Abstract
BACKGROUND
Although clinicians have traditionally used the Finnegan Neonatal Abstinence Scoring Tool to assess the severity of neonatal opioid withdrawal, a newer function-based approach — the Eat, Sleep, Console care approach — is increasing in use. Whether the new approach can safely reduce the time until infants are medically ready for discharge when it is applied broadly across diverse sites is unknown.
METHODS
In this cluster-randomized, controlled trial at 26 U.S. hospitals, we enrolled infants with neonatal opioid withdrawal syndrome who had been born at 36 weeks’ gestation or more. At a randomly assigned time, hospitals transitioned from usual care that used the Finnegan tool to the Eat, Sleep, Console approach. During a 3-month transition period, staff members at each hospital were trained to use the new approach. The primary outcome was the time from birth until medical readiness for discharge as defined by the trial. Composite safety outcomes that were assessed during the first 3 months of postnatal age included in-hospital safety, unscheduled health care visits, and nonaccidental trauma or death.
RESULTS
A total of 1305 infants were enrolled. In an intention-to-treat analysis that included 837 infants who met the trial definition for medical readiness for discharge, the number of days from birth until readiness for hospital discharge was 8.2 in the Eat, Sleep, Console group and 14.9 in the usual-care group (adjusted mean difference, 6.7 days; 95% confidence interval [CI], 4.7 to 8.8), for a rate ratio of 0.55 (95% CI, 0.46 to 0.65; P<0.001). The incidence of adverse outcomes was similar in the two groups.
CONCLUSIONS
As compared with usual care, use of the Eat, Sleep, Console care approach significantly decreased the number of days until infants with neonatal opioid withdrawal syndrome were medically ready for discharge, without increasing specified adverse outcomes. (Funded by the Helping End Addiction Long-term (HEAL) Initiative of the National Institutes of Health; ESC-NOW ClinicalTrials.gov number, NCT04057820.)
Every 18 minutes in the united States, neonatal opioid withdrawal syndrome is diagnosed in at least one newborn as a result of in utero opioid exposure.1 The clinical signs of this syndrome — which include gastrointestinal disturbances, irritability, hypertonia, and seizures2 — necessitate close monitoring and focused care, which subsequently prolong hospitalizations. In addition, without strong evidence to support a standard approach, care for infants with opioid withdrawal is highly varied, which has resulted in differences in the initiation of pharmacologic therapy, a primary driver for length of hospital stay.3
For nearly 50 years, the severity of neonatal opioid withdrawal syndrome has largely been assessed with the use of subjective, observer-rated scales — specifically, the Finnegan Neonatal Abstinence Scoring Tool or a modified version of this tool — and the decision to treat affected infants pharmacologically with opioids and other medications has relied on Finnegan severity thresholds.2,4–9 Despite concerns that this assessment tool overestimates the need for pharmacologic treatment,10,11 clinical management has remained largely dependent on its use in the absence of an evidence-based alternative.12
In 2014, Grossman and colleagues proposed the Eat, Sleep, Console approach for the assessment of infants with opioid withdrawal.13 More recently, the Eat, Sleep, Console approach, along with its associated care tool,14 has been increasing in use.10,15 The Eat, Sleep, Console Care Tool relies on a function-based assessment of withdrawal severity that is focused on an infant’s ability to eat, sleep, and be consoled, along with the use of nonpharmacologic interventions (e.g., low-stimulation environment, skin-to-skin contact, clustered care, and breast-feeding) as the first line of treatment and empowerment of families and caregivers in the care of their infants. This approach has been favorably evaluated by several statewide and regional quality-improvement initiatives, as compared with care using the Finnegan tool, and is consequently being adopted and implemented into clinical practice across the United States and internationally.10,15–19
Although findings from these initiatives appear promising,10,13–15,18,19 the rapid spread of this approach without strong evidence to support its efficacy, safety, or generalizability across diverse populations and varied care settings has caused concerns.20 These concerns include the potential for pharmacologic undertreatment21 and for premature discharge of affected infants, which could place them at increased risk for readmission, nonaccidental trauma, and death.
The Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW) collaborative, which is part of the National Institutes of Health Helping to End Addiction Long-term (HEAL) Initiative, was designed to advance high-quality evidence to inform a standard approach to caring for infants with opioid withdrawal.22 As part of this collaborative, we performed a randomized, controlled trial — Eating, Sleeping, Consoling for Neonatal Opioid Withdrawal (ESC-NOW) — to evaluate the safety, efficacy, and generalizability of the Eat, Sleep, Console approach as compared with usual care with the use of the Finnegan tool.
METHODS
TRIAL DESIGN AND OVERSIGHT
This multicenter, stepped-wedge, cluster-randomized, controlled trial was conducted at 26 U.S. sites in the ACT NOW Collaborative in accordance with Good Clinical Practice guidelines. The University of Arkansas for Medical Sciences served as the central institutional review board with reliance agreements for all trial sites. The in-hospital and initial follow-up portions of the trial were conducted with a waiver of informed consent, as approved by the central institutional review board and in accordance with the Code of Federal Regulations (45 CFR 46.116). An independent data and safety monitoring committee, which was appointed by the director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, oversaw the trial conduct. The first through fourth authors and the last author vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, which has been published previously23 and is available with the full text of this article at NEJM.org.
PATIENTS
To increase generalizability, we selected sites that were geographically diverse and that included both academic centers and community hospitals. The sites were also varied in terms of the volume of infants with opioid withdrawal who were treated, the proportion of infants who received pharmacological treatment, the location of care within the hospital (including open-bay neonatal intensive care units), and the extent of available nonpharmacologic interventions for infants with opioid withdrawal.
Throughout the trial, we enrolled infants who had been born at 36 weeks’ gestation or more, who had been born at or transferred to a trial site within 60 hours after birth, who had evidence of antenatal opioid exposure, and who were being treated for opioid withdrawal. Complete eligibility criteria and a list of participating sites are provided in the Supplementary Appendix, available at NEJM.org.
RANDOMIZATION
The 26 sites were randomly assigned to be included in one of eight blocks on the basis of strata that were defined according to the proportion of infants who were being treated pharmacologically before the trial initiation, a process that resulted in the inclusion of three to four sites per block. The trial blocks were then randomly assigned with respect to the timing of their transition to the Eat, Sleep, Console approach, as described in the Supplementary Appendix.
INTERVENTION
During the first trial period, all the infants with opioid withdrawal were treated according to the usual-care practices at each site, including the use of the Finnegan tool. Then, in the randomly assigned order, each site entered a 3-month transition period, which included training and implementation activities at the site (Fig. 1). Infants were not enrolled during this time. After implementation, the infants with opioid withdrawal were cared for according to the Eat, Sleep, Console approach, including the use of the associated care tool. Details regarding the intervention and the processes for training, implementation, and ensuring fidelity to the trial protocol are provided in Figures S2 through S4 in the Supplementary Appendix and in the Training and Implementation Manual.23
Figure 1. Process for Site Training and Implementation of the Eat, Sleep, Console Approach.
