ABSTRACT
Individuals with cystic fibrosis (CF) suffer from frequent and recurring microbial airway infections. The Gram-negative bacterium Pseudomonas aeruginosa is one of the most common organisms isolated from CF patient airways. P. aeruginosa establishes chronic infections that persist throughout a patient’s lifetime and is a major cause of morbidity and mortality. Throughout the course of infection, P. aeruginosa must evolve and adapt from an initial state of early, transient colonization to chronic colonization of the airways. Here, we examined isolates of P. aeruginosa from children under the age of 3 years old with CF to determine genetic adaptations the bacterium undergoes during this early stage of colonization and infection. These isolates were collected when early aggressive antimicrobial therapy was not the standard of care and therefore highlight strain evolution under limited antibiotic pressure. Examination of specific phenotypic adaptations, such as lipid A palmitoylation, antibiotic resistance, and loss of quorum sensing, did not reveal a clear genetic basis for such changes. Additionally, we demonstrate that the geography of patient origin, within the United States or among other countries, does not appear to significantly influence genetic adaptation. In summary, our results support the long-standing model that patients acquire individual isolates of P. aeruginosa that subsequently become hyperadapted to the patient-specific airway environment. This study provides a multipatient genomic analysis of isolates from young CF patients in the United States and contributes data regarding early colonization and adaptation to the growing body of research about P. aeruginosa evolution in the context of CF airway disease.
IMPORTANCE Chronic lung infection with Pseudomonas aeruginosa is of major concern for patients with cystic fibrosis (CF). During infection, P. aeruginosa undergoes genomic and functional adaptation to the hyperinflammatory CF airway, resulting in worsening lung function and pulmonary decline. All studies that describe these adaptations use P. aeruginosa obtained from older children or adults during late chronic lung infection; however, children with CF can be infected with P. aeruginosa as early as 3 months of age. Therefore, it is unclear when these genomic and functional adaptations occur over the course of CF lung infection, as access to P. aeruginosa isolates in children during early infection is limited. Here, we present a unique cohort of CF patients who were identified as being infected with P. aeruginosa at an early age prior to aggressive antibiotic therapy. Furthermore, we performed genomic and functional characterization of these isolates to address whether chronic CF P. aeruginosa phenotypes are present during early infection.
KEYWORDS: Pseudomonas aeruginosa, cystic fibrosis, airway adaptation, genomics, LPS evolution
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (cftr) gene. Mutations in cftr lead to protein malfunction or loss of function, resulting in defective transport of chloride ions across epithelial surfaces (1, 2). This malfunction alters the nature of the airway surface liquid, leading to impaired mucociliary function, which directly impairs the noninflammatory host defense against inhaled microorganisms. As such, CF patients are more susceptible to microbial colonization and infection and suffer from repeated airway infections throughout their lifetime (1, 3).
The Gram-negative bacterium Pseudomonas aeruginosa is one of the most common causes of chronic infection, with almost one-third of CF patients being colonized with P. aeruginosa by the time they reach the age of 20 (4–6). Despite early and aggressive antibiotic intervention, P. aeruginosa infections persist and can eventually lead to respiratory failure, lung transplantation, and death (7).
Within the CF airway microenvironments, P. aeruginosa genetically adapts to and evolves during chronic infection (4, 8, 9). Longitudinal studies have demonstrated that P. aeruginosa undergoes multiple genomic and phenotypic changes during chronic infection that are characteristic hallmarks of adaptation. These include palmitoylation of the outer membrane molecule lipid A, the loss of quorum sensing (QS), and an increased resistance to antibiotics (9). Although genomic sequencing studies have been conducted with P. aeruginosa CF isolates in the United States (10, 11), none have focused specifically on longitudinal isolates from patients within the first few years of life. Here, we sequenced 128 isolates of P. aeruginosa from the environment, CF patients, non-CF bronchiectasis patients, and patients with acute nonairway P. aeruginosa infection. Of these isolates, 81 are from nine CF patients in early childhood and were collected approximately every 6 months at routine well visits, in addition to any sick visits, over a 3-year period (Fig. 1) (12, 13). These isolates, from patients ranging in age from 3 months to 3 years, represent the earliest isolates from CF patients sequenced to date. Furthermore, these isolates would be difficult to acquire today due to early and aggressive antibiotic therapy, as well as the wide use of highly effective CFTR modulator therapies (e.g., the combination of elexacaftor, tezacaftor, and ivacaftor [ETI]), which improve CFTR function for those with specific cftr mutations and may impact the ability of P. aeruginosa to colonize the airway (12, 13).
