Abstract
Immunotherapy offers survival benefits for patients with advanced gastric cancer, but not all populations can benefit from immunotherapy. Good nutritional status is fundamental to a patient's immune function and may have an impact on the efficacy of immunotherapy. The present study aimed to investigate changes in prognostic nutritional index (PNI), advanced lung cancer inflammation index (ALI) and albumin-globulin ratio (AGR) values before and after immunotherapy in patients with advanced gastric cancer. The study also aimed to determine the potential association of the aforementioned values with patient outcomes and prognosis. Body mass index (BMI), serum albumin, total protein, peripheral blood lymphocyte, neutrophil, carcinoembryonic antigen (CEA), carbohydrate antigen19-9 (CA19-9) and a-fetoprotein (AFP) data were collected from 195 patients with advanced gastric cancer who underwent immunotherapy from January 2020 to October 2021. In addition, PNI, ALI and AGR values were calculated based on variables in blood collected from the patients within 3 days prior to immunotherapy and 3 weeks after immunotherapy. The results demonstrated that low PNI was associated with elevated CEA levels. Moreover, low ALI levels were associated with reduced BMI levels, elevated AFP levels, PD-L1 negative and first-line treatment. Comparison of responding and non-responding groups revealed that patients who responded to immunotherapy had higher PNI and AGR values than patients who did not respond, both before and after treatment, but had lower CEA and CA19-9 levels after treatment. Furthermore, in the non-responding group, PNI and AGR values were decreased and CEA values were increased following treatment compared with those prior to treatment. The objective response and disease control rates were higher in the high PNI and AGR groups compared with the low PNI and AGR groups, respectively. Moreover, PNI and AGR were found to be independent predictors of the short-term efficacy of immunotherapy for advanced gastric cancer, with cut-off values of 47.18 and 1.29, respectively. Univariate analysis revealed that ALI was associated with the progression-free survival (PFS) of patients, while multivariate analysis demonstrated that baseline PNI and AGR were independent predictors of PFS. In conclusion, tumor progression leads to a decline in the nutritional level of patients, and the present study indicated that effective immunotherapy may alleviate this deterioration to a certain extent. Furthermore, PNI and AGR exhibit potential in predicting the efficacy of immunotherapy and the prognosis of patients with advanced gastric cancer, and may exhibit potential as biomarkers in clinical practice.
Keywords: advanced gastric cancer, biomarkers, prognostic nutritional index, advanced lung cancer inflammatory index, albumin-globulin ratio
Introduction
In 2020, it was predicted that there were 1.089 million new cases of gastric cancer and 769,000 gastric cancer-associated deaths worldwide. In addition, gastric cancer exhibited the fifth highest incidence rate and fourth highest mortality rate among malignant tumors (1).
At diagnosis, the majority of patients have an advanced stage of gastric cancer and, therefore, radical surgery is not an option. At present, patients with advanced gastric cancer are mainly treated with a combination of chemotherapy-based regimens. Although the overall survival time of patients with advanced gastric cancer has increased following advances in chemotherapeutic regimens and drugs, the median overall duration of patient survival after chemotherapy remains at <1 year (2). The results of a phase III clinical trial demonstrated that the use of trastuzumab, a targeted therapy for the treatment of gastric cancer, in combination with chemotherapy increased the survival rates of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric or gastro-esophageal cancer when compared with chemotherapy alone (3). However, the majority of clinical trials conducted with other targeted agents in patients with advanced gastric cancer did not meet the primary study endpoint (4). JACOB (5), LOGIC (6), REAL3 (7), GATSBY (8) and a number of other phase III clinical trials all showed that targeted agents did not prolonged advanced overall survival time in patients with gastric cancer.
Programmed cell death 1 (PD-1) is a negative co-stimulatory transmembrane protein expressed on a variety of immune cells that binds to PD ligand-1 (PD-L1), which is expressed on tumor cells and inhibits antitumor immunity (9). Anti-PD-1/PD-L1 therapy blocks the PD-1/PD-L1 pathway and promotes the innate immune response to tumor cells (10). Notably, anti-PD-1/PD-L1 therapy exhibits potential in gastric cancer (11). However, the results of previous clinical trials have demonstrated that only specific patients may benefit from immunotherapy. Therefore, if patients with gastric cancer are indiscriminately treated with PD-1 immunotherapy, certain patients may experience hyper-progression and adverse side effects, including immune-related myocarditis and encephalitis, and incur unnecessary high costs (12,13). Therefore, the selection of patients that are likely to respond to PD-1 immunotherapy is crucial. Moreover, it is necessary to further investigate the specific biomarkers of gastric cancer in order to maximize patient benefits, minimize the risk of toxicity and achieve precision immunotherapy.
The findings of previous studies have demonstrated that the prognostic nutritional index (PNI), advanced lung cancer inflammation index (ALI) and albumin-globulin ratio (AGR) values are nutritional indicators associated with albumin, globulin, neutrophils, lymphocytes and body mass index (BMI), which reflect the systemic nutritional levels of the patient and are associated with the prognosis of patients with gastric cancer (14–16). Therefore, the present study aimed to investigate the potential associations of PNI, AGR and ALI with the efficacy of immunotherapy and the prognosis of patients with advanced gastric cancer. In the present study, χ2 tests were used to analyze the factors associated with PNI, ALI and AGR, and to evaluate the association of PNI, ALI and AGR with the objective response rate (ORR) and disease control rate (DCR) of the patients. In addition, differences in clinical indicators between patients in different efficacy groups before and after treatment were analyzed using mixed ANOVA followed by pairwise comparisons using Bonferroni post hoc tests. Logistic regression analysis was also used to create receiver operating characteristic (ROC) curves to analyze the association of nutritional indicators with the effect of treatment. Survival curves were plotted using the Kaplan-Meier method, and differences between survival curves were examined using a log-rank test. Furthermore, the Cox proportional risk model was used to analyze the associations of PNI, ALI and AGR with progression-free survival (PFS).
Materials and methods
Study population and response assessment
A total of 273 patients with advanced gastric cancer treated with PD-1 inhibitors, who were admitted to the Department of Oncology of The First Hospital of Shanxi Medical University (Taiyuan, China) from January 2020 to October 2021, were screened for inclusion in the present study. The inclusion criteria were as follows: i) Patients diagnosed with clinical stage IV, HER2-negative gastric adenocarcinoma via imaging and histopathological examination; ii) patients who received at least three rounds of PD-1 inhibitor monotherapy, PD-1 inhibitor combined with chemotherapy or PD-1 inhibitor combined with anti-angiogenic agents at the First Hospital of Shanxi Medical University; and iii) patients with complete carcinoembryonic antigen (CEA), carbohydrate antigen19-9 (CA19-9) and a-fetoprotein (AFP) levels, PD-L1 expression level, mismatch repair (MMR) status, peripheral blood lymphocyte, albumin, neutrophil and total protein results, and relevant follow-up test results. Patients were excluded from the present study according to the following criteria: i) Other types of malignancies; ii) HER2-positive gastric adenocarcinoma; iii) acute infections, immunodeficiency and autoimmune diseases; iv) severe underlying diseases, affecting organs such as the heart, lung, liver and kidney; and v) systemic hormone treatment. Among the 273 patients that were screened, 195 patients met the aforementioned criteria and were included in the present study. The excluded patients comprised 27 patients with a lack of lymphocyte subpopulation data, 23 patients with missing PD-L1 scores and MMR test results, 6 patients who had concomitant severe underlying diseases and 22 patients with a lack of follow-up data. Patients included 60 female and 135 males; the mean age of the included population was 61.44 years old, ranging from 29–84 years old. Of these, 166 patients study underwent treatment with PD-1 inhibitors combined with chemotherapy, 24 patients were treated with PD-1 inhibitors alone, and 5 were treated with PD-1 inhibitors with anti-angiogenic.
