ABSTRACT
With limited and often toxic treatment options, carbapenem-resistant Gram-negative infections are associated with significant mortality. Cefepime-zidebactam is a promising antibiotic option undergoing a phase 3 trial that has activity against diverse antibiotic-resistant mechanisms in Gram-negative pathogens due to its β-lactam enhancer mechanism, mediating multiple PBP binding. We report a case of disseminated infection caused by a New Delhi metallo-β-lactamase-producing, extensively drug-resistant Pseudomonas aeruginosa isolate in a patient with acute T-cell leukemia, successfully managed with cefepime-zidebactam as a salvage therapy.
KEYWORDS: salvage therapy, XDR Pseudomonas aeruginosa, polymyxins, metallo-β-lactamase, cefepime-zidebactam, WCK 5222, disseminated infections, drug resistance
CASE PRESENTATION
An 18-year-old male patient with a known case of acute T-cell leukemia presented to us with a 1-day history of fever and loose stools on day 32 post-induction chemotherapy (prednisolone, daunorubicin, vincristine, and pegylated l-asparaginase). On arrival at the emergency room, he was febrile with tachycardia and hypotension. He was started on broad-spectrum antibiotics (meropenem and teicoplanin), along with fluid resuscitation. His hemodynamics improved with aggressive fluid resuscitation in the emergency room. The source of sepsis was suspected via the right chest wall Hickman catheter that had been placed for the chemotherapy. Blood cultures were collected from the catheter, as well as peripheral blood, after which the line was removed.
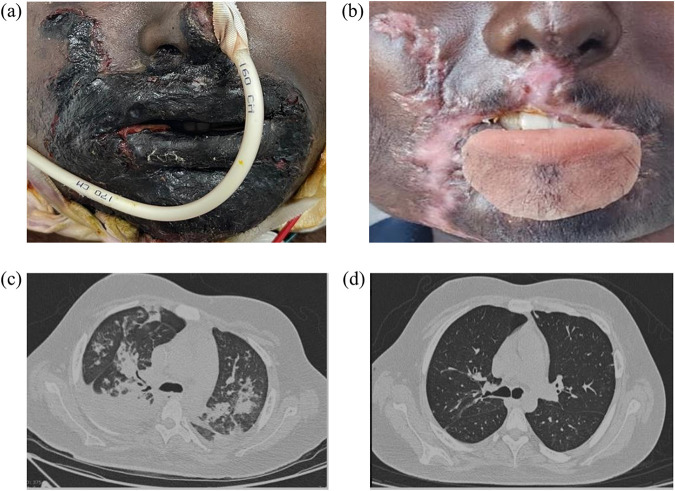
On day 2 of admission, the patient noted a black discoloration at the right angle of the mouth, with associated swelling of the right lower lip. The swelling rapidly progressed over the next 24 h, with black eschar and loss of sensation, indicative of necrotizing fasciitis (Fig. 1a). He underwent debridement and collection of tissue samples, which upon culturing yielded an extensively drug-resistant (XDR) Pseudomonas aeruginosa isolate that was resistant to all antibiotics, including carbapenems, ceftazidime-avibactam, and ceftolozane-tazobactam (colistin susceptibility was determined using the Vitek 2 broth microdilution method). The blood and catheter tip cultures also grew P. aeruginosa cells with the same susceptibility pattern. The P. aeruginosa isolate was positive for New Delhi metallo-β-lactamase (NDM) and negative for other carbapenemases, viz., VIM, OXA-48, KPC, and IMP, tested using the Xpert Carba-R assay (Cepheid, CA, USA).
FIG 1.
(a) Ecthyma gangrenosum involving the face, with significant edema and eschar formation (at presentation); (b) complete healing of the wound and deformed lower lip covered by a removable lower-lip silicone prosthesis (at the time of discharge); (c) bilateral upper-lobe consolidation (day 12 after admission); (d) complete resolution of consolidation after treatment (day 50 of admission).
