as standard drugs were dissolved in dimethylsulfoxide as a solvent to prepare stock solutions (10 mg/100 μL). The standard drugs and extract solutions (10 μL) were individually loaded onto paper disks. Pure dimethylsulfoxide was used as a negative control for the antibacterial assay. All paper disks containing either standard drugs or crude extracts were placed on the prepared MHA plates, which were then incubated at 37 °C overnight. After the incubation period, the diameter of the inhibition zone in each paper disk was measured.
Evaluation of Antioxidant Activities
2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity
Each M. citrifolia extract (20 μL in dimethylsulfoxide) at various concentrations (0.625–5.0 mg/mL) was added to a 96-well plate, followed by addition of 180 μL of a 120 μM 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution in methanol. The plate was incubated in the dark at 37 °C for 30 min. After that, the absorbance was measured using a microplate reader at a wavelength of 517 nm. Quercetin, a stock solution in methanol with a concentration of 100 μg/mL, was used as a positive control. The radical scavenging ability against DPPH radicals was calculated as the inhibition percentage using eq 1.
| 1 |
Nitric Oxide Radical Scavenging Activity
A slightly modified nitric oxide (NO) radical scavenging method27 was used to determine the NO radical scavenging activity using the Griess reaction. NO radicals were initially generated from 10 mM SNP in phosphate buffered saline (PBS) at pH 7.3. Each M. citrifolia extract (10 μL in dimethylsulfoxide) at various concentrations (0.625–5.0 mg/mL) in the 96-well plate was reacted with 10 mM SNP in PBS at pH 7.3 (90 μL), and the reaction mixture was incubated at room temperature for 90 min under visible polychromatic light. Afterward, the pretreated extract was added with 1% sulfanilamide in 5% phosphoric acid (50 μL) and further incubated for 5 min at room temperature in the dark. Next, a solution of 0.1% N-(1-naphtyl)-ethylenediamine (NED) (50 μL) was added to each well followed by incubating for 30 min at room temperature in the dark. The formation of a diazo compound and subsequent coupling with NED afforded a chromophore with magenta color. The absorbance was measured at a wavelength of 540 nm using a microplate reader. Gallic acid, a stock solution in water with a concentration of 1000 μg/mL, was used as a positive control. The percentage of NO radical inhibition rate was calculated using a nitrite ion (NO2–) amount of the SNP-treated group as a control group (eq 2).
| 2 |
Ferric Reducing Ability Power Assay
Each M. citrifolia extract (10 μL in methanol) from the 1.0 mg/mL stock solution was added into a 96-well plate, and a freshly prepared ferric reducing ability power (FRAP) reagent (190 μL) was then added. Afterward, the reaction mixture was incubated for 4 min at room temperature. An intense blue color developed due to the reduction of ferrous ions to ferric ions, and the absorbance was measured at a wavelength of 595 nm. The antioxidant power was determined by calculating the concentration of ferric ions found in the samples relative to a standard ferrous sulfate (FeSO4) solution (FRAP value in μM FeSO4/mg dry weight of extract) using a standard calibration curve (eq 3) as follows:
| 3 |
where C is the concentration of the calibrated sample, V is the volume sample, d is the dilution factor, and c is the concentration of the tested sample.
Phytochemical Analysis
A phytochemical analysis was conducted following previously reported procedures28−31 to identify the bioactive compounds. According to our previous study,24 hexane, dichloromethane, ethyl acetate, and ethanol root extract solutions were analyzed for triterpenes (Salkowski’s and Liebermann–Burchard’s tests), steroids (Salkowski’s and Liebermann–Burchard’s tests), phenolic compounds (ferric chloride test), flavonoids (alkaline test), and tannins (ferric chloride test). Each dried root extract was mixed with water and analyzed for saponins (foam test). The acid layer of each root extract was prepared for the analysis of alkaloids via Dragendorff’s, Wagner’s, and Hager’s tests. Furthermore, the analysis of anthraquinones was conducted using Borntrager’s test. Each crude extract (2.0 mg) was dissolved in dimethylsulfoxide (1.0 mL), and each solution (0.5 mL) was added to a 2 M hydrochloric acid solution (2.0 mL). The mixture was then boiled for 15 min. After cooling, chloroform (2.0 mL) was added and vortexed. The chloroform layer was collected and added with 10% potassium hydroxide solution. The formation of a pink solution indicated the presence of anthraquinone aglycones in the extract.
1H-NMR-Based Screening of Secondary Metabolites
All crude root extracts were dissolved in deuterated solvents including chloroform-d, acetone-d6, and methanol-d4. 1H-NMR spectra of the hexane and dichloromethane extracts (in chloroform-d), the ethyl acetate extract (in acetone-d6), and the ethanolic extract (in methanol-d4) were recorded on a Bruker Avance 300 spectrometer (1H-NMR at 300 MHz). The chemical shifts (δ) are reported in parts per million (ppm).
