Abstract
Many researchers are attempting to identify drugs that can be repurposed as effective therapies for Alzheimer’s disease (AD). Several recent studies have highlighted epidermal growth factor receptor (EGFR) inhibitors approved for use as anti-cancer drugs as potential candidates for repurposing as AD therapeutics. In cancer, EGFR inhibitors target cell proliferation and angiogenesis, and studies in AD mouse models have shown that EGFR inhibitors can attenuate amyloid-beta (Aβ) pathology and improve cognitive function. In this review, we discuss the different functions of EGFR in cancer and AD and the potential of EGFR as a dual molecular target for AD diseases. In addition, we describe the effects of anti-cancer EGFR tyrosine kinase inhibitors (TKIs) on AD pathology and their prospects as therapeutic interventions for AD. By summarizing the physiological functions of EGFR in cancer and AD, this review emphasizes the significance of EGFR as an important molecular target for these diseases.
Keywords: Alzheimer’s disease, EGFR, EGFR inhibitor, cancer, Aβ, learning and memory
1 Introduction
Heredity and aging are common risk factors for cancer and Alzheimer’s disease (AD), which are the leading causes of death worldwide (White et al., 2014; Guerreiro and Bras, 2015; Majd et al., 2019). Numerous anti-cancer therapies are available, but therapeutic drugs for AD are scarce. AD is the most common neurodegenerative disease characterized by amyloid and tau protein aggregation and cognitive decline (Ates et al., 2016). Amyloid and tau protein aggregates are not only pathophysiological biomarkers of AD, but also cause neuronal loss, synapse destruction, and neuroinflammation (Serrano-Pozo et al., 2011; Krstic and Knuesel, 2013). Other potential mechanisms of AD progression are oxidative stress and epigenetic dysfunction.
Several studies have found an inverse association between cancer and AD, but others have argued for a parallel relationship (Majd et al., 2019; Nudelman et al., 2019; Branigan et al., 2021; Zhang et al., 2022). Although the underlying mechanism of the relationship between cancer and AD has not been thoroughly investigated, the diseases share hallmarks and risk factors. Risk factors for both cancer and AD include aging, smoking, obesity, and type 2 diabetes (Cannata et al., 2010; Cataldo et al., 2010; Mayeux and Stern, 2012; White et al., 2014; Emmerzaal et al., 2015). Strikingly, cell-cycle entry, which is required for cancer pathogenesis, is high in patients with AD (Majd et al., 2019). At the cellular level, the pathogenesis of AD and cancer both involve the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway, which regulates cell proliferation, metabolism, growth, and autophagy (Pei and Hugon, 2008; Morgan et al., 2009; Advani, 2010; Talbot et al., 2012; Fumarola et al., 2014; Porta et al., 2014). Abnormal growth suppressor evasion is also observed in both cancer and AD. Specifically, cell growth and division in cancer often occur through inactivating mutations of tumor suppressors such as retinoblastoma transcriptional corepressor 1 (RB1) and TP53 (Hanahan and Weinberg, 2011; Fischer et al., 2016; Robinson et al., 2017). In patients with AD, levels of p27, a critical negative cell cycle regulator, are significantly reduced (Ogawa et al., 2003; Munoz et al., 2008). The systemic dysregulation of the cell cycle in both cancer and AD supports a correlation between these two diseases. Angiogenesis, cell adhesion inhibition, and inflammation are also shared by cancer and AD (Nudelman et al., 2019). Therefore, verifying the commonalities between cancer and AD might contribute to the development of effective therapeutic strategies.
A genome-wide association study found a significant positive genetic correlation between cancer and AD, implying that the pathophysiology of cancer and AD share common genetic variants (Feng et al., 2017). Specifically, super-enhancer, a broad enhancer domain affecting cell type identification and function, exhibits a significant positive genetic correlation with cancer and AD, indicating a potential role of gene expression regulation in the common genetic etiologies of cancer and AD (Feng et al., 2017; Zhao et al., 2022). Several genes [e.g., epidermal growth factor receptor (EGFR) and amyloid precursor protein (APP)] are associated with both cancer and AD, and we and others have recently found that anti-cancer drugs can penetrate the blood-brain barrier (BBB) and modulate AD pathology (Ryu and McLarnon, 2008; Cramer et al., 2012). Specifically, inhibitors of EGFR and other tyrosine kinases, which are multitarget enzymes, may have practical value for treating cancer and AD (Mansour et al., 2021c). This review provides insights into the potential roles of EGFR and EGFR inhibitors in cancer and AD and related therapeutic strategies.
2 EGFR
EGFR is a cell surface growth factor receptor that regulates cell proliferation, differentiation, and survival (Yewale et al., 2013). EGFR was the first receptor tyrosine kinase (RTK) to be discovered and is a member of the ErbB family of RTKs (Wong and Guillaud, 2004; Roskoski, 2019). The EGFR gene contains 31 exons and encodes a 170-kDa transmembrane glycoprotein (Mitsudomi and Yatabe, 2010; Sabbah et al., 2020). EGFR is stimulated by ligands such as epidermal growth factor (EGF) and transforming growth factor-alpha (TGF-α) (Purba et al., 2017). Upon binding to the extracellular domain of EGFR, these ligands induce conformational changes in the receptor that facilitate the formation of receptor dimers or oligomers (Schlessinger, 2002). EGFR dimerization triggers the activation of its intrinsic tyrosine kinase activity and subsequent autophosphorylation of several tyrosine residues in the EGFR C-terminal domain (Yu et al., 2002). These phosphorylated tyrosine residues serve as docking sites for various signaling molecules and initiate canonical EGFR signaling pathways (Lemmon and Schlessinger, 2010). Although numerous reviews have discussed EGFR as a target in cancer, inflammatory diseases, and monogenic diseases, the regulation of EGFR expression or signaling as a multi-disease target requires further investigation.
3 EGFR in various cancers
EGFR plays an essential physiological role in regulating the development of epithelial tissue and homeostasis and hence is also linked to tumorigenesis, including lung cancer, breast cancer, and glioblastoma (Sigismund et al., 2018). EGFR is a critical modulator and a target for developing novel therapeutic strategies in various cancers. EGFR signaling modulates cancer cell proliferation through several metabolic processes (Sigismund et al., 2018). For example, Srivatsa et al. (2017) found that EGFR-expressing myeloid cells are abundant in the colorectal tumor stroma, indicating that EGFR in tumor-associated myeloid cells may be a diagnostic biomarker for colorectal cancer (CRC). CRISPR/Cas9-mediated elimination of EGFR significantly inhibits tumor cell growth and activates the mitogen-activated protein kinase (MAPK) (p-ERK1/2) pathway (Liu et al., 2020). Moreover, Song et al. (2020) identified upregulation of EGFR and phosphorylated signal transducer and activator of transcription 3 (p-STAT3) in breast cancer tissues.
The EGFR pathway is a widely recognized oncogenic pathway for non-small cell lung cancer (NSCLC), which represents approximately 75% of lung cancers (Bethune et al., 2010; Hsu et al., 2019). The EGFR pathway regulates the Bax/B-cell lymphoma 2 (Bcl-2) cascade, which is associated with apoptosis in NSCLC (Alam et al., 2022). Interestingly, a study identified EGFR overexpression or mutations in intracellular EGFR in 43%–89% of NSCLC cases (Gupta et al., 2009). Exon 19 deletion and L858R point mutation are the most frequent EGFR mutations in NSCLC (Khaddour et al., 2021). Activating somatic mutations in exons 18–21 of EGFR in NSCLC can continuously activate the EGFR kinase domain regardless of ligand binding and result in sustained downstream signaling. Several studies have reported that EGFR expression is increased by 40%–89% in NSCLC (Lu et al., 2001; Lynch et al., 2004; Al Olayan et al., 2012). Shao et al. (2022) found that upregulated EGFR signaling induces increased levels of the membrane-bound complement regulatory proteins (mCRPs) CD55 and CD59, thereby promoting tumor immune evasion in lung cancer cells (CD8+T). In addition, Ohsaki et al. (2000) observed shorter survival of NSCLC patients with EGFR overexpression. Selvaggi et al. (2004) found that EGFR expression is significantly increased in stage III of NSCLC, implying that EGFR levels are a potential prognostic factor. Merrick et al. (2006) observed a significantly positive relationship between EGFR expression and the progression of bronchial dysplasia, a precursor of lung carcinoma, indicating a role of EGFR upregulation in lung cancer development and progression. Yang et al. (2015) reported that EGFR mutation-mediated lung cancer is associated with downregulation of cluster of differentiation 82 (CD82), which promotes EGFR expression. Shien et al. (2012) discovered that EGFR silencing by siRNA significantly reduces the cell viability of EGFR-mutant cell lines (PC-9, HCC827, NCI-H820, and NCI-1975), further supporting EGFR as a promising therapeutic target in NSCLC. This critical role of EGFR upregulation in the development, progression, and longevity of lung cancer has led to the development of drugs that control EGFR activity and expression.