Thus, the patient was diagnosed with a bloodstream infection (secondary to a line infection) caused by XDR P. aeruginosa, complicated with necrotizing ecthyma gangrenosum. Given the lack of antibiotic options, and while susceptibility to colistin was being confirmed, a combination of polymyxin B (15x105 IU intravenous [i.v.] loading dose, followed by 7.5 x105 IU every 12 h [q12h]) and high-dose meropenem (2 g q8h, i.v. infusion over 3 h) was administered. However, despite treatment with these last-resort antibiotics, the patient showed persistent fever spikes, with the development of pancytopenia (hemoglobin, 8 g/dL; total leukocyte count, 3,300 cells/mm3; absolute neutrophil count, 3,036 cells/mm3; platelets, 20,000 cells/mm3) and new onset of an oxygen requirement. The computed tomography (CT) chest scan was suggestive of bilateral lung consolidation (Fig. 1c), and the respiratory cultures also showed the presence of XDR P. aeruginosa cells, with a similar antibiotic susceptibility pattern.
On day 5 of the polymyxin B treatment, the patient had difficulty in lifting the bilateral lower limb, with no bowel or bladder incontinence or sensory involvement. The possibilities of septic emboli involving or compressing the cord, critical illness neuromyopathy, polymyxin B-induced neurotoxicity, or leukemic involvement of the cord and nerve roots were considered. A magnetic resonance image (MRI) of the spine was performed and showed normal features, with no evidence of cord compression or radicular involvement.
Due to the patient’s deteriorating clinical condition, alongside the concern about polymyxin-induced neurotoxicity, further treatment options, including investigational antibiotics, were explored. Cefepime-zidebactam is a novel β-lactam and β-lactam enhancer combination currently in a phase 3 trial. Published in vitro and in vivo studies have shown its activity against XDR Gram-negative pathogens, including metallo-β-lactamase (MBL)-producing P. aeruginosa. Antimicrobial susceptibility testing of cefepime-zidebactam (1:1 ratio) was performed both by reference broth microdilution (conducted in triplicate) and Kirby-Bauer disk (30/30 μg) diffusion, as per recommendations from the Clinical and Laboratory Standards Institute (CLSI) (1). Susceptibility testing showed that all isolates (from blood, tissue, and respiratory samples) were susceptible to cefepime-zidebactam, with an MIC of 8 mg/L (below the pharmacokinetic/pharmacodynamic [PK/PD] breakpoint of ≤32 mg/L) (Table 1).
TABLE 1.
Antibiotic susceptibility of the P. aeruginosa isolate recovered from our patienta
| Antimicrobial(s) | MIC (mg/L)b by sample type |
Zone of inhibition (mm) by sample type |
Interpretationc | ||||
|---|---|---|---|---|---|---|---|
| Blood | Tissue | Respiratory | Blood | Tissue | Respiratory | ||
| Cefepime | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Cefepime-zidebactam (1:1) | 8 | 8 | 8 | 25 | 26 | 25 | S d |
| Imipenem | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Meropenem | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Meropenem-EDTA (200 mM) | 32 | 32 | 32 | 0 | 0 | 0 | NAe |
| Aztreonam | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Aztreonam-avibactamf | 128 | 128 | 64 | 0 | 0 | 0 | NA |
| Ceftazidime-avibactam | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Piperacillin-tazobactam | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Ceftolozane-tazobactam | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Colistin | 0.25 | 0.5 | 0.5 | Disk diffusion not recommended | I | ||
| Tobramycin | >128 | >128 | >128 | 0 | 0 | 0 | R |
| Levofloxacin | 64 | 128 | 64 | 0 | 0 | 0 | R |
Bold text indicates the antimicrobials used for successful treatment of our patient.
MIC values of antibiotics against CLSI quality control strains were within the CLSI-defined ranges.
S, susceptible; R, resistant; I, intermediate. Interpretation per CLSI criteria for all antibiotics.
