ABSTRACT
Ceftolozane-tazobactam (C-T) and ceftazidime-avibactam (CAZ-AVI) are two novel antimicrobials that retain activity against resistant Pseudomonas aeruginosa. The comparative effectiveness and safety of C-T versus CAZ-AVI remain unknown. A retrospective, multicenter cohort study was performed in six tertiary centers in Saudi Arabia and included patients who received either C-T or CAZ-AVI for infections due to multidrug-resistant (MDR) P. aeruginosa. Overall in-hospital mortality, 30-day mortality, and clinical cure were the main study outcomes. Safety outcomes were also evaluated. A multivariate analysis using logistic regression was used to determine the independent impact of treatment on the main outcomes of interest. We enrolled 200 patients in the study (100 in each treatment arm). A total of 56% were in the intensive care unit, 48% were mechanically ventilated, and 37% were in septic shock. Approximately 19% of patients had bacteremia. Combination therapy was administered to 41% of the patients. The differences between the C-T and CAZ-AVI groups did not reach statistical significance in the overall in-hospital mortality (44% versus 37%; P = 0.314; OR, 1.34; 95% CI, 0.76 to 2.36), 30-day mortality (27% versus 23%; P = 0.514; OR, 1.24; 95% CI, 0.65 to 2.35), clinical cure (61% versus 66%; P = 0.463; OR, 0.81; 95% CI, 0.43 to 1.49), or acute kidney injury (23% versus 17%; P = 0.289; OR, 1.46; 95% CI, 0.69 to 3.14), even after adjusting for differences between the two groups. C-T and CAZ-AVI did not significantly differ in terms of safety and effectiveness, and they serve as potential options for the treatment of infections caused by MDR P. aeruginosa.
KEYWORDS: ceftazidime-avibactam, ceftolozane-tazobactam, multidrug-resistant, Pseudomonas aeruginosa
INTRODUCTION
As the resistance to antimicrobial therapy increases, resistance in Gram-negative pathogens creates a serious therapeutic challenge. Pseudomonas aeruginosa is one of the most challenging Gram-negative microorganisms, given its capacities to possess intrinsic resistance and acquire a variety of resistance mechanisms (1). According to the SENTRY Antimicrobial Surveillance Program, the global rate of multidrug-resistant (MDR) P. aeruginosa in the last three decades was 24.9% (2). The World Health Organization (WHO) released a global antibiotic-resistant priority pathogens list that categorized carbapenem-resistant P. aeruginosa as a critical priority for research and development (3). In Saudi Arabia, antibiotic-resistant P. aeruginosa is also concerning, with the highest rate being shown in the Makkah region, which is mostly attributed to the high influx of pilgrims (4). Data from the Global Antimicrobial Resistance Surveillance System and data extracted from published literature showed that the rate of carbapenem-resistant P. aeruginosa in Saudi Arabia ranges between 21% and 30% (5, 6).
Worldwide efforts to overcome this challenge led to the development of a few new antimicrobials with activity against these resistant isolates. Ceftolozane-tazobactam (C-T) and ceftazidime-avibactam (CAZ-AVI) are two novel β-lactam-β-lactamase inhibitor combinations with activity against P. aeruginosa, including MDR strains. These agents received approval from the United States Food and Drug Administration (FDA) for treating complicated intraabdominal infections and complicated urinary tract infections in 2014 and 2015, as well as, more recently, for treating hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) (7, 8). Therefore, many clinicians view the two antimicrobials as interchangeable for the treatment of MDR P. aeruginosa. However, remarkable differences exist. Ceftolozane is a novel oxyimino-aminothiazolyl cephalosporin. Compared to ceftazidime, ceftolozane is less prone to hydrolysis by AmpC and is less likely to be affected by porin loss than is ceftazidime, which are two major resistance mechanisms of P. aeruginosa (9). Although it efficiently inhibits AmpC β-lactamases, avibactam can also inhibit other serine β-lactamases of the KPC and OXA-48 families, which makes CAZ-AVI more useful against resistant Enterobacterales (8).
Both C-T and CAZ-AVI serve as front-line treatment options when encountering infections caused by MDR P. aeruginosa. C-T is often preferred, given its selectivity against MDR P. aeruginosa and its ability to overcome multiple resistance mechanisms. However, some settings require the widespread use of CAZ-AVI as an alternative. Several hospitals are unable to adopt both agents in the formulary due to limited resources and get CAZ-AVI alone to cover multiple resistant Gram-negative pathogens. Additionally, the global recall of C-T in December of 2020 led to the widespread use of CAZ-AVI as an alternative, especially in countries where other novel agents against MDR P. aeruginosa are unavailable. Treating clinicians select one over the other without clear evidence in the literature. Some surveillance studies showed that C-T and CAZ-AVI have high and comparable inhibitory activity against P. aeruginosa, including MDR isolates, while other studies showed that one agent could be better than the other (9–15). However, no clinical study has yet to compare them, and one should be prudent in making clinical inferences from these in vitro data. To address this gap, we designed this study to compare the clinical outcomes of CAZ-AVI to C-T in treating infections due to MDR P. aeruginosa.