Data collection
Clinicopathological characteristics of the patients were collected, including diagnosis, biological sex, age, BMI, history of smoking, drinking history, the presence of liver or peritoneal metastases prior to immunotherapy, treatment regimens and the number of treatment lines. Laboratory indices of the patients were also collected, including CEA, CA19-9 and AFP levels, PD-L1 expression levels, MMR status, peripheral blood lymphocyte counts, albumin levels, neutrophil counts and total protein levels. Blood samples were collected from the patients within the 3 days prior to immunotherapy and after 3 cycles of immunotherapy. The PD-1 inhibitors used included pembrolizumab, nivolumab, tislelizumab, toripalimab, camrelizumab and sintilimab. The chemotherapy regimens used included S-1 plus oxaliplatin, oxaliplatin plus capecitabine, docetaxel plus S-1, folinic acid, fluorouracil and oxaliplatin, albumin-bound paclitaxel monotherapy and docetaxel monotherapy. The anti-angiogenic agent used was apatinib. History of drinking was defined as drinking alcohol ≥2 per week.
The efficacy of immunotherapy and the occurrence of liver or peritoneal metastases prior to treatment were assessed based on computed tomography, magnetic resonance imaging or positron emission tomography/computed tomography findings. Short-term efficacy was classified according to the Response Evaluation in Solid Tumors (RECIST 1.1) assessment criteria (17) as follows: Complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Patients with a CR or PR were classified as the responding group, and patients with SD or PD were classified as the non-responding group. Prognosis was assessed using PFS time, which was defined as the time from the initiation of treatment to progression or mortality from any cause. The DCR was defined as the ratio of the sum of CR, PR and SD cases to the total number of cases. The ORR was defined as the ratio of the sum of CR plus PR cases to the total number of cases. The aforementioned medical information and imaging data were collected using the electronic medical record system of The First Hospital of Shanxi Medical University. The reference ranges of the tumor markers were as follows: CEA, 0–3 µg/l; CA19-9, 0–37 U/ml; and AFP, 0–15 µg/l.
Calculation of nutrition-related indicators
ALI, AGR and PNI values are all based on results from peripheral blood. PNI values were calculated using the following formula: PNI=serum albumin (g/l) + 5× total peripheral blood lymphocytes (×109/l). The neutrophil-to-lymphocyte ratio (NLR) was calculated and used in the calculation of ALI values according to the following formula: ALI=BMI × albumin (g/l)/NLR. Total serum protein levels were also obtained. AGR values were calculated using the following formula: AGR=albumin/(total protein-albumin). The reference ranges for each index are as follows: Absolute neutrophil value, 1.9–6.3×109/l; absolute lymphocyte value, 1.1–3.2×109/l; albumin, 40–55 g/l; and total protein, 65–85 g/l.
PD-L1 immunohistochemical assessment
Immunohisto-chemical staining of the tumor tissue was performed on the Ventata BenchMark Ultra system (Roche Tissue Diagnostics) using monoclonal mouse anti-human PD-L1 (clone number, 22C3; 1:50; Dako; Agilent Technologies, Inc.) following the manufacturer's instructions. The tissue specimens were fixed in 10% neutral formalin at room temperature for 6–24 h. The fixed tissue was placed in a tissue dehydrator overnight for dehydration and clearing, then the tissue was paraffin-embedded and sectioned at 3 µm. Embedding protocol included transferring the tissue core into the hole of the receptor wax block and placing it into a constant temperature wax box at 60°C for 10–15 min and then into a refrigerator at 4°C to cool down. Sections were incubated with primary antibody against PD-L1 (clone number, 22C3; 1:50; Dako, Agilent Technologies, Inc.) at 4°C overnight followed by horseradish peroxidase-labeled goat anti-mouse secondary antibody (Roche Diagnostic Products, Shanghai) at 37°C for 30 min and staining with 3,3′-diaminobenzidine (DAB; Roche Diagnostics) at room temperature for 2 min. Observation was under a light microscope. Matching kit was applied (OptiView, Roche Diagnostics). PD-L1-positive immunoreactive staining was localized to the cell membrane and assessed using the combined positive score (CPS), which is calculated as follows: CPS=total number of immunoreactive-stained tumor cells, lymphocytes and macrophages in the 20X objective field/total number of tumor cells in the field at ×100 magnification using a light microscope. PD-L1 CPS ≥5 was considered as PD-L1 positive, while PD-L1 CPS <5 was considered as PD-L1 negative.
MMR immunohistochemical assessment
MMR staining of the tumor tissue was performed on the Leica BOND III platform (Leica Biosystems) following the manufacturer's instructions. The tissue specimens were fixed in 10% neutral formalin at room temperature for 6–24 h. The fixed tissue was placed in a tissue dehydrator overnight for dehydration and clearing, then the tissue was paraffin-embedded and sectioned at 3 µm. Embedding protocol included transferring the tissue core into the hole of the receptor wax block and placing it into a constant temperature wax box at 60°C for 10–15 min and then into a refrigerator at 4°C to cool down. The tissue specimens were fixed in 10% neutral formalin at room temperature for 6–24 h. The fixed tissue was placed in a tissue dehydrator overnight for dehydration and clearing, then the tissue was paraffin-embedded and sectioned at 3 µm. Embedding protocol included transferring the tissue core into the hole of the receptor wax block and placing it into a constant temperature wax box at 60°C for 10–15 min and then into a refrigerator at 4°C to cool down. Sections were incubated with primary antibody against MutS homolog 2 (MSH2; clone no. RED2; 1:5,000; OriGene Technologies, Inc.) and MutS homolog 6 (MSH6; clone no. EP49; 1:100; OriGene Technologies, Inc.), MutL homolog 1 (MLH1; clone no. GM002; 1:500; Gene Tech Biotechnology Co., Ltd.) and postmeiotic segregation increased 2 (PMS2; clone no. EP51; 1:70; Gene Tech Biotechnology Co., Ltd.) at 4°C overnight followed by horseradish peroxidase-labeled goat anti-mouse secondary antibody (Roche Diagnostics) at 37°C for 30 min and staining with 3,3′-diaminobenzidine (DAB; Roche Diagnostics) at room temperature for 2 min. Staining was performed using an automatic immunohistochemical station (BenchMark ULTRA; Roche Diagnostics). Observation was under a light microscope. The platform kit used for staining was BOND Polymer Refine Detection (Leica Biosystems Newcastle Ltd.). The absence of at least one of the MMR proteins, including MLH1, MSH2, MSH6 and PMS2, was defined as MMR deficient (dMMR). All positive MMR protein staining results were defined as MMR proficient (pMMR).
