Abstract
Background
Changes in brain connectivity occur in patients with multiple sclerosis (MS), even in patients under disease-modifying therapies. Using magnetic resonance imaging (MRI) to asses patients treated with disease-modifying therapies, such as natalizumab, can elucidate the mechanisms involved in clinical deterioration in MS.
Objectives
To evaluate differences in resting-state functional connectivity among MS patients treated with natalizumab, MS patients not treated with natalizumab, and controls.
Design
Single-center retrospective cross-sectional study.
Methods
Twenty-three MS patients being treated with natalizumab were retrospectively compared with 23 MS patients who were naïve for natalizumab, and were using first-line medications (interferon-β and/or glatiramer acetate), and 17 gender- and age-matched control subjects. The MS patient groups were also matched for time since diagnosis and hyperintense lesion volume on FLAIR. All participants underwent brain MRI using a 3 Tesla scanner. Independent component analysis and dual regression were used to identify resting-state functional connectivity using the FMRIB Software Library.
Results
In comparison to controls, the MS patients treated with natalizumab presented decreased connectivity in the left orbitofrontal cortex, in the anterior cingulate and orbitofrontal cortex network. The patients not treated with natalizumab presented increased connectivity in the secondary visual, sensorimotor, and ventral attention networks in comparison to controls.
Compared to patients treated with natalizumab, the patients not using natalizumab presented increased connectivity in the left Heschl’s gyrus and in the right superior frontal gyrus in the ventral attention network.
Conclusion
Differences in brain connectivity between MS patients not treated with natalizumab, healthy controls, and patients treated with natalizumab may be secondary to suboptimal neuronal compensation due to prior less efficient treatments, or due to a compensation in response to maladaptive plasticity.
Keywords: Multiple sclerosis, natalizumab, resting-state functional imaging
Introduction
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system (CNS), which leads to widespread brain and spinal cord lesions,1,2 with early inflammation, causing relapsing–remitting disease and delayed neurodegeneration causing nonrelapsing progression. 3 The current therapeutic strategies for MS aims to reduce the risk of relapse and disability progression. 4 In recent years, the number of therapeutic options for MS expanded widely. Several disease-modifying therapies (DMTs) for MS were approved by the European Medicines Agency and U.S. Food and Drug Administration in both injectable and oral formulations. However, the growth in the number of therapies brings new challenges for individualized treatment in terms of the relative efficacy of the drugs available, identifying which patients should receive therapy, and recognizing the optimum time to start treatment. There is currently no consensus among physicians to classify patients into high-risk and low-risk groups to prioritize treatment strategies 5 ; however, early treatment with highly effective DMTs is increasingly recommended. 6
Natalizumab is a humanized monoclonal antibody. It targets the α4-integrin, which is present on white blood cells involved in inflammation, and it prevents the movement of mononuclear leukocytes to inflamed tissues, including those in the CNS. 7 High efficacy DMTs like natalizumab have a higher risk of serious adverse events than lower efficacy medications, such as interferon, such as progressive multifocal leukoencephalopathy, a disease caused by reactivation of the John Cunningham virus (JCV) or de novo infection. 1
Brain MRI is fundamental for MS diagnosis. MRI is used to visualize the distribution of the disease in the CNS over time, to exclude other conditions,2,8 and to monitor disease evolution, and treatment effects. 9 However, the association between conventional neuroradiological markers of MS and clinical disability has been weak due to several factors. 10 These factors include the limited sensitivity of clinical outcome measures, such as the Expanded Disability Status Scale (EDSS), the inability of conventional MRI to measure remyelination, and the emphasis of MRI on detection of focal white matter pathology. 9
The use of functional MRI can provide novel biomarkers to gather important in vivo information on brain activity following tissue injury. 11 In contrast with structural magnetic resonance techniques, which have allowed the extent and severity of MS brain damage to be qualified, functional MRI has demonstrated that cortical reorganization might play a role in the clinical consequences of tissue damage. 12 The scattered nature of MS lesions may be effectively studied using network analyses. Analysis of brain functional networks in MS has led to several observations. For instance, this type of analysis has shown that there is a high likelihood of altered connectivity in basal ganglia, a decrease in brain modularity, hemispheric asymmetries in connectivity alterations, and a correspondence of behavioral symptoms with task-related and task-unrelated networks. 13 Previous studies showed contrasting results in the alterations of resting state networks in MS, related to clinical disability, with both increased and decreased connectivity in the motor areas, cerebellum and other networks, including the default mode and fronto-parietal. This suggests the co-occurrence of adaptive and maladaptive responses within networks, which vary according to patients’ clinical characteristics. Also, both increased and decreased connectivity in the default mode network has been related to cognitive impairment, indicating that hyper- and hypo-connected networks lead to an inefficient cognition. 14
To date, however, no studies have evaluated the effects of natalizumab on brain connectivity of patients with MS, without progressive multifocal leukoencephalopathy. Therefore, the aim of this study is to analyze resting-state functional connectivity in MS patients treated with natalizumab in comparison to patients not treated with natalizumab and control subjects.
