Abstract
Background
The current research aimed to evaluate the relationship between fruit, vegetable (FV), and dairy consumption with the odds of developing hypertension based on nationwide Stepwise approach to surveillance (STEPS) data in Iran.
Methods
This cross-sectional study was accomplished by the research center of non-communicable diseases (NCDs) in Tehran. In total, 29,378 individuals’ data were analyzed. Participants were classified into normal, elevated BP, stage I, and stage II hypertension according to systolic blood pressure (SBP) and diastolic blood pressure (DBP) examinations. Based on the STEPS questionnaire, the consumption of FVs and dairy products was evaluated. Multinomial logistic regression was applied to assess the relationship between the consumption of FVs and dairy products with hypertension.
Results
The findings revealed that only fruit consumption (≥ 2 servings/day) was negatively related to stage I hypertension (odds ratio (OR) = 0.81; 95% confidence interval (CI): 0.69–0.95) in two servings per day and OR = 0.81; 95% CI: 0.68–0.96 in > two servings per day) in the adjusted model. There was no significant relationship between consuming vegetables and dairy products with elevated BP and hypertension.
Conclusion
Our study showed that increasing fruit consumption was related to reducing hypertension odds. Regarding the consumption of dairy products and vegetables, no significant relationship was found with the odds of hypertension. More studies, especially cohorts, are needed to evaluate the impacts of FV and dairy products on the risk of hypertension.
Keywords: Fruit, Vegetable, Dairy, Hypertension, STEPS
Introduction
Hypertension is an important preventable risk factor for cardiovascular diseases (CVDs), including heart failure, stroke, and myocardial infarction [1, 2]. Hypertension is considered as systolic blood pressure (SBP) equal to or more than 140 mmHg or diastolic blood pressure (DBP) equal to or more than 90 mmHg [3]. The hypertension prevalence is rapidly increasing [4]. The prevalence of this disease has been reported at 19.2% in Iranian samples [5].
Risk factors associated with hypertension include genetic factors, dietary factors, physical inactivity, and obesity [6]. Dietary patterns, as one of the lifestyle factors, can have an important effect on the continuous prevalence of hypertension [7].
Fruits and vegetables (FV) are an important part of the diet, containing vitamins, minerals, and folic acid that benefit endothelial function [8, 9]. Endothelial dysfunction is recognized as a hypertension risk factor [10]. Some studies have shown an inverse relationship between FV intake and hypertension [11, 12]. In contrast, other studies failed to show an effect of increased FV consumption on lowering the risk of hypertension [13, 14].
Overall, a healthy diet is vital for preventing hypertension [15]. However, whether dairy products should be included in such a diet is controversial [16]. Some epidemiological studies have shown an inverse relationship between dairy products and hypertension [17, 18]. The protective impacts of dairy products on hypertension can be caused by bioactive peptides such as lactotripeptides and micronutrients such as potassium, calcium, and magnesium [19, 20]. However, some studies have reported no association between dairy consumption and hypertension [21, 22].
Therefore, since there are inconsistent findings regarding the association between the consumption of FVs and dairy products with hypertension in different studies, and considering that no study with a large sample has been conducted on the Iranian population, the current study aimed to evaluate the relationship between the intake of FVs and dairy products with hypertension by applying data from the nationwide Stepwise approach to surveillance (STEPS) survey in Iran in 2016.
Methods
Sampling and design
This cross-sectional study was conducted by the Tehran University of Medical Sciences at the Non-Communicable Diseases (NCDs) Research Center [23]. This study used the STEPS questionnaire adapted from the World Health Organization (WHO) [24]. The validity and reliability of this questionnaire have already been evaluated [23]. This national and sub-national study was done in all provinces of Iran (except Qom) in 2016. The number of 30,541 participants was selected by cluster random sampling method based on each province’s population and fulfilled the STEPS questionnaire in the study’s first phase. The eligible participants selected based on aged ≥ 18 years and residing in Iran at the time of study. Also, participants were selected by postal code and national ID. In the next step, the history of NCD, medical risk factors, and data related to individuals’ lifestyles and socio-demographic characteristics were evaluated. In the next level, after a physical checkup, 30,042 participants were chosen. Ultimately, 29,378 participants were included in the final analysis (those whose SBP and DBP were assessed). In phases 2 and 3, anthropometric information and laboratory tests were evaluated. People over 18 years old were examined in the present study. Our study was confirmed by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.SCHEANUT.REC.1400.035).
Measurements
SBP and DBP were measured in a sitting position for participants after five minutes of rest (BM 20, Beurer, Germany). This evaluation was repeated two times. The mean of evaluations was used for interpretation. Participants were classified into normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated BP (SBP 120 to 129 mmHg or DBP 80 to 84 mmHg), stage I hypertension (SBP 130 to 139 mmHg or DBP 85 to 89 mmHg), and stage II hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) based on SBP and DBP examination [25]. Based on standard methods, height and weight were measured, and body mass index (BMI) was computed based on: weight (kg) / height (m2) [23].
The Global Physical Activity Questionnaire (GPAQ) was used to evaluate the participants’ physical activity levels. The data were converted to the metabolic equivalent of tasks (METs)-minutes per week for physical activity domains based on the GPAQ Instrument [23]. The details of physical activity calculation based on METs have been published in a previous study [26].