Detection and Identification of Alizarin and Scopoletin via UHPLC-QQQ-MS
The detection and identification of alizarin and scopoletin was performed using an Ultimate 3000 UHPLC system (Thermo Fisher Scientific) coupled to a Triple Quad 5500+ mass spectrometer (AB Sciex) (UHPLC-QQQ-MS) equipped with an electrospray ionization source. Stock solutions of alizarin and scopoletin with a concentration of 2.0 mg/mL were prepared in methanol and then diluted in acetonitrile/water (50:50, V/V) to a final concentration of 5.0 μg/mL as stock solutions. Extract samples with a concentration of 5 mg/mL were 10-fold diluted in acetonitrile/water (50:50, V/V) and further mixed and centrifuged at 15,000 × g for 10 min at 4 °C. The supernatant of each sample was used for mass spectrometry analysis.
The chromatographic separation was performed using a Kinetex Core-Shell column (3.0 mm × 50 mm, 2.6 μm), and a mobile phase was consisted of 0.5% formic acid in water (solvent A) and acetonitrile (solvent B). For alizarin, a gradient elution program was performed at 20% solvent B (0–2 min), 80% solvent B (2–8 min), and 20% solvent B (8–9 min) for 9 min with a flow rate of 350 μL/min. On the other hand, a gradient elution for scopoletin was set at 25% solvent B (0–5 min), 80% solvent B (5–8 min), and 25% solvent B (8–9 min) for 9 min with a flow rate of 300 μL/min. The injection volume was 10 μL, and the column temperature was maintained at 35 °C.
Mass spectrometric detection was conducted in the positive mode using multiple reaction monitoring (MRM) of the precursor-to-product ion transitions of m/z 193.0 to 178.0 and m/z 241.0 to 213.0 for scopoletin and alizarin, respectively. The source parameters were optimized as follows: curtain gas pressure, 30 psi; collision gas pressure, 7 psi; ion spray voltage, 5500 V; gas temperature, 600 °C; nebulizer gas pressure, 16 psi; auxiliary gas pressure, 30 psi; interface heater operation. The optimum values for compound-dependent mass spectrometric parameters including declustering potential, collision energy, entrance potential, and cell exit potential were set at 130, 30, 7, and 15 V, respectively, for scopoletin and at 130, 32, 7, and 12 V, respectively, for alizarin. Data acquisition and processing were controlled using the Analyst 1.7.2 software (AB Sciex).
Quantitative Analysis of the Alizarin and Scopoletin Content via RP-HPLC
Preparation of Alizarin and Scopoletin Solutions
Stock solutions of alizarin and scopoletin with a concentration of 100 μg/mL were prepared in methanol and filtered using 0.22 μm nylon membrane syringe filters. Each stock solution was diluted to concentrations of 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL.
Preparation of the Crude Extract Solutions
Stock solutions of hexane and dichloromethane root extracts with a concentration of 1.0 mg/mL were prepared in 1:1 methanol and chloroform, and stock solutions of ethyl acetate and ethanolic root extracts with a concentration of 1.0 mg/mL were prepared in methanol. All solutions were filtered using 0.22 μm nylon membrane syringe filters before the RP-HPLC analysis.
RP-HPLC Conditions
The RP-HPLC analysis of the alizarin and scopoletin contents in the M. citrifolia root extracts was conducted on a Shimadzu HPLC LL-20A system (Shimadzu, Japan) consisting of a controller (CMB-20A), an autosampler (SIL-20A), an online degassing unit (DGU-20A3), a solvent delivery unit (LC-20A), and a photodiode array detector (SPD-M20A). The chromatographic separation was performed using a C18 column (4.6 × 250 mm) coupled with a C18 guard column (4.6 × 10 mm) as a stationary phase. Formic acid (0.5%) in water (solvent A) was filtered through 0.45 μm nylon membrane filters, and acetonitrile (solvent B) was filtered using 0.45 μm PTFE membrane filters. The gradient elution program was set at 25% solvent B (0–15 min), 70% solvent B (15–20 min), 25% solvent B (20–30 min), and 25% solvent B (30–50 min) for 50 min with a flow rate of 1 mL/min. The samples (20 μL) were injected into the column, which was maintained at 35 °C. The chromatographic profile of alizarin, which showed a retention time (RT) of 26.194 min, was recorded at 250 nm, while the detection of scopoletin (RT = 10.613 min) was monitored at 350 nm. Measurements were performed in triplicate.
Statistical Analysis
One-way analysis of variance and subsequent post-hoc Duncan test (SPSS v.22) were performed to evaluate the significance difference between groups.