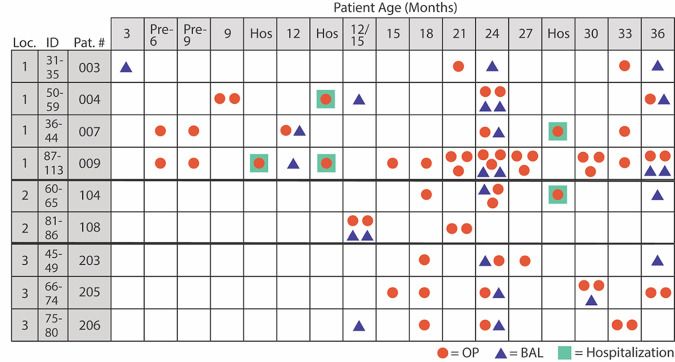
FIG 1.
CF isolates from young children selected for sequencing. Isolates were selected from a total of nine different patients over time. In some cases, multiple isolates from the same time point were collected. OP, collection via oropharyngeal swab; BAL, collection via bronchial alveolar lavage; Hospitalization, samples collected during a hospital visit caused by an exacerbation event; Loc., location (region); Pat., patient. Region numbers: 1, Seattle, WA; 2, Houston, TX; 3, Cleveland, OH.
In the current study, we examined CF airway adaptation phenotypes (e.g., lipid A modification, antibiotic susceptibility) alongside their associated genes. Importantly, P. aeruginosa lipid A structure in chronic CF airway infection is palmitoylated from constitutive activation of the lipid A biosynthetic enzyme PagP (14). As the genetic basis for this adaptive change has not yet been determined, we investigated single nucleotide polymorphism (SNP)-level changes in pagP and examined the genetic divergence in lipid A biosynthetic genes across the sequenced isolates. Furthermore, as these 81 CF isolates from young children were obtained from three distinct geographical regions, we investigated whether location of patient residence influences early genetic adaptation. Additionally, we included 474 previously sequenced CF P. aeruginosa genomes from a cohort in Denmark (8) to determine whether country of residence influences organismal adaptation. Overall, our studies provide insight into previously uninvestigated aspects of P. aeruginosa adaptation and provide a rich resource for researchers in the field.
RESULTS
P. aeruginosa CF isolates are distributed throughout the genomic landscape.
Direct comparison of all isolates revealed an average genome size of 6.545 million bases (Mb) (Table 1). CF and environmental isolate groups had similar genome sizes (6.675 and 6.609 Mb, respectively, compared to an all-isolate average). The GC content was consistent across all isolates (65.99% ± 0.45%). To examine total gene content and therefore, the degree of similarity between the genomes, a large-scale BLAST score ratio (LS-BSR) analysis was conducted (15). A total of 15,538 genes were predicted. The core genome (i.e., the set of genes present in every isolate) was quantified by using genes with an LS-BSR score of 1, representing 100% sequence identity (Table 1). Using the 128 sequenced isolates from this study (see Table S1 in the supplemental material) and two P. aeruginosa reference isolates (accession no. NC_002516.2 for P. aeruginosa PAO1 and NZ_CP017149.1 for P. aeruginosa isolate ATCC 15692), the core genome consisted of 3,645 genes (23.46%). This is a larger predicted core genome than those previously described for P. aeruginosa (between 1% and 15% of the total pangenome) (16, 17). Isolates from CF patients had an increased core genome size (31.84% for all CF infant and adult isolates). This increase in core genome size is unsurprising as each of the compared isolates is from the human airway, likely representing an adaptation to this infectious niche.
TABLE 1.