Dietary factors
The following question was asked to find dietary intakes: “How many servings of dairy products or fruits or vegetables do you usually eat daily?”. One cup of dairy products, half a cup of cooked vegetables or one cup of raw vegetables, and one medium fruit was considered the amount of one serving. The consumption of dairy, vegetables, and fruits was categorized into:
Vegetable intake: less than 2, 2, 3, and more than 3 servings per day.
Fruit intake: less than 1, 1, 2, and > 2 servings per day.
Dairy product intake: <1, 1, 2, and > 2 servings per day [27].
Statistical analysis
SPSS (version 25, SPSS Inc., Chicago IL, USA) was applied to analyze the data. A p-value < 0.05 was defined as statistically significant. Also, R software (version 3.0.2) was used for figures’ depictions. Baseline characteristics of this study were shown as median (interquartile range (IQR)) or percentage and means ± standard deviations (SDs). The chi-square test and analysis of variance (ANOVA) or Kruskal-Wallis test were used for categorical and continuous variables, respectively. Also, multinomial logistic regression was used to assess the relationship between dairy product, vegetable, and fruit consumption with blood pressure (BP) category. Participants were classified into normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated BP (SBP 120 to 129 mmHg or DBP 80 to 84 mmHg), stage I hypertension (SBP 130 to 139 mmHg or DBP 85 to 89 mmHg), and stage II hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) based on SBP and DBP examination. In the adjusted model, gender, education, age, medication, occupation, marital status, area of residency, physical activity, wealth index (WI), and smoking history were controlled. The WI was calculated using the principal component analysis (PCA) method. Bartlett and Kaiser-Meier-Olkin (KMO) tests were utilized to reduce data. The WI was divided into five categories so that the first category represented the poorest group regarding income, and the last category presented the richest one [28].
Results
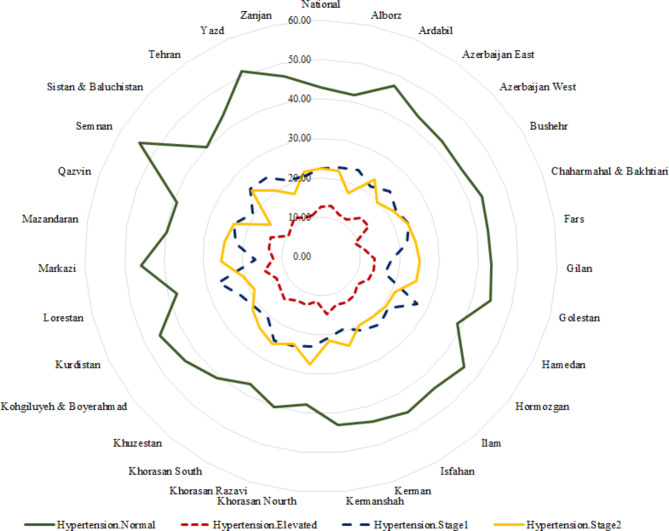
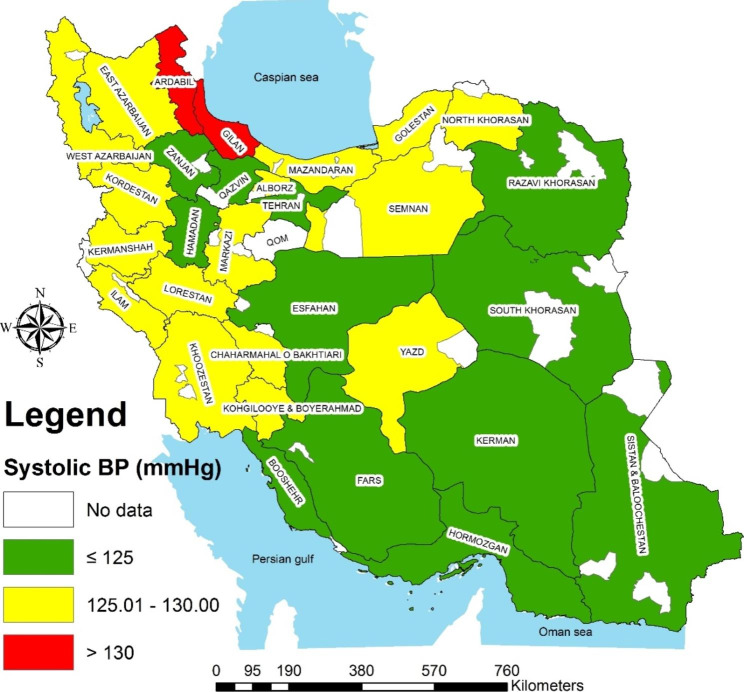
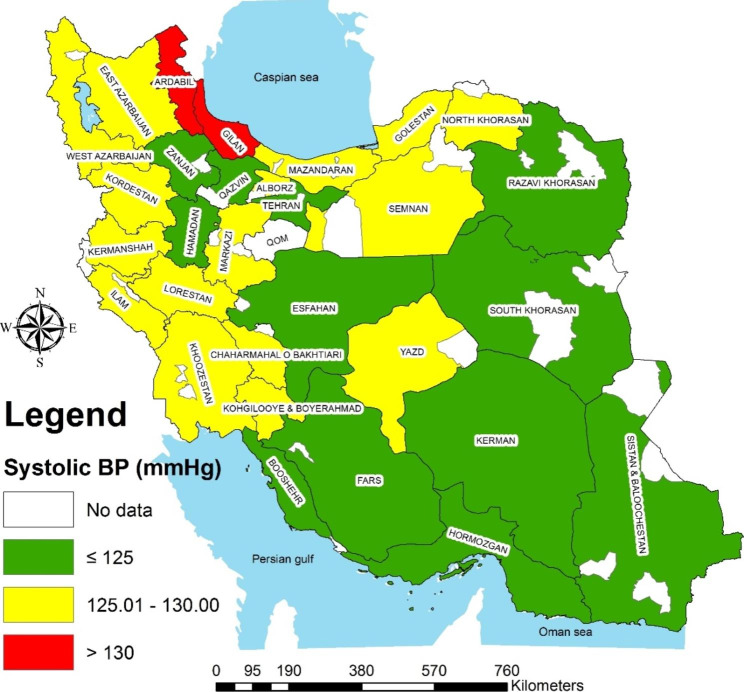