RESULTS
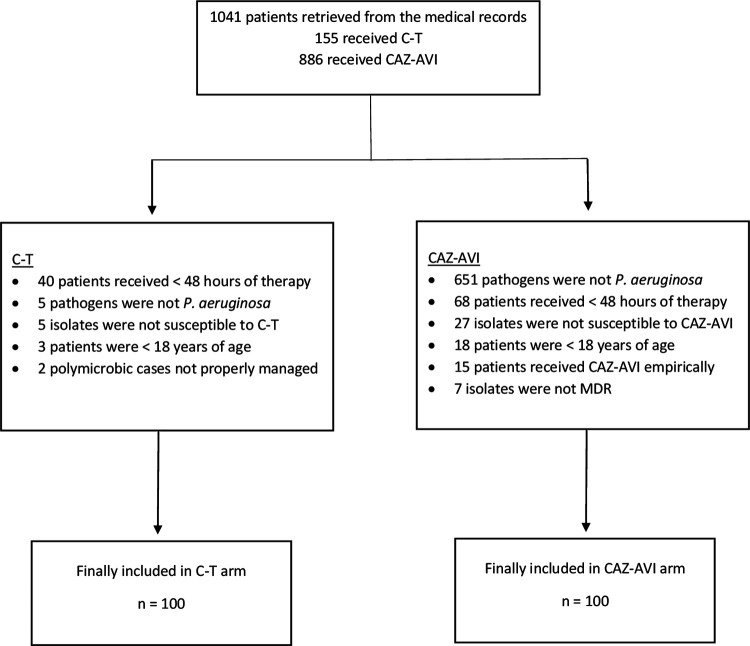
A total of 1,041 patients who received either C-T or CAZ-AVI were retrieved from the medical records, and 200 of these patients were included in this study, with 100 patients in each study arm (Fig. 1). The mean age for the overall study population was 60 ± 19 years, and 132 (66%) were males. The most common comorbidities included diabetes mellitus, hypertension, immunosuppression, moderate to severe chronic renal failure, and cerebrovascular disease, which presented in 58%, 57%, 35%, 30%, and 22% of the study population, respectively. The median interquartile range (IQR) charlson comorbidity index (CCI) was 5 (3 to 8). A total of 112 (56%) patients were in the ICU, and 96 (48%) were on mechanical ventilation. The most common infections were hospital-acquired pneumonia, wound infection, ventilator-associated pneumonia, urinary tract infection, and intraabdominal infection, which presented in 28%, 24%, 21%, 10%, and 8% of the study population, respectively. A total of 37 (19%) patients presented with bacteremia. Polymicrobial infection occurred in 98 (49%) patients. More details are shown in Table 1.
FIG 1.
Patient enrollment and screening for eligibility.
TABLE 1.
Demographic and baseline characteristics of included patientsa
| Characteristic | C-T (n = 100) | CAZ-AVI (n = 100) | P value |
|---|---|---|---|
| Demographic | |||
| Age in yearsb | 60 ± 20 | 60 ± 18 | 0.998 |
| Male | 71 | 61 | 0.136 |
| Comorbidity | |||
| Cerebrovascular disease | 20 | 24 | 0.495 |
| Chronic heart failure | 19 | 20 | 0.858 |
| Chronic obstructive pulmonary disease | 5 | 4 | 0.733 |
| Connective tissue disease | 2 | 5 | 0.248 |
| Dementia | 6 | 6 | 1 |
| Diabetes mellitus | 57 | 59 | 0.774 |
| Hemiplegia or paraplegia | 8 | 8 | 1 |
| History of myocardial infarction | 18 | 12 | 0.235 |
| Hypertension | 53 | 60 | 0.318 |
| Immunosuppressedc | 33 | 37 | 0.553 |
| Liver disease | 9 | 4 | 0.152 |
| Moderate to severe chronic renal failure | 29 | 30 | 0.877 |
| Neurological disease | 16 | 22 | 0.264 |
| Peptic ulcer disease | 0 | 1 | 0.316 |
| Peripheral vascular disease | 10 | 13 | 0.506 |
| Charlson comorbidity indexd | 5 (3 to 8) | 5 (3 to 8) | 0.886 |
| Baseline serum creatinine in μmol/Ld | 82 (52 to 161) | 91 (55 to 200) | 0.423 |
| Baseline creatinine clearance in mL/mind | 65 (32 to 115) | 59 (26 to 114) | 0.463 |
| Indwelling invasive devices | |||
| Central venous catheter | 65 | 57 | 0.282 |
| Foley catheter | 67 | 57 | 0.145 |
| Mechanical ventilation | 50 | 46 | 0.571 |
| Severity of illness | |||
| Intensive care unit at infection onset | 52 | 60 | 0.254 |
| No sepsis | 30 | 38 | 0.232 |
| Sepsis | 31 | 27 | 0.533 |
| Septic shock | 39 | 35 | 0.558 |
| APACHE II scored | 20 (12 to 30) | 19 (13 to 24) | 0.706 |
| Site of infection | |||
| HAP | 25 | 31 | 0.345 |
| Wound | 27 | 20 | 0.234 |
| VAP | 20 | 22 | 0.728 |
| UTI | 11 | 10 | 0.818 |
| Intraabdominal | 6 | 10 | 0.297 |
| CRBSI | 5 | 2 | 0.248 |
| Othere | 6 | 5 | 0.756 |
| Presence of bacteremia | 23 | 14 | 0.101 |
| Polymicrobial infection | 55 | 43 | 0.09 |
| Infectious diseases consultation | 99 | 97 | 0.312 |
| Time to active therapy in hoursd | 47 (10 to 86) | 55 (7 to 90) | 0.97 |
| Time to study drug in hoursd | 88 (37 to 129) | 92 (29 to 134) | 0.73 |
| Combination therapyf | 47 | 35 | 0.084 |
| Combination with more than one agent | 11 | 9 | 0.809 |
| Type of combination therapy | |||
| IV colistin | 22 | 14 | 0.141 |
| Aztreonam | 1 | 15 | <0.001 |
| Inhaled colistin | 9 | 5 | 0.268 |
| Fluoroquinolone | 10 | 3 | 0.045 |
| IV aminoglycoside | 6 | 6 | 1 |
| Carbapenem | 9 | 0 | 0.002 |
| Inhaled aminoglycoside | 1 | 1 | 1 |
| Susceptible to at least one combination agentg | 30 | 22 | 0.928 |
| Duration of therapy in daysd | 10 (7 to 15) | 10 (6 to 14) | 0.226 |
| Overall duration of hospitalization in daysd | 61 (32 to 137) | 59 (30 to 118) | 0.365 |
A χ2 test was applied to compare categorical variables, whereas an independent t test or a Wilcoxon rank-sum test was applied to compare continuous variables. APACHE II, Acute Physiology and Chronic Health Evaluation; CAZ-AVI, ceftazidime-avibactam; C-T, ceftolozane-tazobactam; CRBSI, catheter-related bloodstream infection; ICU, intensive care unit; HAP, hospital-acquired pneumonia; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Mean ± standard deviation.