Analysis of all newly sequenced isolatesa
| Isolate type (n) | Total no. of genes | Avg genome size (Mb) | Avg GC content (%) | Core genes |
Unique genes |
||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| All (130)b | 15,538 | 6.545 | 65.99 | 3,645 | 23.46 | ||
| Environmental (15) | 11,097 | 6.675 | 65.99 | 5,109 | 46.04 | 1,113 | 10.03 |
| PAO1 (11)b | 6,091 | 6.218 | 66.44 | 5,881 | 96.55 | 7 | 0.11 |
| Non-CF bronchiectasis (5) | 7,991 | 6.393 | 66.38 | 5,272 | 65.97 | 224 | 2.80 |
| CF | |||||||
| All (89) | 12,379 | 6.609 | 65.68 | 3,941 | 31.84 | 1,592 | 12.86 |
| Child (81) | 10,811 | 6.485 | 66.29 | 4,162 | 38.50 | 502 | 4.64 |
| Adult (8) | 10,135 | 6.732 | 65.06 | 4,968 | 49.02 | 1,091 | 10.76 |
| Non-CF clinical acute (10) | 10,166 | 6.704 | 66.15 | 5,185 | 51.00 | 660 | 6.49 |
Core genes were identified as having an LS-BSR score of 1 (100% conservation). Unique genes were solely present in the indicated group of isolates.
Two reference isolates were included (P. aeruginosa PAO1 accession no. NC_002516.2 and NZ_CP017149.1). The analysis was not changed when the reference isolates were excluded.
The isolates were separated into seven distinct groups based on source: environmental (15 isolates), PAO1 isolates (9 isolates), non-CF bronchiectasis (5 isolates), child CF (81 isolates), adult CF (8 isolates), all CF (89 isolates), and clinical acute (10 isolates). Two PAO1 reference sequences (accession no. NC_002516.2 and NZ_CP017149.1) were also used in the analysis, as genetic differences among PAO1 strains have been documented (18). Genes unique to only one group of isolates were identified (Table 1; Table S1). Unique genes were defined as genes that had an LS-BSR score of ≥0.8 (approximately ≥80% sequence identity) among the isolates of one group and a score of <0.1 (approximately <10% sequence identity) in all other isolates. The group of all CF isolates had the greatest percentage of group-specific unique genes (12.86%) compared to all other isolate groups. Isolates from adults with chronic CF infection (CF adult group [10.76%]) had a greater percentage of unique genes than isolates from young children (CF child group [4.64%]) (Table 1). Together, this supports the hypothesis that CF isolates acquire greater genetic variation over time as P. aeruginosa adapts to the lung and establishes chronic infection.
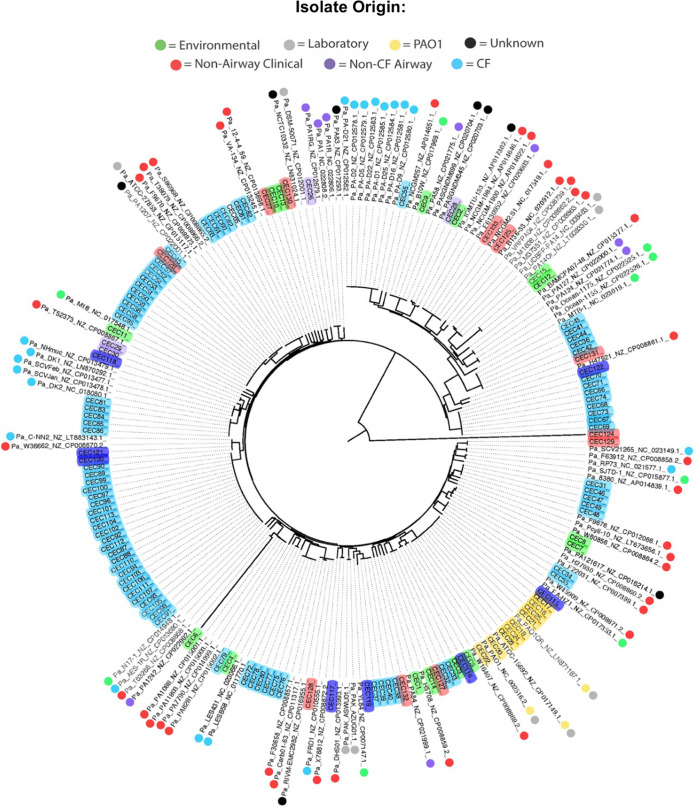
We next conducted phylogenomic analysis to assess how these early CF isolates fit within the P. aeruginosa genomic landscape. Within the inferred phylogeny, several trends were observed (Fig. 2). All PAO1 sublines (yellow) clustered together into a single clade, highlighting their relative genetic similarity compared to the rest of the P. aeruginosa genomic landscape. The environmental isolates (green) were dispersed throughout the phylogeny, indicating they are not genomically distinct from disease-associated isolates. This supports the conclusions of previous studies that have demonstrated similarity between environmental and clinical isolates of P. aeruginosa, highlighting that each individual with CF likely acquires their P. aeruginosa isolate from their local environment (19). In many cases, isolates from a single CF patient (light blue) cluster together on a distinct clade. This observation further supports the understanding that P. aeruginosa acquired by an individual becomes hyperadapted to that specific individual’s airway and becomes increasingly dissimilar to other P. aeruginosa isolates, even during early infection and colonization, as shown in our CF young patient cohort. The adult CF isolates (dark blue) were more distributed throughout the phylogeny, likely because they originated from single isolates from multiple unique patients.