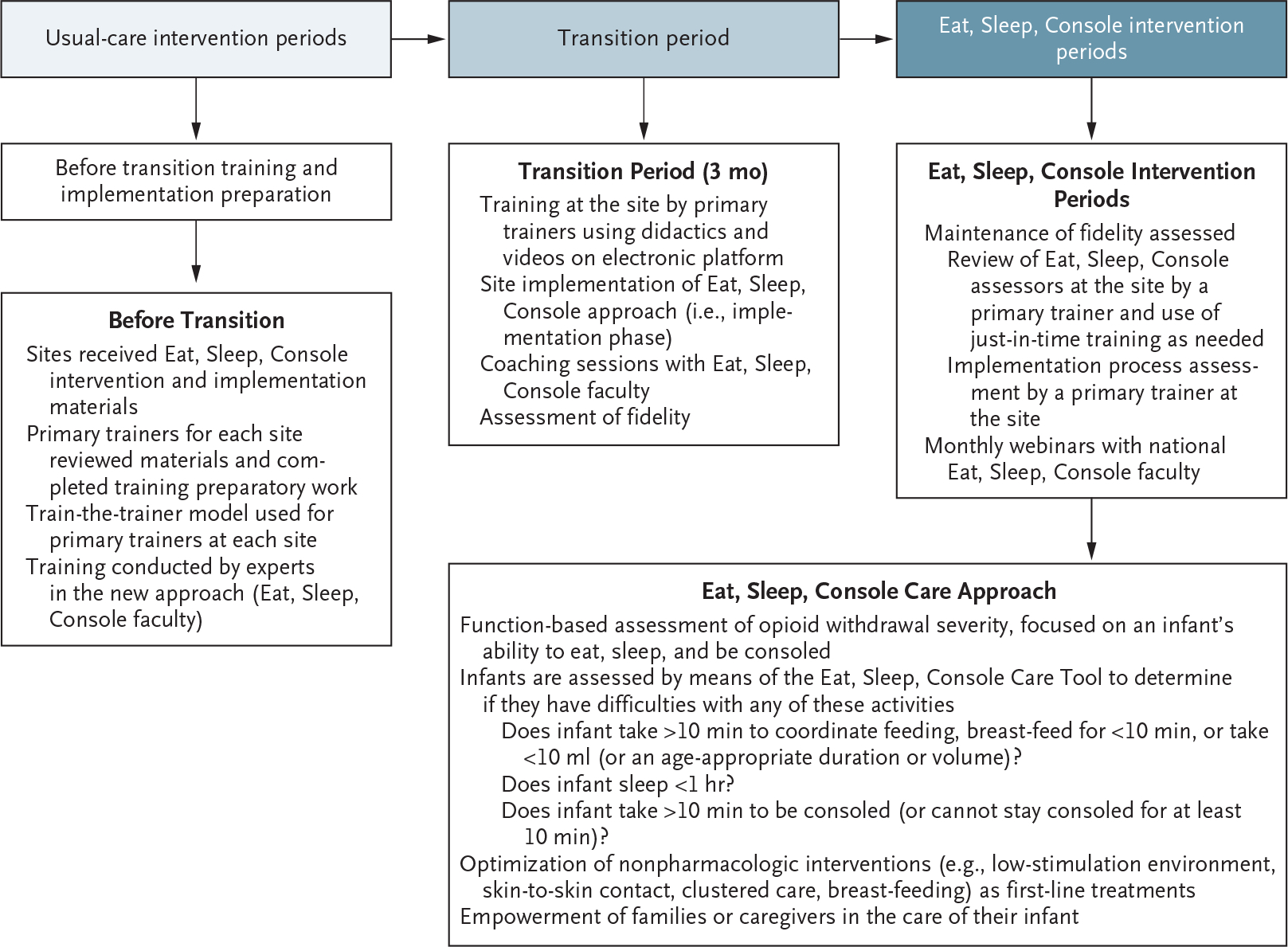
During the first trial period, all the infants with opioid withdrawal were cared for according to the usual-care practices at each site. At a randomly assigned time, each site entered a 3-month transition period, which included training and implementation activities at the site. After this transition, the infants with opioid withdrawal were cared for according to the Eat, Sleep, Console approach along with its care tool. Details regarding the intervention and the processes for training and implementation are provided in Figures S2 through S4 in the Supplementary Appendix.
During the usual-care periods, nonpharmacologic interventions were integrated into the care provided to infants with opioid withdrawal according to usual practice at each site. After implementation of the Eat, Sleep, Console approach, caregivers at each site applied nonpharmacologic interventions as needed for each infant and to the extent possible on the basis of available site resources.
Throughout the trial, sites maintained their local practice for pharmacologic treatment including opioid type, dosing approach, and use of adjuvant medications. Modifications were made to local treatment algorithms only as necessary to allow for their use with the Eat, Sleep, Console Care Tool and did not include the addition of symptom-based dosing. Clinical teams discharged infants according to their usual site practices, independent of the trial criteria for medical readiness for discharge.
OUTCOMES
The primary outcome was the time from birth until the infant was medically ready for discharge. The criteria for medical readiness were prospectively defined as an age of at least 96 hours, a period of at least 48 hours without receipt of an opioid, at least 24 hours with no respiratory support and with 100% oral feeding, and at least 24 hours from initiation of maximum caloric density. This definition of medical readiness for discharge was informed by standards published in 2012 by the American Academy of Pediatrics.24
Key secondary outcomes included the receipt of pharmacologic treatment and hospital length of stay. Safety outcomes included an in-hospital composite safety measure (seizures or accidental trauma [e.g., resulting from a fall] or respiratory insufficiency [documented apnea or need for positive-pressure ventilation or supplemental oxygen] attributed to opioid therapy), a composite safety measure through 3 months of age (any acute or urgent care visit, emergency department visit, or hospital readmission), and a composite critical safety outcome at discharge and through 3 months of age (nonaccidental trauma or death). Outcomes after hospital discharge were assessed prospectively at 3 months of age by means of a review of electronic medical records (including linked medical records) and media review through a search of public records (e.g., news reports, obituaries, and registries).
STATISTICAL ANALYSIS
The individual trial site was the unit of randomization whereas the unit of analysis was the enrolled infant.25 We determined that the enrollment of 864 infants would provide 90% power to detect a between-group difference of 4 days in the mean time from birth until infants were medically ready for hospital discharge with the use of a 0.25 intraclass correlation coefficient and a 0.8 cluster autocorrelation coefficient.25 We performed all the analyses on an intention-to-treat basis using a two-sided type I error of 0.05.