In total, 29,378 individuals’ data were analyzed. Males comprised 52.3% of participants, and 29.6% lived in rural areas. The study participants’ mean age, education, and physical activity were 44.5 years, 8.2 years, and 1,684 MET/min/week, respectively (Table 1). Based on hypertension classification, there were significant differences in gender, area of sedentary, marital status, occupation, WI, age, BMI, physical activity, and education (P<0.001 for all). Also, the prevalence of normal hypertension, elevated, stage I, and II are presented in Fig. 1 (43.0%, 12.5%, 22.4%, and 22.1%, respectively) (distribution of hypertension at the subnational level is shown in Figs. 2 and 3).
Table 1.
Socio-demographic characteristics of the study population
| Variables | Normal (12,657) |
Elevated (3,670) | Stage I (6,554) |
Stage II (6,497) |
Total (29,378) |
P-value |
|---|---|---|---|---|---|---|
| Gender, female, % | 54.0 | 50.5 | 52.3 | 50.2 | 52.3 | <0.001 |
| Area, rural, % | 28.0 | 32.9 | 28.2 | 32.4 | 29.6 | <0.001 |
| Marital status, single, % | 12.5 | 15.8 | 17.2 | 15.8 | 14.7 | <0.001 |
| Occupation | <0.001 | |||||
|
Employee Worker Self-employed housewife Other |
7.9 5.6 21.6 45.1 19.9 |
7.8 7.1 26.9 40.4 17.9 |
8.5 6.8 24.1 42.4 18.2 |
9.1 6.3 26.4 40.1 18.1 |
8.3 6.2 23.9 42.8 18.9 |
|
| Smoking, no, % | 79.7 | 80.2 | 80.6 | 79.3 | 79.9 | 0.256 |
| WI | <0.001 | |||||
|
Poorest Poor Moderate Rich Richest |
19.1 19.7 19.8 19.7 21.6 |
21.8 20.2 18.9 20.6 18.5 |
20.0 20.6 20.7 19.9 18.8 |
19.6 21.0 20.3 20.2 18.8 |
19.7 20.3 20.0 20.0 20.0 |
|
| Age, years | 47.4 ± 17.1 | 42.5 ± 15.3 | 41.6 ± 15.2 | 42.9 ± 15.3 | 44.5 ± 16.2 | <0.001 |
| BMI (kg/m2) | 26.9 ± 5.2 | 26.1 ± 4.9 | 26.2 ± 4.9 | 26.3 ± 5.1 | 26.5 ± 5.1 | <0.001 |
| Physical activity, MET/min/week | 360.0 (1680.0) | 456.0 (2100.0) | 420.0 (1840.0) | 480.0 (2160.0) | 400.0 (1904.0) | <0.001 |
| Education, years | 8.0 (9.0) | 9.0 (7.0) | 10.0 (7.0) | 9.0 (7.0) | 9.0 (7.0) | <0.001 |
BMI, body mass index; MET, metabolic equivalent of task; WI, wealth index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure
Values are mean ± SD for continuous and percentage for categorical variables
BP category: normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated BP (SBP 120 to 129 mmHg or DBP 80 to 84 mmHg), stage I hypertension (SBP 130 to 139 mmHg or DBP 85 to 89 mmHg), and stage II hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg)
Fig. 1.
The prevalence of hypertension classification based on the national and subnational level
Fig. 2.
The distribution of systolic blood pressure at the level of the province
Fig. 3.