FIG 2.
Phylogeny of newly sequenced P. aeruginosa isolates with available complete genomes of P. aeruginosa. All newly sequenced isolates are highlighted according to their sample origin (light blue, child CF isolate; dark blue, adult CF isolate; light purple, non-CF bronchiectasis; red, nonairway clinical disease isolates; yellow, PAO1; green, environmental). Downloaded whole-genome origins are indicated with circles along with their associated NCBI accession numbers.
Regional influence on genetic adaptation is limited.
Several previous studies have analyzed the geographical risk associated with P. aeruginosa acquisition, infection, and subsequent hospitalization in individuals with CF (20–22). However, none have analyzed if geography specifically impacts genetic adaptation in individual P. aeruginosa isolates. Here, we used our cohort of genomes P. aeruginosa isolates from young CF patients to investigate the impact of geography on P. aeruginosa genomic changes early in chronic infection. CF young child isolates (ID no. 31 to 113) were acquired from patients in three distinct geographical regions (collected in a previous study: region 1, Seattle, WA; region 2, Houston, TX; region 3, Columbus, OH) (12, 13). LS-BSR analysis was performed using CF young child and CF adult isolates (89 in total). In total, 13,410 genes were identified, and 9,663 genes were shared among the CF young child isolates, whereas 3,747 genes were specific to the CF adult isolates. These CF adult-specific genes were excluded because we did not have specific geographic locations for all adult samples. Region-specific genes were identified as any genes present (having an LS-BSR score of ≥0.8) in one region and not present (an LS-BSR score of <0.8) in any other region. Genes identified in each region were cross compared with the other regions to determine the amount of overlap and whether each region did or did not have unique genes that were not found in any other region (Table 2 and Fig. 3).
TABLE 2.
Summary of regional overlap region-specific gene frequencya
| Location (n) | No. of unique genes | No. (%) of genes shared with isolates from: |
No. (%) of genes found by no. or % of isolates shown |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Region 1 | Region 2 | Region 3 | 1 isolate | <10% | >50% | >80% | 100% | ||
| Region 1 (49 [4 passages]) | 1,550 | 122 (0.9) | 1,135 (8.5) | 355 (22.9) | 1,032 (66.5) | 177 (11.4) | 4 (0.025) | 0 (0) | |
| Region 2 (12 [2 passages]) | 309 | 122 (0.9) | 140 (1) | 8 (1.9) | 9 (2.2) | 297 (96.1) | 0 (0) | 0 (0) | |
| Region 3 (20 [3 passages]) | 494 | 1,135 (8.5) | 140 (1) | 301 (60.9) | 303 (61.3) | 0 (0) | 0 (0) | 0 (0) | |
Genes that were present in one region and absent in all other regions were identified from the LS-BSR data. Genes present in multiple regions (but not all regions) were also identified. Genes that were present in >50% of the number of isolates from a given region (or >45% for region 3) were removed from subsequent analysis. The majority of genes identified in regions 1 and 3 were in ≤10% of the isolates. Region numbers: 1, Seattle, WA; 2, Houston, TX; 3, Columbus, OH.