Regression models for the primary and all secondary outcomes were adjusted for the stepped-wedge design — with intervention and time as fixed effects and a site-specific random intercept to account for the clustering of infants within sites — and a strata indicator. Multivariable regression models included all prespecified baseline maternal and infant demographic characteristics. For primary and secondary count outcomes, we used a generalized linear mixed model with negative binomial distribution and log-link. We describe the effect of the Eat, Sleep, Console approach on the mean time from birth until medical readiness for discharge and length of stay as an adjusted rate ratio with a 95% confidence interval. For binary secondary outcomes, we used mixed-effect Poisson regression with robust error variance and report adjusted relative risks with 95% confidence intervals. For continuous secondary outcomes, we used a generalized linear mixed model with gamma distribution and log-link or linear mixed-effect model, as appropriate. We examined the heterogeneity of treatment effect across sites and trial periods by including interaction fixed effects in the model. There was no adjustment for multiplicity in analyses of secondary outcomes, so 95% confidence intervals should not be used in place of hypothesis testing.
We conducted a preplanned sensitivity analysis of the primary outcome using a frailty model time-to-event analysis, in which data were included for infants who had been discharged before meeting the trial definition of medical readiness for discharge. In addition, on the basis of the 2020 American Academy of Pediatrics update for the monitoring of this population,26 we performed a post hoc analysis using a modified definition of medical readiness for discharge. Modifications included an age of at least 72 hours and at least 24 hours without receipt of an opioid. This analysis followed the same analytic approach described for the primary outcome.
RESULTS
PATIENTS
From September 2020 through March 2022, of the 1874 infants who underwent screening, 1305 were enrolled (702 during the usual-care periods and 603 during the Eat, Sleep, Console periods) (Fig. 2). Maternal and infant characteristics are provided in Table 1. The characteristics of the groups were balanced at baseline except for the proportion of Hispanic mothers and the proportion residing in metropolitan areas. Differences in these factors, which reflect the timing of site transitions (e.g., sites with larger Hispanic populations transitioned later), were accounted for in all adjusted models. The infants who were included in the trial were largely representative of those with opioid withdrawal in the United States, although non-Hispanic Black and Hispanic infants were slightly overrepresented (Table S1). Each of the 26 sites transitioned to the Eat, Sleep, Console approach at the randomly assigned time and cared for infants according to the site assignment throughout the trial. One site required rerandomization because of sitespecific restrictions associated with coronavirus disease 2019 (Covid-19).
Figure 2. Enrollment, Randomization, and Analytic Sample.
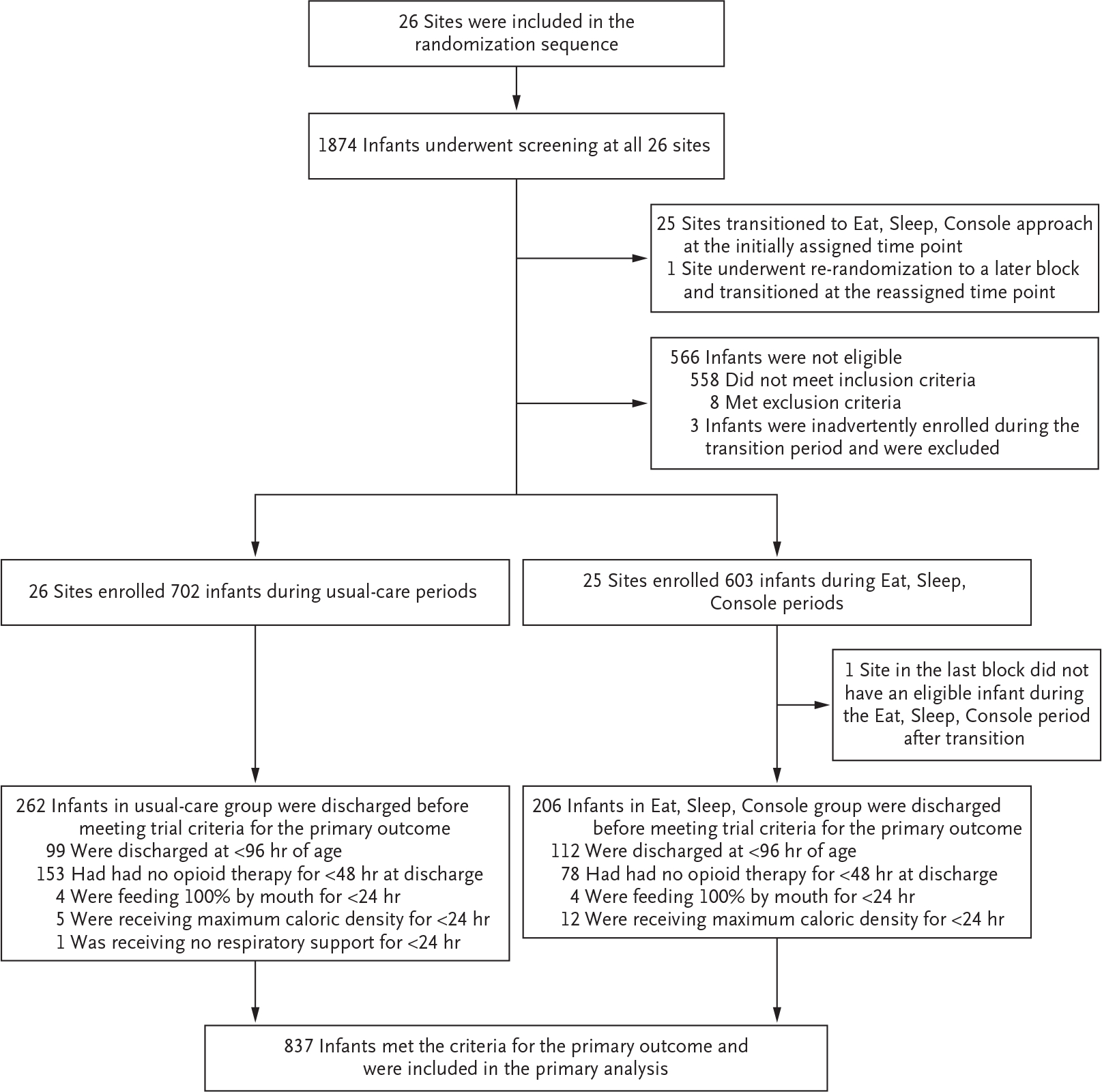
Details regarding eligibility criteria for participation in the trial are provided in Table S1 in the Supplementary Appendix.
Table 1.