The distribution of diastolic blood pressure at the level of the province
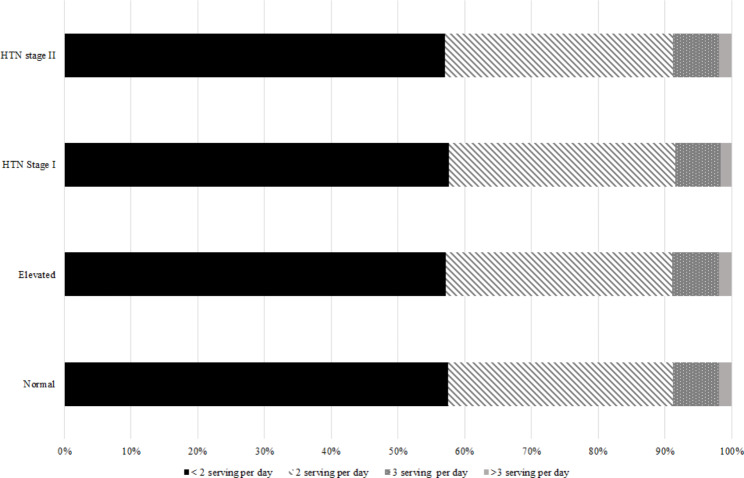
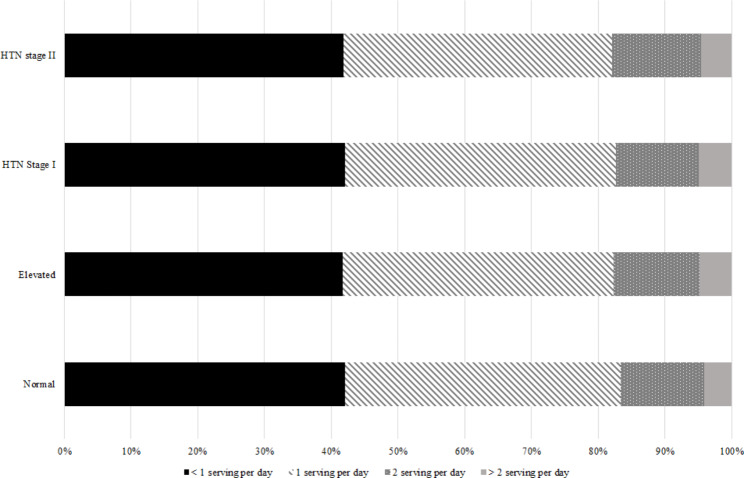
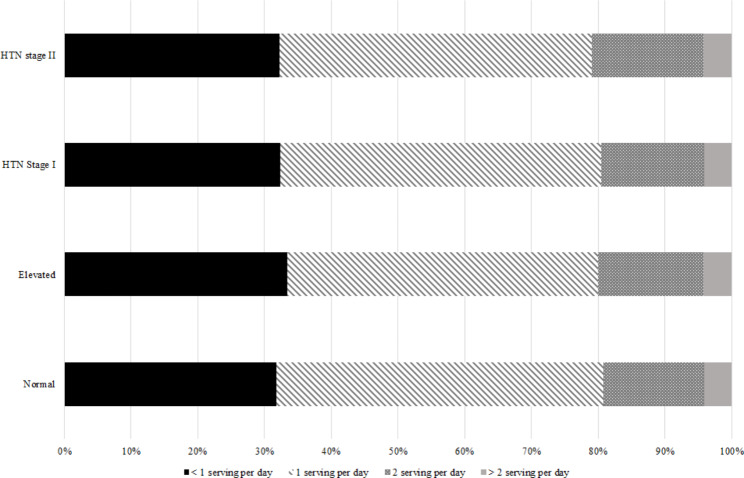
Also, the distribution of FV and dairy product consumption according to the classification of HTN is shown in Fig. 4A, 4B and 4C.
Fig. 4A.
Distribution of vegetable consumption based on hypertension classification
Fig. 4B.
Distribution of fruit consumption based on hypertension classification
Fig. 4C.
Distribution of dairy product consumption based on hypertension classification
According to Table 2 and Table 3, compared to less than one serving of fruit intake/day, we observed a reverse association between consumption of one, two, and more than two servings per day of fruits with the odds of stage I hypertension in the crude model (one serving – odds ratio (OR) = 0.82; 95% confidence interval (CI): 0.71–0.94–2 servings - OR = 0.80; 95% CI: 0.69–0.93 and more than two servings - OR = 0.80; 95% CI: 0.68–0.94). In the adjusted model, the odds of stage I hypertension was significantly decreased in participants who consumed two servings (OR = 0.81; 95% CI: 0.69–0.95) and more than two servings per day of fruits (OR = 0.81; 95% CI: 0.68–0.96) in comparison to less than one serving of fruit consumption. Also, neither crude nor adjusted models found any association between the consumption of vegetables and dairy products and the odds of hypertension.
Table 2.
The association between vegetable, fruit, and dairy product consumption with BP classification in the crude model
| Vegetable Intake | ||||
|---|---|---|---|---|
| Variables | < 2 servings | 2 servings | 3 servings | > 3 servings |
| Elevated vs. normal hypertension* | Ref. | 0.98 (0.74–1.29) | 1.00 (0.76–1.32) | 1.13 (0.84–1.54) |
| Stage I hypertension vs. normal hypertension | Ref. | 1.10 (0.87–1.38) | 1.11 (0.88–1.39) | 1.22 (0.95–1.57) |
| Stage II hypertension vs. normal hypertension | Ref. | 0.97 (0.78–1.21) | 1.00 (0.80–1.25) | 1.11 (0.87–1.42) |
| Fruit Intake | ||||
| Variables | < 1 serving | 1 serving | 2 servings | > 2 servings |
| Elevated vs. normal hypertension | Ref. | 0.85 (0.71–1.02) | 0.85 (0.71–1.02) | 0.88 (0.72–1.08) |
| Stage I hypertension vs. normal hypertension | Ref. | 0.82 (0.71–0.94) | 0.80 (0.69–0.93) | 0.80 (0.68–0.94) |
| Stage II hypertension vs. normal hypertension | Ref. | 0.88 (0.76–1.02) | 0.86 (0.74-1.00) | 0.94 (0.80–1.11) |
| Dairy Product Intake | ||||
| Variables | < 1 serving | 1 serving | 2 servings | > 2 servings |
| Elevated vs. normal hypertension | Ref. | 1.02 (0.84–1.24) | 0.92 (0.76–1.11) | 1.02 (0.83–1.25) |
| Stage I hypertension vs. normal hypertension | Ref. | 1.00 (0.86–1.17) | 0.96 (0.83–1.12) | 1.00 (0.85–1.18) |
| Stage II hypertension vs. normal hypertension | Ref. | 0.96 (0.82–1.12) | 0.91 (0.78–1.06) | 1.05 (0.89–1.23) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure
BP category: normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated BP (SBP 120 to 129 mmHg or DBP 80 to 84 mm Hg), stage I hypertension (SBP 130 to 139 mm Hg or DBP 85 to 89 mmHg), and stage II hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg)
These values are odds ratio (95% CIs) and obtained from multinomial regression
* Reference category
Table 3.