Statistical analysis
SPSS software (version 23.0; IBM Corp.) was used to perform the statistical analysis. Quantitative data are expressed as the mean ± standard deviation, and qualitative data are expressed as the number and percentage of cases, n (%). Median peripheral PNI, AGR and ALI values were used as the critical values for the division of patients into high and low index groups. Differences in the number of patients with various clinicopathological characteristics between groups were analyzed using χ2 tests. Differences in quantitative clinical indicators of patients in different efficacy groups before and after treatment were evaluated using a mixed ANOVA followed by pairwise comparisons using Bonferroni post hoc tests. Independent predictors of immunotherapy efficacy were determined using logistic regression analysis. The potential value of PNI and AGR in the prediction of short-term immunotherapy efficacy in patients with advanced gastric cancer was determined by plotting a ROC curve and calculating the area under the curve (AUC). Kaplan-Meier curves were also plotted and the differences in PFS between groups were compared using log-rank tests. Other variables were determined as independent prognostic factors for PFS using Cox proportional risk model analysis. P<0.05 was considered to indicate a statistically significant result.
Results
Clinicopathological characteristics and factors affecting the PNI, ALI and AGR values of patients
The PNI, ALI and AGR values of patients with advanced gastric cancer prior to immunotherapy were determined, and patients were divided into high and low groups, according to the median value. The median PNI, ALI and AGR values were 45.50, 272.57 and 1.40, respectively. The effects of different clinicopathological characteristics on the nutritional indices of the patients were analyzed. The results demonstrated that low PNI values were associated with elevated CEA levels. Moreover, low ALI levels were associated with reduced BMI values, elevated AFP levels, PD-L1 negative and first-line treatment while high ALI levels were associated with normal or higher BMI values, normal AFP levels, PD-L1 positive and second line and beyond treatment. The results also demonstrated that the AGR levels were not significantly associated with any of the clinical characteristics that were analyzed. The associations between nutritional index levels and clinicopathological characteristics in different groups are displayed in Table I.
Table I.
Association between clinical characteristics and PNI, ALI and AGR values in patients with advanced gastric cancer.
| PNI | ALI | AGR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristic |
|
|
|
||||||||||
| n | <45.5 | ≥45.5 | χ2 | P-value | <272.57 | ≥272.57 | χ2 | P-value | <1.40 | ≥1.40 | χ2 | P-value | |
| Total | 195 | 96 | 99 | 98 | 97 | 100 | 95 | ||||||
| Sex | |||||||||||||
| Female | 60 | 30 (31.3) | 30 (30.3) | 0.021 | 0.886 | 28 (28.6) | 32 (33.0) | 0.447 | 0.504 | 28 (28.0) | 32 (33.7) | 0.739 | 0.390 |
| Male | 135 | 66 (68.8) | 69 (69.7) | 70 (71.4) | 65 (67.0) | 72 (72.0) | 63 (66.3) | ||||||
| Age, years | |||||||||||||
| <65 | 120 | 60 (62.5) | 60 (60.6) | 0.074 | 0.786 | 66 (67.3) | 54 (55.7) | 2.808 | 0.094 | 59 (59.0) | 61 (64.2) | 0.559 | 0.455 |
| ≥65 | 75 | 36 (37.5) | 39 (39.4) | 32 (32.7) | 43 (44.3) | 41 (41.0) | 34 (35.8) | ||||||
| BMI, kg/m2 | |||||||||||||
| <18.5 | 59 | 29 (30.2) | 30 (30.3) | <0.001 | 0.989 | 40 (40.8) | 19 (19.6) | 10.411 | 0.001 | 33 (33.0) | 26 (27.4) | 0.732 | 0.392 |
| ≥18.5 | 136 | 67 (69.8) | 69 (69.7) | 58 (59.2) | 78 (80.4) | 67 (67.0) | 69 (72.6) | ||||||
| Drinking history | |||||||||||||
| No | 159 | 77 (80.2) | 82 (82.8) | 0.222 | 0.637 | 79 (80.6) | 80 (82.5) | 0.112 | 0.728 | 83 (83.0) | 76 (80.0) | 0.291 | 0.589 |
| Yes | 36 | 19 (19.8) | 17 (17.2) | 19 (19.4) | 17 (17.5) | 17 (17.0) | 19 (20.0) | ||||||
| Smoking history | |||||||||||||
| No | 126 | 63 (65.6) | 63 (63.6) | 0.084 | 0.772 | 63 (64.3) | 63 (64.9) | 0.009 | 0.923 | 65 (65.0) | 61 (64.2) | 0.013 | 0.908 |
| Yes | 69 | 33 (34.4) | 36 (36.4) | 35 (35.7) | 34 (35.1) | 35 (35.00) | 34 (35.8) | ||||||
| Liver metastasis | |||||||||||||
| No | 112 | 52 (54.2) | 60 (60.6) | 0.827 | 0.363 | 51 (52.0) | 61 (62.9) | 2.346 | 0.126 | 58 (58.0) | 54 (56.8) | 0.027 | 0.870 |
| Yes | 83 | 44 (45.8) | 39 (39.4) | 47 (48.0) | 36 (37.1) | 42 (42.0) | 41 (43.2) | ||||||
| Peritoneal metastasis | |||||||||||||
| No | 152 | 72 (75.0) | 80 (80.8) | 0.957 | 0.328 | 73 (74.5) | 79 (81.4) | 1.371 | 0.242 | 77 (77.0) | 75 (78.9) | 0.107 | 0.743 |
| Yes | 43 | 24 (25.0) | 19 (19.2) | 25 (25.5) | 18 (18.6) | 23 (23.0) | 20 (21.1) | ||||||
| PD-L1 expression, CPS | |||||||||||||
| <5 | 105 | 54 (56.3) | 51 (51.5) | 0.440 | 0.507 | 62 (63.3) | 43 (44.3) | 7.033 | 0.008 | 60 (60.0) | 45 (47.4) | 3.128 | 0.077 |
| ≥5 | 90 | 42 (43.8) | 48 (48.5) | 36 (36.7) | 54 (55.7) | 40 (40.0) | 50 (52.6) | ||||||
| MMR status | |||||||||||||
| pMMR | 178 | 88 (91.7) | 90 (90.9) | 0.035 | 0.851 | 92 (93.9) | 86 (88.7) | 1.668 | 0.197 | 91 (91.0) | 87 (91.6) | 0.021 | 0.886 |
| dMMR | 17 | 8 (8.3) | 9 (9.1) | 6 (6.1) | 11 (11.3) | 9 (9.0) | 8 (8.4) | ||||||
| Treatment line | |||||||||||||
| First line | 130 | 67 (69.8) | 63 (63.6) | 0.831 | 0.362 | 73 (74.5) | 57 (58.8) | 5.426 | 0.020 | 67 (67.0) | 63 (66.3) | 0.010 | 0.919 |
| Second line and beyond | 65 | 29 (30.2) | 36 (36.4) | 25 (25.5) | 40 (41.2) | 33 (33.0) | 32 (33.7) | ||||||
| CEA, µg/l | |||||||||||||
| <3 | 83 | 34 (35.4) | 49 (49.5) | 3.951 | 0.047 | 35 (35.7) | 48 (49.5) | 3.781 | 0.052 | 38 (38.0) | 45 (47.4) | 1.749 | 0.186 |
| ≥3 | 112 | 62 (64.6) | 50 (50.5) | 63 (64.3) | 49 (50.5) | 62 (62.0) | 50 (52.6) | ||||||
| CA-199, U/ml | |||||||||||||
| <37 | 121 | 53 (55.2) | 68 (68.7) | 3.760 | 0.052 | 61 (62.2) | 60 (61.9) | 0.003 | 0.955 | 62 (62.0) | 59 (62.1) | <0.001 | 0.988 |
| ≥37 | 74 | 43 (44.8) | 31 (31.3) | 37 (37.8) | 37 (38.1) | 38 (38.0) | 36 (37.9) | ||||||
| AFP, µg/l | |||||||||||||
| <15 | 168 | 78 (81.2) | 90 (90.9) | 3.812 | 0.051 | 77 (78.6) | 91 (93.8) | 9.495 | 0.002 | 85 (85.0) | 83 (87.4) | 0.229 | 0.632 |
| ≥15 | 27 | 18 (18.8) | 9 (9.1) | 21 (21.4) | 6 (6.2) | 15 (15.0) | 12 (12.6) | ||||||
PNI, ALI and AGR count data are presented as n (%). PNI, prognostic nutritional index; ALI, advanced lung cancer inflammation index; AGR, albumin-globulin ratio; BMI, body mass index; PD-L1, programmed cell death ligand-1; CPS, combined positive score; MMR, mismatch repair; dMMR, MMR deficient; pMMR, MMR proficient; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen19-9; AFP, α-fetoprotein.