4 EGFR in Alzheimer’s disease (AD)
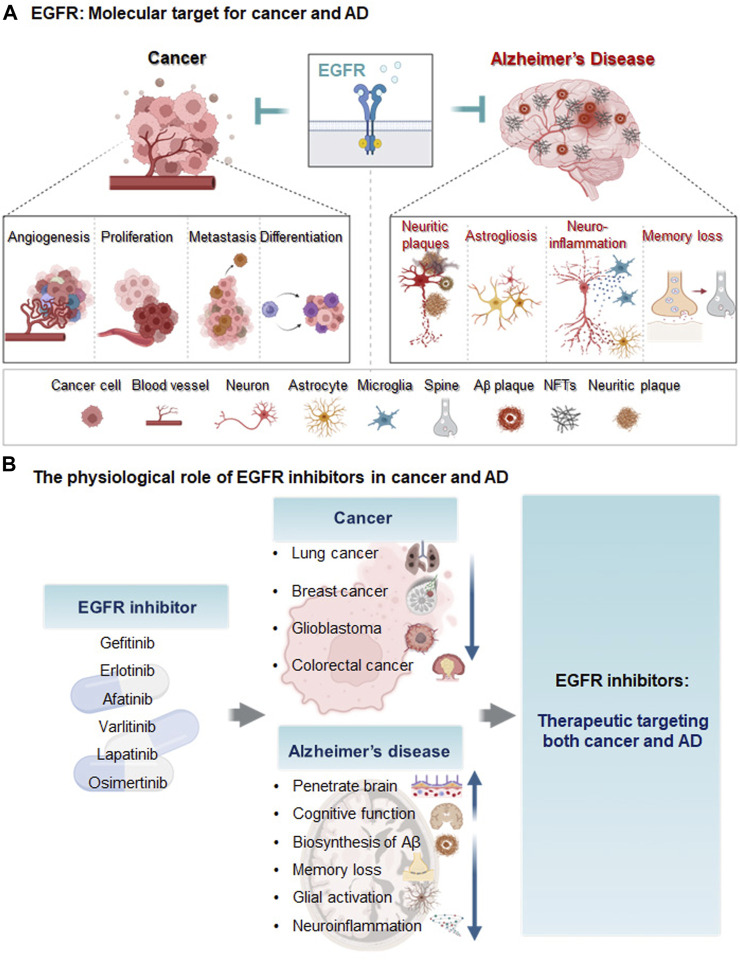
The general functions of EGFR in the central nervous system (CNS) include neural stem cell pool maintenance, astrocyte differentiation and maturation, oligodendrocyte maturation, and neurite outgrowth (Romano and Bucci, 2020). EGFR isoforms are expressed in neurons in the hippocampus, cerebellum, and cerebral cortex (Wong and Guillaud, 2004). Several recent studies have demonstrated that EGFR and its related signaling pathways that it mediates are crucial targets for modulating AD pathology. For instance, Wang et al. (2013) found that EGFR activation (p-EGFR/EGFR) is significantly increased in 8-month-old APP/PS1 mice (a model of AD) compared with wild-type mice. Excessive EGFR expression induces memory impairment in Aβ-overexpressing Drosophila (Prüßing et al., 2013). More importantly, dual overexpression of EGFR and Aβ42 synergistically promotes memory loss, implying that EGFR is upregulated in AD (Chiang et al., 2010). Notably, EGFR upregulation was recently shown to induce Aβ42 neurotoxicity and neuroinflammation and activate astrocytes (Ozbeyli et al., 2017; Chen et al., 2019b). AD patients exhibit neuritic plaques with EGFR expression in the cerebral cortex and hippocampus (Birecree et al., 1988). However, EGF treatment does not alter EGFR or Aβ levels in the brain and prevents cognitive dysfunction in E4FAD mice (Thomas et al., 2016). Taken together, the literature suggests that inhibition of EGFR modulates Aβ plaque accumulation, neuroinflammation, and cognitive function in mouse models of AD. Although there are conflicting reports regarding the role of EGFR in Aβ pathology, there is strong evidence that EGFR is a dual molecular target for cancer and AD (Figure 1A).
FIGURE 1.
Diagram of EGFR as a molecular target and the effects of EGFR inhibitors on cancer and AD. (A) Epidermal growth factor receptor (EGFR) is a transmembrane protein receptor for the epidermal growth factor family that regulates cell growth and proliferation. In cancer, EGFR upregulation increases metastasis, angiogenesis, cancer cell proliferation, differentiation, and cancer viability. In animal models of AD and/or AD patients, EGFR levels are increased, leading to memory loss, astrogliosis, neuroinflammation and Aβ plaque formation. (B) The EGFR inhibitors gefitinib, afatinib, varlitinib, erlotinib, osimertinib, and lapatinib are anti-cancer drugs targeting EGFR. These EGFR inhibitors reduce AD pathology and improve cognitive function and thus may be potential therapeutic agents for cancer and AD.
5 Therapeutic applications of EGFR inhibitors
Despite substantial investments of resources and time in identifying new drugs for AD, clinical trials have produced disappointing results. Thus, the effects of EGFR on Aβ, neuroinflammation, and cognitive function have spurred growing interest in the potential repurposing of EGFR inhibitors used as anti-cancer drugs for the treatment of AD (Mansour et al., 2021c). The molecular mechanisms of EGFR in cancer and AD are also being investigated to develop disease-modifying drugs. Chen et al. (2019b) found that oxygen-glucose deprivation (OGD) increases EGFR phosphorylation and triggers downstream protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) signaling pathways in primary cultured astrocytes and CTX-TNA2 cells. Characterization of EGFR signaling pathways and downstream cascades may reveal promising strategies for utilizing tyrosine kinase inhibitors (TKIs) as disease-modifying therapies in cancer and AD. In addition, EGFR TKIs have greater BBB penetration potential than most intravenous chemotherapies (Ahluwalia et al., 2018). A recent high-performance liquid chromatography (HPLC) analysis showed that ibrutinib can cross the BBB in WT mice (Lee et al., 2021a), and gefitinib, erlotinib, afatinib, varlitinib, lapatinib, and osimertinib are all known to cross the BBB (Lin et al., 2008; Babu et al., 2015; Mansour et al., 2021b; Colclough et al., 2021). Colclough et al. (2021) compared the BBB permeability of EGFR TKIs and found that osimertinib has the highest BBB penetration, with a Kpuu of 0.21, followed by Kpuu values of 0.084 for erlotinib, 0.0092 for gefitinib, and 0.0046 for afatinib. The low BBB permeability of erlotinib and gefitinib means that these drugs do not exhibit significant or persistent effects in the brain (Ahluwalia et al., 2018). Although the abilities of lapatinib, osimertinib, and CL-387,785 to penetrate the BBB have not been determined, lapatinib is expected to cross the BBB due to its low molecular weight and lipophilicity (Mansour et al., 2021b). However, studies of the use of BBB-penetrating EGFR inhibitors to treat AD remain scarce, and the mechanisms of BBB-permeable EGFR TKIs in AD remain to be elucidated. Several anti-cancer EGFR inhibitors that are candidates for AD therapy are described below, and the therapeutic effects, safety, and toxicity profiles of EGFR TKIs in cancer and AD are shown in Tables 1, 2; Figure 1B.
TABLE 1.