Results and Discussion
Antitubercular and Antibacterial Activities
The MIC values of all M. citrifolia root extracts for antitubercular activity were determined using the microplate Alamar Blue assay (Table 1). Initially, the aqueous and ethanolic root extracts were screened for the inhibition of M. tuberculosis. The results showed that both aqueous and ethanolic extracts were inactive against M. tuberculosis. The antitubercular activity of all root extracts was determined according to the gradient maceration. The dichloromethane root extract showed relatively good inhibition of M. tuberculosis growth with an MIC value of 50 μg/mL. However, the hexane, ethyl acetate, and ethanolic root extracts were inactive against M. tuberculosis. Although the dichloromethane root extract was the most active among all the tested extracts, it was less efficient than the standard drug rifampin (MIC = 0.01 μg/mL). These findings are in agreement with previous studies showing that an ethanolic leaf extract of M. citrifolia afforded an 89% inhibition at a concentration of 100 μg/mL7 and that the ethanolic extract of the M. citrifolia fruit was active against the M. tuberculosis H37Rv strain.8
Table 1. Antitubercular and Antibacterial Activities of M. citrifolia Root Extractsa.
| M. citrifolia root extracts/standard drugs | MIC (μg/mL) | inhibition zone (mm) | ||||
|---|---|---|---|---|---|---|
| M. tuberculosisb | S. aureusc | B. cereusd | S. epidermidise | E. colif | P. aeruginosag | |
| individual maceration | ||||||
| aqueous | >100 | 0 | 0 | 0 | 0 | 0 |
| ethanol | >100 | |||||
| gradient maceration | ||||||
| hexane | >100 | 4.0 | 5.0 | 6.5 | 5.0 | 3.0 |
| dichloromethane | 50 | 2.0 | 5.0 | 6.0 | 5.5 | 4.0 |
| ethyl acetate | >100 | 2.5 | 2.0 | 6.5 | 4.5 | 5.5 |
| ethanol | >100 | 0 | 2.0 | 1.0 | 2.5 | 5.0 |
Inactive at >100 μg/mL for M. tuberculosis.
Mycobacterium tuberculosis: MIC (μg/mL) of rifampin (0.01).
Staphylococcus aureus: inhibition zone (mm) of chloramphenicol (14.5) and tetracycline·HCl (15.5).
Bacillus cereus: inhibition zone (mm) of chloramphenicol (19.5) and tetracycline·HCl (17.7).
Staphylococcus epidermidis: inhibition zone (mm) of chloramphenicol (14.7) and tetracycline·HCl (14.0).
Escherichia coli: inhibition zone (mm) of chloramphenicol (17.5) and tetracycline·HCl (16.5).
Pseudomonas aeruginosa: inhibition zone (mm) of chloramphenicol (10.9) and tetracycline·HCl (11.2).
Next, the antibacterial activity of all crude extracts was determined using the disk diffusion method to evaluate the inhibition zone (Table 1), and it was found that all extracts derived from the gradient maceration were active against both Gram-positive and Gram-negative bacteria with a range of inhibition zone of 1.0–6.5 mm. The hexane and ethyl acetate root extracts were the most active against S. epidermidis with an inhibition zone of 6.5 mm.
The hexane root extract showed a relatively good inhibition of S. aureus (4.0 mm) and B. cereus (5.0 mm). The dichloromethane root extract was the most active against E. coli with an inhibition zone of 5.5 mm. The ethyl acetate root extract showed relatively good inhibition of P. aeruginosa (5.5 mm). A previous study using M. citrifolia seed extracts showed that the methanolic extract could inhibit both S. aureus and S. epidermis with an MIC value of 16 mg/mL.32 In addition, leaf, fruit, and seed extracts of M. citrifolia showed inhibition zones ranging from 8.4 to 14.2 mm against E. coli, S. aureus, and Pseudomonas spp.33 However, the inhibitory activity of all M. citrifolia root extracts against the tested bacteria was lower than that of the standard drugs tetracycline hydrochloride and chloramphenicol.
Antioxidant Activities
The hexane, dichloromethane, ethyl acetate, and ethanolic extracts of M. citrifolia derived from the gradient maceration were evaluated for their antioxidant power using DPPH, NO, and FRAP assays (Table 2). The results of the antioxidant activity test against DPPH radicals revealed that the IC50 values ranged between 0.82 and 3.39 mg/mL, while the IC50 values against NO radicals were 0.64–1.88 mg/mL. The FRAP assay showed that the extracts exhibited ferric reducing antioxidant power between 2.35 and 5.02 mM FeSO4/g extract.
Table 2. Antioxidant Activities (DPPH, NO, and FRAP Assays) of M. citrifolia Root Extractsa.
| M. citrifolia root extract | DPPH (IC50, mg/mL) | NO (IC50, mg/mL) | FRAP (mM FeSO4/g extract) |
|---|---|---|---|
| hexane | 3.39 ± 0.34c | 1.87 ± 0.03c | 2.35 ± 0.31a |
| dichloromethane | 1.00 ± 0.08a | 0.64 ± 0.03a | 3.97 ± 0.28b |
| ethyl acetate | 2.56 ± 0.11b | 1.88 ± 0.08c | 3.92 ± 0.14b |
| ethanol | 0.82 ± 0.01a | 0.75 ± 0.07b | 5.02 ± 0.09c |
| quercetin (μg/mL) | 13.6 ± 0.68 | ||
| gallic acid (μg/mL) | 45.6 ± 4.40 |
Values (mean ± SD) in the same column superscripted with different letters are significantly different (p < 0.05).
