Abstract
The type D simian retroviruses cause immunosuppression in macaques and have been reported as a presumptive opportunistic infection in a patient with AIDS. Previous evidence based on viral interference has strongly suggested that the type D simian viruses share a common but unknown cell surface receptor with three type C viruses: feline endogenous virus (RD114), baboon endogenous virus, and avian reticuloendotheliosis virus. Furthermore, the receptor gene for these viruses has been mapped to human chromosome 19q13.1–13.2. We now report the isolation and characterization of a cell surface receptor for this group of retroviruses by using a human T-lymphocyte cDNA library in a retroviral vector. Swiss mouse fibroblasts (NIH 3T3), which are naturally resistant to RD114, were transduced with the retroviral library and then challenged with an RD114-pseudotyped virus containing a dominant selectable gene for puromycin resistance. Puromycin selection yielded 12 cellular clones that were highly susceptible to a β-galactosidase-encoding lacZ(RD114) pseudotype virus. Using PCR primers specific for vector sequences, we amplified a common 2.9-kb product from 10 positive clones. Expression of the 2.9-kb cDNA in Chinese hamster ovary cells conferred susceptibility to RD114, baboon endogenous virus, and the type D simian retroviruses. The 2.9-kb cDNA predicted a protein of 541 amino acids that had 98% identity with the previously cloned human Na+-dependent neutral-amino-acid transporter Bo. Accordingly, expression of the RD114 receptor in NIH 3T3 cells resulted in enhanced cellular uptake of l-[3H]alanine and l-[3H]glutamine. RNA blot (Northern) analysis suggested that the RD114 receptor is widely expressed in human tissues and cell lines, including hematopoietic cells. The human Bo transporter gene has been previously mapped to 19q13.3, which is closely linked to the gene locus of the RD114 receptor.
Retroviral infections are initiated by binding of the viral envelope glycoprotein to a cell surface receptor protein, followed by secondary events that lead to fusion of the viral and cellular membranes. In addition, the envelope glycoprotein-receptor interaction within productively infected cells prevents superinfection by any retrovirus that uses the same receptor, a phenomenon known as interference (12). These observations have been used to classify retroviruses into interference groups that are believed to use a common receptor for infection. For example, 20 retrovirus strains that infect human cells have been classified into only eight interference groups (31). Thus, gibbon ape leukemia virus, feline leukemia virus subgroup B and 10A1 murine leukemia virus (MLV) use a common receptor for infection, as determined by interference studies (38, 41). The receptor for these viruses has been identified as the Na+-dependent phosphate symporter Pit1 (14, 24, 26, 27, 38, 41). A related protein, Pit2, functions as a receptor for amphotropic MLV and 10A1 MLV (23, 24, 41, 42). Xenotropic and polytropic MLVs cross-interfere in some cells (5, 22). Recently, a human cDNA that encodes the receptor for xenotropic/polytropic MLVs was cloned, and its normal cellular function is being investigated (34). Cross-interference has also been observed between different subgroups of avian leukosis/sarcoma viruses (12).
The type D retroviruses, including simian retrovirus type 1 (SRV-1), SRV-2, SRV-4, and SRV-5 and Mason-Pfizer monkey virus (MPMV) (also known as SRV-3) (6, 8, 13, 20, 21, 31, 33), cross-interfere not only with each other but also with three type C retroviruses: feline endogenous virus (RD114), baboon endogenous virus (BaEV), and avian reticuloendotheliosis virus (REV) (16, 31). REV appears to be more related to mammalian viruses than to other avian retroviruses, suggestive of viral transmission from mammals to birds. The type D viruses are of particular interest because they are prevalent in nonhuman primates, where they cause severe immunodeficiencies (8, 11, 13, 20, 21, 33), and because they infect human cells in culture and have been reported as a presumptive opportunistic infection in a human immunodeficiency virus type 1-positive patient with AIDS (10). Consequently, they are of concern as a potential emerging infection in humans. In addition, RD114 infects human cells, including hematopoietic cells, at high efficiency (29) and is resistant to inactivation by human complement, making RD114-based vectors potential candidates for in vivo gene therapy (7, 35). The receptor for this broad interference group of retroviruses has not been identified.
