ABSTRACT
Outer membrane protein A (OmpA) is the most abundant porin in bacterial outer membranes. KJΔOmpA299–356, an ompA C-terminal in-frame deletion mutant of Stenotrophomonas maltophilia KJ, exhibits pleiotropic defects, including decreased tolerance to menadione (MD)-mediated oxidative stress. Here, we elucidated the underlying mechanism of the decreased MD tolerance mediated by ΔompA299–356. The transcriptomes of wild-type S. maltophilia and the KJΔOmpA299–356 mutant strain were compared, focusing on 27 genes known to be associated with oxidative stress alleviation; however, no significant differences were identified. OmpO was the most downregulated gene in KJΔOmpA299–356. KJΔOmpA299–356 complementation with the chromosomally integrated ompO gene restored MD tolerance to the wild-type level, indicating the role of OmpO in MD tolerance. To further clarify the possible regulatory circuit involved in ompA defects and ompO downregulation, σ factor expression levels were examined based on the transcriptome results. The expression levels of three σ factors were significantly different (downregulated levels of rpoN and upregulated levels of rpoP and rpoE) in KJΔOmpA299–356. Next, the involvement of the three σ factors in the ΔompA299–356-mediated decrease in MD tolerance was evaluated using mutant strains and complementation assays. rpoN downregulation and rpoE upregulation contributed to the ΔompA299–356-mediated decrease in MD tolerance. OmpA C-terminal domain loss induced an envelope stress response. Activated σE decreased rpoN and ompO expression levels, in turn decreasing swimming motility and oxidative stress tolerance. Finally, we revealed both the ΔompA299–356-rpoE-ompO regulatory circuit and rpoE-rpoN cross regulation.
IMPORTANCE The cell envelope is a morphological hallmark of Gram-negative bacteria. It consists of an inner membrane, a peptidoglycan layer, and an outer membrane. OmpA, an outer membrane protein, is characterized by an N-terminal β-barrel domain that is embedded in the outer membrane and a C-terminal globular domain that is suspended in the periplasmic space and connected to the peptidoglycan layer. OmpA is crucial for the maintenance of envelope integrity. Stress resulting from the destruction of envelope integrity is sensed by extracytoplasmic function (ECF) σ factors, which induce responses to various stressors. In this study, we revealed that loss of the OmpA-peptidoglycan (PG) interaction causes peptidoglycan and envelope stress while simultaneously upregulating σP and σE expression levels. The outcomes of σP and σE activation are different and are linked to β-lactam and oxidative stress tolerance, respectively. These findings establish that outer membrane proteins (OMPs) play a critical role in envelope integrity and stress tolerance.
KEYWORDS: OmpA, RpoN, sigma factor, outer membrane proteins
INTRODUCTION
Reactive oxygen species (ROS) production, a natural consequence of aerobic metabolism, is inevitable in aerobic bacteria. Aerobic-metabolism-generated ROS mainly include hydrogen peroxide (H2O2), the superoxide radical (O2–•), and the hydroxyl radical (HO•). Pathogens also encounter oxidative stress challenges produced by the host immune system during infection (1). As a result, bacteria use various defense systems to manage oxidative stress (2). Oxidative stress arises due to an imbalance between ROS generation and elimination, causing significant damage to the bacterial cell envelope, lipids, and proteins. Oxidative stress defense systems are diverse and include enzymatic systems (superoxide dismutase [SOD], catalase [Kat], hydroperoxide reductase [Ahp], and glutathione peroxidase [Gpx]) (3), nonenzymatic antioxidants (NADPH, NADH, β-carotene, ascorbic acid, α-tocopherol, and glutathione), and efflux pumps (the EmrBA1-SilC pump of Francisella tularensis, the SCO4121 pump of Streptomyces coelicolor, and the MacAB pump of Salmonella enterica serovar Typhimurium) (4–6). Bacteria can coordinate complex regulatory systems, such as transcriptional regulators, two-component regulatory systems, small RNAs, and σ factors, to generate an effective oxidative stress response (7–15).
Gram-negative bacterial cells are encompassed by a cell envelope that protects them from external stimuli (16). Furthermore, the envelope serves as a gateway for the import and export of various compounds. The envelope stress response (ESR) represents a complex regulatory network that monitors envelope integrity and mounts an appropriate response to mitigate stress (17). The best-characterized ESR consists of the σE-RseA-RseB pathway in Escherichia coli and most Gammaproteobacteria (18). The extracytoplasmic σ factor, σE, is a master regulator of the ESR (19). In normal E. coli cells, σE is mostly inactive owing to RseA sequestration. RseA is an inner membrane anti-σ factor with a cytoplasmic domain that binds to σE to maintain it in an inactive state (20). Under envelope stress, RseA is degraded by two inner membrane proteases, DegS and RseP, and then by the cytoplasmic protease, ClpXP, subsequently releasing free σE (21, 22). RseB functions as a negative regulator of the σE pathway by binding RseA to increase the affinity of the RseA-RseB complex for σE, in turn inhibiting the σE pathway (23). Known σE pathway inducers include misfolded outer membrane protein (OMP), periplasmic lipopolysaccharide accumulation, oxidative stress, heat shock, carbon starvation, biofilm formation, acid stress, UV-A radiation, P22 phage attack, and hypo-osmotic shock (24, 25).
RNA polymerase (RNAP), a DNA-dependent enzyme complex responsible for bacterial transcription, is composed of six subunits, namely, α, α, β, β′, ω, and σ (26). The σ subunit recognizes cognate promoter sequences and ensures adequate gene expression levels. Generally, bacteria harbor several different σ factors that can recognize their own cognate promoter sequences and ensure that all functional genes in the genome are expressed as required (27). σ factors are classified into two structurally and evolutionarily distinct families: σ70 and σ54 families. σ70 family members are further divided into four groups. Group 1 comprises primary or housekeeping σ factors; groups 2 and 3 comprise σ factors that regulate the expression of genes involved in general stress, flagellar structure and function, and chemotaxis; and group 4 (extracytoplasmic function [ECF]) comprises the largest group of σ factors that are involved in a major signaling network that enables bacteria to adapt to various environmental stimuli (28, 29). Bacteria typically harbor several ECF σ factors that induce adaptive responses to environmental changes. ECF σ factor expression and activity can be modulated at many levels but are predominantly controlled by association with the anti-σ factor. Upon signal perception, ECF σ factors are released from the anti-σ factor and assemble with the core RNA polymerase to induce gene expression. Genes encoding the ECF σ factors and their cognate anti-σ factor are located in the same operon (30).