According to our previous study, the ethanolic root extract was seven times less active against DPPH radicals (IC50 = 5.97 ± 0.08 mg/mL) than the ethanolic root extract derived from the gradient maceration.23 The dichloromethane and ethanolic extracts showed relatively good inhibition against NO radicals with IC50 values of 0.64 ± 0.03 and 0.75 ± 0.07 mg/mL, respectively. A previous study showed that an aqueous leaf extract with a concentration of 1 mg/mL afforded 19% inhibition of NO radicals.34 The ethanolic extract showed the highest value in the FRAP assay (5.02 ± 0.09 mM FeSO4/g extract), indicating its reducing power of ferric ions to ferrous ions. Our findings are in accord with a previously reported FRAP assay showing that the 50% ethanolic fruit extract exhibited good antioxidant power.35
Phytochemical Analysis
In our previous report on the phytochemical screening of all root extracts of M. citrifolia, major bioactive compounds including phenolics, flavonoids, tannins, steroids, and triterpenes were detected.24 In addition to these previous results, specific phytochemical details are provided in the present study by comparing the color intensity of the extract samples with that of untreated extract solutions to indicate the relative compound contents (Table 3). The detection of anthraquinones in all root extracts was additionally monitored. The presence of these bioactive compounds varied depending on the polarity of the solvent used for the extraction.
Table 3. Phytochemical Analysis of M. citrifolia Root Extractsa.
| phytochemical tests | M. citrifolia root extracts | |||
|---|---|---|---|---|
| hexane | dichloromethane | ethyl acetate | ethanol | |
| triterpenes | +++ | +++ | +++ | + |
| steroids | +++ | +++ | +++ | + |
| phenolic compounds | + | ++ | +++ | +++ |
| flavonoids | ++ | +++ | +++ | +++ |
| tannins | + | ++ | +++ | +++ |
| saponins | – | – | – | – |
| alkaloids | – | – | + | + |
| anthraquinones | ++ | +++ | ++ | +++ |
+, less present; ++, moderately present; +++, strongly present; −, absent.
The results of the phytochemical analysis showed that all root extracts derived from M. citrifolia contained low-polar secondary metabolites including triterpenes and steroids. Comparatively, the triterpene and steroid contents were higher in the hexane extract than in other extracts owing to the low polarity of these compounds, which are highly soluble in non-polar organic solvents such as hexane and dichloromethane. Meanwhile, saponin, which is as a triterpenoid glycoside, was not found in any of the extracts. All extracts contained phenolic compounds and flavonoids as main bioactive components, with the contents being higher in the dichloromethane, ethyl acetate, and ethanolic extracts than in the hexane extract. This can be attributed to the high polarity of