Material and Methods
Subjects
This study was approved by the Institutional Review Board of the Clementino Fraga Filho University Hospital (research protocol: 169/08), and all participants signed informed consent. Between September 2012 and September 2018, 46 right-handed patients with a diagnosis of relapsing-remitting MS according to the 2017 revision of the McDonald criteria 8 were selected from the Demyelinating Disease Outpatient Clinic of the Clementino Fraga Filho University Hospital. Even patients who were diagnosed before 2017, fulfilled the diagnostic criteria for multiple sclerosis according to the 2017 revision of the McDonald criteria, retrospectively. The patients were all in the clinical remission phase of the disease. The exclusion criteria were the presence of other autoimmune diseases diagnosed by clinical and serological tests, reported use of illicit drugs in the previous year, lesions with contrast enhancement observed in the brain MRI (active lesions), and MRI contraindications.
The MS patients were divided into two groups according to their treatment: MS patients treated with natalizumab and MS patients not treated with natalizumab. Twenty-three MS patients were treated with natalizumab for at least 6 months prior to the date of MRI and were never treated with other DMTs. Patients in this group were administered 300 mg natalizumab intravenously as monotherapy once per month for at least 6 months, and none were positive for JCV antibodies. Twenty-three MS patients were receiving medications other than natalizumab, such as interferon-β and/or glatiramer acetate, when the MRI data were acquired, and they were naïve for other DMTs. In some patients who had been previously treated with corticosteroids to control acute relapse, the MRI scans were performed at least 30 days after the end of this treatment. No participant had active lesions in the MRI. The database also included 17 right-handed healthy controls (11 women and 6 men), who had no clinical or imaging evidence of neurological disease.
Magnetic Resonance Imaging Acquisition
Magnetic resonance imaging was performed with a 3 Tesla scanner (Magneton Trio, Siemens, Erlangen, Germany) using a standard 8-channel phased-array head coil. The conventional MRI protocol included axial FLAIR with the following conditions: repetition time (TR) = 9000 ms, echo time (TE) = 80 ms, inversion time (TI) = 2500 ms, matrix = 208 × 256, and flip angle = 150°. The protocol also included a high-resolution, sagittal, 3D T1 MPRAGE with the following conditions: TR = 2530 ms, TE = 3.45 ms, TI = 1100 ms, matrix = 256 × 256, flip angle = 7°, and voxel size 1.3 mm × 1.0 mm × 1.3 mm. Resting-state functional MRI (RS-fMRI) was acquired using a T2*-weighted gradient spin-echo sequence (TE = 30 ms, TR = 3000 ms, 72 × 72 acquisition matrix, flip angle = 85°) that was sensitive to the blood oxygen level-dependent (BOLD) contrast. In total, 36 interleaved, 124 images, with 3 mm thickness and 30% gap were acquired parallel to the anterior commissure plane, totaling 6 minutes and 18 seconds of scanning. Each subject’s head was stabilized with tape across the forehead and padding around the sides. Earplugs were used to minimize MRI noise interference. All MRI scans were reviewed by an experienced neuroradiologist (10 years of experience) and were considered of good quality for post-processing.
Structural Magnetic Resonance Imaging Analysis
White matter, gray matter and whole brain volumes (white matter + grey matter), as well as thalami volumes were assessed on 3-dimentional (3D) T1 magnetization-prepared rapid gradient echo (MPRAGE), using an automated online MRI brain volumetry system (volBrain - https://www.volbrain.upv.es/). T2-hyperintense lesions volume measurements were performed on fluid-attenuated inversion recovery (FLAIR) images, also in volBrain. This system is primarily based on a multiatlas, patch-based segmentation method and utilizes training libraries implemented in the online platform.15,16 VolBrain has been previously validated, compared with state-of-the-art methods showing very similar results for brain volumetric measures. 16
Processing of RS-fMRI Data
RS-fMRI data were preprocessed with FMRIB Software Library (FSL) version 5.0.9 tools (http://www.fmrib.ox.ac.uk/fsl). To begin, the first 10 volumes were removed to include only steady-state BOLD volumes. Then, before preprocessing, the necessary matrices and warp volumes to register the individual RS-fMRI scan to Montreal Neurological Institute (MNI)-152 standard space were calculated. Next, the remaining 114 images were motion-corrected, and non-brain tissue was removed. In addition, the images were spatially smoothed with a 6-mm full width at half maximum (FWHM) Gaussian kernel and temporally filtered using a high-pass filter of 150 s. After this preprocessing, individual functional scans were non-linearly warped to 4-mm MNI-152 standard space using a 10-mm warp resolution.
To correct for motion, scrubbing was applied. First, based on the raw fMRI scans, the FSL’s Motion Outliers script was used to calculate the volumes to be scrubbed based on the Frame-wise Displacement (FD) and DVARS (D referring to temporal derivative of time-course and VARS to the root mean squared variance over voxels) combined. 17 Next, the 3dTproject script of AFNI (https://afni.nimh.nih.gov/) was used to scrub the preprocessed fMRI scans in 4-mm standard space. Removing volumes leads to fMRI scans of different lengths, which can potentially confound between-group analyses, 18 so we interpolated the values of the to-be scrubbed volumes from the neighboring volumes in time. Therefore, volumes with high motion were censored but not removed. There were no significant differences in motion between the groups (P = .457, and .383, before and after scrubbing respectively; Table 1).
Table 1.