Stenotrophomonas maltophilia, a nonfermenting Gram-negative rod bacterium, is prevalent in nature, in places such as soil, plant roots, animals, and aqueous environments. S. maltophilia is an opportunistic pathogen and a multidrug-resistant organism of concern in hospitals (31). Given its diverse habitats, S. maltophilia uses several stress alleviation systems to ensure survival in different environments. We previously reported several oxidative stress alleviation systems in S. maltophilia, including enzymatic (SOD, Kat, Ahp, Gpx), efflux pump (MasABCsm, SmeYZ, and SmeVWX), and formaldehyde detoxification (FadACB) systems (32–37). Furthermore, similar to the well-studied σE-RseA system of E. coli (18), S. maltophilia harbors an rpoE-rseA-mucD operon (Smlt3555-3553) that is responsible for ESR (38).
Recently, we reported that ompA is highly expressed in logarithmic-phase S. maltophilia KJ cells (38). We characterized an in-frame deletion ompA mutant of S. maltophilia KJ, originally termed KJΔOmpA (39) and later renamed KJΔOmpA299–356 (40). We also reported that the truncated OmpA protein can be stably embedded in the outer membrane but loses contact with peptidoglycan (PG) (40). KJΔOmpA299–356 exhibits decreased conjugation ability and swimming motility (39), as well as increased susceptibility to β-lactams (40). Transcriptome analysis revealed that the expression levels of the three σ factors, rpoN, rpoP, and rpoE, are significantly altered in KJΔOmpA299–356 (39, 40). Notably, rpoN downregulation is the key factor contributing to the swimming defect in KJΔOmpA299–356, and upregulated σP is involved in the ΔompA299–356-mediated increase in β-lactam susceptibility via the σP-NagA-L1/L2 regulatory circuit (39, 40). Here, we aimed to further investigate the effect of ΔompA299–356 on oxidative stress tolerance and elucidate the underlying mechanism.
RESULTS
KJΔOmpA299-356 is more susceptible than KJ to menadione.
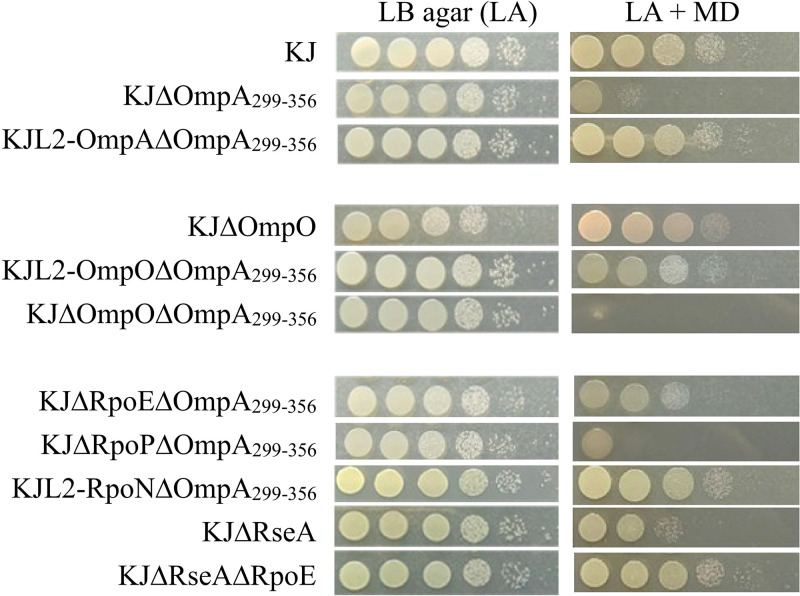
As KJΔOmpA299–356 loses its conjugation ability (39), a plasmid-mediated complementation assay using conjugation is not feasible. Therefore, we previously constructed an alternative ompA complementary strain, KJL2-OmpAΔOmpA299–356, in which the complemented ompA gene was inserted downstream of the L2 gene and was driven by the L2 promoter in KJΔOmpA299–356 (40). The impact of ΔompA299–356 on oxidative stress tolerance was assessed by determining the cell viabilities of KJ, KJΔOmpA299–356, and KJL2-OmpAΔOmpA299–356 in menadione (MD)-containing media. Compared with wild-type KJ, the MD tolerance of KJΔOmpA299–356 was significantly compromised. However, MD tolerance nearly reverted to the wild-type level in the complementation strain, KJL2-OmpAΔOmpA299–356 (Fig. 1).
FIG 1.
Menadione tolerance of wild-type KJ and its derived mutants. Logarithmic-phase bacterial cells (2 × 105 CFU/μL) were 10-fold serially diluted. Then, 5-μL aliquots of the cells were spotted onto Luria-Bertani (LB) agar plates with and without 40 μg/mL menadione (MD). Bacterial growth was observed after 24 h of incubation at 37°C. All experiments were performed at least thrice, and one was selected as a representative experiment.
We previously characterized several oxidative stress alleviation systems in S. maltophilia, including enzymatic and nonenzymatic systems (32–37). To understand the mechanism involved in the ΔompA299–356-mediated decrease in MD tolerance, we analyzed the transcriptome results of KJ and KJΔOmpA299–356 (39), focusing on genes involved in oxidative stress alleviation (see Table S1 in the supplemental material). A change in KJ and KJΔOmpA299–356 gene expression greater than 3-fold was considered statistically significant. Of the 27 genes analyzed, none exhibited significant alterations in transcript levels (Table S2), suggesting that an unidentified determinant is responsible for the ΔompA299–356-mediated decrease in MD tolerance.
OmpO (Smlt0387) expression is downregulated in KJΔOmpA299–356.
To further identify the putative candidates responsible for the ΔompA299–356-mediated decrease in MD tolerance, we rechecked the transcriptome results (39), focusing on the top five upregulated and downregulated genes. Smlt0387 was highly expressed in wild-type KJ and was the most downregulated gene (approximately a 203-fold decrease in expression levels) in KJΔOmpA299–356 (Table 1). Smlt0387 is annotated as a hypothetical protein in several sequenced S. maltophilia genomes. Based on the findings of this study, we designated Smlt0387 as OmpO. OmpO encodes a 190-amino acid (aa) protein. Subcellular location prediction (https://www.psort.org/psortb/) and signal peptide prediction (https://services.healthtech.dtu.dk/service.php?SignalP) indicated that OmpO was an OMP with a 19-aa signal peptide.
TABLE 1.