Maternal and Neonatal Characteristics at Baseline.*
| Characteristic | Usual Care (N = 702) |
Eat, Sleep, Console Care Approach (N=603) |
|---|---|---|
| Maternal | ||
| Median gravidity (IQR) — no. | 3 (2–5) | 4 (2–5) |
| Median parity (IQR) — no. | 3 (2–4) | 3 (2–4) |
| Race or ethnic group — no. (%)† | ||
| Non-Hispanic White | 462 (66) | 447 (74) |
| Non-Hispanic Black | 98 (14) | 71(12) |
| Hispanic | 107 (15) | 33 (5) |
| Other | 25 (4) | 37 (6) |
| Missing data | 10 (1) | 15 (2) |
| Adequate prenatal care — no. (%)‡ | ||
| Yes | 432 (62) | 381 (63) |
| Missing data | 21 (3) | 9 (1) |
| Medication for opioid use disorder — no./total no. (%) | ||
| Any | 512/702 (73) | 451/603 (75) |
| Buprenorphine | 316/512 (62) | 288/451 (64) |
| Methadone | 191/512 (37) | 154/451 (34) |
| Other | 0 | 2/451 (<1) |
| Unknown | 5/512 (1) | 7/451 (2) |
| Missing data | 15/702 (2) | 20/603 (3) |
| Metropolitan residence — no. (%)§ | 586 (83) | 547 (91) |
| Neonatal | ||
| Female sex — no. (%) | 336 (48) | 314 (52) |
| Birth weight — g | 3026.4+455.4 | 3012.8+490.4 |
| Gestational age — wk | 38.6+1.3 | 38.6+1.3 |
| Polysubstance exposure — no. (%)¶ | 420 (60) | 343 (57) |
Plus–minus values are means ±SD. IQR denotes interquartile range.
Race or ethnic group was obtained from the electronic medical record. The category of “other” includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, Asian, and more than one race.
Adequate prenatal care was defined as at least three visits before the start of the third trimester.
Metropolitan residence was determined by means of rural–urban commuting area codes.
Polysubstance exposure included exposure to opioids and an additional psychotropic agent, excluding nicotine. Additional exposures included amphetamines, barbiturates, benzodiazepines, kratom, cocaine, gabapentin, marijuana, methamphetamines, phencyclidine, and selective serotonin reuptake inhibitors.
PRIMARY OUTCOME
The trial definition of medical readiness for discharge was met by 837 of 1305 infants (64%). The most common reasons that infants did not meet the trial definition were discharge before the age of 96 hours (211 infants) and discharge less than 48 hours after the receipt of an opioid (231 infants) (Fig. 2).
Among the 837 infants who met criteria for the primary outcome, the mean length of time from birth until medical readiness for discharge was shorter in the Eat, Sleep, Console group than in the usual-care group (8.2 vs. 14.9 days; adjusted mean difference, 6.7 days; 95% confidence interval [CI], 4.7 to 8.8), for a rate ratio of 0.55 (95% CI, 0.46 to 0.65; P<0.001) (Table 2). The effect of the new approach was consistent over time during the trial, although heterogeneity of treatment effect was seen across sites. Details regarding the results of stratification models according to sites and trial periods are provided in the Supplementary Appendix.
Table 2.
Primary, Secondary, and Safety Outcomes.
| Outcome | Unadjusted Analysis (95% CI)* | Adjusted Analysis (95% CI)† | ||||
|---|---|---|---|---|---|---|
| Usual Care | Eat, Sleep, Console |
Usual Care | Eat, Sleep, Console |
Absolute Difference |
Estimated Effect |
|
| Primary outcome | ||||||
| Mean time until medical readiness for discharge — days‡ | 15.3 (13.3 to 17.3) |
8.0 (7.0 to 9.0) |
14.9 (13.1 to 16.7) |
8.2 (7.2 to 9.2) |
6.7 (4.7 to 8.8) |
Rate ratio, 0.55 (0.46 to 0.65) |
| Secondary outcomes | ||||||
| Mean length of hospital stay — days§ | 13.9 (12.5 to 15.3) |
7.8 (7.0 to 8.5) |
14.0 (12.7 to 15.3) |
7.8 (7.1 to 8.5) |
6.2 (4.6 to 7.7) |
Rate ratio, 0.56 (0.49 to 0.64) |
| Percent who received pharmacologic therapy§ | 53.6 (45.9 to 61.3) |
19.2 (14.0 to 24.4) |
52.0 (45.4 to 58.7) |
19.5 (14.9 to 24.2) |
32.5 (25.9 to 39.0) |
Relative risk, 0.38 (0.30 to 0.47) |
| Mean time until initiation ofopioid replacement — hr¶ | 53.0 (49.1 to 56.8) |
71.4 (61.5 to 81.3) |
53.0 (48.7 to 57.3) |
76.0 (63.0 to 89.0) |
23.0 (8.1 to 37.9) |
Rate ratio, 1.43 (1.16 to 1.77) |
| Percent who received adjuvant therapy || | 21.6 (9.3 to 33.9) |
15.6 (5.8 to 25.3) |
19.4 (8.5 to 30.4) |
15.7 (5.5 to 25.8) |
3.7 (−9.8 to 17.3) |
Relative risk, 0.81 (0.37 to 1.76) |
| Total opioid dose before discharge — mg/kg¶ | 6.9 (4.7 to 9.1) |
5.2 (3.2 to 7.2) |
7.5 (5.0 to 10.1) |
5.3 (3.2 to 7.4) |
2.3 (−0.4 to 4.9) |
Rate ratio, 0.70 (0.46 to 1.06) |
| Maximum percentage weight loss — %§ | 7.5 (7.1 to 7.9) |
8.0 (7.5 to 8.4) |
7.6 (7.2 to 8.0) |
8.0 (7.5 to 8.4) |
0.4 (−0.3 to 1.0) |
NA** |
| Feeding type at discharge —%†† | ||||||
| Exclusive maternal breast milk | 6.6 (2.8 to 10.4) |
13.9 (7.7 to 20.1) |
6.3 (2.7 to 9.8) |
12.1 (7.2 to 17.1) |
5.9 (−0.4 to 12.1) |
Relative risk, 1.94 (0.94 to 3.99) |
| Combination offormula and maternal breast milk | 25.6 (18.4 to 32.9) |
32.1 (23.6 to 40.5) |
26.5 (18.7 to 34.2) |
31.3 (23.6 to 39.0) |
4.8 (−8.3 to 17.9) |
Relative risk, 1.18 (0.75 to 1.87) |
| Exclusive formula | 69.9 (61.4 to 78.4) |
58.2 (50.6 to 65.8) |
68.3 (62.2 to 74.4) |
60.0 (53.2 to 66.8) |
8.2 (−1.9 to 18.4) |
Relative risk, 0.88 (0.75 to 1.03) |
| Any direct breast-feeding at discharge (%)†† | 19.1 (15.2 to 22.9) |
35.3 (24.5 to 46.2) |
19.5 (15.3 to 23.7) |
32.7 (23.2 to 42.2) |
13.2 (2.1 to 24.2) |
Relative risk, 1.68 (1.13 to 2.48) |
| Safety outcome | ||||||
| Composite safety outcome at 3-mo follow-up — %‡‡ | 15.5 (12.8 to 18.3) |
15.0 (10.9 to 19.0) |
15.8 (12.3 to 19.2) |
16.1 (11.6 to 20.5) |
0.3 (−6.2 to 5.5) |
Relative risk, 1.02 (0.71 to 1.47) |
The unadjusted analysis does not include demographic covariates. However, it still accounts for the trial design (i.e., a fixed effect for trial period and random site effect) and randomization stratification indicator (proportion of infants who were treated pharmacologically at each site according to lowest third, middle third, and highest third).