To address these issues, we attempted to clone the human cell surface receptor for RD114. This was done with a human T-lymphocyte cDNA library in the retroviral vector pBabe-X (18), generously donated by Richard Sutton (Baylor College of Medicine, Houston, Tex.). Use of a retroviral vector library has major advantages for this work, as exemplified by its recent use in cloning of cDNAs for simian immunodeficiency virus coreceptors (9) and a human receptor for xenotropic and polytropic MLVs (34).
Cloning of the RD114 receptor (R-receptor).
We have previously described our cloning procedures in detail (34). Briefly, 10 μg of retroviral plasmid library DNA was transfected into Phoenix-Eco packaging cells (Garry Nolan, Stanford University, Stanford, Calif.) (2 × 106 cells in a 100-mm culture plate) by using SuperFect transfection reagent (Qiagen, Valencia, Calif.). Two days after transfection, 10 ml of virus supernatant was harvested and filtered. For this investigation, 0.1 ml of this virus was added with 8 μg of Polybrene per ml to one 100-mm culture plate containing 5 × 105 NIH 3T3 cells. After 16 h, the viral supernatant was replaced with fresh medium. The following day, the transduced NIH 3T3 cells were superinfected with an RD114-pseudotyped virus carrying a puromycin resistance gene (cells producing this pseudotype virus were derived from FLYRD18 cells [7] transfected with the pBabe-puro retroviral expression vector [25]). Selection was initiated 36 h later by adding 5 μg of puromycin per ml to the medium. The selection medium was changed every 2 days until resistant colonies had appeared. These colonies were then isolated and tested for susceptibility to infection by a β-galactosidase-encoding lacZ(RD114) pseudotype virus.
Among 16 resistant colonies that were analyzed, 12 were highly susceptible to lacZ(RD114) infections. Genomic DNA was then isolated from 10 of these clones and was used for PCR amplification with the Expand PCR kit (Boehringer-Mannheim, Indianapolis, Ind.) with primers corresponding to pBabe-X vector sequences, as previously described (34). A common 2.9-kb PCR product was amplified from each of the 10 clones and subsequently cloned into the pCDNA3.1V5His-TOPO mammalian expression vector (Invitrogen, Carlsbad, Calif.). Expression of the 2.9-kb cDNA in Chinese hamster ovary (CHO) cells or in mouse NIH 3T3 fibroblasts resulted in strong susceptibility to lacZ(RD114) infection (see Table 1, experiment 1), indicating that it encodes an R-receptor.
TABLE 1.
Infection of cells expressing the R-receptor by RD114, BaEV, and type D simian retroviruses
| Expt | Target cellsb | Titer of lacZ pseudotype (CFU/ml)a
|
||||
|---|---|---|---|---|---|---|
| RD114 | BaEV | MPMV | SRV-1 | SRV-2 | ||
| 1 | TE671 | 2.1 × 106 | — | — | — | — |
| NIH 3T3 | 7 | — | — | — | — | |
| 3T3R16 | 4.7 × 105 | — | — | — | — | |
| CHO | 0 | — | — | — | — | |
| CHOR16 | 1.5 × 105 | — | — | — | — | |
| 2 | TE671 | 1.3 × 106 | 1.2 × 105 | 1.9 × 103 | 4.5 × 104 | 5.9 × 106 |
| CHO | <1 | 1 | <0.3 | <2 | <2 | |
| CHOR16 | 5.4 × 104 | 4.7 × 103 | 9.8 × 101 | 9.7 × 102 | 2.8 × 105 | |
| NRK | <2 | 1.4 × 104 | <2 | <2 | <2 | |
| NRKR16 | 5.4 × 105 | 3.9 × 104 | 4.6 × 101 | 4.4 × 103 | 3.9 × 105 | |
lacZ(RD114) was produced by TELCeB6/RDF-7 helper-free packaging cells (7). lacZ(BaEV) was rescued by infection of mink Mv-1-Lu cells harboring a lacZ vector (35) with a replication-competent BaEV stock. Although MPMV could not package and rescue an MLV vector in the absence of MLV core proteins (28, 37), lacZ(MPMV) was produced by complementation of MLV core particles with MPMV Env; an MPMV env expression plasmid was constructed by replacing the RD114 env coding sequence in plasmid RDLF (7) with the MPMV env coding sequence derived from pTMOWT (4) (kindly provided by Eric Hunter) and transfected into TELCeB6 cells which express a lacZ vector and MLV Gag-Pol proteins (19a). lacZ(MPMV) was harvested from a pooled population of phleomycin-resistant transfectants. lacZ(SRV-1) and lacZ(SRV-2) were produced from TELCeB6 cells infected with SRV-1 and SRV-2, respectively, while infection of TE671/MFGnlslacZ cells lacking MLV Gag and Pol proteins by either SRV-1 or SRV-2 produced no pseudotype virus (data not shown). Titers are averages of two (experiment 2: RD114, BaEV, SRV-1, and SRV-2), three (experiment 1; RD114), or four (experiment 2; MPMV) infection studies. Titers of MPMV, BaEV, SRV-1, and SRV-2 were not determined in experiment 1, as indicated by dashes.