phenolic compounds and flavonoids owing to the presence of hydroxy groups on the aromatic rings. The results of the analysis of tannins were similar to those of the phenolic compounds in all extracts. In the alkaloid tests, only low amounts of alkaloids were detected in the ethyl acetate and ethanolic extracts. The anthraquinone content was high in the dichloromethane and ethanolic extracts. These results of the phytochemical contents in root extracts were similar to those previously reported for aqueous leaf extracts.34
1H-NMR-Based Secondary Metabolite Screening
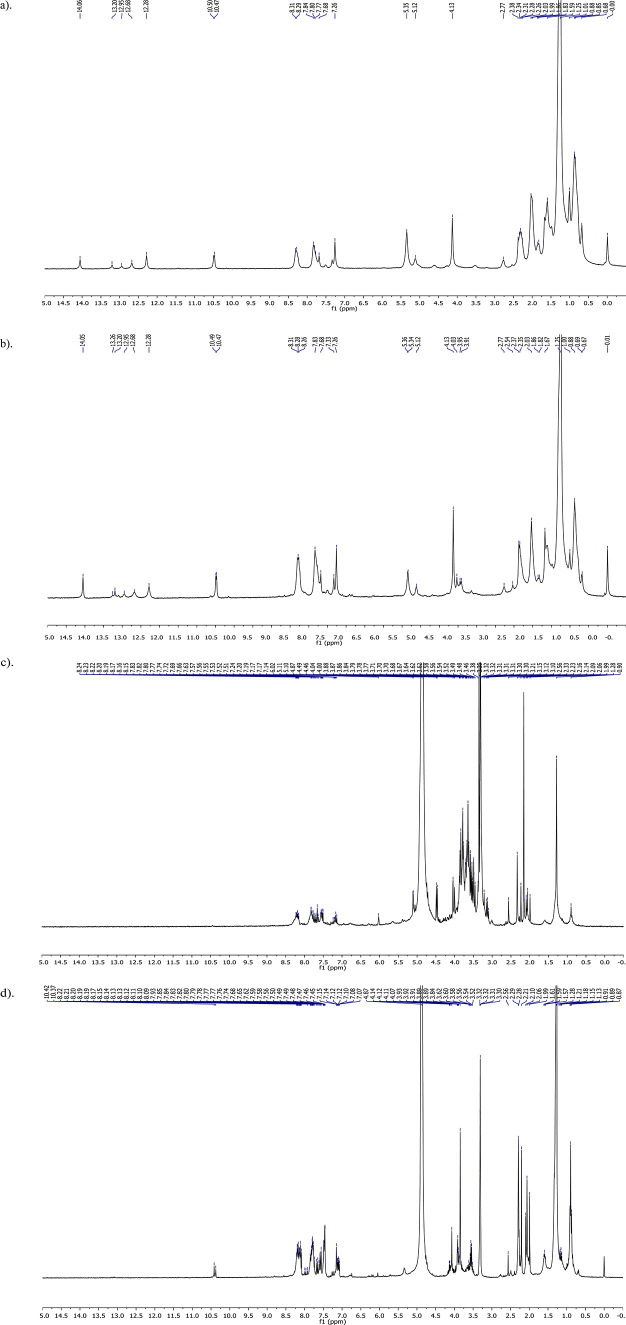
The 1H-NMR analysis of the M. citrifolia extracts (Figure 2) revealed that the hexane and dichloromethane root extracts showed a similar pattern of chemical shifts in the aliphatic (δ 0.68–2.27 ppm), olefinic (δ 5.12–5.35 ppm), and aromatic (δ 7.33–8.31 ppm) regions. The 1H-NMR spectrum of the ethyl acetate root extract showed signals in the aliphatic (δ 1.99–2.56 ppm), O- or N-heteroatomic (δ 3.10–4.49 ppm), and aromatic (δ 7.14–8.24 ppm) regions. Additionally, the 1H-NMR spectrum of the ethanolic root extract showed signals at δ 0.87–2.29, 3.52–4.14, and 7.07–8.22 ppm due to aliphatic, O- or N-heteroatomic, and aromatic protons, respectively.
Figure 2.
1H-NMR spectra of (a) hexane, (b) dichloromethane, (c) ethyl acetate, and (d) ethanolic extracts of M. citrifolia root.
The 1H-NMR spectra of the hexane and dichloromethane root extracts (Figure 2a,b) were in accordance with the presence of triterpenes and steroids (δ 0.68–2.27 ppm) and phenolics, flavonoids, tannins, and anthraquinones (δ 7.33–8.31 ppm). Meanwhile, the signals observed in the 1H-NMR spectra of the ethyl acetate and ethanolic root extracts (Figure 2c,d) were ascribable to triterpenes and steroids (δ 1.99–2.56 and 0.87–2.29 ppm, respectively), glycosides (δ 3.10–4.49 and 3.52–4.14 ppm, respectively), and phenolics, flavonoids, tannins and anthraquinones (δ 7.14–8.24 and 7.07–8.22 ppm, respectively). These results confirmed the detection of bioactive compounds using phytochemical screening tests.
Detection and Identification of Alizarin and Scopoletin via UHPLC-QQQ-MS
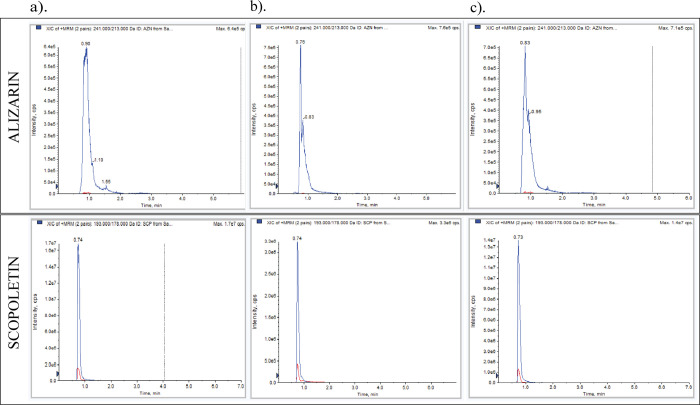
After the prescreening of secondary metabolites via 1H-NMR and phytochemical analyses, the identification and detection of bioactive alizarin and scopoletin were conducted via UHPLC-QQQ-MS analysis in the MRM mode. The mass of alizarin or scopoletin was specific for their detection in M. citrifolia root extracts. The results clearly revealed the presence of both alizarin and scopoletin in dichloromethane, ethyl acetate, and ethanol extracts (Figure 3).
Figure 3.
Detection of alizarin and scopoletin via UHPLC-QQQ-MS in (a) dichloromethane, (b) ethyl acetate, and (c) ethanol extracts of M. citrifolia root.