The model was adjusted for sex, birth weight, gestational age, gravidity, parity, race, adequate prenatal care, medication for opioid use disorder, polysubstance exposures, and rural-urban commuting area code, in addition to the trial design and randomization stratification indicator.
For the primary outcome, the unadjusted analysis included 837 infants. Because of missing data regarding demographic characteristics (4.7%), the adjusted analysis included 798 infants (P<0.001).
The unadjusted analysis included 1305 infants. Because of missing data regarding demographic characteristics (5.9%), the adjusted analysis included 1228 infants.
The unadjusted analysis included 468 infants who received pharmacologic therapy and were not enrolled in other ACT NOW clinical trials. Because of missing data regarding demographic characteristics (7.5%), the adjusted analysis included 433 infants.
This category is reported as the estimated probability of receipt of adjuvant therapy among infants who received pharmacologic therapy.
NA denotes not available because a linear mixed-effects model was used, so only absolute difference could be calculated.
The type of feeding at discharge is reported as the estimated probability of the outcome. Because of missing data regarding the feeding plan (0.15%), the unadjusted analysis included 1303 infants. Because of missing demographic data (5.8%), the adjusted analysis included 1227 infants.
The composite safety outcome was defined as having an acute or urgent care visit, emergency department visit, or hospitalization during the first 3 months of follow-up. The unadjusted analysis included 1305 infants. Because of missing demographic data (5.9%), the adjusted analysis included 1228 infants.
For the primary sensitivity analysis, the proportion of infants with data censoring because of discharge before meeting the trial definition of medical readiness was similar in the two groups (37% [262 of 702] in the usual-care group and 34% [206 of 603] in the Eat, Sleep, Console group) (Table S5). In a post hoc analysis involving 89% of the infants (1164 of 1305) in which the modified definition of medical readiness was used, the adjusted mean between-group difference was 6.4 days (95% CI, 4.7 to 8.0) (Table S6).
SECONDARY AND SAFETY OUTCOMES
The mean length of hospital stay was 7.8 days in the Eat, Sleep, Console group and 14.0 days in the usual-care group (mean difference, 6.2 days; 95% CI, 4.6 to 7.7; rate ratio, 0.56; 95% CI, 0.49 to 0.64) (Table 2). The proportion of infants who received opioid treatment was 52.0% in the usual-care group and 19.5% in the Eat, Sleep, Console group (absolute difference, 32.5 percentage points; relative risk, 0.38; 95% CI, 0.30 to 0.47). The composite measure of infant safety through 3 months of age showed that infants in the Eat, Sleep, Console group had a risk of adverse outcomes that was similar to that in the usual-care group (16.1% and 15.8%, respectively; relative risk, 1.02; 95% CI, 0.71 to 1.47). The composite critical safety outcome at discharge and through 3 months of age was also similar in the two groups (Table 3).
Table 3.
Descriptive Summary of Safety Measures.*
| Variable | Usual Care (N =702) |
Eat, Sleep, Console Care Approach (N = 603) |
|---|---|---|
| number of patients (percent) | ||
| Inpatient outcome | ||
| Composite safety outcome† | 1 (<1) | 2 (<1) |
| Seizures | 1 (<1) | 0 |
| Accidental trauma | 0 | 2 (<1) |
| Outcome at 3 mo | ||
| Composite safety outcome‡ | 113 (16) | 86 (14) |
| Acute or urgent care visit | 40 (6) | 13 (2) |
| Emergency department visit | 66 (9) | 47 (8) |
| Hospitalization§ | 24 (3) | 35 (6) |
| Composite critical safety outcome | 5(1) | 1 (<1) |
| Nonaccidental trauma | 4(1) | 1 (<1) |
| Death | 2 (< 1) | 0 |
Individual components of the composite outcomes are not mutually exclusive.
During the inpatient period, the composite safety outcome was the occurrence of seizures, accidental trauma (e.g., a fall off of a surface), or respiratory insufficiency (apnea or need for positive-pressure ventilation or supplemental oxygen); no patients had respiratory insufficiency during the inpatient period. In addition, no patients had a critical safety outcome, which was defined as nonaccidental trauma (an intentional injury as recorded in the medical record because of a pattern of injury or following formal evaluation) or death during the inpatient period.
The composite safety outcome at 3 months was the only outcome that had sufficient data to perform any statistical modeling or inferential analysis.
Among the hospitalizations, the proportion of infants who were hospitalized for potential diagnoses related to neonatal opioid withdrawal syndrome was 1.9% (13 of 702 infants) in the usual-care group and 2.5% (15 of 603 infants) in the Eat, Sleep, Console group. Classification of diagnoses as potentially related to opioid withdrawal was determined according to the International Classification of Diseases, 10th revision, code review and included codes for failure to thrive, fussy baby, diaper dermatitis, neonatal withdrawal, fever, feeding problems, abnormal weight loss, tachypnea, vomiting, nystagmus, newborn exposure, and severe malnutrition.
The effect of the Eat, Sleep, Console approach was consistent over time and across sites for the proportion of infants receiving opioid treatment and for the composite safety outcome. The treatment effect for length of hospital stay was consistent over time, although the heterogeneity of treatment effect was observed across sites.
DISCUSSION
In this multicenter, stepped-wedge, cluster-randomized, controlled trial, we found that the use of the Eat, Sleep, Console care approach decreased the time until infants with opioid withdrawal were medically ready for hospital discharge by a mean of 6.7 days, as compared with usual care. The use of the approach also decreased the proportion of infants who received pharmacologic treatment by 32.5 percentage points, without increasing specified adverse safety outcomes through 3 months of age.