For cefepime-zidebactam, a PK/PD susceptible breakpoint of ≤32 mg/L was applied.
NA, not applicable (no susceptible breakpoints are available).
For aztreonam-avibactam, avibactam was employed at fixed 4 mg/L.
Written informed consent was obtained from the patient’s father. Ethical approval is not required at our institute to publish anonymous brief reports.
CHALLENGE QUESTION
Which antibiotic regimen would you choose to treat this infection caused by a carbapenem-resistant P. aeruginosa isolate harboring NDM?
Ceftazidime/avibactam plus aztreonam
Cefepime-zidebactam
Colistin plus meropenem
Cefiderocol
After discussing the rationale for choosing cefepime-zidebactam therapy, the patient was counseled and presented with an opportunity for a compassionate use protocol with this investigational agent. After the necessary approvals for compassionate use were obtained, the patient received cefepime-zidebactam (3 g: 2 g cefepime plus 1 g zidebactam, q8h, 1 h infusion) and in the next couple of days, showed signs of clinical improvement. On day 4 after stopping polymyxin B, the patient’s lower limb weakness started improving, further supporting the possibility of polymyxin B-induced neuromyopathy. The patient became afebrile after 10 days of cefepime-zidebactam therapy, blood culture was negative by day 6, and he was off oxygen by day 15. With repeated debridement and source control, along with cefepime-zidebactam treatment (total duration of 28 days), the facial swelling improved gradually (Fig. 1b). Our patient tolerated the 4 weeks of cefepime-zidebactam therapy well, with no drug-related adverse events.
TREATMENT AND OUTCOME
Carbapenem-resistant P. aeruginosa infections are associated with significant mortality and morbidity (2). The management of such infections continues to be challenging, as currently approved β-lactam/β-lactamase inhibitor (BL/BLI) combinations have a limited ability to overcome the various mechanisms of carbapenem resistance. Presently, efficacy- and safety-compromised polymyxins continue to be last-resort drugs. Although ceftolozane-tazobactam, imipenem-relebactam, and ceftazidime-avibactam are effective BL/BLI combinations against certain carbapenem-resistant P. aeruginosa isolates, they are ineffective against MBL-producing isolates. The only approved BL/BLI antibiotic currently accessible to Indian patients is ceftazidime-avibactam, which is of extremely limited utility, as NDM is the predominant mechanism of carbapenem resistance even in P. aeruginosa isolates (3, 4). The combination of ceftazidime-avibactam and aztreonam is frequently used due to its synergistic mechanisms (avibactam inhibits the extended-spectrum β-lactamases OXA-48, KPC, and AmpC, whereas aztreonam is stable to NDM), which are best demonstrated against MBL-producing K. pneumoniae isolates (5, 6). However, this combination has major gaps with respect to treating infections caused by carbapenem-resistant P. aeruginosa (including MBL-producing isolates) and Acinetobacter baumannii isolates, which involve multiple other resistance mechanisms impacting aztreonam, though it is stable to NDM.
Polymyxins have poor pharmacokinetic and pharmacodynamic properties and are associated with significantly high clinical failure rates. Moreover, they are also linked with significant neurotoxicity and nephrotoxicity (7). Nevertheless, due to a lack of treatment options, polymyxins are used as a last-resort therapy for infections caused by MBL-producing P. aeruginosa isolates. Though there is no randomized trial comparing colistin and polymyxin B, the consensus guidance on the use of polymyxins and other reports suggests the advantages of polymyxin B over colistin for infections other than urinary tract infections (8). The addition of carbapenems to polymyxins has not been shown to be beneficial in treating serious carbapenem-resistant Gram-negative infections in randomized controlled studies (9, 10).