Quantitative Analysis of the Alizarin and Scopoletin Content via RP-HPLC
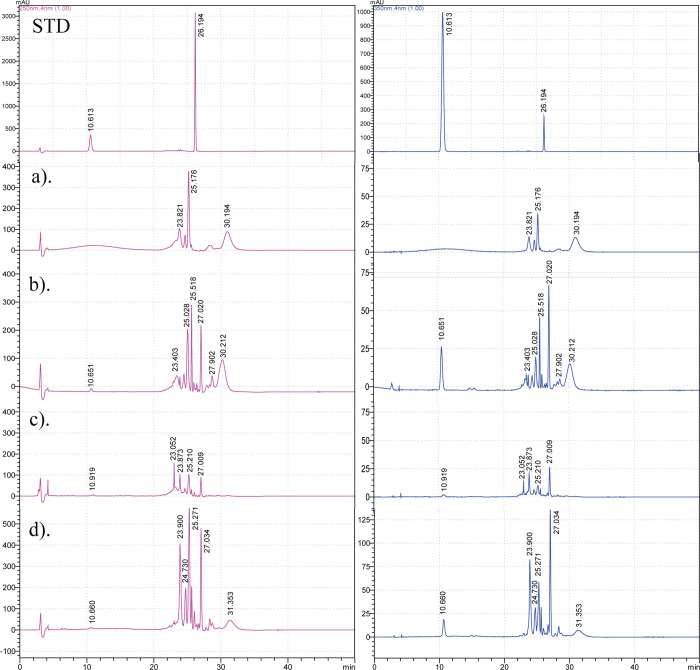
The quantitative analysis of alizarin (RT = 26.194 min, λ = 250 nm) and scopoletin (RT = 10.613 min, λ = 350 nm) in M. citrifolia root extracts was performed via RP-HPLC analysis (Figure 4). Alizarin (RT = 27.009–27.034 min) and scopoletin (RT = 10.651–10.919 min) were detected in all root extracts except for the hexane extract by comparing with the standard compounds.
Figure 4.
RP-HPLC analysis of the alizarin and scopoletin content in (a) hexane, (b) dichloromethane, (c) ethyl acetate, and (d) ethanol M. citrifolia root extracts; STD: standard (RTalizarin = 26.194 min and RTscopoletin = 10.613 min).
Next, the alizarin and scopoletin contents were quantified to be 0.552–3.227 and 0.092–0.554 g/100 g of dry weight in the dichloromethane, ethyl acetate, and ethanolic root extracts, respectively (Table 4). The highest contents of alizarin (3.227 ± 0.003 g/100 g dry weight) and scopoletin (0.554 ± 0.001 g/100 g dry weight) were found in the ethanolic and dichloromethane root extracts, respectively. According to a previous RP-HPLC analysis, a 50% ethanolic leaf extract contained 2.19% scopoletin.36 In addition, scopoletin was mainly found in an aqueous fruit extract along with a lower amount of alizarin.16 Linear regressions of alizarin and scopoletin were expressed as y = 146,124x + 96,843 (R2 = 0.9979) and y = 112,909x – 43,225 (R2 = 0.9979), respectively.
Table 4. RP-HPLC Analysis of the Alizarin and Scopoletin Content in Bioactive M. citrifolia Root Extractsa.
| M. citrifolia root extract | alizarin (g/100 g dry weight) | scopoletin (g/100 g dry weight) |
|---|---|---|
| hexane | nd | nd |
| dichloromethane | 1.631 ± 0.004b | 0.554 ± 0.001c |
| ethyl acetate | 0.552 ± 0.004a | 0.092 ± 0.000a |
| ethanol | 3.227 ± 0.003c | 0.371 ± 0.003b |
nd, not detected. Values (mean ± SD) in the same column superscripted with different letters are significantly different (p < 0.05).
Overall, the dichloromethane extract exhibited relatively strong antitubercular activity, strong antioxidant activities toward NO and DPPH radicals, and relatively high ferric reducing power compared with all the tested extracts. The potency of antitubercular and antibacterial activities of the dichloromethane extract may be attributed to the presence of various bioactive metabolites, in particular scopoletin and alizarin, which was highly found in the extract. Scopoletin is a coumarin phytoalexin essential for the plant defense mechanism. The resistance to microbial attack is correlated with the biosynthesis and accumulation of scopoletin;37 hence, it is a potent antibacterial and antifungal agent.38 A previous study reported that scopoletin isolated from Pelargonium sidoides roots exhibited antitubercular activity toward Mycobacterium smegmatis with an MIC value of 7.81 μg/mL.39 On the other hand, alizarin has been identified as a promising antitubercular agent.40 However, a further study of bioactivity-guided isolation is recommended to find the responsible active compounds against M. tuberculosis and pathogenic bacterial strains.
Conclusions
In this study, we focus on the rarely used part of M. citrifolia by investigating the in vitro antitubercular activity of M. citrifolia root extracts. In conclusion, the dichloromethane root extract derived from gradient maceration showed strong antitubercular activity with an MIC value of 50 μg/mL. The hexane, dichloromethane, ethyl acetate, and ethanolic root extracts showed inhibitory activity against all tested bacteria. Furthermore, the ethanolic and dichloromethane root extracts showed the highest antioxidant power against DPPH (IC50 = 0.82 mg/mL) and NO (IC50 = 0.64 mg/mL) radicals, respectively. Bioactive compounds including triterpenes, steroids, phenolic compounds, flavonoids, tannins, and anthraquinones were found in all root extracts. Additionally, the alizarin and scopoletin contents in the root extracts were quantified via RP-HPLC analysis. These results demonstrate the potential of M. citrifolia root extracts; however, further in vivo studies are essential for their efficacy and safety before implementing treatments for TB and bacterial infections.