Neutropenic, chronic therapy with corticosteroids, active chemotherapy, or solid organ/stem cell transplant patients on immunosuppressants.
Median (interquartile range). Otherwise, the data are presented as n (and percents, as n = 100).
Including five bacteremia of unknown origin, two empyema, two cystic fibrosis, one infective endocarditis, and one meningitis.
Given concurrently with the study drug for at least 48 hours.
Among all isolates for patients who received combination therapy only.
In the CAZ-AVI arm, the median (IQR) time to active therapy and time to study drug were 55 (7 to 90) hours and 92 (29 to 134) hours, respectively, whereas the median times in the C-T arm were 47 (10 to 86) hours and 88 (37 to 129) hours, respectively. These differences were not statistically significant. Combination therapy was more common in the C-T arm (47% versus 35%), but the difference was not statistically significant. Among those cases, the isolates were susceptible in vitro to at least one combination agent in 30% and 22% in the C-T arm and CAZ-AVI arm, respectively. Specific MIC data were available or retrievable in 36 and 43 cases in the C-T and CAZ-AVI groups, respectively. The median MIC in the C-T group was 2 μg/mL (range, 0.5 to 4 μg/mL), whereas the median MIC in the CAZ-AVI group was 4 μg/mL (range, 2 to 8 μg/mL). In the C-T group, the numbers of patients with MICs of 0.5, 1, 1.5, 2, and 4 μg/mL were 2, 6, 4, 17, and 7, respectively. In the CAZ-AVI group, the numbers of patients with MICs of 2, 4, 6, and 8 μg/mL were 18, 13, 1, and 11, respectively.
The two groups were comparable in demographics and clinical characteristics. Significantly more patients were on concomitant carbapenems and fluoroquinolones in the C-T arm, and more patients were on concomitant aztreonam in the CAZ-AVI arm. More details are shown in Table 1.
When comparing the C-T group to the CAZ-AVI group in terms of clinical cure (61% versus 66%; P = 0.463; OR, 0.81; 95% CI, 0.43 to 1.49), in-hospital mortality (44% versus 37%; P = 0.314; OR, 1.34; 95% CI, 0.76 to 2.36), 30-day mortality (27% versus 23%; P = 0.514; OR, 1.24; 95% CI, 0.65 to 2.35), and infection-related mortality (25% versus 19%; P = 0.307; OR, 1.42; 95% CI, 0.72 to 2.79), the differences were not statistically significant, even after adjusting for differences between the two groups. The differences between the two groups in other outcomes, including microbiologic eradication, 30-day readmission, 30-day recurrence, 90-day recurrence, the length of hospital stay and ICU stay from the onset of infection, the duration of mechanical ventilation, acute kidney injury (AKI), and acute liver injury were not statistically significant (Table 2). Two patients in the CAZ-AVI arm developed Clostridioides difficile infections. In the C-T arm, one patient developed a maculopapular rash, one patient developed seizures, and two patients developed diarrhea. AKI developed in 40 (20%) patients. 26 (60%) of these patients received combination therapy, and, among these patients, intravenous (IV) colistin was concurrently given to 16 (40%) of them.
TABLE 2.