Sociodemographic and Clinical Data of MS Patient Groups and Controls Participants.
| Groups | Mean | Standard Deviation | F | P | |
|---|---|---|---|---|---|
| Age in years* | Controls | 44.24 (range 21-52) | 9.54 | 1.71 | .189 |
| MSuNat | 41.13 (range 29-61) | 9.01 | |||
| MSnuNat | 38.73 (range 28-59) | 9.35 | |||
| Sex*1 | Controls | 6 M/11 W | — | — | .447 |
| MSuNat | 4 M/19 W | — | |||
| MSnuNat | 6 M/17 W | — | |||
| Time since diagnosis (months)*2 | Controls | — | — | — | .741 |
| MSuNat | 78.91 (range 12-180) | 50.87 | |||
| MSnuNat | 77.00 (range 7-228) | 58.24 | |||
| RS-fMRI relative displacement ratio before scrubbing* | Controls | .06 (range .02-.17) | .04 | .794 | .457 |
| MSuNat | .05 (range .02-.11) | .02 | |||
| MSnuNat | .05 (range .01-.21) | .04 | |||
| RS-fMRI relative displacement ratio after scrubbing* | Controls | .05 (range .02-.14) | .03 | .976 | .383 |
| MSuNat | .04 (range .01-.17) | .02 | |||
| MSnuNat | .04 (range .01-.17) | .03 | |||
| T2-hyperintense lesion volume seen on FLAIR (absolute volume – cm3)*2 | Controls | — a | — | — | .248 |
| MSuNat | 20.67 (range 1.52-80.01) | 20.84 | |||
| MSnuNat | 19.37 (range .32-85.05) | 24.57 | |||
| T2-hyperintense lesions volume seen on FLAIR (relative volume – normalized by the total intracranial volume - %)*2 | Controls | — a | — | — | .218 |
| MSuNat | 1.50 (range .11-4.92) | 1.40 | |||
| MSnuNat | 1.40 (range .02-5.73) | 1.74 | |||
| Time using natalizumab (months) | Controls | — | — | — | - |
| MSuNat | 16.52 (range 6-48) | 9.91 | |||
| MSnuNat | — | — | |||
| Time elapsed from diagnosis until the start of natalizumab (months) | Controls | — | — | — | - |
| MSuNat | 62.39 (range 0-152) | 45.79 | |||
| MSnuNat | — | — |
M = men; W = women; MSuNat = multiple sclerosis patients using natalizumab; MSnuNat = multiple sclerosis patients not using natalizumab; RS-fMRI = resting state functional magnetic resonance imaging. Statistical analysis used: *: ANOVA with post hoc Bonferroni; *1: Fisher's exact test; *2: Kruskal–Wallis test.
aControl participants had a normal T2-FLAIR or only mild unspecific hyperintensities foci in frontal and parietal white matter. The average absolute volume of these hyperintensities was .21 cm3 (range 0-1.75 cm3), relative volume was .01% (range 0%–.14%).
Independent component analysis was used to identify large-scale functional connectivity patterns. 19 The spatial and temporal information was then back-regressed onto the individual fMRI scans in common space using FSL’s “dual-regression” approach 20 to allow for voxel-wise between-group comparisons. The functional networks were selected by visual inspection and comparison with earlier studies. 21
Statistical Analysis
Demographic information was analyzed using IBM SPSS Statistics version 20 (Chicago, IL, USA) using analysis of variance (ANOVA) with post-hoc Bonferroni or the two-sample t-test for normally distributed variables, the Kruskal–Wallis test for non-normally distributed variables, and Fisher's exact test for categorical variables. For RS-fMRI, FSL permutation analysis of linear models was used to perform two-sided voxel-wise between-group analyses using a non-parametric general linear model with 10 000 permutations. 22 Multiple comparisons were controlled for by applying threshold-free cluster enhancement and family-wise error correction using age and sex as confounding factors. The following comparisons were made: (1) All MS patients (treated and not treated with natalizumab) vs controls, (2) MS patients treated with natalizumab vs controls, (3) MS patients not treated with natalizumab vs controls, and (4) MS patients treated with natalizumab vs MS patients not treated with natalizumab. P < .05 was considered statistically significant.
Results
Participants
The entire MS group included 46 participants (36 women and 10 men). Of the MS patients, 23 were treated with natalizumab (50%), and 23 were not treated with natalizumab (50%). In the MS group not using natalizumab, 10 were using interferon, and 13 were using glatiramer acetate. No participant had a history of using fingolimod or another disease-modifying treatment. The control group included 17 participants (11 women and 6 men). As shown in Table 1, all groups were matched for age and sex. The MS groups were also comparable in terms of disease duration. The MS patients treated with natalizumab had a disease duration of 78.91 ± 50.87 months, and the MS patients not treated with natalizumab had a disease duration of 77.00 ± 58.24 months (P = .741). There was no significant difference in T2-hyperintense lesions volume between the MS patients treated with natalizumab and those not treated with natalizumab (Table 1). Although there was brain atrophy in both MS groups, because of a reduction in white matter and thalami volumes, there was no significant difference in white matter, gray matter, thalami and whole-brain volumes between the two MS groups (Table 2).
Table 2.