Selected transcriptome analysis of wild-type KJ and ompA mutant, KJΔOmpA299–356
| Locus | Protein | TPMa |
Fold changeb | |
|---|---|---|---|---|
| KJ | KJΔOmpA299–356 | |||
| Upregulated genes | ||||
| Smlt0603 | Carboxypeptidase A | 2.86 | 47.24 | +16.55 |
| Smlt0602 | TonB-dependent receptor | 11.79 | 195.12 | +16.54 |
| Smlt3952 | Reductase | 0.98 | 11.00 | +11.20 |
| Smlt3740 | TonB-dependent receptor | 40.37 | 430.30 | +10.66 |
| Smlt0564 | Hypothetical protein | 35.37 | 313.24 | +8.86 |
| Smlt3555 | σE | 434.09 | 2,424.29 | +5.58 |
| Smlt3514 | σP | 49.65 | 156.39 | +3.15 |
| Downregulated genes | ||||
| Smlt0387 | OmpO | 5690.31 c | 27.95 | −203.56 |
| Smlt2730 | Secretory pathway protein | 3.33 | 0.19 | −17.91 |
| Smlt1149 | Hypothetical protein | 31.53 | 2.06 | −15.34 |
| Smlt2317 | Proximal rod protein | 26.61 | 2.19 | −12.16 |
| Smlt2283 | Flagellar basal body protein | 12.18 | 1.17 | −10.38 |
| Smlt2297 | σN | 65.24 | 12.27 | −5.32 |
TPM, transcripts per kilobase million.
Negative fold changes represent genes that were significantly downregulated in KJΔOmpA299–356, whereas positive fold changes represent upregulation in KJΔOmpA299–356.
The bolded values indicate the most downregulated gene in KJΔOmpA299–356.
To verify whether OmpO is indeed an OMP, the OMP profiles of wild-type KJ and KJΔOmpO, an ompO isogenic mutant, were analyzed via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). KJΔOmpO lacked a protein band that was present in KJ (band A in Fig. 2). This band was excised from the gel and characterized using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LC-MS/MS results correlated band A with Smlt0387 (OmpO). The expected mature OmpO protein had an expected molecular weight of 19.0 kDa, which matches the location of band A in the gel (Fig. 2). Furthermore, the fragmentation patterns of band A showed the absence of the predicted signal peptide (1 to 19 aa residues) (Table S3), confirming that OmpO is an OMP with a 19-aa signal peptide. Interestingly, another protein with a molecular weight smaller than OmpO was upregulated in KJΔOmpO (band B in Fig. 2). This band was characterized by LC-MS/MS, and the results correlated band B with Smlt0184 (Table S4).
FIG 2.

Outer membrane protein profiling of KJ and KJΔOmpO. Outer membrane proteins (OMPs) were prepared from logarithmic-phase KJ and KJΔOmpO and separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 5% stacking and 15% separating gels. Lane M, molecular weight standards; lane 1, KJ; lane 2, KJΔOmpO. A and B indicate the proteins that were excised for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
To further clarify the biological significance of OmpO, phylogenetic analysis of OmpO and other OMPs, including TolC family and β-barrel proteins (OmpA, OmpX, phospholipase A, general porins, substrate-specific porins, and TonB-dependent transporters), was conducted. OmpO was phylogenetically closely related to OmpX of E. coli (Fig. 3).
FIG 3.
Phylogenetic analysis of OmpO of Stenotrophomonas maltophilia KJ and comparison with other OMPs. A phylogenic tree was constructed using the neighbor-joining method. Numbers at the branch nodes indicate the bootstrap values as a percentage of 1,000 replications. Accession numbers of the proteins are provided in parenthesis.
Downregulated OmpO expression in KJΔOmpA299–356 is involved in the ΔompA299–356-mediated decrease in MD tolerance.
To clarify the role of downregulated ompO expression in the ΔompA299–356-mediated decrease in MD tolerance, the ompO allele was chromosomally integrated downstream of the L2 gene of KJ cells to form KJL2-OmpO. The ΔompA299–356 allele was then introduced into the chromosome of KJL2-OmpO, generating KJL2-OmpOΔOmpA299–356. L2 and the integrated ompO gene formed an operon-mimic structure; therefore, ompO expression was driven by the L2 gene promoter. L2 expression is upregulated in KJΔOmpA299–356 (39); therefore, KJL2-OmpOΔOmpA299–356 can be an ompO complementation construct of KJΔOmpA299–356. We further constructed an ompO deletion mutant of KJΔOmpA299–356, KJΔOmpOΔOmpA299–356, and determined its MD tolerance capacity. Compared with the parental strain, KJΔOmpA299–356, KJL2-OmpOΔOmpA299–356 showed an MD tolerance level reverted to near the wild-type level, whereas KJΔOmpOΔOmpA299–356 exhibited low MD tolerance (Fig. 1), indicating that the extent of MD tolerance is proportional to the expression level of ompO. Therefore, OmpO downregulation in KJΔOmpA299–356 is involved in the ΔompA299–356-mediated decrease in MD tolerance. As OmpO is related to oxidative stress tolerance, we designated Smlt0387 as OmpO in this study.
The involvement of OmpO levels in MD tolerance was revealed in the ΔompA299–356 genetic background, and we wondered whether a similar effect could be observed in the wild-type KJ genetic background. Thus, we prepared an ompA deletion construct of KJ cells for an MD tolerance assay. KJΔOmpO displayed MD tolerance comparable to that of its parent strain, KJ (Fig. 1).
Upregulated RpoE (Smlt3555) and downregulated rpoN (Smlt2297) levels in KJΔOmpA299–356 are involved in the ΔompA299–356-mediated decrease in MD tolerance.
We recently demonstrated that the expression levels of the three σ factors are significantly altered (upregulated σP and σE levels and downregulated σN levels) in KJΔOmpA299–356 (40). To assess whether the alteration of these σ factor expression levels is associated with the ΔompA299–356-mediated decrease in MD tolerance, KJΔRpoEΔOmpA299–356, KJΔRpoPΔOmpA299–356, and KJL2-RpoNΔOmpA299–356, a strain of KJΔOmpA299–356 complemented with rpoN expression, were subjected to an MD tolerance assay. The MD tolerance assay revealed that KJΔRpoEΔOmpA299–356 and KJL2-RpoNΔOmpA299–356 had an MD tolerance level that was nearly reverted to the wild-type level, whereas KJΔRpoPΔOmpA299–356 exhibited MD tolerance comparable to that of KJΔOmpA299–356 (Fig. 1). These results indicated that rpoE upregulation and rpoN downregulation in KJΔOmpA299–356 are involved in the ΔompA299–356-mediated decrease in MD tolerance.