The effects of EGFR inhibitors on cancer and AD.
| EGFR Inhibitor |
BBB Penetration | Effects in Cancer | Effects in AD | References |
|---|---|---|---|---|
| Gefitinib | 0.0092 Brain Kpuu in WT rats |
- First-generation EGFR TKI for lung cancer treatment | - Ameliorates Aβ-induced memory loss in APP/PS1 transgenic mice and a Drosophila AD model |
Wang et al. (2012), Niu et al. (2014), Arrieta et al. (2020), Colclough et al. (2021), Dhamodharan et al. (2022) |
| - Inhibits extracellular Aβ40/42 levels and reduces β-secretase (BACE-1) activity in APP-overexpressing N2a cells | ||||
| - Improves cognition function in Swiss albino mice | ||||
| Erlotinib | 0.084 Brain Kpuu in WT rats |
- Suppresses tumor growth in the human endometrial adenocarcinoma cell line HEC-1A | - Significantly increases the performance index of Drosophila with Aβ42-induced memory loss |
Wang et al. (2012), Nishimura et al. (2015), Colclough et al. (2021) |
| Afatinib | 0.0046 Brain Kpuu in WT rats |
- Second-generation EGFR-TKI with anti-inflammatory effects | - Prevents astrocyte activation |
Chen et al. (2019a), Chen et al. (2019b), Vengoji et al. (2019), Colclough et al. (2021) |
| - Inhibits the proliferation, migration, and invasion of hepatocellular carcinoma (HCC) cells | ||||
| - Inhibits brain tumor formation by regulating EGFRvIII-cMet signaling when combined with temozolomide in glioblastoma cells | - Reduces proinflammatory cytokine levels and caspase-1 activation in CTXTNA2 cells | |||
| Varlitinib | Crosses BBB | - FDA-approved EGFR/HER2 inhibitor | - Downregulates LPS-mediated neuroinflammatory responses and tau pathology in wild-type and tauoverexpressing PS19 mice |
Babu et al. (2015), Liu et al. (2019), Dokduang et al. (2020), Kim et al. (2022) |
| - Suppresses cell migration, invasion, and mammosphere formation in triple-negative breast cancer (TNBC) cells | ||||
| Lapatinib | Crosses BBB | - Dual TKI targeting EGFR and HER2 | - Decreases Aβ1–42 and p-tau levels and ameliorates cognitive impairment in D-galactose/ovariectomized rats |
Oakman et al. (2010), Matsumoto et al. (2018), Cihan (2019), Mansour et al. (2021b) |
| - Antitumor effects in HER2-positive breast cancer cells | ||||
| Osimertinib | 0.21 Brain Kpuu in WT rats |
- Clinical activity against EGFR-mutant glioblastoma and non-small cell lung cancer (NSCLC) | - No specific studies of osimertinib as an AD therapy |
Makhlin et al. (2019), Colclough et al. (2021), Gen et al. (2022) |
| CL-387,785 | Expected to cross BBB | - Inhibits EGFR mutants more effectively than first/secondgeneration EGFR TKIs | - Decreases C99 and AICD levels in cellular, zebrafish, and mouse models of AD |
Greulich et al. (2005), Kobayashi et al. (2005), Wang et al. (2017) |
| - Rescues cognitive impairment in APP/ PS1 mice |
TABLE 2.
Safety, toxicity profiles, and target cancers of EGFR inhibitors.
| EGFR Inhibitor | Target group | Target cancers | Safety profile | Toxicity profile | References |
|---|---|---|---|---|---|
| Gefitinib | - ATP binding sites of EGFR | - NSCLC | - Treated with optimal biological dosage (250 mg/day) | - Rash |
Noble and Faulds (1999), Van Zandwijk (2003), Jiang et al. (2005), Arrieta et al. (2020) |
| - Diarrhea | |||||
| - Xeroderma | |||||
| - Pruritus | |||||
| Erlotinib | - ATP binding sites of EGFR | - NSCLC | - Tolerate total 1,600 mg weekly dosing for cancer patients | - Skin rash |
Cohen et al., 2005
Smith (2005), Kiyohara et al. (2013), Carter and Tadi (2020) |
| - Xeroderma | |||||
| - Pruritus | |||||
| - Paronychia | |||||
| Afatinib | - EGFR, HER2/ErbB2 and ErbB4 | - NSCLC | - Increased up to a maximum dosage of 50 mg/day | - Skin deformity |
Ingelheim (2016), Lai et al. (2017), Zhang et al. (2017), Tanaka et al. (2019) |
| - Diarrhea | |||||
| - Paronychia | |||||
| - Oral mucositis | |||||
| - Anorexia | |||||
| Varlitinib | - EGFR and HER2/ErbB2 | - Gastric cancer | - Maximum tolerated dosage of 300 mg began twice-daily (BID) | - No toxicity observed in CCA-inoculated mouse |
Hotte et al. (2009), Dokduang et al. (2020) |
| - Pancreatic cancer | |||||
| - Colorectal cancer | |||||
| - Breast cancer | |||||
| Lapatinib | - EGFR and HER2/ErbB2 | - Breast cancer | - Maximum tolerated dosage of 1,500 mg began twice-daily (BID) | - Diarrhea |
Moy and Goss (2007), Oakman et al. (2010), Morikawa et al. (2019) |
| - Rash | |||||
| Osimertinib | - Mutant-selective EGFR (exon 19 deletion EGFR) | - NSCLC | - Optimal toxic limit of 259 ng/mL | - Skin rash |
Jiang and Zhou (2014), Makhlin et al. (2019), Agema et al. (2022) |
| - Paronychia | |||||
| - Acrodermatitis | |||||
| CL-387,785 | - EGFR and mutant EGFR | - NSCLC | - Studies regarding toxicity not reported | - Studies regarding toxicity not reported |
Greulich et al. (2005), Kobayashi et al. (2005), Wang et al. (2017) |
5.1 Gefitinib
The EGFR/HER2 inhibitor Gefitinib is a first-generation EGFR TKI approved for lung cancer treatment (Arrieta et al., 2020). Gefitinib inhibits the binding of EGFR and adenosine triphosphate (ATP), thereby blocking EGFR autophosphorylation and downstream signaling cascades that control cell growth and trigger apoptosis (Jiang et al., 2005). Compared with erlotinib, gefitinib appears to be more effective and safer for treating NSCLC (Zhang et al., 2018).
Several studies have shown that gefitinib can ameliorate AD pathology. Gefitinib can penetrate the brain and thus positively affects non-EGFR targets that participate in AD pathology in E4FAD (APOE4-expressing) AD transgenic (Tg) mice (Thomas et al., 2016). A computational analysis indicated that gefitinib and the hydrophobic pocket of β-site APP cleaving enzyme (BACE) have complementary shapes and binding interactions, suggesting that gefitinib suppresses Aβ40/42 through BACE (Niu et al., 2014). In addition, gefitinib prevents memory loss in an Aβ42-overexpressing Drosophila model and rescues memory impairment in APP/PS1 Tg mice, a model of AD, indicating that EGFR inhibition can potentially improve cognitive function (Wang et al., 2012). In AD-induced Swiss albino mice, gefitinib attenuates hippocampal-dependent memory impairment as assessed by the Morris water maze (MWM) test and reduces acetylcholinesterase (AChE) activity (Dhamodharan et al., 2022). The ability of gefitinib to reduce Aβ-mediated AChE levels and attenuate cognitive impairments supports its potential as an AD treatment, but whether gefitinib directly affects other AD-associated factors (e.g., tau pathology) and its molecular mechanisms of action on AD pathology remain to be clarified.
5.2 Erlotinib
The EGFR-TKI erlotinib is an FDA-approved drug used to treat patients with NSCLC with mutations in the ATP-binding pocket of EGFR (Cohen et al., 2005; Smith, 2005; Nishimura et al., 2015; Lee et al., 2021b). Erlotinib suppresses the tumor growth of the human endometrial adenocarcinoma cell line HEC-1A, which expresses high levels of EGFR (Nishimura et al., 2015). Deep learning and machine learning algorithms predict that erlotinib is a BBB-permeable compound (Jang et al., 2022). Erlotinib blocks lipopolysaccharide (LPS)-induced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-dependent cytokine production in C57BL/6J mice, implying that erlotinib modulates neuroinflammatory responses in the brain (De et al., 2015). However, due to their low BBB penetration, the effects of erlotinib and gefitinib on brain metastasis are neither significant nor persistent (Ahluwalia et al., 2018).