Mean Absolute and Normalized Brain Volume of the Participants. Note that Although there was Brain Atrophy in MS Patients, Compared to Controls, Because of a Reduction in White Matter and Thalami Volumes, there was No Significant Difference in White Matter, Gray Matter, Thalami and Whole-Brain Volumes Between the Two MS Groups.
| Volume | Groups | Mean (Standard Deviation) | P | Pairwise Comparisons (P) |
|---|---|---|---|---|
| White matter (absolute volume – cm3)* | Controls | 487.10 (72.55) | .025 | Controls vs MSuNat – .022 |
| MSuNat | 425.75 (69.76) | Controls vs MSnuNat – .672 | ||
| MSnuNat | 467.97 (73.78) | MSuNat vs MSnuNat – .152 | ||
| White matter (normalized by the total cranial volume - %)* | Controls | 37.20 (2.47) | <.001 | Controls vs MSuNat - <.001 |
| MSuNat | 31.70 (3.90) | Controls vs MSnuNat – .003 | ||
| MSnuNat | 33.47 (3.84) | MSuNat vs MSnuNat – .256 | ||
| Gray matter (absolute volume – cm3)* | Controls | 626.02 (75.30) | .299 | Controls vs MSuNat – .993 |
| MSuNat | 627.34 (59.75) | Controls vs MSnuNat - .372 | ||
| MSnuNat | 653.82 (64.49) | MSuNat vs MSnuNat – .407 | ||
| Gray matter (normalized by the total cranial volume - %)* | Controls | 48.05 (3.96) | .388 | Controls vs MSuNat – .415 |
| MSuNat | 46.85 (2.67) | Controls vs MSnuNat - .429 | ||
| MSnuNat | 46.87 (2.56) | MSuNat vs MSnuNat – .999 | ||
| White matter + gray matter (absolute volume – cm3)* | Controls | 1113.13 (132.36) | .159 | Controls vs MSuNat – .491 |
| MSuNat | 1054.65 (119.86) | Controls vs MSnuNat – .776 | ||
| MSnuNat | 1147.88 (214.96) | MSuNat vs MSnuNat – .170 | ||
| White matter + gray matter (normalized by the total cranial volume - %)* | Controls | 85.26 (3.79) | <.001 | Controls vs MSuNat – <.001 |
| MSuNat | 78.56 (5.10) | Controls vs MSnuNat – .004 | ||
| MSnuNat | 80.35 (5.05) | MSuNat vs MSnuNat – .452 | ||
| Right thalamus volume (absolute volume – cm3)* | Controls | 5.15 (.50) | <.001 | Controls vs MSuNat – <.001 |
| MSuNat | 4.06 (.99) | Controls vs MSnuNat – .02 | ||
| MSnuNat | 4.42 (.87) | MSuNat vs MSnuNat – .36 | ||
| Right thalamus volume (normalized by the total cranial volume - %)* | Controls | .39 (.03) | <.001 | Controls vs MSuNat – <.001 |
| MSuNat | .30 (.06) | Controls vs MSnuNat – <.001 | ||
| MSnuNat | .31 (.06) | MSuNat vs MSnuNat – .63 | ||
| Left thalamus volume (absolute volume – cm3)* | Controls | 5.15 (.49) | .004 | Controls vs MSuNat – .02 |
| MSuNat | 4.15 (1.08) | Controls vs MSnuNat – .025 | ||
| MSnuNat | 4.38 (.97) | MSuNat vs MSnuNat – .70 | ||
| Left thalamus volume (normalized by the total cranial volume - %)* | Controls | .39 (.03) | <.001 | Controls vs MSuNat – <.001 |
| MSuNat | .30 (.06) | Controls vs MSnuNat – <.001 | ||
| MSnuNat | .31 (.06) | MSuNat vs MSnuNat – .93 |
MSuNat = multiple sclerosis patients using natalizumab; MSnuNat = multiple sclerosis patients not using natalizumab. Statistical analysis used: *: ANOVA with post hoc Bonferroni.
Functional Connectivity at Rest
Independent component analysis identified 37 components of which 15 coincided with resting-state networks described in previous studies. These 15 resting-state networks were the following: primary visual, secondary visual, anterior default mode (with hippocampus), posterior default mode (without hippocampus), dorsal attention, frontal, left frontoparietal, right frontoparietal, cerebellar, auditory, primary sensorimotor, anterior cingulate and orbitofrontal cortex, limbic, basal ganglia, and ventral attention networks.
All Multiple Sclerosis Patients vs Controls
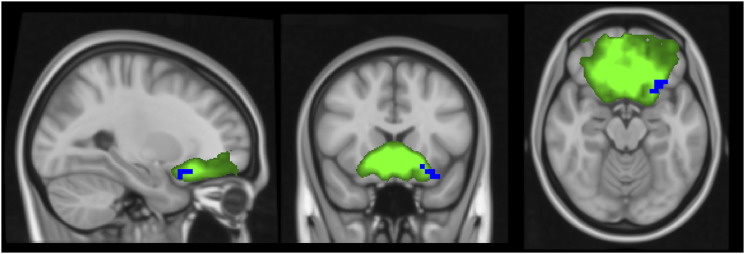
Compared to controls, the MS patients (those treated and not treated with natalizumab) presented decreased connectivity in voxels in the left orbitofrontal cortex in the anterior cingulate and orbitofrontal cortex network (Figure 1). The other resting-state networks did not show significant differences in connectivity in this comparison.
Figure 1.
Independent component analysis map of the anterior cingulate and orbitofrontal cortex network in green on a 1-mm MNI152 standard brain, for visual purpose. Corrected RS-fMRI maps in the sagittal, coronal, and axial planes that compare all the MS patients (treated and not treated with natalizumab) vs the control group. Blue voxels represent areas with decreased connectivity in the MS group compared to controls present in the left orbitofrontal cortex.