Changes in nutritional indicators and tumor markers in patients before and after treatment
The 195 patients with advanced gastric cancer in the present study had all undergone immunotherapy, and based on the assessment of immunotherapy efficacy after three cycles, the patients were divided into a responding group (n=68; CR, 3; PR, 65) and a non-responding group (n=127; SD, 74; PD, 53). The proportion of patients in the responding group was 34.9%. Comparison of the two groups revealed that patients in the responding group had higher PNI and AGR values than those in the non-responding group, both before and after treatment, but had lower CEA and CA19-9 levels than the non-responding group after treatment. The other indicators examined, namely ALI and AFP, exhibited no differences between the two groups both before and after treatment. In a comparison of pre- and post-treatment results, no significant changes were observed in PNI, ALI, AGR, CEA, CA19-9 and AFP in the responding group after treatment compared with the pre-treatment period. In the non-responding group, PNI and AGR values were decreased, CEA values were increased, and ALI, CA19-9 and AFP values were not significantly changed following treatment, compared with the respective values in the pre-treatment period. Comparisons between PNI, ALI, AGR, CEA, CA19-9 and AFP values before and after immunotherapy in the responding and non-responding groups are displayed in Table II.
Table II.
Comparison of PNI, ALI, AGR, CEA, CA19-9 and AFP values before and after immunotherapy in responding and non-responding groups.
| Between time points | Between groups | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Responding group | Non-responding group | P-value |
|
|
|
|||
| F-value | P-value | F-value | P-value | F-value | P-value | ||||
| PNI | |||||||||
| Before immunotherapy | 48.08±5.95 | 43.81±5.93 | <0.001 | 11.879 | 0.001 | 40.874 | <0.001 | 1.933 | 0.166 |
| After immunotherapy | 47.05±5.89 | 41.38±6.61 | <0.001 | ||||||
| P-value | 0.204 | <0.001 | |||||||
| ALI | |||||||||
| Before immunotherapy | 384.08±271.45 | 355.78±300.63 | 0.518 | 0.781 | 0.378 | 0.616 | 0.434 | 0.001 | 0.981 |
| After immunotherapy | 363.03±237.36 | 335.86±287.83 | 0.506 | ||||||
| P-value | 0.574 | 0.468 | |||||||
| AGR | |||||||||
| Before immunotherapy | 1.50±0.22 | 1.31±0.30 | <0.001 | 14.561 | <0.001 | 45.239 | <0.001 | 8.592 | 0.043 |
| After immunotherapy | 1.48±0.29 | 1.18±0.28 | <0.001 | ||||||
| P-value | 0.584 | <0.001 | |||||||
| CEA | |||||||||
| Before immunotherapy | 28.71±51.06 | 69.48±407.03 | 0.412 | 0.942 | 0.333 | 2.771 | 0.098 | 3.941 | 0.049 |
| After immunotherapy | 16.62±24.01 | 104.68±234.65 | 0.002 | ||||||
| P-value | 0.530 | 0.013 | |||||||
| CA19-9 | |||||||||
| Before immunotherapy | 122.66±323.63 | 133.10±282.47 | 0.816 | 0.023 | 0.879 | 3.830 | 0.052 | 4.336 | 0.039 |
| After immunotherapy | 27.03±28.55 | 215.70±680.91 | 0.024 | ||||||
| P-value | 0.168 | 0.104 | |||||||
| AFP | |||||||||
| Before immunotherapy | 41.43±156.59 | 221.78±1066.02 | 0.168 | 1.266 | 0.262 | 1.930 | 0.166 | 1.092 | 0.297 |
| After immunotherapy | 48.98±345.97 | 425.67±2331.69 | 0.187 | ||||||
| P-value | 0.960 | 0.068 | |||||||
Data are expressed as the mean ± standard deviation. PNI, prognostic nutritional index; ALI, advanced lung cancer inflammation index; AGR, albumin-globulin ratio; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen19-9; AFP, α-fetoprotein.
Analysis of treatment efficacy in different groups
Analysis of treatment efficacy in patients in the low and high PNI, ALI and AGR groups according to RECIST 1.1 criteria is displayed in Table III. The ORR and DCR of patients in the low PNI group were 24.0 and 64.6%, respectively, compared with 45.5 and 80.8%, respectively, in the high PNI group. The results revealed that the ORR and DCR of patients in the low PNI group were significantly lower than those in the high PNI group. The ORR and DCR of patients in the low ALI group were 31.6 and 69.4%, respectively, while those in the high ALI group were 38.1 and 76.3%, respectively. Notably, these rates were not found to be statistically significant between the two groups. The ORR and DCR of patients in the low AGR group were 23.0 and 60.0%, respectively, compared with 47.4 and 86.3%, respectively, in the high AGR group. The ORR and DCR of patients in the low AGR group were significantly lower than those in the high AGR group.
Table III.
Analysis of treatment efficacy in patients according to PNI, ALI and AGR values.