Importantly, erlotinib rescues memory deficits in APP/PS1 Tg mice as assessed by the MWM test, suggesting that erlotinib can modulate cognitive function (Wang et al., 2012). Although research has focused on the potential utility of erlotinib in treating AD, the effects of erlotinib on Aβ/tau pathology and its mechanisms of action in mouse models of AD require further study.
5.3 Afatinib
Afatinib is a second-generation EGFR-TKI with anti-inflammatory effects and is widely used to treat NSCLC (Chen et al., 2019b; Moosavi and Polineni, 2023). Afatinib also inhibits the migration, proliferation, and invasion of hepatocellular carcinoma (HCC) cells, implying anti-tumorigenic effects (Chen et al., 2019a). Treatment with a combination of afatinib and temozolomide suppresses brain tumor formation by inhibiting crosstalk between EGFRvIII, a constitutively active EGFR mutant, and the RTK cross-activation of tyrosine kinase receptor (cMet) (Vengoji et al., 2019). Interestingly, afatinib (1 or 10 nM) inhibits OGD-induced EGFR phosphorylation, astrocyte activation, and reduced proinflammatory cytokine levels in CTX-TNA2 cells (Chen et al., 2019b). These results suggest that afatinib has anti-inflammatory effects on OGD-induced neuroinflammation. However, whether afatinib regulates AD pathology and cognitive function in mouse models of AD is unknown. Although the direct effects of afatinib on AD pathology have not been comprehensively investigated, the anti-cancer and anti-inflammatory effects of afatinib indicate promising potential for repurposing as an AD therapy.
5.4 Varlitinib
Studies have examined the effects of varlitinib, an FDA-approved EGFR/HER2 inhibitor, on various cancers, including gastric, pancreatic, colorectal, and breast cancers (Dokduang et al., 2020). Varlitinib can penetrate the BBB and suppresses cell migration, invasion, and mammosphere formation through ERK/AKT signaling in triple-negative breast cancer (TNBC) cells (Babu et al., 2015; Liu et al., 2019). In addition, EGFR/HER2 inhibition by varlitinib has therapeutic effects on cholangiocarcinoma (CCA) (Dokduang et al., 2020). Whereas other EGFR inhibitors have side effects, varlitinib does not have significant toxicity in CCA-inoculated mice (Dokduang et al., 2020). Importantly, we recently demonstrated that varlitinib downregulates LPS-mediated neuroinflammation and tau pathology through dual specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A), a tau kinase (Kim et al., 2022). In addition, we found that varlitinib significantly diminishes LPS-induced neuroinflammation in both BV2 microglial cells and primary astrocytes, suggesting that varlitinib has therapeutic effects on neuroinflammation and tau pathology (Kim et al., 2022). In contrast to its effects on cancer, the impact of varlitinib on AD pathology (including Aβ pathology and its mechanism of action) is poorly understood; thus, further studies are needed to evaluate the feasibility of using varlitinib for the treatment of AD.
5.5 Lapatinib
Like varlitinib, lapatinib is a dual TKI targeting both EGFR and HER2 and is currently used to treat cancer (Oakman et al., 2010). Lapatinib is a low-molecular-weight and lipophilic molecule and can penetrate the BBB (Mansour et al., 2021b). In HER2-positive breast cancer cells, a combination of lapatinib and capecitabine has synergistic anti-tumor effects (Matsumoto et al., 2018).
Lapatinib has been shown to ameliorate autoimmune encephalomyelitis, a functional disorder of the CNS (Akama-Garren et al., 2015). In addition, lapatinib has neuroprotective effects against neuronal ferroptosis, indicating the involvement of ferroptosis in AD pathologies (Jia et al., 2020; Chen et al., 2021). A study of the effects of lapatinib on cognitive function in vivo found that lapatinib rescues short/long-term recognition memory impairment by activating the PI3K/AKT/glycogen synthase kinase-3 beta (GSK-3β) pathway in D-galactose/ovariectomized (D-gal/OVX) rats (Mansour et al., 2021b). The same study demonstrated that lapatinib decreases Aβ1-42 and p-tau levels and suppresses HER2 expression in D-gal/OVX rats (Mansour et al., 2021b). Thus, inhibition of HER2 by lapatinib promotes autophagy and reduces Aβ1–42 and p-tau levels, consistent with the results of previous studies of the role of EGFR/HER2 in autophagy (Mansour et al., 2021a; Mansour et al., 2022). Overall, lapatinib is a promising candidate anti-cancer drug for repositioning as an AD therapeutic. However, further studies of the effects of lapatinib on AD pathophysiology are needed.
5.6 Osimertinib
Osimertinib is an irreversible EGFR inhibitor and a third-generation TKI with high brain penetration (Makhlin et al., 2019). Osimertinib targets EGFR T790, which is resistant to most first- and second-generation EGFR TKIs (Janjigian et al., 2014; Jiang and Zhou, 2014; Barbuti et al., 2019). Osimertinib is known for its clinical activities in glioblastoma and NSCLC (Makhlin et al., 2019; Gen et al., 2022). In vitro, osimertinib has higher affinity for EGFR L858R/T790M than for wild-type EGFR (Kuijper et al., 2015; Greig, 2016). Interestingly, a recent report indicated that osimertinib is also highly effective against CNS metastasis (Liam, 2019). Furthermore, Ge et al. (2017) observed a higher probability and frequency of EGFR mutations in NSCLC patients with brain metastasis than in NSCLC patients without brain metastasis. In addition, the incidence of brain metastasis is higher in NSCLC patients with mutated EGFR than in NSCLC patients with wild-type EGFR, implying a correlation between EGFR mutation and brain metastasis (Ge et al., 2017). Whether the potential effectiveness of osimertinib in the CNS extends to AD pathology is unknown.
The relationships between specific EGFR mutations (exon 19 deletions and exon 21 L858R) and AD have not been examined. Jolly et al. (2022) investigated the associations of the locations of mutations in EGF-like repeats (EGFr) with vascular cognitive impairment (VCI) in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), one of the most common forms of stroke and early-onset dementia, and found no significant relationship (Jolly et al., 2022). Although these results are not consistent with the mechanism of osimertinib, different results might be obtained for the relationships between EGFR mutations and ADs. Osimertinib may have utility as an EGFR inhibitor for treating AD pathology due to its high brain penetration, but the impact of osimertinib on AD has not been studied.
5.7 CL-387,785
CL-387,785 is an irreversible selective EGFR inhibitor designed to specifically inhibit EGFR autophosphorylation and tumor cell proliferation (Discafani et al., 1999). CL-387,785 inhibits not only wild-type EGFR but also EGFR T790M, which is resistant to EGFR TKIs such as erlotinib, gefitinib, and afatinib (Kobayashi et al., 2005; Yun et al., 2008; Mok et al., 2009; Rosell et al., 2012; Zhang et al., 2012; Chi et al., 2013; Sequist et al., 2013; Niu and Wu, 2014). Thus, CL-387,785 is expected to solve the cause of drug resistance. Although CL-387,785 is only approved for research purposes, several studies have indicated therapeutic effects of CL-387,785 on lung cancer. For instance, CL-387,785 inhibits colony formation by lung cancer cells expressing an EGFR missense or deletion mutant more effectively than gefitinib and erlotinib, suggesting that CL-387,785 may be a good therapeutic for lung cancer with exon 20 insertion mutations of EGFR (Greulich et al., 2005). In addition, CL-387,785 inhibits the proliferation and apoptosis of NSCLC H1975 cells expressing EGFR T790M, indicating that CL-387,785 can restrict the invasion and metastasis of NSCLC H1975 cells (Cai et al., 2023).
With respect to potential effects on AD, CL-387,785 significantly reduces C99-CTF (c-terminal fragment) and APP intracellular domain (AICD) levels in C99-YFP–overexpressing HEK293 cells and C99 CTF-expressing zebrafish (Wang et al., 2017). More importantly, CL-387,785 rescues spatial learning and memory and reduces Aβ levels in APP/PS1 Tg mice (Wang et al., 2017). CL-387,785 also reduces the LC3-II/LC3-I ratio, which is crucial for activating autophagy, promoting the clearance of Aβ40 and Aβ42, and improving memory (Wang et al., 2017). Although the effects of CL-387,785 on AD pathology (i.e., tau) and its mechanism of action require further investigation, CL-387,785 can be considered a potential EGFR TKI for both cancer and AD.