Multiple Sclerosis Patients Treated With Natalizumab vs Controls
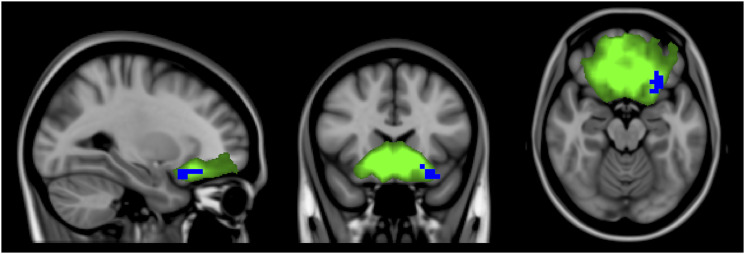
Compared to controls, MS patients treated with natalizumab presented decreased connectivity in voxels in the left orbitofrontal cortex in the anterior cingulate and orbitofrontal cortex network (Figure 2). The other resting-state networks did not show significant differences in connectivity in this comparison.
Figure 2.
Independent component analysis map of the anterior cingulate and orbitofrontal cortex network in green on a 1-mm MNI152 standard brain, for visual purpose. Corrected RS-fMRI maps in the sagittal, coronal, and axial planes that compare MS patients treated with natalizumab vs the control group. Blue voxels represent areas with decreased connectivity in the MS group compared to controls present in the left orbitofrontal cortex. Note the similarity of the altered voxels of MS patients using natalizumab vs controls in relation with the altered voxels in the comparison of all MS patients vs controls.
Multiple Sclerosis Patients Not Treated With Natalizumab vs Controls
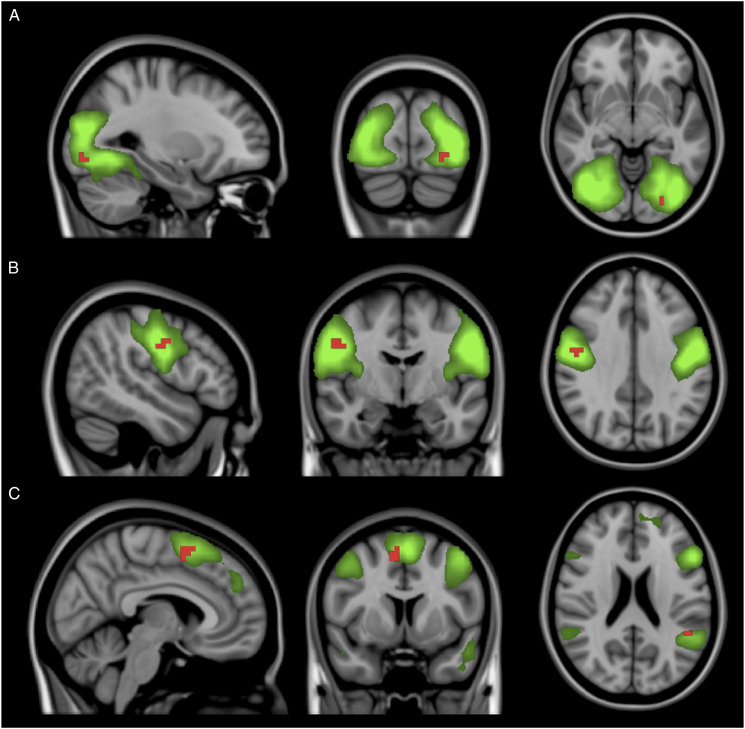
Compared to controls, MS patients not treated with natalizumab presented increased connectivity in voxels in the left fusiform gyrus in the secondary visual network, in the right precentral gyrus in the sensorimotor network, and in the left supramarginal and right frontal superior gyri in the ventral attention network (Figure 3). The other resting-state networks did not show significant differences in connectivity in this comparison.
Figure 3.
Independent component analysis maps of the secondary visual network (A), sensorimotor network (B), and ventral attention network (C) in green on a 1-mm MNI152 standard brain, for visual purpose. Corrected RS-fMRI maps in the sagittal, coronal, and axial planes that compare MS patients not using natalizumab vs the control group. Red voxels represent areas with increased connectivity in the MS group not using natalizumab compared to the controls. These areas include the left fusiform gyrus in panel A (secondary visual network), right precentral gyrus in panel B (sensorimotor network), and left supramarginal and right frontal superior gyri in panel C (ventral attention network).
Multiple Sclerosis Patients Not Treated With Natalizumab vs Multiple Sclerosis Patients Treated With Natalizumab
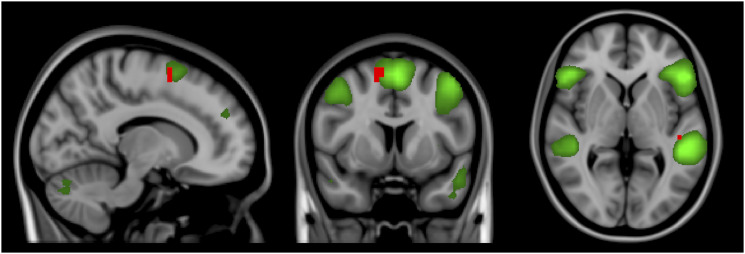
In comparison to MS patients using natalizumab, MS patients not treated with natalizumab presented increased connectivity in voxels in the left Heschl’s gyrus and in the right superior frontal gyrus in the ventral attention network (Figure 4). The other resting-state networks did not show significant differences in connectivity in this comparison.