We chose the time until medical readiness for discharge as the primary outcome because issues unrelated to opioid withdrawal often prolong hospital stays for these infants. This choice also guarded against possible bias in favor of the Eat, Sleep, Console approach that might result from the premature discharge of infants, a concern that has previously been raised about this approach.7,20 We used standards endorsed by the American Academy of Pediatrics24 to guide our definition of medical readiness for discharge. Given the large proportion of infants who were discharged before meeting these criteria, we conducted a post hoc analysis using modifications to our original definition based on the 2020 update to the standards,26 and the results were similar to those for the primary analysis. Lengths of stay for both groups were consistent with those that have been historically reported27–31 and in keeping with outcomes from quality-improvement initiatives that have assessed the Eat, Sleep, Console approach.10,13–15
Infants in the Eat, Sleep, Console group were treated with opioids less often than those receiving usual care. This finding supports the premise that the new approach facilitates a more judicious use of medication for these infants. The proportions of infants who received pharmacologic therapy in the Eat, Sleep, Console group (19.5%) and in the usual-care group (52.0%) are consistent with those reported previously.3,10,15,32
Receipt of pharmacologic treatment is the primary driver for an increased length of hospital stay for infants with opioid withdrawal,10,13,15,31,33 which makes the judicious use of such treatment an important step toward improving short-term outcomes. However, exactly when pharmacologic treatment is indicated in these infants remains unclear. Results from the ongoing assessment of longer-term neurodevelopmental and behavioral outcomes and assessment of family and infant well-being in a subgroup of trial patients will be critical to informing this practice.
Short-term adverse safety events were rare throughout the trial, and no material differences were found between groups for specified safety outcomes through 3 months of age. Thus, the Eat, Sleep, Console approach appears to be as safe as usual care for infants with opioid withdrawal through early infancy.
Limitations of the trial include the unmasked nature of the stepped-wedge design and its vulnerability to treatment contamination and temporal trends. Known temporal trends during the trial included changes in visitation policies and earlier newborn discharges during the Covid-19 pandemic,34 nurse staffing shortages,35 and updates made to the recommendations26 for monitoring infants with opioid withdrawal before discharge. The potential for contamination was addressed by limiting access to intervention-specific materials until sites neared their transition period. The chosen primary outcome limited the potential effect of earlier discharges of newborns. We speculate that visitation restrictions and nurse shortages would make implementation and use of the Eat, Sleep, Console approach more challenging and that these changes would attenuate the treatment effect. Postdischarge safety outcomes were limited to the first 3 months of age and relied on electronic medical records from the enrolling hospital, linked medical records when available, and media review. Although this approach may not have captured all outcomes, it facilitated assessment of short-term safety across all trial patients. Planned longer-term follow-up includes reassessment of critical safety outcomes for all enrolled infants at 2 years of age.
The observed treatment effect for this trial, which was greater than hypothesized, supports the generalizability of the Eat, Sleep, Console approach across diverse sites and varied populations, including those not previously represented in the literature. Although heterogeneity in treatment effect was anticipated, given the variation known to exist across hospitals,3 further study of potential contributors (e.g., site variation in population, location of care, and use of nonpharmacologic interventions as part of usual care) is warranted and will further inform the use of this care approach.
In this multicenter, randomized, controlled trial, the Eat, Sleep, Console approach substantially decreased the time until infants with opioid withdrawal were medically ready for hospital discharge, without evidence of short-term harms. Long-term follow-up is critical to further inform the safety of this approach.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health, including the Helping to End Addiction Long-term (HEAL) Initiative, through grants (UG1-HD21364, UG1-HD27853, UG1-HD36790, UG1-HD40492, UG1-HD53089, UG1-HD53109, UG1-HD68244, UG1-HD68278, UG1-HD68263, and UG1-HD87226) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and grants (UL1-TR41, UL1-TR42, UL1-TR105, UL1-TR442, and UL1 TR1117) from the National Center for Advancing Translational Sciences for the Neonatal Research Network; and by grants (2UG10-D024947-03, UG1-OD024958, 3U2C-OD023375-06S1, 3U2CO-D023375-07S2, UG1-OD024945, UG1-OD024947, UG1-OD024948, UG1-OD024954, UG1-OD024950, UG1-OD024955, UG1-OD024956, U24-OD024957, UG1-OD024959, UG1-OD024943, UG1-OD024942, and UG1-OD024953) from the Institutional Development Awards (IDeA) Program of the States Pediatric Clinical Trials Network of the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) Program.
Appendix
The authors’ full names and academic degrees are as follows: Leslie W. Young, M.D., Songthip T. Ounpraseuth, Ph.D., Stephanie L. Merhar, M.D., Zhuopei Hu, M.S., Alan E. Simon, M.D., Andrew A. Bremer, M.D., Ph.D., Jeannette Y. Lee, Ph.D., Abhik Das, Ph.D., Margaret M. Crawford, B.S., Rachel G. Greenberg, M.D., P. Brian Smith, M.D., Brenda B. Poindexter, M.D., Rosemary D. Higgins, M.D., Michele C. Walsh, M.D., Ward Rice, M.D., Ph.D., David A. Paul, M.D., Jessie R. Maxwell, M.D., Sucheta Telang, M.D., Camille M. Fung, M.D., Tanner Wright, M.D., Anne Marie Reynolds, M.D., Devon W. Hahn, M.D., Julie Ross, M.D., Jennifer M. McAllister, M.D., Moira Crowley, M.D., Sophie K. Shaikh, M.D., Karen M. Puopolo, M.D., Ph.D., Lori Christ, M.D., Jaime Brown, M.D., Julie Riccio, M.D., Kara Wong Ramsey, M.D., Akshatha, M.D., Erica F. Braswell, M.D., Lauren Tucker, M.D., Karen R. McAlmon, M.D., Krishna Dummula, M.D., Julie Weiner, M.D., Jessica R. White, M.D., Meghan P. Howell, M.D., Sarah Newman, A.P.R.N., D.N.P., Jessica N. Snowden, M.D., and Lori A. Devlin, D.O.