The association between vegetable, fruit, and dairy product consumption with BP classification in the adjusted model
| Vegetable Intake | ||||
|---|---|---|---|---|
| Variables | < 2 servings | 2 servings | 3 servings | > 3 servings |
| Elevated vs. normal hypertension* | Ref. | 0.92 (0.68–1.26) | 0.92 (0.68–1.26) | 1.09 (0.78–1.53) |
| Stage I hypertension vs. normal hypertension | Ref. | 0.99 (0.77–1.28) | 0.99 (0.76–1.28) | 1.11 (0.84–1.47) |
| Stage II hypertension vs. normal hypertension | Ref. | 0.90 (0.70–1.16) | 0.91 (0.71–1.17) | 1.04 (0.79–1.38) |
| Fruit Intake | ||||
| Variables | < 1 serving | 1 serving | 2 servings | > 2 servings |
| Elevated vs. normal hypertension | Ref. | 0.90 (0.74–1.10) | 0.88 (0.72–1.07) | 0.89 (0.71–1.10) |
| Stage I hypertension vs. normal hypertension | Ref. | 0.85 (0.73-1.00) | 0.81 (0.69–0.95) | 0.81 (0.68–0.96) |
| Stage II hypertension vs. normal hypertension | Ref. | 0.93 (0.79–1.10) | 0.90 (0.76–1.06) | 0.97 (0.81–1.16) |
| Dairy Product Intake | ||||
| Variables | < 1 serving | 1 serving | 2 servings | > 2 servings |
| Elevated vs. normal hypertension | Ref. | 1.09 (0.88–1.36) | 1.01 (0.81–1.25) | 1.02 (0.81–1.29) |
| Stage I hypertension vs. normal hypertension | Ref. | 1.03 (0.86–1.23) | 0.97 (0.81–1.16) | 1.00 (0.83–1.21) |
| Stage II hypertension vs. normal hypertension | Ref. | 1.03 (0.86–1.23) | 0.95 (0.80–1.14) | 1.05 (0.87–1.27) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure
BP category: normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated BP (SBP 120 to 129 mmHg or DBP 80 to 84 mmHg), stage I hypertension (SBP 130 to 139 mmHg or DBP 85 to 89 mmHg), and stage II hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg)
Adjusted for gender, age, BMI, marital status, physical activity, education, occupation, area of residency, wealth index, and smoking history
These values are odds ratio (95% CIs) and obtained from multinomial regression
* Reference category
Discussion
In the crude model, we found that the odds of developing stage I hypertension was lower in people who consumed more fruits (more than one serving). Also, in the adjusted model, only fruit consumption (≥ 2 servings/day) was negatively associated with stage I hypertension. Moreover, there was no significant relationship between consuming vegetables and dairy products with elevated BP and hypertension.
The results of a cross-sectional study indicated that a plant-based diet had a beneficial effect on hypertension [7]. There are several mechanisms for the association between FV intake and hypertension risk. FV are high in vitamin C, magnesium, folic acid, potassium, carotenoid, and flavonoid, which can help reduce BP [6]. Also, FV intake cause an increasing intake of fiber and decreasing fat intake [29]. It has been shown that people who consumed more dietary fiber were less prone to hypertension [30]. Moreover, increased consumption of FV may decrease BP by improving anthropometric parameters of the body, such as reducing overweight or obesity [31].
The findings showed the inverse relationship between fruit consumption and hypertension. The current study’s findings were consistent with some other studies. A study by Kim et al. revealed that a higher fruit intake was inversely correlated to hypertension risk in older and middle-aged adults [32]. Also, Borgi et al. reported that higher whole fruit intake decreased the hypertension risk [33]. Moreover, a systematic review and meta-analysis study showed that increasing fruit consumption by almost 300 gr/day reduced hypertension risk by 7% [34].
As previously mentioned, there was no correlation between the consumption of vegetables and hypertension odds in the current study. A study by Kim et al. proved no significant association between vegetable intake and hypertension [32]. Also, three prospective cohort studies showed no significant association between vegetable consumption and hypertension risk [33]. Contrary to the present study’s findings, Wang et al. indicated a negative relationship between vegetable intake and hypertension [35]. Further, Utsugi et al. reported a negative relationship between vegetable consumption and hypertension [11]. The difference in the population, samples, and definitions of hypertension in the mentioned studies and the present study can be the reason for their inconsistent results with the current research.