Figure 4.
Independent component analysis map of the ventral attention network in green on a 1-mm MNI152 standard brain, for visual purpose. Corrected RS-fMRI maps in the sagittal, coronal, and axial planes that compare MS patients not using natalizumab vs MS patients using natalizumab. Red voxels represent areas with increased connectivity in the MS group not using natalizumab compared to the MS group using natalizumab. These areas are present in the left Heschl’s gyrus and the right superior frontal gyrus.
Discussion
In this study, we used a voxel-wise method to assess RS-fMRI networks through independent component analysis in 23 MS patients treated with natalizumab, 23 MS patients not treated with natalizumab, and 17 control subjects. Our results showed that compared to controls, the group formed by all the MS patients presented decreased connectivity in the anterior cingulate and orbitofrontal cortex network. Furthermore, the MS patients using natalizumab also presented decreased connectivity in the anterior cingulate and orbitofrontal cortex network compared to controls. In contrast, MS patients not using natalizumab presented increased connectivity in the secondary visual, sensorimotor, and ventral attention networks compared to controls, but no differences in the orbitofrontal cortex network. Therefore, at a group level, the decreased anterior cingulate and orbitofrontal cortex network connectivity was entirely driven by those patients using natalizumab. In addition, MS patients not treated with natalizumab presented increased connectivity in the ventral attention network compared to the patients using natalizumab.
The inflammatory process of MS leads to tissue damage within the lesions. In parallel to the disease process, remyelination is possible and can repair damaged tissue to some extent and is observable in all disease stages. 3 In addition to remyelination, there is evidence of reorganization of brain function, secondary to cortical plasticity, which consists of a reorganization of the functional activation of cortical regions to maintain clinical function.1,23 However, this functional reorganization can be maladaptive and contribute to disability. To date, the effects of DMTs on the mechanisms of brain plasticity are not fully understood. 23 Switching from MS treatment with interferon or glatiramer acetate to natalizumab results in superior outcomes, including a reduced risk of relapse, longer time to relapse, reduced treatment discontinuation events, and reduced disability worsening.24-26 The escalation treatment approach is presumed to be safer, but carries a risk of suboptimal control of neuroinflammation and subsequent irreversible long-term neurodegeneration. In contrast, early use of DMT might generate better long-term outcomes.27,28 Guidelines from the American Academy of Neurology, European Committee of Treatment and Research in Multiple Sclerosis, and European Academy of Neurology encourage early initiation of DMT in patients with active relapsing-remitting MS. 27
The MS patients in the current study had been diagnosed with MS for an average of 78.91 months for those treated with natalizumab and 77 months for those not treated with natalizumab. Some of the patients had been diagnosed with MS for more than 20 years, and were diagnosed prior to the approval of natalizumab for clinical use in 2006. Thus, the MS patients using natalizumab had a history of receiving medications that were less effective, such as interferon, prior to their treatment with natalizumab. One hypothesis for our results is that some participants in the group using natalizumab had their treatments switched to more effective drugs because they initially had poor clinical outcomes with less effective drugs. Meanwhile, participants in the group not treated with natalizumab were not switched to a more effective treatment because they had good responses to interferon or glatiramer. This heterogeneous response to treatment with less effective drugs can occur due to genetic and environmental factors, which are not yet fully known.
The group treated with natalizumab may have lower connectivity in some brain networks compared to the group not using natalizumab and the controls because they had worse clinical conditions prior to the use of natalizumab. The clinical condition of these patients may be secondary to poor responses to less effective treatments prior to their treatment with natalizumab. This reduction in functional connectivity in the group treated with natalizumab may have occurred due to previous progressive accumulation of structural damage. The patients using natalizumab had been diagnosed with MS for an average of 78.91 months, but were only using natalizumab for 16.52 months. Therefore, these patients may have started an optimal treatment with natalizumab too late to adequately compensate for neuronal damage.
There is an alternative hypothesis to explain our results. Although increased functional connectivity is generally considered an adaptation to compensate for tissue damage, it can also represent maladaptive plasticity secondary to disease activity. 29 Therefore, the increased connectivity seen in patients not using natalizumab may represent a failed attempt to compensate for brain lesions due to neuroinflammation and be the result of suboptimal treatment. However, on the other hand, it should also be considered that recent reviews described that several studies have found increased connectivity to be related with cognitive impairment, whereas other studies have found decreased connectivity to be related with cognitive impairment in MS.14,30 Additionally, other studies found both increased and decreased connectivity in different brain regions to be related to poor cognitive and motor performances.14,30 In the review by Jandric et al., 30 there was no clear relation between increased connectivity and earliest stages of the disease, as a compensatory phenomenon, and reduced connectivity in the late phases of the disease, as a result of structural damage progression, 30 as previously thought. 29 Therefore, the causes of decreases or increases in functional connectivity in MS are as yet unclear, mostly because of substantial heterogeneity in studies’ methodology and the wide spectrum of clinical characteristics. 30
Therefore, the results of previously RS-fMRI studies in MS are somewhat discordant. Liu et al. 31 reported decreased connectivity in several brain regions in patients with clinical isolated syndrome, demonstrating that the dynamics of cortical reorganization is more complex than simple compensation in the early phases of the disease. Alterations in resting-state functional connectivity have been observed throughout the brain in MS patients. Some studies showed a significant increase in global connectivity,32-35 and others reported decreased connectivity.33,35 In addition, Tona et al. 36 and Bonavita et al. 37 showed both increased and decreased connectivity in the thalamocortical network and default mode network, respectively. These results demonstrate that the redistribution of cortical connectivity is more complex than a simple trend of increased or decreased connectivity as a compensatory phenomenon or due to damage progression.