The linkage between rpoE upregulation and the ΔompA299–356-mediated decrease in MD tolerance highly suggested the occurrence of ESR in KJΔOmpA299–356. The involvement of the rpoE-rseA system in ESR has been characterized in several bacteria (18), including the rpoE-rseA-mucD operon in S. maltophilia (38). RseA functions as an anti-σE factor; thus, the loss of RseA function induces σE activation, mimicking ESR (18). To assess the relationship between ESR and MD tolerance, the MD tolerance assay was performed with KJΔRseA and KJΔRseAΔRpoE. Compared to wild-type KJ, KJΔRseA exhibited a decreased MD tolerance and KJΔRseAΔRpoE showed an MD tolerance level that had reverted to the wild-type level (Fig. 1), indicating that an ESR system reduces the resistance of bacteria to MD-induced oxidative stress.
σE activation downregulates ompO expression.
The next question was whether σE upregulation in KJΔOmpA299–356 imposes a negative effect on ompO expression. Thus, the ompO transcript levels in KJ, KJΔOmpA299–356, and KJΔRpoEΔOmpA299–356 were compared by quantitative reverse transcription PCR (qRT-PCR). The ompO transcript was downregulated in KJΔOmpA299–356 and reverted to near wild-type levels in KJΔRpoEΔOmpA299–356 (Fig. 4A). We further determined the impact of σE activation on ompO expression in the wild-type KJ. Plasmid pOmpOxylE, containing a PompO-xylE transcriptional fusion construct, was introduced into KJ, KJΔRseA, and KJΔRseAΔRpoE to generate KJ(pOmpOxylE), KJΔRseA(pOmpOxylE), and KJΔRseAΔRpoE(pOmpOxylE), respectively. KJ(pOmpOxylE) exhibited significantly higher catechol 2,3-dioxygenase (C23O) activity than the vector-only control strain, KJ(pRKXylE) (Fig. 4), indicating that the ompO gene is highly expressed in logarithmic-phase KJ cells, consistent with the transcriptome results (Table 1). KJΔRseA(pOmpOxylE) showed lower C23O activity than KJ(pOmpOxylE). However, C23O activity was comparable in KJΔRseAΔRpoE(pOmpOxylE) and KJ(pOmpOxylE) (Fig. 4B), indicating that σE activation has a negative effect on ompO expression.
FIG 4.

Roles of σE and σN in ompO expression. The data represent the means from three independent experiments. Error bars represent the standard deviations for triplicate samples. *, P < 0.01, calculated via Student’s t test. (A) Impacts of σE activation on ompO expression in KJΔOmpA299–356. Overnight-cultured bacterial cells were inoculated into fresh LB with an initial optical density at 450 nm (OD450) of 0.15. After 5 h of aerobic culture, ompO transcript levels were determined by qRT-PCR. All values were normalized to the transcript levels of KJ cells. (B) Roles of σE and σN in ompO expression in wild-type KJ. Overnight cultures of tested S. maltophilia strains were inoculated into fresh LB medium at an initial OD450 of 0.15. After 7 h of culture, catechol 2,3-dioxygenase (C23O) activities were determined.
A similar strategy was used to investigate the regulatory effect of σN on ompO expression. The rpoN expression levels of KJ cells under our test condition were first verified by RT-PCR (data not shown). C23O activity was then determined in KJΔRpoN(pOmpOxylE). The results demonstrated that rpoN barely affected the expression level of the ompO gene (Fig. 4B).
σE activation negatively regulates σN expression.
The involvement of σE and σN in the ΔompA299–356-mediated decrease in MD tolerance was previously established. Next, we explored whether there is an interplay between σE and σN. First, the rpoN transcripts in KJ, KJΔOmpA299–356, and KJΔRpoEΔOmpA299–356 were determined by qRT-PCR to determine whether activated σE downregulates rpoN expression in KJΔOmpA299–356. Compared to wild-type KJ, KJΔOmpA299–356 had decreased rpoN transcription levels (Fig. 5A). We also observed that the rpoN transcript level in KJΔRpoEΔOmpA299–356 had reverted to a level higher than the level in wild-type KJ (Fig. 5A). Next, we used a PrpoN-xylE transcriptional fusion construct (pRpoNxylE) to investigate the role of σE activation in rpoN expression in wild-type KJ. Compared to wild-type KJ, KJΔRpoE(pRpoNxylE) displayed increased C23O activity (Fig. 5B), in turn indicating that, under a σE-nonactivated condition, free-form σE exists and exerts a negative impact on rpoN expression. We also observed that C23O activity was lower in KJΔRseA(pRpoNxylE) than wild-type KJ. Furthermore, the C23O activity of KJΔRseAΔRpoE(pRpoNxylE) had reverted to a level even higher than that of wild-type KJ (Fig. 5B), indicating that σE activation attenuates rpoN expression. Collectively, the rpoN transcript level was inversely proportional to the free-form σE level.
FIG 5.
Interplay between σE and σN. The data represent the means from three independent experiments. Error bars represent the standard deviations for triplicate samples. *, P < 0.01, calculated via Student’s t test. (A) Impact of σE activation on rpoN expression in KJΔOmpA299–356. Overnight-cultured bacterial cells were inoculated into fresh LB with an initial OD450 of 0.15. After 5 h of aerobic culture, rpoN transcript levels were determined by qRT-PCR. All values were normalized to the transcript levels of KJ cells. (B) Impact of σE activation on rpoN expression. Overnight cultures of tested S. maltophilia strains were inoculated into fresh LB medium at an initial OD450 of 0.15. After 7 h of culture, their C23O activities were determined. (C) Impact of σE activation on swimming motility. Logarithmic-phase bacterial suspensions were inoculated into swimming agar (1% tryptone, 0.5% NaCl, and 0.15% agar). The swimming zones were recorded after 48 h of incubation at 37°C. (D) Role of σN in rpoE expression. Overnight cultures of tested S. maltophilia strains were inoculated into fresh LB medium at an initial OD450 of 0.15. After 7 h of culture, their C23O activities were determined.
To further investigate the correlation between the rpoN transcript level and swimming motility, the swimming motilities of KJ, KJΔRpoE, KJΔRseA, and KJΔRseAΔRpoE were evaluated. The swimming motility of KJΔRpoE was slightly, but not significantly, higher than that of wild-type KJ (Fig. 5C). However, KJΔRseA exhibited decreased swimming motility, and rpoE deletion from the chromosome of KJΔRseA reverted the swimming motility to the wild-type level (Fig. 5C). Based on these results, we concluded that σE activation-mediated rpoN downregulation results in compromised swimming motility.