Outcomes in patients receiving ceftolozane-tazobactam versus ceftazidime-avibactam
| Outcomea | C-T (n = 100) | CAZ-AVI (n = 100) | P Value | Odds Ratio (95% CI) | Adjusted Oddsb Ratio (95% CI) |
|---|---|---|---|---|---|
| Clinical cure | 61 | 66 | 0.463 | 0.81 (0.43 to 1.49) | 0.92 (0.41 to 2.05) |
| In-hospital mortality | 44 | 37 | 0.314 | 1.34 (0.76 to 2.36) | 1.13 (0.52 to 2.48) |
| 30-day mortality | 27 | 23 | 0.514 | 1.24 (0.65 to 2.35) | 1.20 (0.48 to 3.00) |
| Infection-related mortality | 25 | 19 | 0.307 | 1.42 (0.72 to 2.79) | 1.00 (0.40 to 2.52) |
| Microbiologic outcomec | |||||
| Eradication | 46 | 43 | 0.843 | 0.94 (0.46 to 1.89) | |
| Persistence | 32 | 28 | |||
| 30-day readmissiond | 11 | 14 | 0.73 | ||
| 30-day readmission due to infectiond | 5 | 8 | 0.511 | ||
| 30-day recurrenced | 8 | 13 | 0.364 | ||
| 90-day recurrenced | 14 | 16 | 0.96 | ||
| Length of hospital stay from onset of infection (days) | 30 (20 to 75) | 32 (17 to 66) | 0.61 | ||
| Length of ICU stay from onset of infection (days)e | 25 (9 to 44) | 24 (14 to 40) | 0.829 | ||
| Duration of mechanical ventilation (days)f | 23 (7 to 45) | 21 (8 to 42) | 0.874 | ||
| Acute kidney injury | 23 | 17 | 0.289 | 1.46 (0.69 to 3.14) | 1.74 (0.66 to 4.59) |
| Risk | 6 | 8 | |||
| Injury | 7 | 1 | |||
| Failure | 6 | 7 | |||
| RRT | 4 | 1 | |||
| Acute liver injury | 3 | 1 | |||
The data are presented as n (and percents, as n = 100) or as the median (IQR). Either a χ2 test or a Fisher’s exact test was used to compare categorical variables, whereas either an independent t test or a Wilcoxon rank-sum test was used to compare continuous variables. Abbreviations: CAZ-AVI, ceftazidime-avibactam; C-T, ceftolozane-tazobactam; CI, confidence interval; ICU, intensive care unit; RRT, renal replacement therapy.
Adjusted for gender, the presence of bacteremia, polymicrobial infections, combination therapy, liver disease, Foley catheter, time to active therapy, and duration of therapy.
Only including patients who had repeated cultures (n = 78 in the ceftolozane-tazobactam arm, and n = 71 in the ceftazidime-avibactam arm).
Only including patients who survived (n = 56 in the ceftolozane-tazobactam arm, and n = 63 in the ceftazidime-avibactam arm).
Only including patients who were in the ICU at infection onset.
Only including patients who were mechanically ventilated during the infection episode.
After conducting further analysis, 20 patients in the C-T arm received 1.5 gram-based dosing for HAP or VAP, among which clinical failure, in-hospital mortality, and 30-day mortality occurred in 12, 12, and 8 patients, respectively. After excluding those patients, the rates of clinical cure in the C-T and CAZ-AVI groups did not change (61% versus 66%). However, the rates of in-hospital mortality became closer (40% versus 37%), and the rates of 30-day mortality became similar (23% in both groups) between the two groups.
Further, among cases with available MICs, the differences were not significant between those who received C-T and CAZ-AVI in clinical cure (61% versus 70%; P = 0.419), in-hospital mortality (42% versus 35%; P = 0.536), 30-day mortality (25% versus 21%; P = 0.668), infection-related mortality (25% versus 16%; P = 0.874), and microbiologic eradication (56% versus 74%; P = 0.135).
DISCUSSION
This study showed that C-T and CAZ-AVI did not significantly differ in overall in-hospital mortality, 30-day mortality, and clinical cure at the end of the treatment when used for the treatment of infections caused by MDR P. aeruginosa. In addition, no difference was found in the other outcomes, including the infection-related mortality, 30-day readmission, 30-day recurrence, 90-day recurrence, microbiologic eradication, length of stay, duration of mechanical ventilation, AKI, or acute liver injury. Although C-T and CAZ-AVI are available options for these cases, C-T could have been prioritized for resistant P. aeruginosa in clinical practice, given the utility of CAZ-AVI against carbapenem-resistant Enterobacterales, for which β-lactamases and carbapenemases are the predominant resistance determinants. However, our study showed insignificant trends toward a higher rate of clinical cure and a lower mortality rate in patients who received CAZ-AVI versus those who received C-T. However, it should be noted that more isolates with available MICs were at borderline susceptibility in the C-T arm (59%), compared to the CAZ-AVI arm (25%), and 20% of patients in the C-T arm did not receive the appropriate dose per indication, which might contribute to the insignificant trends toward the slightly better outcomes in the CAZ-AVI arm. Besides the insignificant differences in clinical outcomes, other factors may be considered, including the local epidemiology and the cost, with CAZ-AVI currently being more affordable in the Kingdom of Saudi Arabia.
Data regarding the clinical effectiveness of C-T against infections caused by resistant P. aeruginosa came from real-world case series (16, 17) or comparative cohort studies, as opposed to more traditional antipseudomonal agents (18, 19). These studies showed clinical success rates of approximately 70% to 80% as well as a mortality of 20%. Our study showed less successful outcomes, which could be due to the differences in the disease severity of the patients (higher rate of septic shock in our study). Additionally, more isolates with available MICs were at borderline susceptibility (59% in the C-T arm and 25% in the CAZ-AVI arm). Data investigating the clinical outcomes of CAZ-AVI versus comparators against infections caused by MDR P. aeruginosa are lacking and are limited to pooled data from randomized clinical trials (20). These data showed a clinical and microbiologic success of 57%, which was comparable to our findings. The existing data comparing the activity of C-T to CAZ-AVI against P. aeruginosa, including MDR isolates, are limited to surveillance studies (9–15). Although earlier studies showed that C-T has better in vitro activity (9, 10, 12), later studies showed contrary or similar activity (13–15). Our study is the first to compare the two agents in terms of clinical outcomes. Although AKI occurred in 40 (20%) patients in this study, 26 (60%) of those patients received combination therapy in which IV colistin was concurrently given to 16 (40%) patients. This is expected, as previous studies showed that the rate of AKI in patients who received IV polymyxin was 30% to 43% (18, 21).