In this study, we demonstrated that despite having the disease for a similar length of time, patients using natalizumab had reduced connectivity compared to MS patients who never used this therapy. This difference is potentially due to the late initiation of the natalizumab or maladaptive plasticity. To the best of our knowledge, this is the first study to evaluate resting-state connectivity in MS patients using natalizumab in comparison to controls and MS patients who were never treated with natalizumab.
Several resting-state networks have been shown to be affected in MS patients. The most commonly affected networks are the visual, sensorimotor, and default mode networks. 13 Tommasin et al. 38 found higher connectivity in the default mode, executive control, and bilateral frontoparietal networks, of MS patients, compared to controls. Also, these authors found reduced connectivity between the anterior cerebellar network and executive control network and between the anterior cerebellar network and salience network, in MS patients, compared to controls. In the current study, we found that MS patients not using natalizumab had alterations in the secondary visual network, regardless of whether the participants had active optic neuritis or not, as in previous studies.35,39,40 Additionally, we found changes in the sensorimotor network in MS patients not using natalizumab, as observed in several previous studies.32,35,40 Although, we did not find alterations in the default mode network, other researchers also did not observe these differences.39,40 which may be due to differences in disease duration, MS phenotype, level of cognitive impairment, and treatment adequacy between the studies.
The limitations of this study include its relatively small sample size. Although we were able to demonstrate abnormalities in resting-state connectivity, studies with a larger number of participants may expand the results. As the study was retrospective, we were unable to assess the clinical functional status of the participants at the time of the examination. Therefore, we could not consider clinical characteristics, such as the EDSS in this study. However, we ensured that the groups of MS patients were matched for sex, age, and time from diagnosis, as well as for the hyperintense lesion volume seen on FLAIR, and white matter, gray matter, including thalami, and whole brain volumes. Also, none of the participants had active lesions or were using corticosteroids at the time of the study.
Conclusion
Patients with MS using natalizumab showed decreased connectivity in some resting-state networks when compared to controls and patients not using natalizumab. This decreased connectivity might be due to the late use of a highly efficacious drug, a poor response to other less efficacious drugs, a compensation in response to maladaptive plasticity. Future studies should consider the longitudinal evolution of brain functional connectivity in MS patients using natalizumab since their initial diagnosis.
Footnotes
Author contributions: Diogo Goulart Corrêa: Made a substantial contribution to the design of the work, drafted the article, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
Eelco van Duinkerken: Made a substantial contribution to the design of the work, drafted the article, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
João Gabriel Dib Farinhas: Made a substantial contribution to the acquisition of data, drafted the article, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
Valéria Coelho Pereira: Made a substantial contribution to the acquisition of data, drafted the article, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
Emerson Leandro Gasparetto: Made a substantial contribution to interpretation of data, revised the article critically for important intellectual content, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
Soniza Vieira Alves-Leon: Made a substantial contribution to analysis of data, revised the article critically for important intellectual content, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
Fernanda Cristina Rueda Lopes: Made a substantial contribution to analysis of data, revised the article critically for important intellectual content, approved the version to be published, take public responsibility for appropriate portions of the content of the article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author’s note: Diogo Goulart Correa: Conceptualization, Methodology, Writing – original draft, Eelco van Duinkerken: Data curation, Formal analysis, Methodology, Software, Writing – original draft, Joao Gabriel Dib Farinhas: Formal analysis, Investigation, Methodology, Writing – original draft, Valeria Coelho Pereira: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Emerson Leandro Gasparetto: Conceptualization, Formal analysis, Investigation, Project administration, Supervision, Writing – review & editing, Soniza Vieira Alves-Leon: Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review & editing, Fernanda Cristina Rueda Lopes: Formal analysis, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Ethics Approval and Consent to Participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of the Clementino Fraga Filho University Hospital (research protocol: 169/08) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all the participants included in the study.
Consent for Publication: Not applicable.
Availability of Data and Material: Available upon relevant requests.