Next, we investigated whether rpoN regulates rpoE expression. The plasmid pRpoExylE, a PrpoE-xylE transcriptional fusion construct, was transfected into KJ and KJΔRpoN to evaluate C23O activity. KJΔRpoN(pRpoExylE) and KJ(pRpoExylE) exhibited comparable C23O activities (Fig. 5D).
DISCUSSION
ECF σ factors are crucial in bacterial signaling networks, as they allow bacteria to recognize external signals. ECF σ factors remain in an inactive state under normal conditions, via either nonexpression or functional restriction by the anti-σ factor. In response to stress signals, ECF σ factors are activated by upregulating their expression or sequestering them from the anti-σ factor. Free ECF σ factors recruit the associated RNAP core enzyme, drive the RNAP holoenzyme to bind to specific promoters, and induce gene expression. In the best-known ECF-mediated regulatory circuits, the genes responsible for stress alleviation are generally not expressed under normal conditions, but are upregulated under stressed conditions via ECF σ factor-mediated transcription. In this study, we revealed a particular regulatory circuit mediated by σE. Transcriptome and promoter assays (Table 1; Fig. 4B) revealed that ompO is intrinsically expressed. Intrinsic expression of ompO should be driven by the housekeeping σ factor, σD. ΔompA299–356-mediated stress induced the upregulation of σE, which drove ompO expression. σE-mediated ompO expression levels may be lower than those mediated by σD. Therefore, ompO downregulation in KJΔOmpA299–356 may be due to the σ factor switch from σD to σE.
Based on their structures, OMPs can be mainly classified into two types, namely, the TolC family and β-barrel proteins. The TolC family comprises proteins with an α-helical trans-periplasmic tunnel embedded in the outer membrane via a contiguous β-barrel channel. TolC-like proteins, which are inner membrane-associated periplasmic proteins, usually assemble with integral inner membrane proteins to form a tripartite efflux pump, which is involved in chemical export (41). β-Barrel OMPs, which include OmpA, OmpX, phospholipase A, general porins, substrate-specific porins, and TonB-dependent transporters (42), generally function as channels for the influx or efflux of hydrophilic molecules. Of these β-barrel proteins, OmpA is particularly important, as it tightly attaches the outer membrane to the PG layer via its periplasmic domain (43) and, thus, plays a critical role in envelope stability. Unlike the OmpA protein, most β-barrel OMPs, such as OmpO investigated in this study, are devoid of the periplasmic domain; therefore, most β-barrel OMPs participate in molecule transportation.
A correlation between OMP deletion and a decrease in oxidative stress tolerance has been reported in some bacteria, but most such instances have involved TolC-like OMPs, such as those in Salmonella enterica, Acinetobacter baumannii, Cronobacter sakazakii, and Pseudomonas syringae (44–47). This is likely due to the deleted TolC-like OMPs being members of the tripartite efflux pumps, which extrude the toxic compounds generated during oxidative stress. Therefore, TolC-like OMP deletion compromises oxidative stress tolerance due to the accumulation of toxic compounds. In this study, we found that KJΔOmpA299–356 is more susceptible to oxidative stress than its parental strain, KJ. These results seem to indicate that OmpA is an outlet for oxidative-stress-mediating toxic compounds. However, the exact determinant leading to the decrease in oxidative stress tolerance in KJΔOmpA299–356 was the downregulation of the expression of another OMP, OmpO, supporting that the biological significance of OmpA is envelope stabilization, rather than molecular transport. OmpA defects cause ESR and σE activation. OmpO, an intrinsically highly expressed OMP, is a member of the σE regulon, and its expression is downregulated upon σE activation. We further established a novel regulatory circuit of ΔompA299–356-σE-ompO involved in the ΔompA299–356-mediated decrease in oxidative stress tolerance.
The association between ECF and oxidative stress adaptation has been widely reported in several microorganisms, such as Porphyromonas gingivalis (σE), Bacteroides fragilis (EcfO), Bradyrhizobium japonicum (CarQ), and Shewanella oneidensis (σE2) (13, 48–50). ECF acts as a positive regulator that protects bacteria from oxidative stress. Under conditions of oxidative stress, ECF is activated and induces ECF regulon expression to address the oxidative stress. Therefore, the loss of ECF function is generally linked to a decrease in oxidative stress tolerance. Here, we demonstrated that ΔompA299–356-mediated upregulation of rpoE expression exerted a negative effect on oxidative stress tolerance mediated by the downregulation of rpoN and ompO. OmpO is a novel OMP that is associated with oxidative stress tolerance. RpoN is a σ54 family σ factor involved in nitrogen assimilation, flagellar motility, type III and VI secretion systems, biofilm formation, and environmental adaptation (51–54). The role of rpoN in oxidative stress adaptation has rarely been reported, except in Labrenzia aggregata and Edwardsiella tarda (54, 55). Here, we found that activated σE had a negative impact on the expression of rpoN. Interplay among σ factors is important and widely studied. For example, σE regulates rpoN, rpoH, and rpoD expression levels in E. coli (24, 56, 57), σT controls rpoU and rpoR expression levels in Caulobacter crescentus (15), and rpoHII expression in Rhodobacter sphaeroides is σE-dependent (58).
The truncated OmpA protein expressed by KJΔOmpA299–356 can be stably embedded in the outer membrane but fails to contact with PG (40), which endows KJΔOmpA299–356 with pleiotropic defects, including compromised swimming motility (39), increased β-lactam susceptibility (40), and decreased oxidative stress tolerance. Based on our previous study (40) and the novel findings of this study, we conclude that KJΔOmpA299–356 experiences PG and envelope stress, leading to upregulation of the σ factors, rpoP and rpoE, respectively. σP upregulates the expression of nagA, which decreases L1/L2 expression levels and increases bacterial susceptibility to β-lactams (40). In contrast, σE-mediated ESR downregulates rpoN and ompO expression. OmpO downregulation results in a decrease in oxidative stress tolerance. Furthermore, downregulation of σN decreases swimming motility (39) and oxidative stress tolerance. Multidrug resistance is a challenging issue for the treatment of S. maltophilia infections, and OmpA represents the most abundant OMP in S. maltophilia. Blocking the interaction between OmpA and PG may present an alternative strategy for S. maltophilia infection control.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and primers used in this study are listed in Table S5.
Outer membrane protein preparation and SDS-PAGE.