Conclusion and future directions
Several recent studies have revealed associations of EGFR with cancer and AD; thus, regulating EGFR expression may be a strategy for treating both diseases. However, comprehensive studies of the roles of EGFR and EGFR inhibitors (TKIs) in cancer and AD are not available. In addition, although EGFR is a potential target for AD treatment, the effectiveness of major anti-cancer EGFR TKIs as AD therapeutics has received little attention. This review highlights the functional roles of EGFR and EGFR TKIs in cancer and AD. Specifically, EGFR upregulation induces various types of cancer and promotes Aβ pathology. EGFR inhibition has promising effects on both diseases, including inhibiting cancer cell migration and AD pathology (e.g., Aβ, neuroinflammation, and cognitive function). The literature and our findings suggest that anti-cancer drugs can be regarded as candidates for repurposing as AD treatments. However, the direct relationship between EGFR and AD, the effects of EGFR on tau pathology in mouse models of AD, and the mechanisms of action of EGFR in the brain are still unclear. Moreover, the effects of EGFR TKIs on AD pathology have not been well examined. Further studies are required to address these issues and may provide significant insights into cancer therapy and AD progression.
Acknowledgments
We thank neurodegenerative diseases lab members for editing and valuable comments on our manuscript. The figures were created in BioRender.com.
Funding Statement
This work was supported by the National Research Foundation of Korea (grant number 2021R1F1A1057865, JK) and the KBRI basic research program through KBRI funded by the Ministry of Science, ICT and Future Planning (grant numbers 23-BR-02-03, 23-BR-02-12, 23-BR-03-07, 23-BR-03-01, 23-BR-05-02, H-SH) and a National Research Council of Science and Technology (NST) grant funded by the Korean government (CCL22061-100, H-SH).
Author contributions
H-JC, YJJ, JK, and H-SH wrote the manuscript. JK and H-SH conceived the study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Advani S. (2010). Targeting mTOR pathway: A new concept in cancer therapy. Indian J. Med. Paediatr. Oncol. 31, 132–136. 10.4103/0971-5851.76197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agema B. C., Veerman G. D. M., Steendam C. M. J., Lanser D. a. C., Preijers T., Van Der Leest C., et al. (2022). Improving the tolerability of osimertinib by identifying its toxic limit. Ther. Adv. Med. Oncol. 14, 17588359221103212. 10.1177/17588359221103212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia M. S., Becker K., Levy B. P. (2018). Epidermal growth factor receptor tyrosine kinase inhibitors for central nervous system metastases from non-small cell lung cancer. Oncologist 23, 1199–1209. 10.1634/theoncologist.2017-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama-Garren E., Swanson C., Robinson W. H. (2015). Epidermal growth factor receptor inhibition treats experimental autoimmune Encephalomyelitis. MURJ 22, 20–27. [Google Scholar]
- Al Olayan A., Al Hussaini H., Jazieh A. R. (2012). The roles of epidermal growth factor receptor (EGFR) inhibitors in the management of lung cancer. J. Infect. public health 5, S50–S60. 10.1016/j.jiph.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Alam M., Alam S., Shamsi A., Adnan M., Elasbali A. M., Al-Soud W. A., et al. (2022). Bax/Bcl-2 cascade is regulated by the EGFR pathway: Therapeutic targeting of non-small cell lung cancer. Front. Oncol. 12, 869672. 10.3389/fonc.2022.869672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta O., Catalan R., Guzman-Vazquez S., Barron F., Lara-Mejia L., Soto-Molina H., et al. (2020). Cost-effectiveness analysis of first and second-generation EGFR tyrosine kinase inhibitors as first line of treatment for patients with NSCLC harboring EGFR mutations. BMC Cancer 20, 829. 10.1186/s12885-020-07329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M. P., Karaman Y., Guntekin S., Ergun M. (2016). Analysis of genetics and risk factors of Alzheimer’s Disease. Neuroscience 325, 124–131. 10.1016/j.neuroscience.2016.03.051 [DOI] [PubMed] [Google Scholar]
- Babu S. K., Prabhakar V., Ravindranath L., Latha J., Hari K. (2015). Nagamaddaiah Quinazoline derivatives and its biological significance–a review. Int. J. Curr. Trends Pharm. Res. 3, 997–1010. [Google Scholar]
- Barbuti A. M., Zhang G. N., Gupta P., Narayanan S., Chen Z. S. (2019). “EGFR and HER2 inhibitors as sensitizing agents for cancer chemotherapy,” in Protein kinase inhibitors as sensitizing agents for chemotherapy 4, 1–11. [Google Scholar]
- Bethune G., Bethune D., Ridgway N., Xu Z. (2010). Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2, 48–51. [PMC free article] [PubMed] [Google Scholar]
- Birecree E., Whetsell W. O., Jr., Stoscheck C., King L. E., Jr., Nanney L. B. (1988). Immunoreactive epidermal growth factor receptors in neuritic plaques from patients with Alzheimer's disease. J. Neuropathol. Exp. Neurol. 47, 549–560. 10.1097/00005072-198809000-00006 [DOI] [PubMed] [Google Scholar]
- Branigan G. L., Torrandell‐Haro G., Brinton R. D. (2021). Breast cancer therapies reduce risk of Alzheimer’s disease and dementia: A claims‐based retrospective study with clinic to bench implications. Alzheimer's Dementia 17, e055602. 10.1002/alz.055602 [DOI] [Google Scholar]
- Cai Y., Sheng Z., Dong Z., Wang J. (2023). EGFR inhibitor CL-387785 suppresses the progression of lung adenocarcinoma. Curr. Mol. Pharmacol. 16, 211–216. 10.2174/1874467215666220329212300 [DOI] [PubMed] [Google Scholar]
- Cannata D., Fierz Y., Vijayakumar A., Leroith D. (2010). Type 2 diabetes and cancer: What is the connection? Mt. Sinai J. Med. A J. Transl. Personalized Med. A J. Transl. Personalized Med. 77, 197–213. 10.1002/msj.20167 [DOI] [PubMed] [Google Scholar]
- Carter J., Tadi P. (2020). Erlotinib. Treasure Island: StatPearls. [Google Scholar]
- Cataldo J. K., Prochaska J. J., Glantz S. A. (2010). Cigarette smoking is a risk factor for alzheimer's disease: An analysis controlling for tobacco industry affiliation. J. Alzheimer's Dis. 19, 465–480. 10.3233/JAD-2010-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Jiang X., Wu M., Cao X., Bao W., Zhu L.-Q. (2021). Ferroptosis, a potential therapeutic target in Alzheimer’s disease. Front. Cell Dev. Biol. 9, 704298. 10.3389/fcell.2021.704298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen X., Ding X., Wang Y. (2019a). Afatinib, an EGFR inhibitor, decreases EMT and tumorigenesis of Huh-7 cells by regulating the ERK-VEGF/MMP9 signaling pathway. Mol. Med. Rep. 20, 3317–3325. 10.3892/mmr.2019.10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. J., Hsu C. C., Shiao Y. J., Wang H. T., Lo Y. L., Lin A. M. Y. (2019b). Anti-inflammatory effect of afatinib (an EGFR-TKI) on OGD-induced neuroinflammation. Sci. Rep. 9, 2516. 10.1038/s41598-019-38676-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi A., Remick S., Tse W. (2013). EGFR inhibition in non-small cell lung cancer: Current evidence and future directions. Biomark. Res. 1, 2–10. 10.1186/2050-7771-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H. C., Wang L., Xie Z., Yau A., Zhong Y. (2010). PI3 kinase signaling is involved in Abeta-induced memory loss in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 107, 7060–7065. 10.1073/pnas.0909314107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihan Y. B. (2019). Lapatinib? Or radiotherapy? In cranial metastasis of breast cancer. Eur. J. Breast Health 15, 205–206. 10.5152/ejbh.2019.4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. H., Johnson J. R., Chen Y.-F., Sridhara R., Pazdur R. (2005). FDA drug approval summary: Erlotinib (Tarceva®) tablets. Oncol. 10, 461–466. 10.1634/theoncologist.10-7-461 [DOI] [PubMed] [Google Scholar]
- Colclough N., Chen K., Johnstrom P., Strittmatter N., Yan Y., Wrigley G. L., et al. (2021). Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin. Cancer Res. 27, 189–201. 10.1158/1078-0432.CCR-19-1871 [DOI] [PubMed] [Google Scholar]