TE671 cells are human rhabdomyosarcoma cells. CHOR16 and NRKR16 cells are pooled populations of G418-resistant CHO and rat NRK cells, respectively, transfected with the R-receptor gene. 3T3R16 cells are NIH 3T3 cells trasduced with the R-receptor gene.
The R-receptor protein.
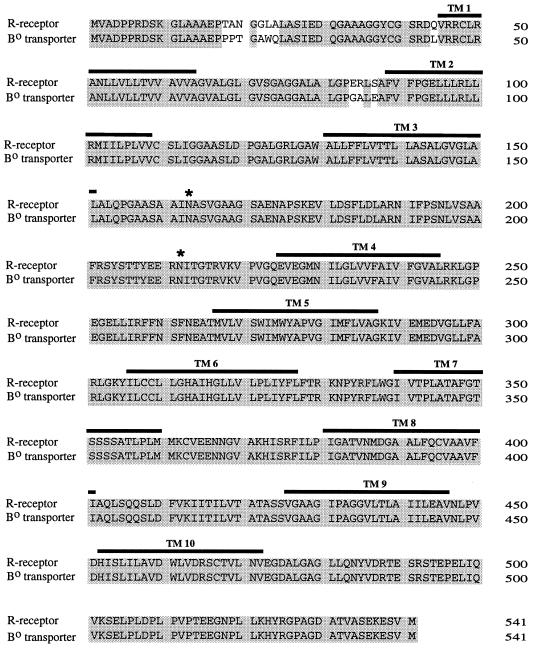
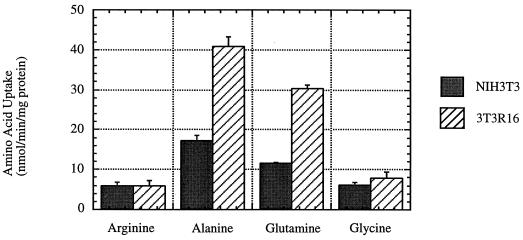
The nucleotide sequence of the 2.9-kb R-receptor cDNA and subsequent BLAST (2) searches of databases indicated 98% identity with a previously cloned cDNA from human placental choriocarcinoma cells that encodes the broad-specificity neutral-amino-acid transporter Bo, so designated to indicate these transport characteristics (15). Figure 1 shows a comparison of the predicted amino acid sequences of the human R-receptor and the previously reported human Bo transporter. Also indicated are the 10 hydrophobic potential transmembrane regions and the two potential sites for N-linked glycosylation that were predicted for the Bo transporter. The Bo transporter is a Na+-dependent transporter or exchanger with broad specificity for neutral amino acids, including alanine, glutamine, and possibly glycine (15). Compatible with this relationship, NIH 3T3 cells transduced with the R-receptor had highly elevated transport activity for l-[3H]alanine and l-[3H]glutamine in comparison with untransduced control cells; however, no significant transport activity was observed for l-[3H]glycine or l-[3H]arginine (Fig. 2). Similar results were obtained with independent clones of NIH 3T3 cells that expressed the R-receptor. We are quantitatively analyzing the transport activity of the R-receptor by electrophysiological methods in Xenopus oocytes (19b).
FIG. 1.
Amino acid sequence comparison of human R-receptor and human neutral-amino-acid transporter Bo. The R-receptor and Bo transporter have 98% sequence identity. Common amino acids are shaded, transmembrane (TM) sequences are shown as lines over the amino acid sequence, and potential N-linked glycosylation sites are shown by asterisks.