The authors’ affiliations are as follows: the Larner College of Medicine, University of Vermont, Burlington (L.W.Y.); the Departments of Biostatistics (S.T.O., Z.H., J.Y.L.) and Pediatrics (J.N.S.), University of Arkansas for Medical Sciences, Little Rock; the University of Cincinnati College of Medicine and Perinatal Institute and the Division of Neonatology, Cincinnati Children’s Hospital Medical Center, Cincinnati (S.L.M., W.R., J.M.M.), the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Case Western Reserve University, Cleveland (M.C.), and the Department of Pediatrics, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus (E.F.B.); the Institutional Development Awards Program of the States Pediatric Clinical Trials Network, Environmental Influences on Child Health Outcomes (ECHO) Program, National Institutes of Health, Rockville (A.E.S.), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda (A.A.B., R.D.H., M.C.W.) — both in Maryland; the Social, Statistical, and Environmental Sciences Unit, RTI International, Research Triangle Park (A.D., M.M.C.), and the Duke Clinical Research Institute, Duke University School of Medicine (R.G.G., P.B.S.), and the Department of Pediatrics, Duke University (S.K.S.), Durham — all in North Carolina; Emory University School of Medicine, Department of Pediatrics, Children’s Healthcare of Atlanta, Atlanta (B.B.P.); the Office of Research and Sponsored Programs, Florida Gulf Coast University, Fort Myers (R.D.H.), and the Department of Pediatrics, University of South Florida, Tampa (T.W.); St. Elizabeth Healthcare, Edgewood (W.R.), and the Department of Pediatrics, University of Louisville, Louisville (S.T., L.A.D.) — both in Kentucky; the Division of Neonatology, Department of Pediatrics, ChristianaCare, Newark, DE (D.A.P.); the University of New Mexico School of Medicine, Albuquerque (J.R.M.); the Department of Pediatrics, Division of Neonatology, University of Utah School of Medicine, Salt Lake City (C.M.F.); the Department of Pediatrics, University at Buffalo, Buffalo (A.M.R.), and the University of Rochester School of Medicine and Dentistry, Rochester (J. Riccio) — both in New York; the Oklahoma University Health Sciences Center, Oklahoma City (D.W.H.); the Medical University of South Carolina, Health Shawn Jenkins Children’s Hospital, Charleston (J. Ross), and the Department of Pediatrics, Spartanburg Regional Medical Center, Spartanburg (J.B.) — both in South Carolina; the Section on Newborn Medicine, Pennsylvania Hospital (K.M.P.), and the Hospital of the University of Pennsylvania (L.C.), Philadelphia; the Kapiolani Medical Center for Women and Children, Honolulu (K.W.R., A.); the Department of Pediatrics, University of Mississippi Medical Center, Jackson (L.T.); Winchester Hospital, Winchester, MA (K.R.M.); the Department of Pediatrics, University of Kansas Medical Center (K.D.), and Children’s Mercy Hospital (J.W.) — both in Kansas City, MO; Sanford Health, Sioux Falls, SD (J.R.W.); Tulane University School of Medicine, New Orleans (M.P.H.); and the University of Nebraska Medical Center, Omaha (S.N.).
Footnotes
Disclosure forms as provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
A list of members of ACT NOW, a collaboration between the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network and the Institutional Development Awards (IDeA) Program of the States Pediatric Clinical Trials Network of the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) Program, is provided in the Supplementary Appendix, available at NEJM.org.
Contributor Information
L.W. Young, Larner College of Medicine, University of Vermont, Burlington.
S.T. Ounpraseuth, Department of Biostatistics, University of Arkansas for Medical Sciences, Little Rock.
S.L. Merhar, University of Cincinnati College of Medicine and Perinatal Institute and the Division of Neonatology, Cincinnati Children’s Hospital Medical Center, Cincinnati.
Z. Hu, Department of Biostatistics, University of Arkansas for Medical Sciences, Little Rock.
A.E. Simon, Institutional Development Awards Program of the States Pediatric Clinical Trials Network, Environmental Influences on Child Health Outcomes (ECHO) Program, National Institutes of Health, Rockville.
A.A. Bremer, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
J.Y. Lee, Department of Biostatistics, University of Arkansas for Medical Sciences, Little Rock.
A. Das, Social, Statistical, and Environmental Sciences Unit, RTI International, Research Triangle Park, Durham, North Carolina.
M.M. Crawford, Social, Statistical, and Environmental Sciences Unit, RTI International, Research Triangle Park, Durham, North Carolina.
R.G. Greenberg, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina.
P.B. Smith, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina.
B.B. Poindexter, Emory University School of Medicine, Department of Pediatrics, Children’s Healthcare of Atlanta, Atlanta.
R.D. Higgins, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland; Office of Research and Sponsored Programs, Florida Gulf Coast University, Fort Myers.
M.C. Walsh, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
W. Rice, University of Arkansas for Medical Sciences, Little Rock; the University of Cincinnati College of Medicine and Perinatal Institute and the Division of Neonatology, Cincinnati Children’s Hospital Medical Center, Cincinnati; St. Elizabeth Healthcare, Edgewood, University of Louisville, Louisville.
D.A. Paul, Division of Neonatology, Department of Pediatrics, ChristianaCare, Newark, DE.
J.R. Maxwell, University of New Mexico School of Medicine, Albuquerque.
S. Telang, Department of Pediatrics, University of Louisville, Louisville, Kentucky.
C.M. Fung, Department of Pediatrics, Division of Neonatology, University of Utah School of Medicine, Salt Lake City.
T. Wright, Department of Pediatrics, University of South Florida, Tampa.
A.M. Reynolds, Department of Pediatrics, University at Buffalo, Buffalo, New York.
D.W. Hahn, Oklahoma University Health Sciences Center, Oklahoma City.
J. Ross, Medical University of South Carolina, Health Shawn Jenkins Children’s Hospital, Charleston, South Carolina.
J.M. McAllister, University of Arkansas for Medical Sciences, Little Rock; the University of Cincinnati College of Medicine and Perinatal Institute and the Division of Neonatology, Cincinnati Children’s Hospital Medical Center, Cincinnati.
M. Crowley, Department of Pediatrics, Rainbow Babies and Children’s Hospital, Case Western Reserve University, Cleveland.
S.K. Shaikh, Department of Pediatrics, Duke University, Durham, North Carolina.
K.M. Puopolo, Section on Newborn Medicine, Pennsylvania Hospital, Philadelphia.
L. Christ, Hospital of the University of Pennsylvania, Philadelphia.
J. Brown, Department of Pediatrics, Spartanburg Regional Medical Center, Spartanburg, South Carolina.
J. Riccio, University of Rochester School of Medicine and Dentistry, Rochester, New York.
K. Wong Ramsey, Kapiolani Medical Center for Women and Children, Honolulu.
Akshatha, Kapiolani Medical Center for Women and Children, Honolulu.
E.F. Braswell, Department of Pediatrics, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus.
L. Tucker, Department of Pediatrics, University of Mississippi Medical Center, Jackson.
K.R. McAlmon, Winchester Hospital, Winchester, MA.
K. Dummula, Department of Pediatrics, University of Kansas Medical Center, Kansas City, MO.
J. Weiner, Children’s Mercy Hospital Kansas City, MO.
J.R. White, Sanford Health, Sioux Falls, SD Kansas City, MO.
M.P. Howell, Tulane University School of Medicine, New Orleans.
S. Newman, University of Nebraska Medical Center, Omaha.
J.N. Snowden, Departments of Pediatrics, University of Arkansas for Medical Sciences, Little Rock.