A study by Muslimi et al. on Iranian samples showed that 53.33% of the participants fried vegetables moderately [36]. Also, Djazayery et al. showed that 77% of Iranian women fried or boiled vegetables for a long time [37]. On the other hand, Miglio et al. indicated that frying vegetables reduced their antioxidant compounds. Also, their results showed that frying and boiling vegetables decreased their nutritional quality [38]. Cooking practices of vegetables in Iran may be the reason for the present study’s lack of a relationship between vegetable consumption and hypertension.
The present study found no significant relationship between dairy product consumption and elevated BP and hypertension. In line with our results, research by Villaverde et al. demonstrated no significant association between the overall intake of dairy products and hypertension in a prospective cohort study [39]. Also, Heraclides et al. reported that specific dairy subgroups (fermented, low-fat, and full-fat) or total dairy products were unrelated to BP and hypertension after ten years of follow-up [40]. While in a systematic review and meta-analysis study on cohort studies, an inverse and significant relationship was found between the consumption of low-fat dairy products and hypertension risk. Also, a study found a nonlinear relationship between total dairy and hypertension [41]. The relationship between different types of dairy products and hypertension risk has been examined. In a 15-year prospective study by Steffen et al., cheese consumption did not significantly correlate with hypertension [42]. However, a study found an inverse relationship between yogurt consumption and hypertension risk [43]. Besides recommendations on other food groups’ intake, the Dietary Approaches to Stop Hypertension (DASH) diet has emphasized intaking more than two servings/day of low-fat dairy products [44]. Most clinical trial studies have shown the role of the DASH diet in lowering BP [45, 46]. The present study assessed total dairy product consumption without separating low-fat versus high-fat dairy products. It may be the reason for the non-association between dairy products and hypertension. Dairy products are the primary sources of calcium, which has a beneficial role in reducing BP in patients with hypertension [47, 48]. A systematic review study by Cormick et al. revealed that by increasing calcium intake, SBP and DBP decreased by 1.37 mm Hg and 1.45 mmHg, respectively. This effect was greater with doses of calcium > 1000 mg daily [49]. However, the impact of dairy products on hypertension is not apparent [50, 51], especially in the elderly, as the BP-lowering response to dairy consumption may vary with age [52].
Among the strengths of the current research, we can mention the large sample size and the adjustment of the effect of confounders. Since this study was a cross-sectional study, it is impossible to discuss the effect mechanisms of fruit and dairy product intake on hypertension. Also, due to the unavailability of data on low-fat and high-fat dairy products, it was impossible to investigate their effect separately on hypertension. In addition, the unavailability of data on energy and salt intake was another limitation of the present study.
Conclusion
In conclusion, our study showed that increasing fruit consumption was related to reducing hypertension odds. Regarding the consumption of dairy products and vegetables, no significant relationship was found with the odds of hypertension. More studies, especially cohorts, are needed to assess the impacts of FV and dairy products on the risk of hypertension.
Acknowledgements
Not applicable.
Author contributions
M.N, Z.S and M.V; Contributed to writing the first draft. M.N and M.V; Contributed to all data, statistical analysis, and interpretation of data. S.F.; Contributed to the research concept, supervised the work, and revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data Availability
To protect study participant privacy, data cannot be shared openly, but data are available through a reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was approved by the Medical Research and Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.SCHEANUT.REC.1400.035), and all participants completed the informed consent. Also, we confirmed all the methods included in this study are in accordance with the declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. The Lancet. 2018;392(10159):1923–94. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, De Ferranti S, Després J-P, Fullerton HJ. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of Population-Based Studies from 90 countries. Circulation. 2016;134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermansen K. Diet, blood pressure and hypertension. Br J Nutr. 2000;83(S1):113–S119. doi: 10.1017/s0007114500001045. [DOI] [PubMed] [Google Scholar]
- 5.Najafipour H, Nasri HR, Rostamzadeh F, Amirzadeh R, Shadkam M, Mirzazadeh A. Prevalence and incidence of pre-hypertension and hypertension (awareness/control) in Iran: findings from Kerman coronary artery diseases risk factors study 2 (KERCADRS) J Hum Hypertens. 2022;36(5):461–72. doi: 10.1038/s41371-020-00392-5. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Li F, Wang L, Zhang D. Fruit and vegetables consumption and risk of hypertension: a meta-analysis. J Clin Hypertens. 2016;18(5):468–76. doi: 10.1111/jch.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuven Res. 2007;10(2):225–34. doi: 10.1089/rej.2006.0529. [DOI] [PubMed] [Google Scholar]