Acknowledgments
We would like to sincerely thank the College of Public Health Sciences, Chulalongkorn University and all collaborators participating in this project. Additionally, we are grateful to the Chulabhorn Research Institute for the instruments and the National Omics Center for the mass spectrometry analysis and appreciate P. Poolchanuan’s assistance and advice on antibacterial activities. This work was supported by the Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (DNS 66_004_53_002_3 and DNS 63_039_53_002_1) and the Research and Development Institute Pibulsongkram Rajabhat University (RDI-2-66-64).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03656.
Radical scavenging inhibition data including DPPH, NO, and FRAP assays of M. citrifolia root extracts; RP-HPLC calibration curves of alizarin and scopoletin; and 1H-NMR spectra and UHPLC-QQQ-MS chromatograms of M. citrifolia root extracts (PDF)
Author Contributions
P.S. and A.P. contributed equally to this work. P.S. contributed to data curation, methodology, investigation, analysis, and writing—review and editing. A.P. contributed to conceptualization, data curation, methodology, investigation, analysis, funding acquisition, and writing—original draft preparation. D.L. contributed to methodology, investigation, and visualization. S.S. contributed to methodology, investigation, and visualization. P.H. contributed to supervision. P.K. contributed to supervision and writing—review and editing.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
Supplementary Material
References
- Li K.; Chung-Davidson Y. W.; Bussy U.; Li W. Recent advances and applications of experimental technologies in marine natural product research. Mar. Drugs 2015, 13, 2694–2713. 10.3390/md13052694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T.; Reker D.; Schneider P.; Schneider G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- Shen B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. Noni seed handling and seedling production. Fruits Nuts 2005, 10, 8–11. [Google Scholar]
- Almeida É. S.; de Oliveira D.; Hotza D. Properties and Applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. 10.1111/1541-4337.12456. [DOI] [PubMed] [Google Scholar]
- Torres M. A. O.; Magalhães I. d. F. B.; Mondêgo-Oliveira R.; de Sá J. C.; Rocha A. L.; Abreu-Silva A. L. One Plant, Many Uses: A Review of the Pharmacological Applications of Morinda citrifolia. Phytother. Res. 2017, 31, 971–979. 10.1002/ptr.5817. [DOI] [PubMed] [Google Scholar]
- Saludes J. P.; Garson M. J.; Franzblau S. G.; Aguinaldo A. M. Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn. (Rubiaceae). Phytother. Res. 2002, 16, 683–685. 10.1002/ptr.1003. [DOI] [PubMed] [Google Scholar]
- Mauliku N. E.; W H.; Saputro S. H.; Kristina T. N. Anti-tubercular activity of extract and compounds of noni (Morinda citrifolia Linn). Int. J. Pharm. Pharm. Sci. 2017, 9, 105–109. 10.22159/ijpps.2017v9i12.19841. [DOI] [Google Scholar]
- Singh D. R. Morinda citrifolia L. (Noni): A review of the scientific validation for its nutritional and therapeutic properties. J. Diabetes Endocrinol. 2012, 3, 77–91. 10.5897/JDE10.006. [DOI] [Google Scholar]
- World Health Organization . Global Tuberculosis Report, 2022, https://www.who.int/publications/i/item/9789240061729 (accessed 04 April 2023).
- Khawbung J. L.; Nath D.; Chakraborty S. Drug resistant Tuberculosis: A review. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101574 10.1016/j.cimid.2020.101574. [DOI] [PubMed] [Google Scholar]
- Xi Y.; Zhang W.; Qiao R.-J.; Tang J. Risk factors for multidrug-resistant tuberculosis: A worldwide systematic review and meta-analysis. PLoS One 2022, 17, e0270003 10.1371/journal.pone.0270003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sina H.; Dramane G.; Tchekounou P.; et al. Phytochemical composition and in vitro biological activities of Morinda citrifolia fruit juice. Saudi J. Biol. Sci. 2021, 28, 1331–1335. 10.1016/j.sjbs.2020.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.-M.; Wang W.; Zhang J.-J.; et al. Antibacterial Constituents of Hainan Morinda citrifolia (Noni) Leaves. J. Food Sci. 2016, 81, M1192–M1196. 10.1111/1750-3841.13302. [DOI] [PubMed] [Google Scholar]
- Pawlus A. D.; Kinghorn D. A. Review of the ethnobotany, chemistry, biological activity and safety of the botanical dietary supplement Morinda citrifolia (noni). J. Pharm. Pharmacol. 2007, 59, 1587–1609. 10.1211/jpp.59.12.0001. [DOI] [PubMed] [Google Scholar]