The purification of outer membrane proteins was carried out as described previously (40). The OMPs were separated by discontinuous SDS-PAGE with a 5% stacking gel and a 15% separating gel. Bands were visualized by staining with 0.1% Coomassie brilliant blue R250 (Bio-Rad) and de-staining with 40% methanol/10% glacial acetic acid.
Construction of deletion mutant KJΔOmpO.
The deletion mutants were obtained using the double homologous recombination method as described previously (59). The upstream and downstream DNA fragments of ompO were gotten by PCR using the primer pairs OmpON-F/OmpON-R and OmpOC-F/OmpOC-R (Table S5). Next, the two PCR amplicons were subsequently cloned into pEX18Tc to yield plasmid pΔOmpO (Table S5). Plasmid pΔOmpO was transferred into S. maltophilia KJ by conjugation. The plasmid’s conjugation, the transconjugants’ selection, and the mutant’s confirmation were carried out as described previously (59).
Construction of KJL2-OmpOΔOmpA299–356 and KJL2-RpoNΔOmpA299–356.
Given OmpA is critical outer membrane protein for conjugation, KJΔOmpA299-356 failed to obtain a complementation plasmid by conjugation (39). To get the ompO and rpoN complementation strains of KJΔOmpA299-356, KJL2-OmpOΔOmpA299-356 and KJL2-RpoNΔOmpA299-356 were constructed. Plasmid pEXHH1 (Table S5), constructed in our previous study (40), is designed for cloning the exotic gene intended to be expressed under the L2 promoter drive. The intact ompO and rpoN genes were amplified by PCR using the primers pairs OmpO-F/OmpO-R and RpoN-F/RpoN-R, and the PCR amplicons were cloned into pEXHH1 to obtain pEXHH1-OmpO and pEXHH1-RpoN, respectively. Plasmids pEXHH1-OmpO and pEXHH1-RpoN were transported into wild-type KJ by conjugation. The ompO and rpoN genes in pEXHH1-OmpO and pEXHH1-RpoN were inserted into a chromosome downstream from the L2 gene via double homologous recombination to yield KJL2-OmpO and KJL2-RpoN, respectively. The chromosomal ompA gene was then deleted from KJL2-OmpO and KJL2-RpoN by double homologous recombination, and KJL2-OmpOΔOmpA299–356 and KJL2-RpoNΔOmpA299–356 were obtained.
Construction of the PompO-xylE transcriptional fusion plasmid, pOmpOxylE.
The DNA fragment containing the promoter region of the ompO gene was obtained by PCR using primer pair OmpON-F/OmpON-R (Table S5). The 365-bp PCR amplicon was cloned into pRKxylE, a xylE reporter plasmid, yielding pOmpOxylE.
Catechol 2,3-dioxygenase activity determination.
Catechol 2,3-dioxygenase (C23O) is encoded by the xylE gene. The C23O activity was measured using 100 mM catechol as the substrate, as described previously (60). The hydrolysis rate of catechol was calculated using 44,000 M−1cm−1 as the extinction coefficient. One unit of C23O activity (U) was defined as the enzyme amount that converts 1 nmol of substrate per minute. The specific activity was expressed as U/optical density at 450 nm (OD450).
Swimming motility.
The mid-logarithmic-phase bacterial cells were inoculated onto the 0.15% semisolid swimming agar (1% tryptone, 0.5% NaCl, and 0.15% agar). After incubation at 37°C for 48 h, the diameters of the swimming zones (mm) were recorded.
Statistical analysis.
Student’s t test was used for comparison of means between the groups. The Bonferroni correction method was applied to adjust the P values.
Data availability.
The RNA-seq data have been deposited in GenBank under BioProject accession number PRJNA876818.
ACKNOWLEDGMENTS
This work was supported by the National Science and Technology Council, Taiwan (grant number 111-2320-B-A49-025-MY3), the Taipei Veterans General Hospital (grant number V112C-227), and the Professor Tsuei-Chu Mong Merit Scholarship (grant number 310260016).
Footnotes
Supplemental material is available online only.
Contributor Information
Tsuey-Ching Yang, Email: tcyang@nycu.edu.tw.
Silvia T. Cardona, University of Manitoba
REFERENCES
- 1.Ezraty B, Gennaris A, Barras F, Collet JF. 2017. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15:385–396. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 2.Seixas AF, Quendera AP, Sousa JP, Silva AFQ, Arraiano CM, Andrade JM. 2021. Bacterial response to oxidative stress and RNA oxidation. Front Genet 12:821535. doi: 10.3389/fgene.2021.821535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra S, Imlay J. 2012. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch Biochem Biophys 525:145–160. doi: 10.1016/j.abb.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Z, Banik S, Rane H, Mora VT, Rabadi SM, Doyle CR, Thanassi DG, Bakshi CS, Malik M. 2014. EmrA1 membrane fusion protein of Francisella tularensis LVS is required for resistance to oxidative stress, intramacrophage survival and virulence in mice. Mol Microbiol 91:976–995. doi: 10.1111/mmi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nag A, Mehra S. 2021. A major facilitator superfamily (MFS) efflux pump, SCO4121, from Streptomyces coelicolor with roles in multidrug resistance and oxidative stress tolerance and its regulation by a MarR regulator. Appl Environ Microbiol 87:e02238-20. doi: 10.1128/AEM.02238-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogomolnaya LM, Tilvawala R, Elfenbein JR, Cirillo JD, Andrews-Polymenis HL. 2020. Linearized siderophore products secreted via MacAB efflux pump protect Salmonella enterica serovar Typhimurium from oxidative stress. mBio 11:e00528-20. doi: 10.1128/mBio.00528-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu H, Yuan J, Gao H. 2015. Microbial oxidative stress response: novel insights from environmental facultative anaerobic bacteria. Arch Biochem Biophys 584:28–35. doi: 10.1016/j.abb.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Dubbs JM, Mongkolsuk S. 2012. Peroxide-sensing transcriptional regulators in bacteria. J Bacteriol 194:5495–5503. doi: 10.1128/JB.00304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta V, Jain K, Garg R, Malik A, Gulati P, Bhatnagar R. 2018. Characterization of a two component system, Bas1213-1214, important for oxidative stress in Bacillus anthracis. J Cell Biochem 119:5761–5774. doi: 10.1002/jcb.26751. [DOI] [PubMed] [Google Scholar]