Our patient had an extensively drug-resistant, NDM-producing P. aeruginosa isolate, presenting us with limited treatment options. The patient’s clinical condition worsened upon administration of polymyxin B, along with a high-dose extended infusion of meropenem. Further, the patient developed significant neurotoxicity in the form of bilateral lower limb weakness, which resolved after stopping polymyxin B. As the isolate from our patient was an NDM producer, ceftazidime-avibactam was not considered an option for our patient. Cefiderocol could have been considered an option, as it is known to be effective against Gram-negative bacteria producing MBLs; however, it is not available for clinical use in India. Regardless, in our view, cefiderocol would not have been an ideal antibiotic option, considering its elevated MICs against NDM-producing P. aeruginosa isolates, surpassing FDA breakpoints coupled with susceptibility testing challenges (11, 12). Thus, in this case, compassionate use of the novel β-lactam/β-lactam enhancer antibiotic cefepime-zidebactam was considered the best option, based on its previously reported consistent activity against XDR P. aeruginosa isolates, including MBL producers (13).
Cefepime-zidebactam is a unique combination of cefepime with a bicyclo-acyl hydrazide zidebactam, which acts as a β-lactam enhancer. Zidebactam, besides protecting cefepime from hydrolysis by certain serine-β-lactamases, also has standalone antibacterial activity, due to its potent PBP2 binding in all Gram-negative bacteria (14). Moreover, in combination with cefepime, zidebactam helps overcome other nonenzymatic resistance mechanisms, such as upregulated efflux and diminished or nonfunctional OprD (15). Several studies have established the potent activity of cefepime-zidebactam against a range of carbapenem-resistant pathogens, including VIM/NDM-expressing P. aeruginosa isolates coharboring efflux and impermeability (16, 17). Therefore, with such a pathogen/resistance mechanism coverage profile, cefepime-zidebactam seems to be a promising antibiotic option, especially in countries like India, with its high burden of carbapenem resistance mediated by NDM.
Septic patients with hematological malignancies tend to deteriorate rapidly and succumb to sepsis. Our patient was managed by a multidisciplinary team, including hematologists, critical care physicians, orofaciomaxillary and plastic surgeons, and infectious disease physicians. With aggressive source control measures and with cefepime-zidebactam treatment, our patient improved and was ultimately discharged home. It is noteworthy that cefepime-zidebactam showed a good safety profile in our patient, with no drug-related adverse events.
In summary, cefepime-zidebactam appears to be a safe option for the treatment of XDR P. aeruginosa infections, with broader coverage of resistance mechanisms. It may be considered a last-line treatment for XDR P. aeruginosa isolates suspected of harboring increasingly more common MBL enzymes, such as NDM. This was our first experience using cefepime-zidebactam for the management of an XDR P. aeruginosa infection in cancer patients.
ACKNOWLEDGMENTS
We declare that there are no conflicts of interest regarding the publication of this article.
P. K. Tirlangi: Conceptualization, Writing – Original draft, Writing – Review and editing; B. S. Wanve: Conceptualization, Writing – Original draft, Writing – Review and editing; R. R. Dubbudu: Conceptualization, Writing – Review and editing; B. S. Yadav: Writing – Review and editing; L. S. Kumar: Conceptualization, Writing – Review and editing; A. Gupta: Writing – Review and editing; R. A. Sree: Writing – Review and editing; H. P. R. Challa: Writing – Review and editing; P. N. Reddy: Conceptualization, Writing – Review and editing.
This journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. Expert clinicians then provide a commentary on the case.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, 31st ed. Document M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.Reyes J, Komarow L, Chen L, Ge L, Hanson BM, Cober E, Herc E, Alenazi T, Kaye KS, Garcia-Diaz J, Li L, Kanj SS, Liu Z, Oñate JM, Salata RA, Marimuthu K, Gao H, Zong Z, Valderrama-Beltrán SL, Yu Y, Tambyah P, Weston G, Salcedo S, Abbo LM, Xie Q, Ordoñez K, Wang M, Stryjewski ME, Munita JM, Paterson DL, Evans S, Hill C, Baum K, Bonomo RA, Kreiswirth BN, Villegas MV, Patel R, Arias CA, Chambers HF, Fowler VG, Doi Y, van Duin D, Satlin MJ. Antibacterial Resistance Leadership Group and Multi-Drug Resistant Organism Network Investigators. 2023. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): a prospective cohort study. Lancet Microbe 4:e159–e170. doi: 10.1016/S2666-5247(22)00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pragasam AK, Veeraraghavan B, Anandan S, Narasiman V, Sistla S, Kapil A, Mathur P, Ray P, Wattal C, Bhattacharya S, Deotale V, Subramani K, Peter JV, Hariharan TD, Ramya I, Iniyan S, Walia K, Ohri VC. 2018. Dominance of international high-risk clones in carbapenemase-producing Pseudomonas aeruginosa: multicentric molecular epidemiology report from India. Indian J Med Microbiol 36:344–351. doi: 10.4103/ijmm.IJMM_18_294. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y-L, Hsueh P-R. 2023. Poor in vitro activity of ceftazidime/avibactam, ceftolozane/tazobactam, and meropenem/vaborbactam against carbapenem-resistant Pseudomonas aeruginosa in India: results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) program, 2018–2021. J Infect doi: 10.1016/j.jinf.2023.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG, Jr, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayol A, Nordmann P, Poirel L, Dubois V. 2018. Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother 73:542–544. doi: 10.1093/jac/dkx393. [DOI] [PubMed] [Google Scholar]
- 7.Wagenlehner F, Lucenteforte E, Pea F, Soriano A, Tavoschi L, Steele VR, Henriksen AS, Longshaw C, Manissero D, Pecini R, Pogue JM. 2021. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin Microbiol Infect 27:671–686. doi: 10.1016/j.cmi.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. 2018. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 10.Kaye KS, Marchaim D, Thamlikitkul V, Carmeli Y, Chiu C-H, Daikos G, Dhar S, Durante-Mangoni E, Gikas A, Kotanidou A, Sims M. 2022. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evid 2. doi: 10.1056/EVIDoa2200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. 2020. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant Gram-negative bacteria. Antimicrob Agents Chemother 64:e01582-20. doi: 10.1128/AAC.01582-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simner PJ, Palavecino EL, Satlin MJ, Mathers AJ, Weinstein MP, Lewis JS, Jr, Humphries R. 2023. Potential of inaccurate cefiderocol susceptibility results: a CLSI AST subcommittee advisory. J Clin Microbiol 61:e0160022. doi: 10.1128/jcm.01600-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlowsky JA, Hackel MA, Bouchillon SK, Sahm DF. 2020. In vitro activity of WCK 5222 (cefepime-zidebactam) against worldwide collected Gram-negative bacilli not susceptible to carbapenems. Antimicrob Agents Chemother 64:e01432-20. doi: 10.1128/AAC.01432-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd JM, Abdelraouf K, Nicolau DP. 2020. Efficacy of human-simulated bronchopulmonary exposures of cefepime, zidebactam and the combination (WCK 5222) against MDR Pseudomonas aeruginosa in a neutropenic murine pneumonia model. J Antimicrob Chemother 75:149–155. doi: 10.1093/jac/dkz414. [DOI] [PubMed] [Google Scholar]
- 15.Moya B, Bhagwat S, Cabot G, Bou G, Patel M, Oliver A. 2020. Effective inhibition of PBPs by cefepime and zidebactam in the presence of VIM-1 drives potent bactericidal activity against MBL-expressing Pseudomonas aeruginosa. J Antimicrob Chemother 75:1474–1478. doi: 10.1093/jac/dkaa036. [DOI] [PubMed] [Google Scholar]
- 16.Mullane EM, Avery LM, Nicolau DP. 2020. Comparative evaluation of the in vitro activities of WCK 5222 (cefepime-zidebactam) and combination antibiotic therapies against carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:e01669-19. doi: 10.1128/AAC.01669-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]