Around 45% of patients with HAP or VAP in the C-T arm received 1.5 gram-based dosing, among which clinical failure, in-hospital mortality, and 30-day mortality occurred in 60%, 60%, and 40% of patients, respectively. Compared to all patients who received C-T, these outcomes occurred less frequently: 39%, 44%, and 27%, respectively. In a simulated probability of target attainment (PTA) of ceftolozane in epithelial lining fluid, with a ≥50% fT/MIC target and a MIC of up to 8, the PTA values were approximately 90% and 59% with a 3 g dose and a 1.5 g dose, respectively (22). Our results emphasize the importance of 3 gram-based dosing for nosocomial pneumonia.
The concept of “difficult-to-treat” resistance (DTR) was proposed earlier (23) and was recently used in the latest IDSA guidelines to support the use of C-T and CAZ-AVI for P. aeruginosa-related infections (24). Although we included patients based on the presence of MDR isolates, these isolates can be considered difficult-to-treat, given the internal protocols for the participating hospitals to allow the use of C-T or CAZ-AVI only if the isolate is not susceptible to all traditional antipseudomonal β-lactam and antipseudomonal fluoroquinolones (the definition of P. aeruginosa with difficult-to-treat resistance) (24).
This study has several limitations. Given its retrospective observational design, the study was subject to selection bias, inclusion bias, healthcare access bias, data incompleteness, and failure to control residual confounding. Screening for carbapenemase genes was not performed, although 18% of the carbapenem-resistant P. aeruginosa in Saudi Arabia were carbapenemase producers (25). In the C-T arm, 20% of patients did not receive the appropriate dose per indication, which might contribute to the insignificant trends toward slightly better outcomes in the CAZ-AVI arm. Although isolates confirmed as not susceptible to study drugs were excluded, the MIC data were unavailable for many included ones. Limited local and regional data showed that P. aeruginosa susceptibility to C-T and CAZ-AVI ranges between 63% and 97% (11, 15, 26). Additionally, data on source control, which might have influenced the prognosis, were not documented. Further, although deferred renal dose reduction in patients with AKI is recommended to improve the outcomes (27), we were unable to document these data. We included different sources of infections, which may create heterogeneity in the results. The sample size was relatively small. However, this is not surprising, as infections caused by MDR P. aeruginosa are not common, and C-T and CAZ-AVI were introduced to the Kingdom of Saudi Arabian hospitals in 2017. Nevertheless, it is a large comparative study that was conducted across six tertiary care hospitals.
In conclusion, this study showed that C-T and CAZ-AVI did not significantly differ in terms of safety and effectiveness and serve as potential options for the treatment of infections caused by MDR P. aeruginosa. These novel agents should be preferred over more traditional antipseudomonal agents, including IV colistin and aminoglycosides.
MATERIALS AND METHODS
Study design and setting.
This retrospective, multicenter cohort study was conducted from January of 2017 to June of 2022, and it included hospitalized patients who underwent treatment with either C-T or CAZ-AVI for MDR P. aeruginosa infections. The study was conducted at six tertiary centers in Saudi Arabia: King Saud University Medical City (KSUMC), a 1,500-bed academic medical center in Riyadh; King Faisal Specialist Hospital and Research Center (KFSHRC), a 1,600-bed specialist hospital in Riyadh; Prince Sultan Military Medical City (PSMMC), a 1,200-bed hospital in Riyadh; King Faisal Specialist Hospital and Research Center (KFSHRC), a 500-bed specialist hospital in Jeddah; King Fahad Medical City (KFMC), a 1,200-bed hospital in Riyadh; and King Abdulaziz Medical City (KAMC), a 750-bed hospital in Jeddah. Data from the included cohort were retrieved from electronic health records. Eligible patients were those aged ≥18 years who developed MDR P. aeruginosa infections and were treated with either CAZ-AVI or C-T for ≥48 h. The exclusion criteria were as follows: if the microbiologic data showed that the isolated strain was not susceptible to the study drug being evaluated or if concomitant or polymicrobial infections existed and were improperly treated. Proper treatment was defined as an appropriate, in vitro active antibiotic against the causative pathogen with an appropriate dose and duration of therapy, based on the recommendations from the guidelines of the concomitant infections. Patients with reported colonization, rather than infection, were excluded. In cases of multiple events of infection due to MDR P. aeruginosa, only the first event was included. The primary outcomes of this study were overall in-hospital mortality, 30-day mortality, and clinical cure at the end of treatment. Secondary outcomes included microbiologic eradication, infection-related mortality, the length of the hospital stay, the length of the intensive-care unit stay from the onset of the infection, 30-day readmission, 30-day recurrence, 90-day recurrence, and the duration of mechanical ventilation. Safety outcomes were also investigated, with special attention given to acute liver injury, AKI, and Clostridioides difficile infections up to two months after the last dose or discharge, whichever came first. CAZ-AVI was dosed as 2.5 g, intravenously administered over 2 h, given every 8 h, and adjusted per renal function. C-T was dosed as 3 g or 1.5 g, intravenously administered over 1 h, and given every 8 h, depending on the indication and adjusted per renal function. However, before the FDA approval for HAP and VAP, some patients might have received a 1.5 g dose for these indications. Ethical reviews and approvals were obtained from the ethics committees of all participating sites (IRB Approval of Research Project No. E-22-6628).