- 10.Pardo-Esté C, Hidalgo AA, Aguirre C, Briones AC, Cabezas CE, Castro-Severyn J, Fuentes JA, Opazo CM, Riedel CA, Otero C, Pacheco R, Valvano MA, Saavedra CP. 2018. The ArcAB two-component regulatory system promotes resistance to reactive oxygen species and systemic infection by Salmonella Typhimurium. PLoS One 13:e0203497. doi: 10.1371/journal.pone.0203497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bensig EO, Valadez-Cano C, Kuang Z, Freire IR, Reyes-Prieto A, MacLellan SR. 2022. The two-component regulatory system CenK-CenR regulates expression of a previously uncharacterized protein required for salinity and oxidative stress tolerance in Sinorhizobium meliloti. Front Microbiol 13:1020932. doi: 10.3389/fmicb.2022.1020932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fröhlich KS, Gottesman S. 2018. Small regulatory RNAs in the Enterobacterial response to envelope damage and oxidative stress. Microbiol Spectr 6:6.4.03. doi: 10.1128/microbiolspec.RWR-0022-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaweethawakorn A, Parks D, So JS, Chang WS. 2015. Role of the extracytoplasmic function sigma factor CarQ in oxidative response of Bradyrhizobium japonicum. J Microbiol 53:526–534. doi: 10.1007/s12275-015-5308-9. [DOI] [PubMed] [Google Scholar]
- 14.Tran HT, Bonilla CY. 2021. SigB-regulated antioxidant functions in gram-positive bacteria. World J Microbiol Biotechnol 37:38. doi: 10.1007/s11274-021-03004-7. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Martinez CE, Lourenço RF, Baldini RL, Laub MT, Gomes SL. 2007. The ECF sigma factor sigma(T) is involved in osmotic and oxidative stress responses in Caulobacter crescentus. Mol Microbiol 66:1240–1255. doi: 10.1111/j.1365-2958.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- 16.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabowicz M, Silhavy TJ. 2017. Envelope stress responses: an interconnected safety net. Trends Biochem Sci 42:232–242. doi: 10.1016/j.tibs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dartigalongue C, Missiakas D, Raina S. 2001. Characterization of the Escherichia coli sigma E regulon. J Biol Chem 276:20866–20875. doi: 10.1074/jbc.M100464200. [DOI] [PubMed] [Google Scholar]
- 19.Guo MS, Gross CA. 2014. Stress-induced remodeling of the bacterial proteome. Curr Biol 24:R424–R434. doi: 10.1016/j.cub.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Missiakas D, Mayer MP, Lemaire M, Georgopoulos C, Raina S. 1997. Modulation of the Escherichia coli σE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol 24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 21.Alba BM, Leeds JA, Onufryk C, Lu CZ, Gross CA. 2002. DegS and YaeL participate sequentially in the cleavage of RseA to activate the σE-dependent extracytoplasmic stress response. Genes Dev 16:2156–2168. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn JM, Neher SB, Kim Y-I, Sauer RT, Baker TA. 2003. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol Cell 11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 23.Chaba R, Alba BM, Guo MS, Sohn J, Ahuja N, Sauer RT, Gross CA. 2011. Signal integration by DegS and RseB governs the σE-mediated envelope stress response in Escherichia coli. Proc Natl Acad Sci USA 108:2106–2111. doi: 10.1073/pnas.1019277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowley G, Spector M, Kormanec J, Roberts M. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat Rev Microbiol 4:383–394. doi: 10.1038/nrmicro1394. [DOI] [PubMed] [Google Scholar]
- 25.Amar A, Pezzoni M, Pizarro RA, Costa CS. 2018. New envelope stress factors involved in σE activation and conditional lethality of rpoE mutations in Salmonella enterica. Microbiology (Reading) 164:1293–1307. doi: 10.1099/mic.0.000701. [DOI] [PubMed] [Google Scholar]
- 26.Darst SA. 2001. Bacterial RNA polymerase. Curr Opin Struct Biol 11:155–162. doi: 10.1016/s0959-440x(00)00185-8. [DOI] [PubMed] [Google Scholar]
- 27.Feklístov A, Sharon BD, Darst SA, Gross CA. 2014. Bacterial σ factors: a historical, structural, and genomic perspective. Annu Rev Microbiol 68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 28.Helmann JD. 2002. The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol 46:47–110. doi: 10.1016/S0065-2911(02)46002-X. [DOI] [PubMed] [Google Scholar]
- 29.Staroń A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. 2009. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol Microbiol 74:557–581. doi: 10.1111/j.1365-2958.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 30.Marcos-Torres FJ, Moraleda-Muñoz A, Contreras-Moreno FJ, Muñoz-Dorado J, Pérez J. 2022. Mechanisms of action of non-canonical ECF sigma factors. Int J Mol Sci 23:3601. doi: 10.3390/ijms23073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majumdar R, Karthikeyan H, Senthilnathan V, Sugumar S. 2022. Review on Stenotrophomonas maltophilia: An emerging multidrug-resistant opportunistic pathogen. Recent Pat Biotechnol 16:329–354. doi: 10.2174/1872208316666220512121205. [DOI] [PubMed] [Google Scholar]
- 32.Lin YT, Huang YW, Liou RS, Chang YC, Yang TC. 2014. MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J Antimicrob Chemother 69:3221–3226. doi: 10.1093/jac/dku317. [DOI] [PubMed] [Google Scholar]
- 33.Lin YT, Huang YW, Chen SJ, Chang CW, Yang TC. 2015. The SmeYZ efflux pump of Stenotrophomonas maltophilia contributes to drug resistance, virulence-related characteristics, and virulence in mice. Antimicrob Agents Chemother 59:4067–4073. doi: 10.1128/AAC.00372-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu CJ, Chiu TT, Lin YT, Huang YW, Li LH, Yang TC. 2018. Role of smeU1VWU2X operon in the alleviation of oxidative stresses and occurrence of sulfamethoxazole-trimethoprim-resistant mutants in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 62:e02114-17. doi: 10.1128/AAC.02114-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jair HW, Lu HF, Huang YW, Pan SY, Lin IL, Huang HH, Yang TC. 2019. Roles of the two-MnSOD system of Stenotrophomonas maltophilia in the alleviation of superoxide stress. Int J Mol Sci 20:1770. doi: 10.3390/ijms20071770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li LH, Shih YL, Huang JY, Wu CJ, Huang YW, Huang HH, Tsai YC, Yang TC. 2020. Protection from hydrogen peroxide stress relies mainly on AhpCF and KatA2 in Stenotrophomonas maltophilia. J Biomed Sci 27:37. doi: 10.1186/s12929-020-00631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li LH, Wu CM, Lin YT, Pan SY, Yang TC. 2020. Roles of FadRACB system in formaldehyde detoxification, oxidative stress alleviation and antibiotic susceptibility in Stenotrophomonas maltophilia. J Antimicrob Chemother 75:2101–2109. doi: 10.1093/jac/dkaa173. [DOI] [PubMed] [Google Scholar]