FIG. 2.
Uptake of l-[3H]arginine, l-[3H]alanine, l-[3H]glutamine, and l-[3H]glycine by NIH 3T3 cells and NIH 3T3 cells that express the human R-receptor. Amino acid uptake was analyzed as previously described (39). NIH 3T3 cells transduced with the R-receptor gene (3T3R16) had highly elevated levels of l-[3H]alanine and l-[3H]glutamine uptake compared to parental NIH 3T3 cells. No significant uptake was observed for l-[3H]glycine or for the cationic amino acid l-[3H]arginine.
Distribution in tissue of R-receptor expression.
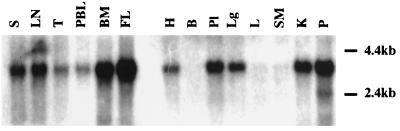
The previously characterized human Bo transporter cDNA was isolated from a placental choriocarcinoma cDNA library and was found to hybridize to a 2.9-kb mRNA that was most abundant in epithelial tissues, including lung, kidney, and intestines (15). Figure 3 shows RNA blot (Northern) analysis in which we have used the 2.9-kb R-receptor cDNA as a probe. These results identify a more broadly expressed mRNA of 2.9 kb that is present in many tissues and is highly expressed in hematopoietic tissues, including fetal liver, bone marrow, peripheral blood lymphocytes, thymus, lymph node, and spleen. This distribution of expression is compatible with derivation of our clone from a T-lymphocyte cDNA library, with induction of immunodeficiency by type D retroviruses (8, 11, 13, 20, 21, 33), and with efficient infection of hematopoietic cells by RD114 (29).
FIG. 3.
Northern blot analysis of poly(A)+ RNA from various human tissues. Multiple-tissue Northern blots containing approximately 2 μg of poly(A)+ RNA (Clontech, Palo Alto, Calif.) were probed with the 2.9-kb 32P-labeled R-receptor cDNA. S, spleen; LN, lymph node; T, thymus; PBL, peripheral blood lymphocytes; BM, bone marrow; FL, fetal liver; H, heart; B, brain; Pl, placenta; Lg, lung; L, liver; SM, skeletal muscle; K, kidney; P, pancreas.
The R-receptor mediates infections of BaEV and type D simian retroviruses.
Previous studies have suggested that RD114 cross-interferes with BaEV and type D simian retroviruses. Because mouse and rat cells can be infected by BaEV (32), we analyzed the susceptibilities of CHO cells that express the human R-receptor to BaEV and type D simian retroviruses infections. As shown in Table 1 (experiment 2), a heterogeneous population of CHO cells that had been transfected with the R-receptor cDNA (CHOR16 cells) differed from control untransfected cells in being susceptible to infections by lacZ(BaEV), lacZ(MPMV), lacZ(SRV-1), and lacZ(SRV-2) in addition to lacZ(RD114). The titers obtained in the population of CHOR16 cells were approximately 20- to 45-fold lower than the titers of the same viruses in the highly susceptible human cell line TE671. In contrast, lacZ pseudotypes bearing envelopes derived from gibbon ape leukemia virus, xenotropic MLV, or pig endogenous retrovirus class A, B, or C (36) did not plate on either parental CHO or CHOR16 cells (data not shown). Similarly, expression of the R-receptor in normal rat kidney (NRK) cells, which are naturally susceptible to BaEV, conferred susceptibility to infections by RD114, MPMV, SRV-1, and SRV-2 (Table 1, experiment 2).
Our results strongly suggest that the cloned cDNA encodes a receptor for RD114, BaEV, and type D simian retroviruses. This receptor, which is broadly expressed in human tissues (Fig. 3), is highly related to the previously cloned neutral-amino-acid transporter Bo (15), and we have shown that it functions in accordance with this predicted activity (Fig. 2). The Bo transporter gene has been previously mapped to human chromosome 19q13.3 (15), which is consistent with the approximate localization of the RD114 receptor gene to chromosome 19q13.1-13.2 (32). Further studies will be needed to determine whether the sequence differences between the R-receptor and the previously cloned Bo transporter (Fig. 1) represent slight differences in the human Bo transporter genes or mutations in the cDNA clone. However, our sequence was present in independent cDNA clones. The R-receptor appears to belong to a family of transporters that includes glutamate transporters; the amino acid transporter for alanine, serine, and cysteine, termed ASCT; the insulin-activatable neutral/anionic amino acid transporter, and the Bo transporter (3, 15, 19, 30). We recently isolated a cDNA that encodes the mouse homologue of the human R-receptor. Mice are susceptible to BaEV entry (32), implying that there may be a sequence difference in the mouse protein that permits cellular penetration of BaEV but not of RD114. It is intriguing that all of the cloned receptors for type C and D mammalian retroviruses have multiple transmembrane domains and have been identified as transporters that are widely expressed in different tissues (1, 14, 17, 23, 26, 27, 34, 40, 42).