- Cramer P. E., Cirrito J. R., Wesson D. W., Lee C. Y., Karlo J. C., Zinn A. E., et al. (2012). ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506. 10.1126/science.1217697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S., Zhou H., Desantis D., Croniger C. M., Li X., Stark G. R. (2015). Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc. Natl. Acad. Sci. U. S. A. 112, 9680–9685. 10.1073/pnas.1511794112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamodharan J., Sekhar G., Muthuraman A. (2022). Epidermal growth factor receptor kinase inhibitor ameliorates beta-amyloid oligomer-induced alzheimer disease in Swiss albino mice. Molecules 27, 5182. 10.3390/molecules27165182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discafani C. M., Carroll M. L., Floyd M. B., Jr, Hollander I. J., Husain Z., Johnson B. D., et al. (1999). Irreversible inhibition of epidermal growth factor receptor tyrosine kinase with in vivo activity by N-[4-[(3-bromophenyl) amino]-6-quinazolinyl]-2-butynamide (CL-387,785). Biochem. Pharmacol. 57, 917–925. 10.1016/s0006-2952(98)00356-6 [DOI] [PubMed] [Google Scholar]
- Dokduang H., Jamnongkarn W., Promraksa B., Suksawat M., Padthaisong S., Thanee M., et al. (2020). In vitro and in vivo anti-tumor effects of pan-HER inhibitor varlitinib on cholangiocarcinoma cell lines. Drug Des. Devel Ther. 14, 2319–2334. 10.2147/DDDT.S250061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerzaal T. L., Kiliaan A. J., Gustafson D. R. (2015). 2003-2013: A decade of body mass index, alzheimer's disease, and dementia. J. Alzheimer's Dis. 43, 739–755. 10.3233/JAD-141086 [DOI] [PubMed] [Google Scholar]
- Feng Y.-C. A., Cho K., Lindstrom S., Kraft P., Cormack J., Liang L., et al. (2017). Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics. Hum. Genet. 136, 1341–1351. 10.1007/s00439-017-1831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Grossmann P., Padi M., Decaprio J. A. (2016). Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F target gene analyses identifies cell cycle gene regulatory networks. Nucleic acids Res. 44, 6070–6086. 10.1093/nar/gkw523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumarola C., Bonelli M. A., Petronini P. G., Alfieri R. R. (2014). Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 90, 197–207. 10.1016/j.bcp.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Ge M., Zhuang Y., Zhou X., Huang R., Liang X., Zhan Q. (2017). High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J. neuro-oncology 135, 413–418. 10.1007/s11060-017-2590-x [DOI] [PubMed] [Google Scholar]
- Gen S., Tanaka I., Morise M., Koyama J., Kodama Y., Matsui A., et al. (2022). Clinical efficacy of osimertinib in EGFR-mutant non-small cell lung cancer with distant metastasis. BMC Cancer 22, 654. 10.1186/s12885-022-09741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig S. L. (2016). Brodalumab: First global approval. Drugs 76, 1403–1412. 10.1007/s40265-016-0634-8 [DOI] [PubMed] [Google Scholar]
- Greulich H., Chen T.-H., Feng W., Jänne P. A., Alvarez J. V., Zappaterra M., et al. (2005). Oncogenic transformation by inhibitor-sensitive and-resistant EGFR mutants. PLoS Med. 2, e313. 10.1371/journal.pmed.0020313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R., Bras J. (2015). The age factor in Alzheimer's disease. Genome Med. 7, 106. 10.1186/s13073-015-0232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Dastane A. M., Forozan F., Riley-Portuguez A., Chung F., Lopategui J., et al. (2009). Evaluation of EGFR abnormalities in patients with pulmonary adenocarcinoma: The need to test neoplasms with more than one method. Mod. Pathol. 22, 128–133. 10.1038/modpathol.2008.182 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: The next generation. Cell 144, 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hotte S. J., Hirte H. W., Turner S., Jonker D., Fortin C., Barrett E., et al. (2009). Abstract B54: A phase 1 study to assess the safety, tolerability and pharmacokinetics of the ErbB‐family inhibitor ARRY‐334543 in patients with advanced solid tumors. Mol. Cancer Ther. 8, B54. 10.1158/1535-7163.targ-09-b54 [DOI] [Google Scholar]
- Hsu P.-C., Jablons D. M., Yang C.-T., You L. (2019). Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int. J. Mol. Sci. 20, 3821. 10.3390/ijms20153821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelheim B. (2016). Gilotrif [prescribing information](2014). Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. [Google Scholar]
- Jang H. Y., Oh J. M., Kim I. W. (2022). Drug repurposing using meta-analysis of gene expression in Alzheimer's disease. Front. Neurosci. 16, 989174. 10.3389/fnins.2022.989174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjigian Y. Y., Smit E. F., Groen H. J., Horn L., Gettinger S., Camidge D. R., et al. (2014). Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 4, 1036–1045. 10.1158/2159-8290.CD-14-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.-N., Yin X.-X., Li Q., Guan Q.-W., Yang N., Chen K.-N., et al. (2020). Neuroprotective effects of the anti-cancer drug lapatinib against epileptic seizures via suppressing glutathione peroxidase 4-dependent ferroptosis. Front. Pharmacol. 11, 601572. 10.3389/fphar.2020.601572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Greulich H., Janne P. A., Sellers W. R., Meyerson M., Griffin J. D. (2005). Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 65, 8968–8974. 10.1158/0008-5472.CAN-05-1829 [DOI] [PubMed] [Google Scholar]
- Jiang T., Zhou C. (2014). Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients with EGFR inhibitor-resistant non-small cell lung cancer. Transl. Lung Cancer Res. 3, 370–372. 10.3978/j.issn.2218-6751.2014.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly A. A., Nannoni S., Edwards H., Morris R. G., Markus H. S. (2022). Prevalence and predictors of vascular cognitive impairment in patients with CADASIL. Neurology 99, e453–e461. 10.1212/WNL.0000000000200607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaddour K., Jonna S., Deneka A., Patel J. D., Abazeed M. E., Golemis E., et al. (2021). Targeting the epidermal growth factor receptor in EGFR-mutated lung cancer: Current and emerging therapies. Cancers 13, 3164. 10.3390/cancers13133164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim S. J., Jeong H. R., Park J. H., Moon M., Hoe H. S. (2022). Inhibiting EGFR/HER-2 ameliorates neuroinflammatory responses and the early stage of tau pathology through DYRK1A. Front. Immunol. 13, 903309. 10.3389/fimmu.2022.903309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara Y., Yamazaki N., Kishi A. (2013). Erlotinib-related skin toxicities: Treatment strategies in patients with metastatic non-small cell lung cancer. J. Am. Acad. Dermatol 69, 463–472. 10.1016/j.jaad.2013.02.025 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Boggon T. J., Dayaram T., Jänne P. A., Kocher O., Meyerson M., et al. (2005). EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792. 10.1056/NEJMoa044238 [DOI] [PubMed] [Google Scholar]
- Krstic D., Knuesel I. (2013). Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 9, 25–34. 10.1038/nrneurol.2012.236 [DOI] [PubMed] [Google Scholar]
- Kuijper M., Van Hoften G., Janssen B., Geurink R., De Carlo S., Vos M., et al. (2015). FEI’s direct electron detector developments: Embarking on a revolution in cryo-TEM. J. Struct. Biol. 192, 179–187. 10.1016/j.jsb.2015.09.014 [DOI] [PubMed] [Google Scholar]
- Lai W.-C. V., Lebas L., Milia J., Barnes T. A., Gautschi O., Peters S., et al. (2017). Afatinib in patients with metastatic HER2-mutant lung cancers: An international multicenter study. United States: American Society of Clinical Oncology. [Google Scholar]