ORCID iDs
Diogo G. Correa https://orcid.org/0000-0003-4902-0021
Joao Gabriel D. Farinhas https://orcid.org/0000-0001-8246-7891
References
- 1.Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet . 2018;391:1622-1636. [DOI] [PubMed] [Google Scholar]
- 2.Radü E-W, Mueller-Lenke N, Thoeni A, et al. MRI in multiple sclerosis: description of lesions and guidelines for standardized MRI Examinations. NeuroRadiol J. 2009;22(1 suppl):43-50. [Google Scholar]
- 3.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol . 2019;26:27-40. [DOI] [PubMed] [Google Scholar]
- 4.Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol . 2015;25(Suppl 2):157-165. [DOI] [PubMed] [Google Scholar]
- 5.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler . 2018;24:96-120. [DOI] [PubMed] [Google Scholar]
- 6.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol . 2018; 31: 233-243. [DOI] [PubMed] [Google Scholar]
- 7.Schreiner TL, Miravalle A. Current and emerging therapies for the treatment of multiple sclerosis: focus on cladribine. J Cent Nerv Syst Dis . 2012;4:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol . 2018;17:162-173. [DOI] [PubMed] [Google Scholar]
- 9.Oreja-Guevara C. Overview of magnetic resonance imaging for management of relapsing-remitting multiple sclerosis in everyday practice. Eur J Neurol . 2015;222:22-27. [DOI] [PubMed] [Google Scholar]
- 10.Schneider R, Matusche B, Genç E, et al. Microstructural white matter alterations in cognitively impaired patients at early stages of multiple sclerosis. Clin Neuroradiol . 2021;31:993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna N, Altmeyer W, Zhuo J, et al. Functional neuroimaging: fundamental principles and clinical applications. NeuroRadiol J 2015;28:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi M, Agosta F, Spinelli EG, et al. Imaging resting state brain function in multiple sclerosis. J Neurol . 2013;260:1709-1713. [DOI] [PubMed] [Google Scholar]
- 13.Tahedl M, Levine SM, Greenlee MW, et al. Functional connectivity in multiple sclerosis: recent findings and future directions. Front Neurol . 2018;9:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca MA, Schoonheim MM, Valsasina P, et al. Task- and resting-state fMRI studies in multiple sclerosis: from regions to systems and time-varying analysis. Current status and future perspective. Neuroimage Clin. 2022;35:103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh MK, Singh KK. A review of publicly available automatic brain segmentation methodologies, machine learning models, recent advancements, and their comparison. Ann Neurosci . 2021;28:82-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manjón JV, Coupé P. volBrain: an online MRI brain volumetry system. Front Neuroinform . 2016;27;10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage . 2012;59:2142-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage . 2015;105:536-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2017;360:1001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickerson LD, Smith SM, Öngür D, et al. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front Neurosci . 2017;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MH, Smyser CD, Shimony J. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol . 2013;34:1866-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage . 2014;92:381-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomassini V, Matthews PM, Thompson AJ, et al. Neuroplasticity and functional recovery in multiple sclerosis. Nat Rev Neurol . 2012;8:635-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Río J, Tintoré M, Sastre-Garriga J, et al. Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol . 2012;19:899-904. [DOI] [PubMed] [Google Scholar]
- 25.Spelman T, Kalincik T, Zhang A, et al. Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol . 2015;2:373-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prosperini L, Giannì C, Leonardi L, et al. Escalation to natalizumab or switching among immunomodulators in relapsing multiple sclerosis. Mult Scler . 2012;18:64-71. [DOI] [PubMed] [Google Scholar]
- 27.Jalkh G, Abi Nahed R, Macaron G, et al. Safety of newer disease modifying therapies in multiple sclerosis. Vaccines (Basel) . 2020;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoepner R, Faissner S, Salmen A, Gold R, Chan A. Efficacy and side effects of natalizumab therapy in patients with multiple sclerosis. J Cent Nerv Syst Dis . 2014;6:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbardella E, Petsas N, Tona F, et al. Resting-State fMRI in MS: general concepts and brief overview of its application. BioMed Res Int . 2022;2015:212693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jandric D, Doshi A, Scott R, et al. A systematic review of resting-state functional MRI connectivity changes and cognitive impairment in multiple sclerosis. Brain Connect . 2022;12:112-133. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Duan Y, Liang P, et al. Baseline brain activity changes in patients with clinically isolated syndrome revealed by resting-state functional MRI. Acta Radiol . 2012;53:1073-1078. [DOI] [PubMed] [Google Scholar]
- 32.Faivre A, Rico A, Zaaraoui W, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler . 2012;18:1251-1258. [DOI] [PubMed] [Google Scholar]
- 33.Dogonowski AM, Siebner HR, Soelberg Sørensen P, et al. Resting-state connectivity of pre-motor cortex reflects disability in multiple sclerosis. Acta Neurol Scand . 2013;128:328-335. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Liang P, Duan Y, et al. Brain plasticity in relapsing-remitting multiple sclerosis: evidence from resting-state fMRI. J Neurol Sci . 2011;304:127-131. [DOI] [PubMed] [Google Scholar]
- 35.Rocca MA, Valsasina P, Martinelli V, et al. Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology . 2012;79:1449-1457. [DOI] [PubMed] [Google Scholar]
- 36.Tona F, Petsas N, Sbardella E, et al. Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology . 2014;271:814-821. [DOI] [PubMed] [Google Scholar]
- 37.Bonavita S, Gallo A, Sacco R, et al. Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler . 2011;17:411-422. [DOI] [PubMed] [Google Scholar]
- 38.Tommasin S, De Giglio L, Ruggieri S, et al. Multi-scale resting state functional reorganization in response to multiple sclerosis damage. Neuroradiology. 2020;62:693-704. [DOI] [PubMed] [Google Scholar]
- 39.Sbardella E, Tona F, Petsas N, et al. Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing-remitting multiple sclerosis. Mult Scler . 2015;21:1681-1692. [DOI] [PubMed] [Google Scholar]
- 40.Janssen AL, Boster A, Patterson BA, et al. Resting-state functional connectivity in multiple sclerosis: an examination of group differences and individual differences. Neuropsychologia . 2013;51:2918-2929. [DOI] [PubMed] [Google Scholar]