Nucleotide sequence accession number.
The nucleotide sequence of the 2.9-kb R-receptor cDNA has been assigned GenBank accession no. AF105423.
Acknowledgments
We are grateful to Richard Sutton for generously supplying the retroviral cDNA library, to Lisa Rosenblum for providing SRV-1, to Susan L. Kozak for help with Northern blot analysis, to William Andrews for assistance with MPMV and BaEV infection assays, to Mariana Marin for helpful discussions, and to Robin Weiss for encouragement and helpful criticism of the manuscript.
This research was supported by NIH grant CA25810, by The Wellcome Trust, and by the Medical Research Council. C.S.T. is a Wellcome Trust International Prize Fellow.
REFERENCES
- 1.Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Arriza J L, Kavanaugh M P, Fairman W A, Wu Y N, Murdoch G H, North R A, Amara S G. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 4.Brody B A, Rhee S S, Hunter E. Postassembly cleavage of a retroviral glycoprotein cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. J Virol. 1994;68:4620–4627. doi: 10.1128/jvi.68.7.4620-4627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesebro B, Wehrly K. Different murine cell lines manifest unique patterns of interference to superinfection by murine viruses. Virology. 1985;141:119–129. doi: 10.1016/0042-6822(85)90188-6. [DOI] [PubMed] [Google Scholar]
- 6.Chopra H C, Mason M M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970;30:2081–2086. [PubMed] [Google Scholar]
- 7.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel M D, King N W, Letvin N L, Hunt R D, Sehgal P K, Desrosiers R C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984;223:602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature (London) 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 10.Ford R J, Donehower L A, Bohannon R C. Studies on a type D retrovirus isolated from an AIDS patient lymphoma. AIDS Res Hum Retroviruses. 1992;8:742–751. [PubMed] [Google Scholar]
- 11.Gardner M B, Endres M, Barry P. The simian retroviruses: SIV and SRV. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1994. pp. 133–276. [Google Scholar]
- 12.Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–120. [PubMed] [Google Scholar]
- 13.Jensen E M, Zelljadt I, Chopra H C, Mason M M. Isolation and propagation of a virus from a spontaneous mammary carcinoma of a rhesus monkey. Cancer Res. 1970;30:2388–2393. [PubMed] [Google Scholar]
- 14.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kekuda R, Prasad P D, Fei Y J, Torres-Zamorano V, Sinha S, Yang-Feng T L, Leibach F H, Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 16.Kewalramani V N, Panganiban A T, Emerman M. Spleen necrosis virus, an avian immunosuppressive retrovirus, shares a receptor with the type D simian retroviruses. J Virol. 1992;66:3026–3031. doi: 10.1128/jvi.66.5.3026-3031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J W, Closs E I, Albritton L M, Cunningham J M. Transport of cationic amino acids by the mouse ecotropic receptor. Nature (London) 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan G P. Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci USA. 1995;92:9146–9150. doi: 10.1073/pnas.92.20.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao K, Lane M D. Expression of a novel insulin-activated amino acid transporter gene during differentiation of 3T3-L1 preadipocytes into adipocytes. Biochem Biophys Res Commun. 1995;208:1008–1015. doi: 10.1006/bbrc.1995.1434. [DOI] [PubMed] [Google Scholar]
- 19a.Liong, S. H., and Y. Takeuchi. Unpublished results.
- 19b.Madani, N., C. S. Tailor, D. Kabat, and M. P. Kavanaugh. Unpublished results.