| Variable | n | CR | PR | SD | PD | ORR | DCR |
|---|---|---|---|---|---|---|---|
| PNI | |||||||
| Low | 96 | 1 (1.0) | 22 (22.9) | 39 (40.6) | 34 (35.4) | 23 (24.0) | 62 (64.6) |
| High | 99 | 2 (2.0) | 43 (43.4) | 35 (35.4) | 19 (19.2) | 45 (45.5) | 80 (80.8) |
| χ2 | 9.916 | 6.482 | |||||
| P-value | 0.002 | 0.011 | |||||
| ALI | |||||||
| Low | 98 | 0 (0.0) | 31 (31.6) | 37 (37.8) | 30 (30.6) | 31 (31.6) | 68 (69.4) |
| High | 97 | 3 (3.1) | 34 (35.1) | 37 (38.1) | 23 (23.7) | 37 (38.1) | 74 (76.3) |
| χ2 | 0.910 | 1.173 | |||||
| P-value | 0.340 | 0.279 | |||||
| AGR | |||||||
| Low | 100 | 0 (0.0) | 23 (23.0) | 37 (37.0) | 40 (40.0) | 23 (23.0) | 60 (60.0) |
| High | 95 | 3 (3.2) | 42 (44.2) | 37 (38.9) | 13 (13.7) | 45 (47.4) | 82 (86.3) |
| χ2 | 12.738 | 17.046 | |||||
| P-value | <0.001 | <0.001 |
Data are presented as n (%). PNI, prognostic nutritional index; ALI, advanced lung cancer inflammation index; AGR, albumin-globulin ratio; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Association between baseline nutritional indicators and short-term patient outcomes
The aforementioned analysis of PNI, ALI and AGR values prior to treatment between the 68 patients in the responding group and the 127 patients in the non-responding group highlighted that the PNI and AGR values were higher in the responding group than in the non-responding group, while no significant difference in ALI values was detected between the responding and non-responding groups. Differences in PD-L1 expression, MMR status, sex, age, BMI, smoking history, alcohol consumption and liver or peritoneal metastasis were compared between the responding and non-responding groups using χ2 tests (Table IV). The results reveal that the proportion of dMMR- and PD-L1-positive patients in the responding group was higher than that in the non-responding group, and the remaining indices did not differ between the two groups. The inclusion of PNI, AGR, PD-L1 and MMR status as variables in a logistic regression model demonstrated that PNI [OR, 0.890; 95% confidence interval (CI), 0.830–0.955; P=0.001], AGR (OR, 0.109; 95% CI, 0.027–0.444; P=0.002), PD-L1 levels (OR, 0.150; 95% CI, 0.071–0.317; P<0.001) and MMR status (OR, 0.212; 95% CI, 0.064–0.708; P=0.012) were independent predictors of the short-term efficacy of immunotherapy in patients with advanced gastric cancer. In addition, when ROC curves for PNI and AGR values in patients prior to treatment were plotted, the AUCs were 0.699 (P<0.001) and 0.696 (P<0.001), respectively (Fig. 1), which highlights that these factors are independent predictors of treatment efficacy. Notably, the cut-off value was 47.18 for PNI (sensitivity, 0.603; specificity, 0.764) and 1.29 for AGR (sensitivity, 0.853; specificity, 0.496).
Table IV.
Comparison of PD-L1 expression, MMR status, sex, age, BMI, smoking history, alcohol consumption, liver and peritoneal metastasis between responding and non-responding patients.
| Variable | n | Responding group | Non-responding group | χ2 | P-value |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 60 | 25 (36.8) | 35 (27.6) | 1.762 | 0.184 |
| Male | 135 | 43 (63.2) | 92 (72.4) | ||
| Age, years | |||||
| <65 | 120 | 45 (66.2) | 75 (59.1) | 0.949 | 0.330 |
| ≥65 | 75 | 23 (33.8) | 52 (40.9) | ||
| BMI, kg/m2 | |||||
| <18.5 | 59 | 23 (33.8) | 36 (28.3) | 0.630 | 0.428 |
| ≥18.5 | 136 | 45 (66.2) | 91 (71.7) | ||
| Drinking history | |||||
| No | 159 | 58 (85.3) | 101 (79.5) | 0.978 | 0.323 |
| Yes | 36 | 10 (14.7) | 26 (20.5) | ||
| Smoking history | |||||
| No | 126 | 46 (67.6) | 80 (63.0) | 0.420 | 0.517 |
| Yes | 69 | 22 (32.4) | 47 (37.0) | ||
| Liver metastasis | |||||
| No | 112 | 37 (54.4) | 75 (59.1) | 0.391 | 0.531 |
| Yes | 83 | 31 (45.6) | 52 (40.9) | ||
| Peritoneal metastasis | |||||
| No | 152 | 57 (83.8) | 95 (74.8) | 2.096 | 0.148 |
| Yes | 43 | 11 (16.2) | 32 (25.2) | ||
| PD-L1 expression, CPS | |||||
| <5 | 105 | 19 (27.9) | 86 (67.7) | 28.193 | <0.001 |
| ≥5 | 90 | 49 (72.1) | 41 (32.3) | ||
| MMR status | |||||
| pMMR | 178 | 57 (83.8) | 121 (95.3) | 7.299 | 0.007 |
| dMMR | 17 | 11 (16.2) | 6 (4.7) |
Data are presented as n (%). BMI, body mass index; PD-L1, programmed cell death ligand-1; CPS, combined positive score; MMR, mismatch repair; dMMR, MMR deficient; pMMR, MMR proficient.
Figure 1.
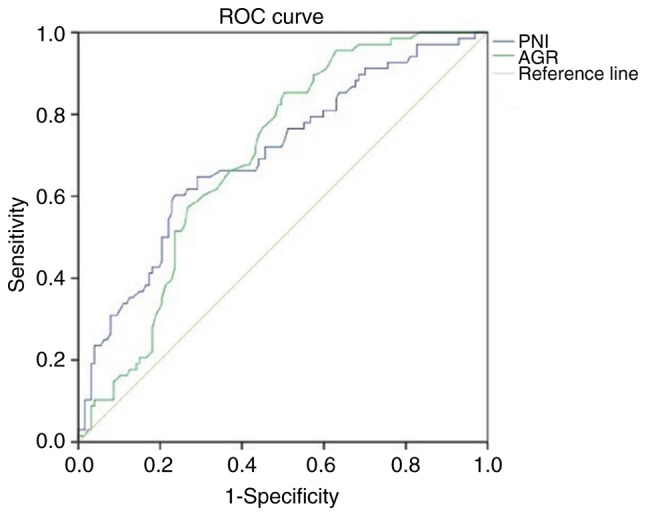
ROC curves of PNI and AGR values prior to immunotherapy. PNI: area under the curve, 0.699; P<0.001; cut-off value, 47.18; sensitivity, 0.603; specificity, 0.764. AGR: area under the curve, 0.696; P<0.001; cut-off value, 1.29; sensitivity, 0.853; specificity, 0.496. Diagonal segments in the curves are produced by ties. ROC, receiver operating characteristic; PNI, prognostic nutritional index; AGR, albumin-globulin ratio.