- Lee H.-K., Noh M. H., Hong S.-W., Kim S.-M., Kim S. H., Kim Y. S., et al. (2021a). Erlotinib activates different cell death pathways in EGFR-mutant lung cancer cells grown in 3D versus 2D culture systems. Anticancer Res. 41, 1261–1269. 10.21873/anticanres.14883 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Jeon S. G., Kim J., Kang R. J., Kim S. M., Han K. M., et al. (2021b). Ibrutinib modulates Aβ/tau pathology, neuroinflammation, and cognitive function in mouse models of Alzheimer's disease. Aging Cell 20, e13332. 10.1111/acel.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J. (2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. 10.1016/j.cell.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liam C. K. (2019). Central nervous system activity of first-line osimertinib in epidermal growth factor receptor-mutant advanced non-small cell lung cancer. Ann. Transl. Med. 7, 61. 10.21037/atm.2018.12.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N. U., Carey L. A., Liu M. C., Younger J., Come S. E., Ewend M., et al. (2008). Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2–positive breast cancer. J. Clin. Oncol. 26, 1993–1999. 10.1200/JCO.2007.12.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Diaz Arguello O. A., Chen D., Chen S., Saber A., Haisma H. J. (2020). CRISPR-mediated ablation of overexpressed EGFR in combination with sunitinib significantly suppresses renal cell carcinoma proliferation. PLoS One 15, e0232985. 10.1371/journal.pone.0232985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Y., Chu P. Y., Huang C. T., Chen J. L., Yang H. P., Wang W. L., et al. (2019). Varlitinib downregulates HER/ERK signaling and induces apoptosis in triple negative breast cancer cells. Cancers (Basel) 11, 105. 10.3390/cancers11010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Jiang G., Blume-Jensen P., Hunter T. (2001). Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell. Biol. 21, 4016–4031. 10.1128/MCB.21.12.4016-4031.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T. J., Bell D. W., Sordella R., Gurubhagavatula S., Okimoto R. A., Brannigan B. W., et al. (2004). Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- Majd S., Power J., Majd Z. (2019). Alzheimer's disease and cancer: When two monsters cannot Be together. Front. Neurosci. 13, 155. 10.3389/fnins.2019.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlin I., Salinas R. D., Zhang D., Jacob F., Ming G. L., Song H., et al. (2019). Clinical activity of the EGFR tyrosine kinase inhibitor osimertinib in EGFR-mutant glioblastoma. CNS Oncol. 8, CNS43. 10.2217/cns-2019-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H. M., Fawzy H. M., El-Khatib A. S., Khattab M. M. (2021a). Inhibition of mitochondrial pyruvate carrier 1 by lapatinib ditosylate mitigates Alzheimer's-like disease in D-galactose/ovariectomized rats. Neurochem. Int. 150, 105178. 10.1016/j.neuint.2021.105178 [DOI] [PubMed] [Google Scholar]
- Mansour H. M., Fawzy H. M., El-Khatib A. S., Khattab M. M. (2021b). Lapatinib ditosylate rescues memory impairment in D-galactose/ovariectomized rats: Potential repositioning of an anti-cancer drug for the treatment of Alzheimer's disease. Exp. Neurol. 341, 113697. 10.1016/j.expneurol.2021.113697 [DOI] [PubMed] [Google Scholar]
- Mansour H. M., Fawzy H. M., El-Khatib A. S., Khattab M. M. (2021c). Potential repositioning of anti-cancer EGFR inhibitors in alzheimer's disease: Current perspectives and challenging prospects. Neuroscience 469, 191–196. 10.1016/j.neuroscience.2021.06.013 [DOI] [PubMed] [Google Scholar]
- Mansour H. M., Fawzy H. M., El-Khatib A. S., Khattab M. M. (2022). Repurposed anti-cancer epidermal growth factor receptor inhibitors: Mechanisms of neuroprotective effects in alzheimer's disease. Neural Regen. Res. 17, 1913–1918. 10.4103/1673-5374.332132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Hayashida T., Takahashi M., Jinno H., Kitagawa Y. (2018). Antitumor effect of lapatinib and cytotoxic agents by suppression of E2F1 in HER2-positive breast cancer. Mol. Med. Rep. 18, 958–964. 10.3892/mmr.2018.9068 [DOI] [PubMed] [Google Scholar]
- Mayeux R., Stern Y. (2012). Epidemiology of alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006239. 10.1101/cshperspect.a006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D. T., Kittelson J., Winterhalder R., Kotantoulas G., Ingeberg S., Keith R. L., et al. (2006). Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: Evaluation of potential targets for chemoprevention of lung cancer. Clin. Cancer Res. 12, 2281–2288. 10.1158/1078-0432.CCR-05-2291 [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Yatabe Y. (2010). Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 277, 301–308. 10.1111/j.1742-4658.2009.07448.x [DOI] [PubMed] [Google Scholar]
- Mok T. S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.-T., Saijo N., et al. (2009). Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- Moosavi L., Polineni R. (2023). “Afatinib,” in StatPearls. (Treasure Island (FL) ineligible companies. Disclosure: Rahul Polineni declares no relevant financial relationships with ineligible companies (United States: StatPearls Publishing; ). [Google Scholar]
- Morgan T. M., Koreckij T. D., Corey E. (2009). Targeted therapy for advanced prostate cancer: Inhibition of the PI3K/Akt/mTOR pathway. Curr. cancer drug targets 9, 237–249. 10.2174/156800909787580999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa A., De Stanchina E., Pentsova E., Kemeny M. M., Li B. T., Tang K., et al. (2019). Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin. Cancer Res. 25, 3784–3792. 10.1158/1078-0432.CCR-18-3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy B., Goss P. E. (2007). Lapatinib-associated toxicity and practical management recommendations. Oncologist 12, 756–765. 10.1634/theoncologist.12-7-756 [DOI] [PubMed] [Google Scholar]
- Munoz U., Bartolomé F., Bermejo F., Martín-Requero Ã. (2008). Enhanced proteasome-dependent degradation of the CDK inhibitor p27kip1 in immortalized lymphocytes from Alzheimer's dementia patients. Neurobiol. aging 29, 1474–1484. 10.1016/j.neurobiolaging.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Nakamura K., Yamashita S., Ikeda S., Kigure K., Minegishi T. (2015). Effect of the molecular targeted drug, erlotinib, against endometrial cancer expressing high levels of epidermal growth factor receptor. BMC Cancer 15, 957. 10.1186/s12885-015-1975-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu F.-Y., Wu Y.-L. (2014). Novel agents and strategies for overcoming EGFR TKIs resistance. Exp. Hematol. Oncol. 3, 2–5. 10.1186/2162-3619-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Hu J., Wu S., Xiaoe Z., Xu H., Zhang Y., et al. (2014). Structural bioinformatics-based identification of EGFR inhibitor gefitinib as a putative lead compound for BACE. Chem. Biol. Drug Des. 83, 81–88. 10.1111/cbdd.12200 [DOI] [PubMed] [Google Scholar]
- Noble S., Faulds D. (1999). Management of advanced non-small cell lung cancer: The potential role of gemcitabine. Dis. Manag. Health Outcomes 5, 167–179. 10.2165/00115677-199905030-00005 [DOI] [Google Scholar]
- Nudelman K. N., Mcdonald B. C., Lahiri D. K., Saykin A. J. (2019). Biological hallmarks of cancer in Alzheimer’s disease. Mol. Neurobiol. 56, 7173–7187. 10.1007/s12035-019-1591-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman C., Pestrin M., Zafarana E., Cantisani E., Di Leo A. (2010). Role of lapatinib in the first-line treatment of patients with metastatic breast cancer. Cancer Manag. Res. 2, 13–25. 10.2147/CMAR.S8951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa O., Lee H. G., Zhu X., Raina A., Harris P. L., Castellani R. J., et al. (2003). Increased p27, an essential component of cell cycle control, in Alzheimer's disease. Aging Cell 2, 105–110. 10.1046/j.1474-9728.2003.00042.x [DOI] [PubMed] [Google Scholar]
- Ohsaki Y., Tanno S., Fujita Y., Toyoshima E., Fujiuchi S., Nishigaki Y., et al. (2000). Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol. Rep. 7, 603–607. 10.3892/or.7.3.603 [DOI] [PubMed] [Google Scholar]
- Ozbeyli D., Sari G., Ozkan N., Karademir B., Yuksel M., Cilingir Kaya O. T., et al. (2017). Protective effects of different exercise modalities in an Alzheimer's disease-like model. Behav. Brain Res. 328, 159–177. 10.1016/j.bbr.2017.03.044 [DOI] [PubMed] [Google Scholar]