Data collection.
The following variables were electronically retrieved: demographics, dosages of study drugs, source of infection, time to appropriate antibiotic (any antibiotic with in vitro activity), time to study drug (C-T or CAZ-AVI), duration of treatment, microbial susceptibility to study drugs, MICs, concurrent antibiotics and the susceptibility data, serum creatinine at baseline and at after treatment, presence of comorbidities, CCI, Acute Physiology and Chronic Health Evaluation (APACHE II) score, placement of artificial devices, occurrence of polymicrobial infections, setting of admission, immune status, severity of illness, mechanical ventilation, infectious diseases consultation, and duration of hospital stay. Clinical, microbiological, and safety outcomes were also collected.
Microbiological testing.
Isolates of P. aeruginosa were identified, and antibiotic susceptibility testing was conducted using automated systems, based on each hospital’s own protocol: a MicroScan WalkAway 96 Plus (Beckman Coulter, Inc., Brea, CA, USA), a Vitek 2 system (bioMérieux, Marcy-l'Étoile, France), or a BD Phoenix M50 (Becton, Dickinson Diagnostic Systems, Sparks, MD, USA). The C-T breakpoints followed the guidelines from the Clinical and Laboratory Standards Institute (CLSI): ≤4/4 was susceptible, 8/4 was intermediate, and ≥16/4 was resistant (28). The CAZ-AVI breakpoints followed the guidelines from CLSI: ≤8/4 was susceptible, and ≥16/4 was resistant (28). The susceptibilities for C-T and CAZ-AVI were tested using gradient diffusion by either Etest (bioMérieux, Marcyl'Étoile, France) or MIC test strip (Liofilchem, Roseto degli Abruzzi, Italy).
Definitions.
Sepsis was defined as life-threatening organ dysfunction as a response to infection, which can be identified as an increased SOFA score of ≥2 from the baseline (29). Septic shock was defined as sepsis with consistent hypotension that required vasopressors and a serum lactate level of >2 mmol/L, despite proper volume resuscitation (29). APACHE II is a general measure of disease severity and a mortality estimation score that is commonly used in critically ill patients (30). CCI is a validated and readily applicable method of comorbid conditions that can be used to estimate the one-year mortality of medical inpatients (31). MDR P. aeruginosa was defined as P. aeruginosa that is not susceptible to at least one agent in ≥3 antimicrobial categories (32). The time to active therapy was defined as the time to initiate any antibiotic with in vitro susceptibility from the time the cultures were collected. The time to study drug was defined as the time to initiate the study of the antimicrobial (C-T or CAZ-AVI) from the time the cultures were collected.
Clinical cure was defined as the resolution of the infection manifestations after the use of the study drug, without therapy needing to have been modified due to failure or toxicity, whereas clinical failure was defined as the persistence of infection manifestations, despite adequate antimicrobial therapy. Clinical, laboratory, and radiologic findings were used in the evaluation of the clinical outcomes, including fever, pulse rate, white blood cells, arterial blood gas, C-reactive protein, procalcitonin, and imaging studies, when applicable. The assessment of clinical cure was performed by using a categorical variable with values of “yes” or “no”. Each participating site had one to two data collectors who were clinical pharmacists with Pharm.D. degrees or the equivalent and had specialized training in infectious diseases. They reviewed the electronic records of the included patients by using the previously mentioned criteria as well as the documented progress reports that were provided by the treating clinicians. In each site, at least one clinical pharmacist is classified as a “consultant”, per the Saudi Commission for Health Specialties. Difficult cases or cases with disagreement were resolved via open discussion to develop a consensus.
In-hospital mortality was defined as death due to any cause within the current admission. This mortality was considered infection-related if patients had ongoing, unequivocal clinical and/or biochemical signs of infection at the time of death. Likewise, difficult cases or cases with disagreement were resolved via open discussion to develop a consensus. 30-day mortality was defined as death that occurred within 30 days of the index culture.
Microbiologic eradication was defined as the absence of microbial growth, and microbiologic failure was defined as the persistence of positive cultures of the etiologic microorganisms at the same infection site, despite the clinical outcome of the infection (evaluated only if repeated cultures were available). Indeterminate was selected if no repeated microbiologic evaluation was available.
Polymicrobial infection was defined as an additional pathogen during the same infection episode, except for possible contaminants.
Recurrence (30-day or 90-day) of infection was defined as positive cultures of P. aeruginosa and susceptibility that was similar to that observed for the index culture after evidence of at least one negative growth within 30 or 90 days of the primary infection episode.
For the assessment of renal function, the “RIF” components of the RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) criteria were used, as long-term assessments were not conducted. We considered the patient to have AKI if any of the previous categories occurred during the course of treatment (33). We also included whether patients received renal replacement therapy due to AKI. The assessment of acute liver injury was performed in accordance with the Drug-Induced Liver Injury Network (DILIN) (34).