- 8.Tamai Y, Wada K, Tsuji M, Nakamura K, Sahashi Y, Watanabe K, Yamamoto K, Ando K, Nagata C. Dietary intake of vitamin B12 and folic acid is associated with lower blood pressure in japanese preschool children. Am J Hypertens. 2011;24(11):1215–21. doi: 10.1038/ajh.2011.133. [DOI] [PubMed] [Google Scholar]
- 9.May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28(9):1421–9. doi: 10.1016/s0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 10.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed research international 2014, 2014. [DOI] [PMC free article] [PubMed]
- 11.Utsugi MT, Ohkubo T, Kikuya M, Kurimoto A, Sato RI, Suzuki K, Metoki H, Hara A, Tsubono Y, Imai Y. Fruit and vegetable consumption and the risk of hypertension determined by self measurement of blood pressure at home: the Ohasama study. Hypertens Res. 2008;31(7):1435–43. doi: 10.1291/hypres.31.1435. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin P-H, Svetkey LP. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA: J Am Med Association 2003. [DOI] [PubMed]
- 13.Berry SE, Mulla UZ, Chowienczyk PJ, Sanders TA. Increased potassium intake from fruit and vegetables or supplements does not lower blood pressure or improve vascular function in UK men and women with early hypertension: a randomised controlled trial. Br J Nutr. 2010;104(12):1839–47. doi: 10.1017/S0007114510002904. [DOI] [PubMed] [Google Scholar]
- 14.McCall DO, McGartland CP, McKinley MC, Patterson CC, Sharpe P, McCance DR, Young IS, Woodside JV. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation. 2009;119(16):2153–60. doi: 10.1161/CIRCULATIONAHA.108.831297. [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: mendelian randomization study. BMJ 2017, 356. [DOI] [PMC free article] [PubMed]
- 17.Wang H, Fox CS, Troy LM, Mckeown NM, Jacques PF. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: the Framingham Heart Study. Br J Nutr. 2015;114(11):1887–99. doi: 10.1017/S0007114515003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong G, Sun Q, Yu D, Zhu J, Sun L, Ye X, Li H, Jin Q, Zheng H, Hu FB. Dairy consumption, type 2 diabetes, and changes in cardiometabolic traits: a prospective cohort study of middle-aged and older chinese in Beijing and Shanghai. Diabetes Care. 2014;37(1):56–63. doi: 10.2337/dc13-0975. [DOI] [PubMed] [Google Scholar]
- 19.McGrane MM, Essery E, Obbagy J, Lyon J, MacNeil P, Spahn J, Van Horn L. Dairy consumption, blood pressure, and risk of hypertension: an evidence-based review of recent literature. Curr Cardiovasc risk Rep. 2011;5(4):287–98. doi: 10.1007/s12170-011-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-León E-E, Román-Vinas B, Serra-Majem L. Dairy products and health: a review of the epidemiological evidence. Br J Nutr. 2006;96(S1):94–S99. doi: 10.1079/bjn20061709. [DOI] [PubMed] [Google Scholar]
- 21.Samara A, Herbeth B, Ndiaye NC, Fumeron F, Billod S, Siest G, Visvikis-Siest S. Dairy product consumption, calcium intakes, and metabolic syndrome–related factors over 5 years in the STANISLAS study. Nutrition. 2013;29(3):519–24. doi: 10.1016/j.nut.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Snijder MB, Van Dam RM, Stehouwer CD, Hiddink GJ, Heine RJ, Dekker JM. A prospective study of dairy consumption in relation to changes in metabolic risk factors: the Hoorn Study. Obesity. 2008;16(3):706–9. doi: 10.1038/oby.2007.93. [DOI] [PubMed] [Google Scholar]
- 23.Djalalinia S, Modirian M, Sheidaei A, Yoosefi M, Zokaiee H, Damirchilu B, Mahmoudi Z, Mahmoudi N, Hajipour MJ, Peykari N. Protocol design for large–scale cross–sectional studies of surveillance of risk factors of non–communicable diseases in Iran: STEPs 2016. Arch Iran Med. 2017;20(9):–. [PubMed]
- 24.Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes: implications for clinical practice. Prim Care: Clin Office Pract. 1999;26(4):771–90. doi: 10.1016/s0095-4543(05)70130-5. [DOI] [PubMed] [Google Scholar]
- 25.Cardiology ACo. : New ACC/AHA high blood pressure guidelines lower definition of hypertension. ACC News Story 2017.
- 26.Mohebi F, Mohajer B, Yoosefi M, Sheidaei A, Zokaei H, Damerchilu B, Mehregan A, Shahbal N, Rezaee K, Khezrian M. Physical activity profile of the iranian population: STEPS survey, 2016. BMC Public Health. 2019;19(1):1–17. doi: 10.1186/s12889-019-7592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajipour M, Zamaninour N, Yoosefi M, Soleimanzadehkhayat M, Pazhuheian F. Distribution of dietary risk factors in Iran: National and sub-national burden of disease. Arch Iran Med. 2021;24(1):48–57. doi: 10.34172/aim.2021.08. [DOI] [PubMed] [Google Scholar]
- 28.Djalalinia S, Saeedi Moghaddam S, Sheidaei A, Rezaei N, Naghibi Iravani SS, Modirian M, Zokaei H, Yoosefi M, Gohari K, Kousha A. Patterns of obesity and overweight in the iranian population: findings of steps 2016. Front Endocrinol. 2020;11:42. doi: 10.3389/fendo.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, XI Z, Zuo Q, Pang Z, LI Z. Risk factors in rural residents with essential hypertension of different social economic levels. Chin J Hypertens 2006.