- 38.Huang YW, Liou RS, Lin YT, Huang HH, Yang TC. 2014. A linkage between SmeIJK efflux pump, cell envelope integrity, and σE-mediated envelope stress response in Stenotrophomonas maltophilia. PLoS One 9:e111784. doi: 10.1371/journal.pone.0111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao CH, Chang CL, Huang HH, Lin YT, Li LH, Yang TC. 2021. Interplay between OmpA and RpoN regulates flagellar synthesis in Stenotrophomonas maltophilia. Microorganisms 9:1216. doi: 10.3390/microorganisms9061216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li LH, Wu CM, Chang CL, Huang HH, Wu CJ, Yang TC. 2022. σP-NagA-L1/L2 regulatory circuit involved in ΔompA299–356-mediated increase in β-Lactam susceptibility in Stenotrophomonas maltophilia. Microbiol Spectr 10:e0279722. doi: 10.1128/spectrum.02797-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delmar JA, Su CC, Yu EW. 2014. Bacterial multidrug efflux transporters. Annu Rev Biophys 43:93–117. doi: 10.1146/annurev-biophys-051013-022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koebnik R, Locher KP, van Gelder V. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol 37:239–253. doi: 10.1046/j.1365-2958.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- 43.Park JS, Lee WC, Yeo KJ, Ryu KS, Kumarasiri M, Hesek D, Lee M, Mobashery S, Song JH, Kim SI, Lee JC, Cheong C, Jeon YH, Kim HY. 2012. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the Gram-negative bacterial outer membrane. FASEB J 26:219–228. doi: 10.1096/fj.11-188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JJ, Wu YC, Kuo CJ, Hsuan SL, Chen TH. 2016. TolC is important for bacterial survival and oxidative stress response in Salmonella enterica serovar Choleraesuis in an acidic environment. Vet Microbiol 193:42–48. doi: 10.1016/j.vetmic.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan VB, Vaidyanathan V, Rajamohan G. 2015. AbuO, a TolC-like outer membrane protein of Acinetobacter baumannii, is involved in antimicrobial and oxidative stress resistance. Antimicrob Agents Chemother 59:1236–1245. doi: 10.1128/AAC.03626-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Gao J, Ling N, Zeng H, Tong L, Zhang M, Zhang J, Wu Q, Ye Y. 2019. Short communication: Roles of outer membrane protein W on survival, cellular morphology, and biofilm formation of Cronobacter sakazakii in response to oxidative stress. J Dairy Sci 102:2017–2021. doi: 10.3168/jds.2018-14643. [DOI] [PubMed] [Google Scholar]
- 47.Cao B, Liu J, Qin G, Tian S. 2012. Oxidative stress acts on special membrane proteins to reduce the viability of Pseudomonas syringae pv tomato. J Proteome Res 11:4927–4938. doi: 10.1021/pr300446g. [DOI] [PubMed] [Google Scholar]
- 48.Dou Y, Rutanhira H, Chen X, Mishra A, Wang C, Fletcher HM. 2018. Role of extracytoplasmic function sigma factor PG1660 (RpoE) in the oxidative stress resistance regulatory network of Porphyromonas gingivalis. Mol Oral Microbiol 33:89–104. doi: 10.1111/omi.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndamukong IC, Gee J, Smith CJ. 2013. The extracytoplasmic function sigma factor EcfO protects Bacteroides fragilis against oxidative stress. J Bacteriol 195:145–155. doi: 10.1128/JB.01491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai J, Wei H, Tian C, Damron FH, Zhou J, Qiu D. 2015. An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis. BMC Microbiol 15:34. doi: 10.1186/s12866-015-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kustu S, Santero E, Keener J, Popham D, Weiss D. 1989. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev 53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao B, Mo Z-L, Xiao P, Pan H-J, Lan X, Li G-Y. 2013. Role of alternative sigma factor 54 (RpoN) from Vibrio anguillarum M3 in protease secretion, exopolysaccharide production, biofilm formation, and virulence. Appl Microbiol Biotechnol 97:2575–2585. doi: 10.1007/s00253-012-4372-x. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Shi H, Wang Z, Huang X, Zhang X. 2018. Pleiotropic control of antibiotic biosynthesis, flagellar operon expression, biofilm formation, and carbon source utilization by RpoN in Pseudomonas protegens H78. Appl Microbiol Biotechnol 102:9719–9730. doi: 10.1007/s00253-018-9282-0. [DOI] [PubMed] [Google Scholar]
- 54.Xu T, Yu M, Liu J, Lin H, Liang J, Zhang XH. 2019. Role of RpoN from Labrenzia aggregata LZB033 (Rhodobacteraceae) in formation of flagella and biofilms, motility, and environmental adaptation. Appl Environ Microbiol 85:e02844-18. doi: 10.1128/AEM.02844-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F, Yang CL, Wang LL, Zhong NQ, Wu XM, Han LB, Xia GX. 2012. Heterologous expression of a chloroplast outer envelope protein from Suaeda salsa confers oxidative stress tolerance and induces chloroplast aggregation in transgenic Arabidopsis plants. Plant Cell Environ 35:588–600. doi: 10.1111/j.1365-3040.2011.02438.x. [DOI] [PubMed] [Google Scholar]
- 56.Alba BM, Gross CA. 2004. Regulation of the Escherichia coli σE-dependent envelope stress response. Mol Microbiol 52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- 57.Rhodius VA, Suh W, Nonaka G, West J, Gross CA. 2005. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol 4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anthony J, Warczak KL, Donohue TJ. 2005. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc Natl Acad Sci USA 102:6502–6507. doi: 10.1073/pnas.0502225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang TC, Huang YW, Hu RM, Huang SC, Lin YT. 2009. AmpDI is involved in expression of the chromosomal L1 and L2 beta-lactamases of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 53:2902–2907. doi: 10.1128/AAC.01513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu RM, Huang KJ, Wu LT, Hsiao YJ, Yang TC. 2008. Induction of L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 52:1198–1200. doi: 10.1128/AAC.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S5. Download spectrum.01080-23-s0001.docx, DOCX file, 6.0 MB (6MB, docx)
Data Availability Statement
The RNA-seq data have been deposited in GenBank under BioProject accession number PRJNA876818.