Association between pre-treatment nutrition-associated indicators and the PFS of patients
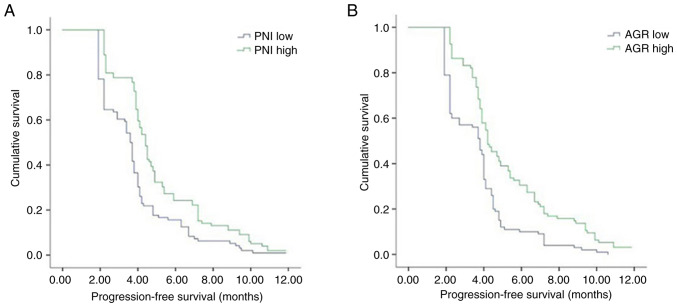
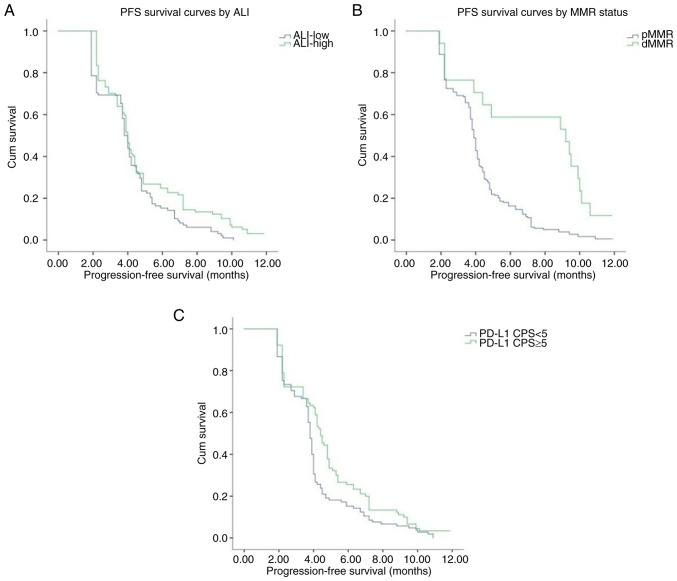
The median PFS time of the patients with advanced gastric cancer was 4.0 months in the present study. Results of the univariate analysis demonstrated no significant association of age, sex, BMI, smoking history, alcohol consumption, liver metastasis or peritoneal metastasis with PFS. Notably, PD-L1 level, MMR status, and PNI, ALI and AGR values exhibited an association with PFS. Moreover, patients in the PD-L1-positive, dMMR, high PNI, high ALI and high AGR groups exhibited significantly longer PFS times than patients in the PD-L1-negative, pMMR, low PNI, low ALI and low AGR groups, respectively. The median PFS durations in the patients in the pMMR, low PNI, low ALI and low AGR groups were 3.9, 3.6, 3.8 and 3.8 months, respectively, while the median PFS durations in patients in the dMMR, high PNI, high ALI and high AGR groups were 9.2, 4.4, 4.0 and 4.2 months, respectively. The results of the Cox proportional risk model analysis revealed that PNI [hazard ratio (HR), 0.949; 95% CI, 0.924–0.975; P<0.001], AGR (HR, 0.516; 95% CI, 0.318–0.836; P=0.007), MMR status (HR, 0.337; 95% CI, 0.191–0.597; P<0.001) and PD-L1 levels (HR, 0:685; 95% CI, 0.509–0.921; P=0.012) were independent prognostic factors for PFS. Kaplan-Meier curves of PFS also demonstrated that patients in the high PNI and AGR groups exhibited a prolonged PFS time compared with patients in the low PNI and AGR groups (Fig. 2). Kaplan-Meier curves also showed that patients with high ALI, dMMR and PD-L1 positive had higher PFS times compared with those with low ALI, pMMR and PD-L1 negative (Fig. 3). The differences in PFS between patients grouped according to various characteristics as analyzed using univariate analysis are displayed in Table V.
Figure 2.
Kaplan-Meier analysis of the progression-free survival of patients with gastric cancer treated with immunotherapy. Stratification by (A) PNI (P<0.001) and (B) AGR values (P<0.001) showed that patients with high PNI and AGR values had higher progression-free survival times compared with those with low PNI and AGR values. PNI, prognostic nutritional index; AGR, albumin-globulin ratio.
Figure 3.
Kaplan-Meier analysis of the progression-free survival of patients with gastric cancer treated with immunotherapy. Stratification by (A) ALI (P=0.023) (B) MMR status (P<0.001), (C) PD-L1 expression levels (P=0.007) showed that patients with high ALI, dMMR and PD-L1 positive had higher progression-free survival times compared with those with low ALI, pMMR and PD-L1 negative. ALI, advanced lung cancer inflammation index; MMR, mismatch repair; dMMR, MMR deficient; pMMR, MMR proficient; PD-L1, programmed cell death ligand-1; CPS, combined positive score.
Table V.
Univariate analysis of the association between baseline variables and PFS in 195 patients with gastric cancer.
| Variable | PFS time (95% CI), months | Log-rank | P-value |
|---|---|---|---|
| Age, years | 0.141 | 0.707 | |
| <65 | 4.00 (3.82–4.18) | ||
| ≥65 | 4.00 (3.78–4.2) | ||
| Sex | 2.620 | 0.106 | |
| Female | 4.10 (3.68–4.52) | ||
| Male | 3.90 (3.73–4.07) | ||
| BMI, kg/m2 | 0.075 | 0.784 | |
| <18.5 | 3.90 (3.60–4.20) | ||
| ≥18.5 | 4.00 (3.85–4.15) | ||
| Smoking history | 0.258 | 0.612 | |
| No | 4.00 (3.79–4.21) | ||
| Yes | 3.90 (3.63–4.17) | ||
| Drinking history | 0.110 | 0.740 | |
| No | 4.00 (3.82–4.18) | ||
| Yes | 3.90 (3.55–4.25) | ||
| Liver metastasis | 0.036 | 0.850 | |
| No | 3.90 (3.68–4.12) | ||
| Yes | 4.00 (3.80–4.20) | ||
| Peritoneal metastasis | 0.011 | 0.915 | |
| No | 3.90 (3.72–4.08) | ||
| Yes | 4.00 (3.79–4.21) | ||
| PD-L1 expression, CPS | 7.220 | 0.007 | |
| <5 | 3.80 (3.68–3.93) | ||
| ≥5 | 4.40 (4.04–4.76) | ||
| MMR status | 16.475 | <0.001 | |
| pMMR | 3.90 (3.76–4.05) | ||
| dMMR | 9.20 (3.15–15.25) | ||
| PNI | 15.936 | <0.001 | |
| Low | 3.60 (3.37–3.83) | ||
| High | 4.40 (4.12–4.68) | ||
| ALI | 5.201 | 0.023 | |
| Low | 3.80 (3.59–4.01) | ||
| High | 4.00 (3.82–4.18) | ||
| AGR | 18.915 | <0.001 | |
| Low | 3.80 (3.39–4.21) | ||
| High | 4.20 (3.65–4.75) |
PFS, progression-free survival; CI, confidence interval; BMI, body mass index; PD-L1, programmed cell death ligand-1; CPS, combined positive score; MMR, mismatch repair; dMMR, MMR deficient; pMMR, MMR proficient; PNI, prognostic nutritional index; ALI, advanced lung cancer inflammation index; AGR, albumin-globulin ratio.
Discussion
Following the success of immunotherapy trials in numerous solid tumors (18), immunotherapy and combination therapies are included in the National Comprehensive Cancer Network (NCCN) guidelines as the standard of care for advanced gastric cancer (19). In various authoritative guidelines, including the NCCN and Chinese Society of Clinical Oncology guidelines, anti-PD-1 drugs have become the standard palliative immunotherapy regimen for gastric cancer, while anti-PD-L1 drugs have not (19,20); therefore, anti-PD-1 therapy rather than anti-PD-L1 therapy was selected for evaluation in the present study. However, the benefits of immunotherapy are limited to certain populations of patients; thus, further investigations of potential biomarkers are required to predict patient prognosis and maximize patient benefits. Malignant tumors are characterized by the high consumption of nutrients, and the development of tumors is often accompanied by a decline in nutritional levels. Furthermore, as nutritional levels decrease, treatment tolerance and survival rates also decrease (21). As gastric cancer affects the digestive system, the nutritional intake and absorption in patients with gastric cancer may be worse than those of other malignancies. Notably, if PNI, ALI and AGR values were found to be independent predictors of gastric cancer, this would exhibit advantages compared with the conventional use of PD-L1 score and MMR status. For example, PNI, ALI and AGR are determined via the analysis of peripheral blood; therefore, they are less invasive to investigate and are inexpensive for patients.
The results of the present study demonstrated that a low PNI was associated with higher than normal CEA levels. Moreover, a low ALI was associated with a low BMI, elevated AFP levels, PD-L1 negative and first-line treatment. These factors are associated with deterioration in the nutritional status of patients with a higher tumor load following disease progression. Moreover, when the patients in the responding and non-responding groups were compared, the patients in the responding group had higher PNI and AGR values than those in the non-responding group both before and after treatment, but had lower CA19-9 and CEA levels than those in the non-responding group after treatment. In a comparison of before and after treatment results in the non-responding group, PNI and AGR levels were decreased and CEA values were increased following treatment compared with those prior to treatment. These results further indicate that the control of tumor progression and reduction of tumor load may alleviate issues with eating and nutrition absorption, and improve the nutritional status of patients to a certain extent.