- 20.Marx P A, Bryant M L, Osborn K G, Maul D H, Lerche N W, Lowenstine L J, Kluge J D, Zaiss C P, Henrickson R V, Shiigi S M, et al. Isolation of a new serotype of simian acquired immune deficiency syndrome type D retrovirus from Celebes black macaques (Macaca nigra) with immune deficiency and retroperitoneal fibromatosis. J Virol. 1985;56:571–578. doi: 10.1128/jvi.56.2.571-578.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx P A, Maul D H, Osborn K G, Lerche N W, Moody P, Lowenstine L J, Henrickson R V, Arthur L O, Gilden R V, Gravell M, et al. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984;223:1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- 22.Miller A D, Wolgamot G. Murine retroviruses use at least six different receptors for entry into Mus dunni cells. J Virol. 1997;71:4531–4535. doi: 10.1128/jvi.71.6.4531-4535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller D G, Miller A D. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Hara B, Johann S V, Klinger H P, Blair D G, Rubinson H, Dunn K J, Sass P, Vitek S M, Robbins T. Characterization of the human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990;1:119–127. [PubMed] [Google Scholar]
- 27.Olah Z, Lehel C, Anderson W B, Eiden M V, Wilson C A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 28.Patience C, Takeuchi Y, Cosset F L, Weiss R A. Packaging of endogenous retroviral sequences in retroviral vectors produced by murine and human packaging cells. J Virol. 1998;72:2671–2676. doi: 10.1128/jvi.72.4.2671-2676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter C D, Collins M K L, Tailor C S, Parkar M H, Cosset F L, Weiss R A, Takeuchi Y. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Hum Gene Ther. 1996;7:913–919. doi: 10.1089/hum.1996.7.8-913. [DOI] [PubMed] [Google Scholar]
- 30.Shafqat S, Tamarappoo B K, Kilberg M S, Puranam R S, McNamara J O, Guadano-Ferraz A, Fremeau R T., Jr Cloning and expression of a novel Na(+)-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J Biol Chem. 1993;268:15351–15355. [PubMed] [Google Scholar]
- 31.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 32.Sommerfelt M A, Williams B P, McKnight A, Goodfellow P N, Weiss R A. Localization of the receptor gene for type D simian retroviruses on human chromosome 19. J Virol. 1990;64:6214–6220. doi: 10.1128/jvi.64.12.6214-6220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stromberg K, Benveniste R E, Arthur L O, Rabin H, Giddens W E, Jr, Ochs H D, Morton W R, Tsai C C. Characterization of exogenous type D retrovirus from a fibroma of a macaque with simian AIDS and fibromatosis. Science. 1984;224:289–292. doi: 10.1126/science.6200929. [DOI] [PubMed] [Google Scholar]
- 34.Tailor C S, Nouri A, Lee C G, Kozak C, Kabat K. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi Y, Cosset F L, Lachmann P J, Okada H, Weiss R A, Collins M K L. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J Virol. 1994;68:8001–8007. doi: 10.1128/jvi.68.12.8001-8007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi Y, Patience C, Magre S, Weiss R A, Banerjee P T, Le Tissier P, Stoye J P. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998;72:9986–9991. doi: 10.1128/jvi.72.12.9986-9991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi Y, Simpson G, Vile R G, Weiss R A, Collins M K. Retroviral pseudotypes produced by rescue of a Moloney murine leukemia virus vector by C-type, but not D-type, retroviruses. Virology. 1992;186:792–794. doi: 10.1016/0042-6822(92)90049-u. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi Y, Vile R G, Simpson G, O’Hara B, Collins M K L, Weiss R A. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992;66:1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Dechant E, Kavanaugh M, North R A, Kabat D. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J Biol Chem. 1992;267:23617–23624. [PubMed] [Google Scholar]
- 40.Wang H, Kavanaugh M P, North R A, Kabat D. Cell surface receptor for ecotropic murine retroviruses is a basic amino acid transporter. Nature (London) 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- 41.Wilson C A, Farrell K B, Eiden M V. Properties of a unique form of the murine amphotropic leukemia virus receptor expressed on hamster cells. J Virol. 1994;68:7697–7703. doi: 10.1128/jvi.68.12.7697-7703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeijl M V, Johann S V, Cross E, Cunningham J, Eddy R, Shows T B, O’Hara B. An amphotropic virus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]