- Porta C., Paglino C., Mosca A. (2014). Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 4, 64. 10.3389/fonc.2014.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J. J., Hugon J. (2008). mTOR-dependent signalling in Alzheimer's disease. J. Cell. Mol. Med. 12, 2525–2532. 10.1111/j.1582-4934.2008.00509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüßing K., Voigt A., Schulz J. B. (2013). Drosophila melanogaster as a model organism for Alzheimer’s disease. Mol. Neurodegener. 8, 35–12. 10.1186/1750-1326-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba E. R., Saita E.-I., Maruyama I. N. (2017). Activation of the EGF receptor by ligand binding and oncogenic mutations: The “rotation model”. Cells 6, 13. 10.3390/cells6020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. R., Wu Y.-M., Lonigro R. J., Vats P., Cobain E., Everett J., et al. (2017). Integrative clinical genomics of metastatic cancer. Nature 548, 297–303. 10.1038/nature23306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano R., Bucci C. (2020). Role of EGFR in the nervous system. Cells 9, 1887. 10.3390/cells9081887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. lancet Oncol. 13, 239–246. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr. (2019). Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol. Res. 139, 395–411. 10.1016/j.phrs.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Ryu J. K., Mclarnon J. G. (2008). Thalidomide inhibition of perturbed vasculature and glial-derived tumor necrosis factor-alpha in an animal model of inflamed Alzheimer's disease brain. Neurobiol. Dis. 29, 254–266. 10.1016/j.nbd.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Sabbah D. A., Hajjo R., Sweidan K. (2020). Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr. Top. Med. Chem. 20, 815–834. 10.2174/1568026620666200303123102 [DOI] [PubMed] [Google Scholar]
- Schlessinger J. (2002). Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110, 669–672. 10.1016/s0092-8674(02)00966-2 [DOI] [PubMed] [Google Scholar]
- Selvaggi G., Novello S., Torri V., Leonardo E., De Giuli P., Borasio P., et al. (2004). Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann. Oncol. 15, 28–32. 10.1093/annonc/mdh011 [DOI] [PubMed] [Google Scholar]
- Sequist L. V., Yang J. C.-H., Yamamoto N., O'byrne K., Hirsh V., Mok T., et al. (2013). Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31, 3327–3334. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189. 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F., Gao Y., Wang W., He H., Xiao L., Geng X., et al. (2022). Silencing EGFR-upregulated expression of CD55 and CD59 activates the complement system and sensitizes lung cancer to checkpoint blockade. Nat. Cancer 3, 1192–1210. 10.1038/s43018-022-00444-4 [DOI] [PubMed] [Google Scholar]
- Shien K., Ueno T., Tsukuda K., Soh J., Suda K., Kubo T., et al. (2012). Knockdown of the epidermal growth factor receptor gene to investigate its therapeutic potential for the treatment of non-small-cell lung cancers. Clin. Lung Cancer 13, 488–493. 10.1016/j.cllc.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Sigismund S., Avanzato D., Lanzetti L. (2018). Emerging functions of the EGFR in cancer. Mol. Oncol. 12, 3–20. 10.1002/1878-0261.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. (2005). Erlotinib: Small-molecule targeted therapy in the treatmentof non-small-cell lung cancer. Clin. Ther. 27, 1513–1534. 10.1016/j.clinthera.2005.10.014 [DOI] [PubMed] [Google Scholar]
- Song X., Liu Z., Yu Z. (2020). EGFR promotes the development of triple negative breast cancer through JAK/STAT3 signaling. Cancer Manag. Res. 12, 703–717. 10.2147/CMAR.S225376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsa S., Paul M. C., Cardone C., Holcmann M., Amberg N., Pathria P., et al. (2017). EGFR in tumor-associated myeloid cells promotes development of colorectal cancer in mice and associates with outcomes of patients. Gastroenterology 153, 178–190.e10. 10.1053/j.gastro.2017.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K., Wang H.-Y., Kazi H., Han L.-Y., Bakshi K. P., Stucky A., et al. (2012). Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. investigation 122, 1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Taima K., Itoga M., Ishioka Y., Baba K., Shiratori T., et al. (2019). Real-world study of afatinib in first-line or re-challenge settings for patients with EGFR mutant non-small cell lung cancer. Med. Oncol. 36, 57. 10.1007/s12032-019-1278-9 [DOI] [PubMed] [Google Scholar]
- Thomas R., Zuchowska P., Morris A. W., Marottoli F. M., Sunny S., Deaton R., et al. (2016). Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol. Commun. 4, 111. 10.1186/s40478-016-0387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zandwijk N. (2003). Tolerability of gefitinib in patients receiving treatment in everyday clinical practice. Br. J. Cancer 89, S9–S14. 10.1038/sj.bjc.6601477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengoji R., Macha M. A., Nimmakayala R. K., Rachagani S., Siddiqui J. A., Mallya K., et al. (2019). Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J. Exp. Clin. Cancer Res. 38, 266. 10.1186/s13046-019-1264-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.-J., Her G. M., Hu M.-K., Chen Y.-W., Tung Y.-T., Wu P.-Y., et al. (2017). ErbB2 regulates autophagic flux to modulate the proteostasis of APP-CTFs in Alzheimer’s disease. Proc. Natl. Acad. Sci. 114, E3129–E3138. 10.1073/pnas.1618804114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chiang H.-C., Wu W., Liang B., Xie Z., Yao X., et al. (2012). Epidermal growth factor receptor is a preferred target for treating Amyloid-β–induced memory loss. Proc. Natl. Acad. Sci. 109, 16743–16748. 10.1073/pnas.1208011109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liang B., Zhong Y. (2013). Reduced EGFR level potentially mediates the Aβ42-induced neuronal loss in transgenic fruit fly and mouse. Protein Cell 4, 647–649. 10.1007/s13238-013-3043-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. C., Holman D. M., Boehm J. E., Peipins L. A., Grossman M., Henley S. J. (2014). Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 46, S7–S15. 10.1016/j.amepre.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. W., Guillaud L. (2004). The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 15, 147–156. 10.1016/j.cytogfr.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Chou H. C., Fu Y. N., Yeh C. L., Cheng H. W., Chang I. C., et al. (2015). EGFR over-expression in non-small cell lung cancers harboring EGFR mutations is associated with marked down-regulation of CD82. Biochim. Biophys. Acta 1852, 1540–1549. 10.1016/j.bbadis.2015.04.020 [DOI] [PubMed] [Google Scholar]
- Yewale C., Baradia D., Vhora I., Patil S., Misra A. (2013). Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials 34, 8690–8707. 10.1016/j.biomaterials.2013.07.100 [DOI] [PubMed] [Google Scholar]
- Yu X., Sharma K. D., Takahashi T., Iwamoto R., Mekada E. (2002). Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol. Biol. Cell 13, 2547–2557. 10.1091/mbc.01-08-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.-H., Mengwasser K. E., Toms A. V., Woo M. S., Greulich H., Wong K.-K., et al. (2008). The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. 105, 2070–2075. 10.1073/pnas.0709662105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.-D., Ou Y.-N., Yang L., Ma Y.-H., Tan L., Feng J.-F., et al. (2022). Investigating the association between cancer and dementia risk: A longitudinal cohort study. Alzheimer's Res. Ther. 14, 146–212. 10.1186/s13195-022-01090-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Wei Y., Yu D., Xu J., Peng J. (2018). Gefitinib provides similar effectiveness and improved safety than erlotinib for east asian populations with advanced non–small cell lung cancer: A meta-analysis. BMC cancer 18, 780–814. 10.1186/s12885-018-4685-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Miao S., Wang F., Fang W., Chen G., Chen X., et al. (2017). The efficacy and toxicity of afatinib in advanced EGFR-positive non-small-cell lung cancer patients after failure of first-generation tyrosine kinase inhibitors: A systematic review and meta-analysis. J. Thorac. Dis. 9, 1980–1987. 10.21037/jtd.2017.06.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lee J. C., Lin L., Olivas V., Au V., Laframboise T., et al. (2012). Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 44, 852–860. 10.1038/ng.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang M., Bishop J., Teng Y., Cao Y., Beall B. D., et al. (2022). Identification and functional validation of super-enhancers in Arabidopsis thaliana . Proc. Natl. Acad. Sci. 119, e2215328119. 10.1073/pnas.2215328119 [DOI] [PMC free article] [PubMed] [Google Scholar]