PNI is a marker used to assess inflammation and nutritional status, which is based on serum lymphocyte count and albumin levels. PNI was initially used to assess the risks associated with gastrointestinal surgery (22), and is also widely used in the prognostic assessment of a variety of malignancies (23–25). The results of a previous study demonstrated a significant association of PNI with disease-free and overall survival following radical gastric cancer surgery (26). Moreover, PNI has been demonstrated to be an independent predictor of prognosis in non-small cell lung cancer (27), melanoma (28) and uroepithelial carcinoma (29). The results of the present study suggest that ineffective treatment may lead to a reduction in PNI in patients with advanced gastric cancer. These results indicate that effective immunotherapy may, to some extent, alleviate deterioration of the nutritional status and clinical symptoms of patients, such as difficulty in eating. The ORR and DCR were higher in the high PNI group compared with those in the low PNI group. In addition, PNI was found to be an independent predictor of the short-term efficacy of immunotherapy and prognosis of patients with advanced gastric cancer. This is consistent with previous results in uroepithelial carcinoma, in which patients with a high PNI exhibited significantly higher ORR and DCR than those with a low PNI (29).
The ALI is a combined indicator of nutrition and inflammation. The results of a previous study demonstrated that the prognostic ability of ALI was superior to that of other inflammatory or nutrition-based indices in a cohort of patients with various types and all stages of lung cancer (30). Notably, ALI has been shown to exhibit prognostic significance in numerous types of cancer, including bile duct cancer (31), head and neck cancer (32), and colorectal cancer (33). In addition, ALI has been demonstrated to be an independent predictor of prognosis in patients undergoing radical surgery for gastric cancer (34). The results of the univariate analysis in the present study revealed a longer PFS time in the high ALI group compared with that in the low ALI group. However, multivariate analysis revealed no statistically significant association of ALI with short-term patient outcomes and prognosis. A previous study on advanced non-small cell lung cancer demonstrated that ALI is a prognostic and predictive biomarker for patients treated with PD-L1 inhibitors alone but not in combination with chemotherapy (35). Notably, the majority of patients in the present study underwent treatment with PD-1 inhibitors combined with chemotherapy, and fewer patients were treated with PD-1 inhibitors alone. Due to the retrospective nature of the present study, further investigations are required to clarify the correlation of ALI with the efficacy of immunotherapy and the prognosis of patients with advanced gastric cancer. In addition, further stratification of the patients and additional ALI cut-off values may be required.
Albumin levels are key nutritional indicators that may also reflect the levels of inflammation in patients (36). Globulin is a cortisol-binding protein associated with immunity and inflammation levels in patients (37,38). Research has focused on the clinical application of AGR as a prognostic marker for tumors, and as a serological indicator of nutritional status and systemic inflammation. Previous studies have provided results indicating that AGR may be an independent prognostic indicator in patients with cancer cachexia (39), and that AGR exhibits prognostic significance in metastatic prostate (40), rectal (41), gastric (15), cervical (42) and nasopharyngeal cancer (43). Furthermore, Liu et al (44) demonstrated that AGR affects the PD-1/PD-L1 pathway in breast cancer, with implications for immunotherapy. Notably, the present study indicated that treatment progression may contribute to a reduction in AGR, and that patients with a high AGR exhibited higher ORR and DCR than those with a low AGR. In addition, it demonstrated that AGR may be an independent predictor of both the short-term efficacy of immunotherapy and prognosis in patients with advanced gastric cancer. These results are consistent with those of previous studies conducted in multiple tumor types (36). However, the cut-off value of AGR varies among studies, which may be due to differences in sample size and detection instruments (45). Thus, further investigations into the use of AGR as a biomarker of immunotherapy efficacy in patients with advanced gastric cancer are required, with increased sample sizes and improved standardization of detection instruments.
The present study exhibits numerous limitations. For example, the overall survival rates of patients were not investigated, and further investigations are required to determine the predictive ability of PNI, ALI and AGR in the efficacy of immunotherapy and the prognosis of patients with advanced gastric cancer. In addition, patients treated with immunotherapeutic monotherapy, immunotherapy combined with chemotherapy and immunotherapy combined with anti-angiogenic agents were included in the present study. Thus, further subgroup analyses are required to evaluate the nutritional status of patients following different treatment regimens. Further subgroup analyses are also required to verify the predictive ability of nutrition-associated indicators for different treatment regimens. Moreover, the present study included a widely varied patient population from a single institution, including patients with different lines of therapy and different combinations of immunotherapy, the majority of whom were Asian individuals. Thus, further studies with larger sample sizes are required.
In conclusion, the present study indicates that tumor progression may lead to a decline in the nutritional levels of patients, and that effective immunotherapy may alleviate the deterioration of nutritional status in patients, to some extent.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XW was responsible for data curation. XL and HD were responsible for investigations and for selecting resources. XW and JJ were responsible for the methodology. HD and JJ supervised the study. XW was responsible for writing the original draft of the manuscript, and XW, XL, HD and JJ were responsible for reviewing and editing the manuscript. XW and JJ confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Due to the retrospective nature of the present study, patients were contacted via telephone to provide informed consent and telephone recordings were obtained. Ethics approval was obtained from the Ethics Committee of The First Hospital of Shanxi Medical University (approval no. KYLL-2023-012).
Patient consent for publication
Not applicable.