L.A. Devlin, Department of Pediatrics, University of Louisville, Louisville, Kentucky.
References
- 1.Agency for Healthcare Research and Quality. HCUP Fast Stats. May 2022. (https://hcup-us.ahrq.gov/faststats/landing.jsp). [Google Scholar]
- 2.Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis 1975;2:141–58. [PubMed] [Google Scholar]
- 3.Young LW, Hu Z, Annett RD, et al. Site-level variation in the characteristics and care of infants with neonatal opioid withdrawal. Pediatrics 2021;1 47(1):e2020008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int J Clin Pharmacol Biopharm 1975;12:19–32. [PubMed] [Google Scholar]
- 5.Gomez Pomar E, Finnegan LP, Devlin L, et al. Simplification of the Finnegan neonatal abstinence scoring system: retrospective study of two institutions in the USA. BMJ Open 2017;7(9):e016176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin LA, Breeze JL, Terrin N, et al. Association of a simplified Finnegan neonatal abstinence scoring tool with the need for pharmacologic treatment for neonatal abstinence syndrome. JAMA Netw Open 2020;3(4):e 202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R, Davis JM. Escaping the Finnegan — is it time? Semin Fetal Neonatal Med 2021;26:101218. [DOI] [PubMed] [Google Scholar]
- 8.Davis JM, Shenberger J, Terrin N, et al. Comparison of safety and efficacy of methadone vs morphine for treatment of neonatal abstinence syndrome: a randomized clinical trial. JAMA Pediatr 2018;172:7 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chervoneva I, Adeniyi-Jones SC, Blanco F, Kraft WK. Development of an abbreviated symptom score for the neonatal abstinence syndrome. J Perinatol 2020;40:1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachman EM, Houghton M, Melvin P, et al. A quality improvement initiative to implement the eat, sleep, console neonatal opioid withdrawal syndrome care tool in Massachusetts’ PNQIN collaborative. J Perinatol 2020;40:1560–9. [DOI] [PubMed] [Google Scholar]
- 11.Grossman MR, Lipshaw MJ, Osborn RR, Berkwitt AK. A novel approach to assessing infants with neonatal abstinence syndrome. Hosp Pediatr 2018;8:1–6. [DOI] [PubMed] [Google Scholar]
- 12.Reddy UM, Davis JM, Ren Z, Greene MF. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol 2017;130:10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman MR, Berkwitt AK, Osborn RR, et al. An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Pediatrics 2017;139(6):e 20163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wachman EM, Grossman M, Schiff DM, et al. Quality improvement initiative to improve inpatient outcomes for neonatal abstinence syndrome. J Perinatol 2018;38:1114–22. [DOI] [PubMed] [Google Scholar]
- 15.Hwang SS, Weikel B, Adams J, et al. The Colorado Hospitals Substance Exposed Newborn Quality Improvement Collaborative: standardization of care for opioid-exposed newborns shortens length of stay and reduces number of infants requiring opiate therapy. Hosp Pediatr 2020;10:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whalen BLMK Grossman MR, Whatley C, et al. Inter- and intra-rater reliability of the eating, sleeping, consoling (ESC) care tool for neonatal abstinence syndrome (NAS). In: Proceedings from the 2018 Annual Meeting of the Pediatric Academic Societies, May 5–8, 2018. Toronto: Pediatric Academic Societies, 2018. [Google Scholar]
- 17.Whalen BL MK, Flanagen VA, Picarillo A. NNEPQIN NAS initiative: implementation of the eat, sleep, console care tool across a regional neonatal quality improvement network. In: Proceedings from the 2019 Annual Meeting of the Pediatric Academic Societies, April 27–30, 2019. Baltimore: Pediatric Academic Societies, 2019. [Google Scholar]
- 18.Blount T, Painter A, Freeman E, Grossman M, Sutton AG. Reduction in length of stay and morphine use for NAS with the “eat, sleep, console” method. Hosp Pediatr 2019;9:615–23. [DOI] [PubMed] [Google Scholar]
- 19.Achilles JS, Castaneda-Lovato J. A quality improvement initiative to improve the care of infants born exposed to opioids by implementing the eat, sleep, console assessment tool. Hosp Pediatr 2019;9:6 24–31. [DOI] [PubMed] [Google Scholar]
- 20.Jansson LM, Velez ML. Optimal care for NAS: are we moving in the wrong direction? Hosp Pediatr 2019;9:655–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camerota M, Davis JM, Dansereau LM, Oliveira EL, Padbury JF, Lester BM. Effects of pharmacologic treatment for neonatal abstinence syndrome on DNA methylation and neurobehavior: a prospective cohort study. J Pediatr 2022; 243:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health. Advancing clinical trials in neonatal opioid withdrawal (ACT NOW). September 2022. (https://heal.nihgov/research/infants-and-children/act-now ) . [Google Scholar]
- 23.Young LW, Ounpraseuth S, Merhar SL, et al. Eating, sleeping, consoling for neonatal opioid withdrawal (ESC-NOW): a function-based assessment and management approach study protocol for a multicenter, stepped-wedge randomized controlled trial. Trials 2022;23:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudak ML, Tan RC, Frattarelli DAC, et al. Neonatal drug withdrawal. Pediatrics 2012;129(2):e540–e560. [DOI] [PubMed] [Google Scholar]
- 25.Stedman MR, Gagnon DR, Lew RA, Jung S-H, Losina E, Brookhart MA. A SAS macro for a clustered logrank test. Comput Methods Programs Biomed 2011;104:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrick SW, Barfield WD, Poindexter BB, et al. Neonatal opioid withdrawal syndrome. Pediatrics 2020;146(5):e2020029074. [DOI] [PubMed] [Google Scholar]
- 27.Strahan AE, Guy GP Jr, Bohm M, Frey M, Ko JY. Neonatal abstinence syndrome incidence and health care costs in the United States, 2016. JAMA Pediatr 2020;174:200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and costs of neonatal abstinence syndrome among infants with medicaid: 2004–2014. Pediatrics 2018;141(4):e20173520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA 2021;325:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 2015;35:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome: advances in diagnosis and treatment. JAMA 2018;319:1362–74. [DOI] [PubMed] [Google Scholar]
- 32.Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract 2014;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraft WK, Adeniyi-Jones SC, Chervoneva I, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med 2017;376:2341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harriel KL, Nolt D, Moore S, Kressly S, Bernstein HH. Management of neonates after postpartum discharge and all children in the ambulatory setting during the coronavirus disease 2019 (COVID-19) pandemic. Curr Opin Pediatr 2020;32:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez V, Anderson J, West S, Cleary M. Does the COVID-19 pandemic further impact nursing shortages? Issues Ment Health Nurs 2022;43:293–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