Statistical analysis.
Descriptive statistics were used to summarize the data. Bivariate comparisons were performed for the baseline characteristics and the outcomes of interest between patients treated with C-T and CAZ-AVI. Categorical variables were presented as numbers and percentages and were compared between treatment groups by using either a χ2 test or a Fisher’s exact test. Continuous variables were presented as the mean ± standard deviation (SD) with an independent t test if normally distributed. If not normally distributed, the median and the IQR were used with a Wilcoxon rank-sum test. The analyses were conducted with the level of significance set at P < 0.05. A multivariate analysis using logistic regression was used to determine the independent impact of treatment on the outcomes of interest (overall in-hospital mortality, 30-day mortality, infection-related mortality, clinical cure, and AKI).
Along with the treatment groups, baseline characteristics associated with a difference at a P value of ≤0.20 were eligible for inclusion into the model. Then, the adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for treatment with C-T were calculated for each clinical outcome. All of the statistical analyses were performed using STATA 15.1 (StataCorp LP, College Station, TX, USA).
ACKNOWLEDGMENTS
We thank King Saud University, Riyadh, Saudi Arabia, for supporting this research project (RSP2023R74).
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
The work described has not been published previously, and it is not under consideration for publication elsewhere.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We declare that we have no conflict of interest.
Yazed Saleh Alsowaida, Department of Clinical Pharmacy, College of Pharmacy, Hail University, Hail 81442, Saudi Arabia, is an alternative author.
REFERENCES
- 1.O'Donnell JN, Bidell MR, Lodise TP. 2020. Approach to the treatment of patients with serious multidrug-resistant Pseudomonas aeruginosa infections. Pharmacotherapy 40:952–969. doi: 10.1002/phar.2449. [DOI] [PubMed] [Google Scholar]
- 2.Shortridge D, Gales AC, Streit JM, Huband MD, Tsakris A, Jones RN. 2019. Geographic and temporal patterns of antimicrobial resistance in Pseudomonas aeruginosa over 20 years from the SENTRY antimicrobial surveillance program, 1997-2016. Open Forum Infect Dis 6:S63–s68. doi: 10.1093/ofid/ofy343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2017. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Available at: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed 18 June 2022.
- 4.Alhifany AA, Alqurashi AF, Al-Agamy MH, Alkhushaym N, Alhomoud F, Alhomoud FK, Almangour TA. 2020. Employment of mapping technology in antimicrobial resistance reporting in Saudi Arabia. Geospat Health 15. doi: 10.4081/gh.2020.868. [DOI] [PubMed] [Google Scholar]
- 5.Al Salman J, Al Dabal L, Bassetti M, Alfouzan WA, Al Maslamani M, Alraddadi B, Elhoufi A, Enani M, Khamis FA, Mokkadas E, Romany I, Somily A, Kanj S. 2020. Management of infections caused by WHO critical priority Gram-negative pathogens in Arab countries of the Middle East: a consensus paper. Int J Antimicrob Agents 56:106104. doi: 10.1016/j.ijantimicag.2020.106104. [DOI] [PubMed] [Google Scholar]
- 6.Moghnieh RA, Kanafani ZA, Tabaja HZ, Sharara SL, Awad LS, Kanj SS. 2018. Epidemiology of common resistant bacterial pathogens in the countries of the Arab League. Lancet Infect Dis 18:e379–e394. doi: 10.1016/S1473-3099(18)30414-6. [DOI] [PubMed] [Google Scholar]
- 7.Zerbaxa. 2014. US FDA. Zerbaxa (ceftolozane and tazobactam) for injection, for intravenous use: US prescribing information. Available at: https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf. Accessed 22 June 2022.
- 8.Avycaz. US FDA. Avycaz (ceftazidime and avibactam) for injection, for intravenous use: US prescribing information. 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206494s005,s006lbl.pdf. Accessed 18 June 2022.