- Jamaludin R.; Kim D.-S.; Salleh L. M.; Lim S.-B. Kinetic Study of Subcritical Water Extraction of Scopoletin, Alizarin, and Rutin from Morinda citrifolia. Foods 2021, 10, 2260. 10.3390/foods10102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Jiang J.-G. Health functions and structure–activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6063–6080. 10.1039/C8FO01569D. [DOI] [PubMed] [Google Scholar]
- Lee J.-H.; Kim Y.-G.; Yong Ryu S.; Lee J. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. 10.1038/srep19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holiyachi M.; Shastri S. L.; Chougala B. M.; et al. Design, Synthesis and Structure-Activity Relationship Study of Coumarin Benzimidazole Hybrid as Potent Antibacterial and Anticancer Agents. ChemistrySelect 2016, 1, 4638–4644. 10.1002/slct.201600665. [DOI] [Google Scholar]
- Dixon A. R.; McMillen H.; Etkin N. L. Ferment this: The transformation of Noni, a traditional polynesian medicine (Morinda Citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. 10.1007/BF02860792. [DOI] [Google Scholar]
- Kerr PG. Plants and Tuberculosis. Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Academic Press: San Diego, 2013, 45–64. [Google Scholar]
- McClatchey W. From Polynesian healers to health food stores: changing perspectives of Morinda citrifolia (Rubiaceae). Integr. Cancer Ther. 2002, 1, 110–120. 10.1177/1534735402001002002. [DOI] [PubMed] [Google Scholar]
- Sam-ang P.; Surangkul D.; Savaspun K.; Phanumartwiwath A. Antioxidant and acetylcholinesterase inhibitory activities of Morinda citrifolia L. extracts. Agric. Nat. Resour. 2020, 54, 173–179. [Google Scholar]
- Phanumartwiwath A.; Boonchaisri S.; Savaspun K.; Chodnakarin A.; Sam-ang P. Potential alpha-glucosidase inhibitory activity of root extracts from Morinda citrifolia L. Agric. Nat. Resour. 2022, 56, 815–824. [Google Scholar]
- Collins L.; Franzblau S. G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. 10.1128/AAC.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschek E.; Brown D. F. J.; Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- Thadhani V. M.; Choudhary M. I.; Ali S.; Omar I.; Siddique H.; Karunaratne V. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. 10.1080/14786419.2010.529546. [DOI] [PubMed] [Google Scholar]
- Gul R.; Jan S. U.; Faridullah S.; Sherani S.; Jahan N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. ScientificWorldJournal 2017, 2017, 5873648 10.1155/2017/5873648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB. Phytochemical Methods. Second ed.; Springer: Dordrecht, 1984. [Google Scholar]
- Yadav M.; Chatterji S.; Gupta S. K.; Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci. 2014, 6, 539–542. [Google Scholar]
- Sakulpanich A.; Gritsanapan W. Extraction Method for High Content of Anthraquinones from Cassia fistula Pods. J. Health Res. 2018, 22, 167–172. [Google Scholar]
- De La Cruz-Sánchez N. G.; Gómez-Rivera A.; Alvarez-Fitz P.; et al. Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb. Pathog. 2019, 128, 347–353. 10.1016/j.micpath.2019.01.030. [DOI] [PubMed] [Google Scholar]
- Sunder J.; Singh D. R.; Sakthivel J.; Kundu A.; Srivastava R. Antimicrobial activity of Morinda citrifolia solvent extracts. Indian Vet. J. 2012, 89, 9–11. [Google Scholar]
- Serafini M. R.; Santos R. C.; Guimarães A. G.; et al. Morinda citrifolia Linn Leaf Extract Possesses Antioxidant Activities and Reduces Nociceptive Behavior and Leukocyte Migration. J. Med. Food 2011, 14, 1159–1166. 10.1089/jmf.2010.0254. [DOI] [PubMed] [Google Scholar]
- Vennila S.; Brindha D. Antioxidant and free radical scavenging effect of Morinda citrifolia fruit extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 55–59. [Google Scholar]
- Lim S.-L.; Goh Y.-M.; Noordin M. M.; et al. Morinda citrifolia edible leaf extract enhanced immune response against lung cancer. Food Funct. 2016, 7, 741–751. 10.1039/C5FO01475A. [DOI] [PubMed] [Google Scholar]
- Gnonlonfin G. J. B.; Sanni A.; Brimer L. Review Scopoletin – A Coumarin Phytoalexin with Medicinal Properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. 10.1080/07352689.2011.616039. [DOI] [Google Scholar]
- Antika L. D.; Tasfiyati A. N.; Hikmat H.; Septama A. W. Scopoletin: a review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforsch. C 2022, 77, 303–316. 10.1515/znc-2021-0193. [DOI] [PubMed] [Google Scholar]
- Mativandlela S. P. N.; Meyer J. J. M.; Hussein A. A.; Lall N. Antitubercular Activity of Compounds Isolated from Pelargonium sidoides. Pharm. Biol. 2007, 45, 645–650. 10.1080/13880200701538716. [DOI] [Google Scholar]
- Zhu M.; Zhu L. The research progress in drug resistant mechanisms of Mycobacterium tuberculosis and its L-form and antibacterial effect of alizarin. Chin. J. Microecol. 2013, 25, 97–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.