- 30.Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen SM, Tran HTT, Tran BQ, Van Hoang M, Truong BD, Nguyen LT, Tran PD, Lai TD, Van Tran T, Shu X-O. Compliance to dietary guidelines on fruit and vegetable intake and prevalence of hypertension among vietnamese adults, 2015. Eur J Prev Cardiol. 2020;27(1):39–46. doi: 10.1177/2047487319867500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Kim J. Association between fruit and vegetable consumption and risk of hypertension in middle-aged and older korean adults. J Acad Nutr Dietetics. 2018;118(8):1438–49. doi: 10.1016/j.jand.2017.08.122. [DOI] [PubMed] [Google Scholar]
- 33.Borgi L, Muraki I, Satija A, Willett WC, Rimm EB, Forman JP. Fruit and vegetable consumption and the incidence of hypertension in three prospective cohort studies. Hypertension. 2016;67(2):288–93. doi: 10.1161/HYPERTENSIONAHA.115.06497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. doi: 10.3945/an.117.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Manson JE, Gaziano JM, Buring JE, Sesso HD. Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am J Hypertens. 2012;25(2):180–9. doi: 10.1038/ajh.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moslemi M, Mahdavi-Roshan M, Joukar F, Naghipour M, Mansour-Ghanaei F. Food Behaviors and its Association with Hypertension and Cardiovascular Diseases in Sowme’eh Sara (North of Iran): the PERSIAN Guilan Cohort Study (PGCS) Prev Nutr Food Sci. 2021;26(3):262. doi: 10.3746/pnf.2021.26.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djazayery A, Siassi F, Kholdi N. Food behaviour and consumption patterns in rural areas of Sirjan, Iran. 1. Dietary patterns, energy and nutrient intakes and food ideology. Ecol Food Nutr. 1992;28(1–2):105–17. [Google Scholar]
- 38.Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J Agric Food Chem. 2008;56(1):139–47. doi: 10.1021/jf072304b. [DOI] [PubMed] [Google Scholar]
- 39.Villaverde P, Lajous M, MacDonald C-J, Fagherazzi G, Boutron-Ruault M-C, Bonnet F. Dairy product consumption and hypertension risk in a prospective french cohort of women. Nutr J. 2020;19(1):1–8. doi: 10.1186/s12937-020-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heraclides A, Mishra GD, Hardy RJ, Geleijnse JM, Black S, Prynne CJ, Kuh D, Soedamah-Muthu SS. Dairy intake, blood pressure and incident hypertension in a general british population: the 1946 birth cohort. Eur J Nutr. 2012;51(5):583–91. doi: 10.1007/s00394-011-0242-z. [DOI] [PubMed] [Google Scholar]
- 41.Feng Y, Zhao Y, Liu J, Huang Z, Yang X, Qin P, Chen C, Luo X, Li Y, Wu Y. Consumption of dairy products and the risk of overweight or obesity, hypertension, and type 2 diabetes Mellitus: a dose-response Meta-analysis and systematic review of Cohort Studies. Advances in Nutrition; 2022. [DOI] [PMC free article] [PubMed]
- 42.Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR., Jr Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the coronary artery Risk Development in Young adults (CARDIA) study. Am J Clin Nutr. 2005;82(6):1169–77. doi: 10.1093/ajcn/82.6.1169. [DOI] [PubMed] [Google Scholar]
- 43.Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–7. doi: 10.1161/HYPERTENSIONAHA.112.195206. [DOI] [PubMed] [Google Scholar]
- 44.Challa HJ, Ameer MA, Uppaluri KR. DASH diet to stop hypertension. StatPearls [Internet]. edn.: StatPearls Publishing; 2021. [PubMed]
- 45.Wang T, Heianza Y, Sun D, Huang T, Ma W, Rimm EB, Manson JE, Hu FB, Willett WC, Qi L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. bmj 2018, 360. [DOI] [PMC free article] [PubMed]
- 46.Mahdavi R, Bagheri asl A, Abadi MAJ, Namazi N. Perceived barriers to following dietary recommendations in hypertensive patients. J Am Coll Nutr. 2017;36(3):193–9. doi: 10.1080/07315724.2014.966176. [DOI] [PubMed] [Google Scholar]
- 47.Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane database of systematic reviews 2018(10). [DOI] [PMC free article] [PubMed]
- 48.Hilpert KF, West SG, Bagshaw DM, Fishell V, Barnhart L, Lefevre M, Most MM, Zemel MB, Chow M, Hinderliter AL. Effects of dairy products on intracellular calcium and blood pressure in adults with essential hypertension. J Am Coll Nutr. 2009;28(2):142–9. doi: 10.1080/07315724.2009.10719765. [DOI] [PubMed] [Google Scholar]
- 49.Cormick G, Ciapponi A, Cafferata ML, Cormick MS, Belizán JM. Calcium supplementation for prevention of primary hypertension. Cochrane Database of Systematic Reviews 2021(8). [DOI] [PMC free article] [PubMed]
- 50.Drouin-Chartier J-P, Gigleux I, Tremblay AJ, Poirier L, Lamarche B, Couture P. Impact of dairy consumption on essential hypertension: a clinical study. Nutr J. 2014;13(1):1–9. doi: 10.1186/1475-2891-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maki KC, Rains TM, Schild AL, Dicklin MR, Park KM, Lawless AL, Kelley KM. Effects of low-fat dairy intake on blood pressure, endothelial function, and lipoprotein lipids in subjects with prehypertension or stage 1 hypertension. Vasc Health Risk Manag. 2013;9:369. doi: 10.2147/VHRM.S45684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To protect study participant privacy, data cannot be shared openly, but data are available through a reasonable request from the corresponding author.