- 9.Humphries RM, Hindler JA, Wong-Beringer A, Miller SA. 2017. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against beta-lactam-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 61. doi: 10.1128/AAC.01858-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupper M, Sutherland C, Nicolau DP. 2017. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible Pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob Agents Chemother 61. doi: 10.1128/AAC.00875-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alatoom A, Elsayed H, Lawlor K, AbdelWareth L, El-Lababidi R, Cardona L, Mooty M, Bonilla MF, Nusair A, Mirza I. 2017. Comparison of antimicrobial activity between ceftolozane-tazobactam and ceftazidime-avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis 62:39–43. doi: 10.1016/j.ijid.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Buehrle DJ, Shields RK, Chen L, Hao B, Press EG, Alkrouk A, Potoski BA, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 60:3227–3231. doi: 10.1128/AAC.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill CM, Aktaþ E, Alfouzan W, Bourassa L, Brink A, Burnham CD, Canton R, Carmeli Y, Falcone M, Kiffer C, Marchese A, Martinez O, Pournaras S, Satlin M, Seifert H, Thabit AK, Thomson KS, Villegas MV, Nicolau DP, ERACE-PA Global Study Group . 2021. The ERACE-PA Global Surveillance Program: ceftolozane/tazobactam and ceftazidime/avibactam in vitro activity against a global collection of carbapenem-resistant Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 40:2533–2541. doi: 10.1007/s10096-021-04308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sader HS, Carvalhaes CG, Streit JM, Doyle TB, Castanheira M. 2021. Antimicrobial activity of ceftazidime-avibactam, ceftolozane-tazobactam and comparators tested against Pseudomonas aeruginosa and Klebsiella pneumoniae isolates from United States medical centers in 2016–2018. Microb Drug Resist 27:342–349. doi: 10.1089/mdr.2020.0217. [DOI] [PubMed] [Google Scholar]
- 15.Sid Ahmed MA, Abdel Hadi H, Hassan AAI, Abu Jarir S, Al-Maslamani MA, Eltai NO, Dousa KM, Hujer AM, Sultan AA, Soderquist B, Bonomo RA, Ibrahim EB, Jass J, Omrani AS. 2019. Evaluation of in vitro activity of ceftazidime/avibactam and ceftolozane/tazobactam against MDR Pseudomonas aeruginosa isolates from Qatar. J Antimicrob Chemother 74:3497–3504. doi: 10.1093/jac/dkz379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher JC, Satlin MJ, Elabor A, Saraiya N, McCreary EK, Molnar E, El-Beyrouty C, Jones BM, Dixit D, Heil EL, Claeys KC, Hiles J, Vyas NM, Bland CM, Suh J, Biason K, McCoy D, King MA, Richards L, Harrington N, Guo Y, Chaudhry S, Lu X, Yu D. 2018. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis 5:ofy280. doi: 10.1093/ofid/ofy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassetti M, Castaldo N, Cattelan A, Mussini C, Righi E, Tascini C, Menichetti F, Mastroianni CM, Tumbarello M, Grossi P, Artioli S, Carannante N, Cipriani L, Coletto D, Russo A, Digaetano M, Losito AR, Peghin M, Capone A, Nicolè S, Vena A, CEFTABUSE Study Group . 2019. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents 53:408–415. doi: 10.1016/j.ijantimicag.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Pogue JM, Kaye KS, Veve MP, Patel TS, Gerlach AT, Davis SL, Puzniak LA, File TM, Olson S, Dhar S, Bonomo RA, Perez F. 2020. Ceftolozane/tazobactam vs polymyxin or aminoglycoside-based regimens for the treatment of drug-resistant Pseudomonas aeruginosa. Clin Infect Dis 71:304–310. doi: 10.1093/cid/ciz816. [DOI] [PubMed] [Google Scholar]
- 19.Almangour TA, Aljabri A, Al Musawa M, Almohaizeie A, Almuhisen S, Damfu N, Alfozan A, Alraddadi BM, Alattas M, Qutub M, Alhameed AF, Khuwaja M, Alghamdi A, Binkhamis KM, Alfahad W, AlShahrani FS. 2022. Ceftolozane-tazobactam vs. colistin for the treatment of infections due to multidrug-resistant Pseudomonas aeruginosa: a multicentre cohort study. J Glob Antimicrob Resist 28:288–294. doi: 10.1016/j.jgar.2022.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Stone GG, Newell P, Gasink LB, Broadhurst H, Wardman A, Yates K, Chen Z, Song J, Chow JW. 2018. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J Antimicrob Chemother 73:2519–2523. doi: 10.1093/jac/dky204. [DOI] [PubMed] [Google Scholar]
- 21.Oliota AF, Penteado ST, Tonin FS, Fernandez-Llimos F, Sanches AC. 2019. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis 94:41–49. doi: 10.1016/j.diagmicrobio.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Xiao AJ, Miller BW, Huntington JA, Nicolau DP. 2016. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol 56:56–66. doi: 10.1002/jcph.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, Palmore TN, Rhee C, Klompas M, Dekker JP, Powers JH, 3rd, Suffredini AF, Hooper DC, Fridkin S, Danner RL, National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI) . 2018. Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2022. Infectious Diseases Society of America 2022 guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P aeruginosa). Clin Infect Dis 75:187–212. doi: 10.1093/cid/ciac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doumith M, Alhassinah S, Alswaji A, Alzayer M, Alrashidi E, Okdah L, Aljohani S, Balkhy HH, Alghoribi MF, NGHA AMR Surveillance Group . 2021. Genomic characterization of carbapenem-non-susceptible Pseudomonas aeruginosa clinical isolates from Saudi Arabia revealed a global dissemination of GES-5-producing ST235 and VIM-2-producing ST233 sub-lineages. Front Microbiol 12:765113. doi: 10.3389/fmicb.2021.765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahabri NM, Al-Alawi MM, Qutub MO, Tashkandi WA, AlTurki R, Janah SS, Ali HE, Almutairi AF, Khalil S. 2022. In-vitro activity of ceftolozane/tazobactam against recent clinical bacterial isolates from two Saudi Arabian hospitals. J Infect Public Health 15:486–490. doi: 10.1016/j.jiph.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Crass RL, Rodvold KA, Mueller BA, Pai MP. 2019. Renal dosing of antibiotics: are we jumping the gun? Clin Infect Dis 68:1596–1602. doi: 10.1093/cid/ciy790. [DOI] [PubMed] [Google Scholar]
- 28.CLSI. Clinical and Laboratory Standards Institute. 2018. CaLS. Performance standards for antimicrobial susceptibility testing, 28th ed Clinical and Laboratory Standards Institute, Wayne, PA. 2018. [Google Scholar]
- 29.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 33.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, the Aw . 2004. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J, DILIN Study Group . 2009. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]