Authors' information
ORCID of Dr Xinyan Wang: 0000-0001-9816-8773. ORCID of Dr Junmei Jia: 0000-0003-1132-7191.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ricci AD, Rizzo A, Brandi G. DNA damage response alterations in gastric cancer: Knocking down a new wall. Future Oncol. 2021;17:865–868. doi: 10.2217/fon-2020-0989. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Ricci AD, Rizzo A, Rojas Llimpe FL, Di Fabio F, De Biase D, Rihawi K. Novel HER2-directed treatments in advanced gastric carcinoma: AnotHER paradigm shift? Cancers (Basel) 2021;13:1664. doi: 10.3390/cancers13071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K, Kang YK. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–1384. doi: 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- 6.Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC-A randomized phase III Trial. J Clin Oncol. 2016;34:443–451. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 7.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G, Shitara K, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640–653. doi: 10.1016/S1470-2045(17)30111-0. [DOI] [PubMed] [Google Scholar]
- 9.Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: Novel translational implications. Int J Mol Sci. 2021;22:3805. doi: 10.3390/ijms22083805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. doi: 10.3389/fimmu.2022.964442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viscardi G, Tralongo AC, Massari F, Lambertini M, Mollica V, Rizzo A, Comito F, Di Liello R, Alfieri S, Imbimbo M, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: A systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi: 10.1016/j.ejca.2022.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidi A, Violati M, Blasi M, Ferrari E, Luciani A, Codecà C, Ferrari D. Autoimmune-related encephalitis during treatment with nivolumab for advanced head and neck cancer: A case report. Tumori. 2020;106:NP23–NP28. doi: 10.1177/0300891620951262. [DOI] [PubMed] [Google Scholar]
- 14.Ding P, Guo H, Sun C, Yang P, Kim NH, Tian Y, Liu Y, Liu P, Li Y, Zhao Q. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: A prospective study. BMC Gastroenterol. 2022;22:121. doi: 10.1186/s12876-022-02199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhu JY, Zhou LN, Tang M, Chen MB, Tao M. Predicting the prognosis of gastric cancer by albumin/globulin ratio and the prognostic nutritional index. Nutr Cancer. 2020;72:635–644. doi: 10.1080/01635581.2019.1651347. [DOI] [PubMed] [Google Scholar]
- 16.Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, Imaoka Y, Yasuda H, Ohi M, Kusunoki M. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr. 2021;40:1130–1136. doi: 10.1016/j.clnu.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol Cancer. 2022;21:28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 20.Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 2021;41:747–795. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bossi P, Delrio P, Mascheroni A, Zanetti M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: A narrative review. Nutrients. 2021;13:1980. doi: 10.3390/nu13061980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. (In Japanese) [PubMed] [Google Scholar]
- 23.Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271:693–700. doi: 10.1097/SLA.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, Zhou L. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. 2021;25:421–427. doi: 10.1007/s11605-019-04492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen F, Ma Y, Guo W, Li F. Prognostic value of geriatric nutritional risk index for patients with non-small cell lung cancer: A systematic review and meta-analysis. Lung. 2022;200:661–669. doi: 10.1007/s00408-022-00567-6. [DOI] [PubMed] [Google Scholar]
- 26.Nogueiro J, Santos-Sousa H, Pereira A, Devezas V, Fernandes C, Sousa F, Fonseca T, Barbosa E, Barbosa JA. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbecks Arch Surg. 2022;407:2703–2714. doi: 10.1007/s00423-022-02627-0. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara T, Takamori S, Haratake N, Toyozawa R, Miura N, Shimokawa M, Yamaguchi M, Seto T, Takenoyama M. The impact of immune-inflammation-nutritional parameters on the prognosis of non-small cell lung cancer patients treated with atezolizumab. J Thorac Dis. 2020;12:1520–1528. doi: 10.21037/jtd.2020.02.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guven DC, Aktepe OH, Taban H, Aktas BY, Guner G, Yildirim HC, Sahin TK, Aksun MS, Dizdar O, Aksoy S, et al. Lower prognostic nutritional index is associated with poorer survival in patients receiving immune checkpoint inhibitors. Biomark Med. 2021;15:1123–1130. doi: 10.2217/bmm-2020-0674. [DOI] [PubMed] [Google Scholar]
- 29.Ishiyama Y, Kondo T, Nemoto Y, Kobari Y, Ishihara H, Tachibana H, Yoshida K, Hashimoto Y, Takagi T, Iizuka J, Tanabe K. Predictive impact of prognostic nutritional index on pembrolizumab for metastatic urothelial carcinoma resistant to platinum-based chemotherapy. Anticancer Res. 2021;41:1607–1614. doi: 10.21873/anticanres.14922. [DOI] [PubMed] [Google Scholar]
- 30.Song M, Zhang Q, Song C, Liu T, Zhang X, Ruan G, Tang M, Xie H, Zhang H, Ge Y, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. 2022;13:2504–2514. doi: 10.1002/jcsm.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Ding F, Lin M, Shi Z, Mei Z, Chen S, Jiang C, Qiu H, Zheng Z, Chen Y, Zhao P. Use of the advanced lung cancer inflammation index as a prognostic indicator for patients with cholangiocarcinoma. Front Surg. 2022;9:801767. doi: 10.3389/fsurg.2022.801767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudioso P, Borsetto D, Tirelli G, Tofanelli M, Cragnolini F, Menegaldo A, Fabbris C, Molteni G, Marchioni D, Nicolai P, et al. Advanced lung cancer inflammation index and its prognostic value in HPV-negative head and neck squamous cell carcinoma: A multicentre study. Support Care Cancer. 2021;29:4683–4691. doi: 10.1007/s00520-020-05979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pian G, Hong SY, Oh SY. Prognostic value of advanced lung cancer inflammation index in patients with colorectal cancer liver metastases undergoing surgery. Tumori. 2022;108:56–62. doi: 10.1177/0300891620983465. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Wang D, Sun T, Li W, Dang C. Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer. 2022;22:684. doi: 10.1186/s12885-022-09774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountzios G, Samantas E, Senghas K, Zervas E, Krisam J, Samitas K, Bozorgmehr F, Kuon J, Agelaki S, Baka S, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open. 2021;6:100254. doi: 10.1016/j.esmoop.2021.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guven DC, Aktepe OH, Aksun MS, Sahin TK, Kavgaci G, Ucgul E, Cakir IY, Yildirim HC, Guner G, Akin S, et al. The association between albumin-globulin ratio (AGR) and survival in patients treated with immune checkpoint inhibitors. Cancer Biomark. 2022;34:189–199. doi: 10.3233/CBM-210349. [DOI] [PubMed] [Google Scholar]
- 37.Hill LA, Bodnar TS, Weinberg J, Hammond GL. Corticosteroid-binding globulin is a biomarker of inflammation onset and severity in female rats. J Endocrinol. 2016;230:215–225. doi: 10.1530/JOE-16-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae YJ, Kratzsch J. Corticosteroid-binding globulin: Modulating mechanisms of bioavailability of cortisol and its clinical implications. Best Pract Res Clin Endocrinol Metab. 2015;29:761–772. doi: 10.1016/j.beem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Xie HL, Zhang Q, Ruan GT, Ge YZ, Hu CL, Song MM, Song CH, Zhang X, Zhang XW, Li XR, et al. Evaluation and validation of the prognostic value of serum albumin to globulin ratio in patients with cancer cachexia: Results from a large multicenter collaboration. Front Oncol. 2021;11:707705. doi: 10.3389/fonc.2021.707705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salciccia S, Frisenda M, Bevilacqua G, Viscuso P, Casale P, De Berardinis E, Di Pierro GB, Cattarino S, Giorgino G, Rosati D, et al. Prognostic value of albumin to globulin ratio in non-metastatic and metastatic prostate cancer patients: A meta-analysis and systematic review. Int J Mol Sci. 2022;23:11501. doi: 10.3390/ijms231911501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong G, Liu S, Xi C, Wang C, Deng J, Qin T. Correlation between preoperative serum albumin-globulin ratio and prognosis of patients undergoing low rectal cancer surgery. Clin Lab. 2022;68 doi: 10.7754/Clin.Lab.2021.210213. [DOI] [PubMed] [Google Scholar]
- 42.Oymak E, Guler OC, Onal C. Prognostic significance of albumin and globulin levels in cervical cancer patients treated with chemoradiotherapy. Int J Gynecol Cancer. 2023;33:19–25. doi: 10.1136/ijgc-2022-003768. [DOI] [PubMed] [Google Scholar]
- 43.Gundog M, Basaran H. Pretreatment low prognostic nutritional index and low albumin-globulin ratio are predictive for overall survival in nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2019;276:3221–3230. doi: 10.1007/s00405-019-05595-2. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Wang W, Meng X, Sun B, Cong Y, Liu J, Wang Q, Liu G, Wu S. Albumin/globulin ratio is negatively correlated with PD-1 and CD25 mRNA levels in breast cancer patients. Onco Targets Ther. 2018;11:2131–2139. doi: 10.2147/OTT.S159481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: A meta-analysis. Clin Chim Acta. 2018;476:81–91. doi: 10.1016/j.cca.2017.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.