FIG 3.
Visualization of location/region-specific genes. Raw LS-BSR values for region-specific genes were visualized in a heat map. Specific gene annotations are listed in Table S3 in the supplemental material. Many of the region-specific genes that were identified appear to also be patient associated.
To further define how geographic region may influence adaptation, these genes were examined to determine the extent of their regional specificity. Table 2 summarizes the number of gene calls conserved among isolates in each region. Interestingly, not a single gene with 80% sequence identity was found in 100% of the isolates in any given region. Regions 2 and 3 did not have any genes that were present in 80% or more of the isolates from their respective regions either. Region 1 did have four genes that were conserved in 80% or more of the isolates, possibly due to the increased sample size for this location (Table 2).
The workflow to identify geographic-specific genes is represented in Fig. S1 in the supplemental material. This process resulted in 51 genes (25 specific to region 1, 21 specific to region 2, and 5 specific to region 3). Gene annotations were used to classify their predicted function and determine whether specific gene classes were implicated in regional adaptation. The gene calls, their functional annotations, and their functional classes are listed in Table S2. A heat map was used to visualize the LS-BSR values of region-specific genes (Fig. 3). Hypothetical genes were most frequently implicated in region-specific adaptation (9 in region 1 and 11 in region 2).
Finally, the majority of genomic studies on P. aeruginosa from CF patients have focused on isolates within one specific country. The largest of these studies sequenced 474 longitudinally collected isolates of P. aeruginosa from CF patients in Denmark (8). To determine whether country of residence significantly impacts P. aeruginosa adaptation, we conducted phylogenomic analysis of our sequenced CF isolates (all from the United States) and the sequenced isolates from Denmark to determine the degree of similarity between the isolates. We observed that isolates from the United States were generally distributed throughout the inferred phylogeny (Fig. S2 [U.S. isolates indicated with red dots]), suggesting that isolates originating from Denmark or the United States are not genetically dissimilar enough to be separated during phylogenetic analysis. Isolates originating from the same patient mostly clustered together (indicated in Fig. S2 by patient number), supporting our conclusion that the individual patient has a greater impact on genetic alteration than geography. Together, our data suggest geographic region and country of residence do not significantly induce specific genome-level alterations during P. aeruginosa adaptation.
Pathoadaptive genes are variably represented throughout CF isolates.
Previous whole-genome sequencing studies of P. aeruginosa isolates from CF patients have identified many gene alterations thought to be selected for during chronic infection of and adaptation to the CF airway (23–25). In these studies, computational methods based on gene sequence dissimilarity were used to determine “pathoadaptive” genes in CF isolates compared to non-CF isolates. Notably, the gene lists in these independent studies were nonoverlapping, highlighting the individual and independent nature of adaptation (23–25). Using our unique cohort of young CF isolates, we queried for these previously identified pathoadaptive genes and used LS-BSR analysis to determine their presence or absence in each genome (Fig. S3). We selected specific genes to investigate based on phenotype data associated with the young child CF isolates obtained during the original study (12, 13) and their documented involvement in P. aeruginosa adaptation, including antibiotic resistance, quorum sensing, and lipid A modification. Our data indicate that many CF-related genes lack large-scale sequence divergence among the CF isolates from young patients. Adult CF isolates had slightly more genetic divergence across the queried genes (Fig. S3).
Several genes had multiple gene alleles, indicating that isolates had alternate forms of the same gene that arose via mutation but reside at the same locus in the genome. Most notably, dnaX had six alleles (Fig. S3 [dnaX alleles are marked with dots]). The dnaX gene encodes the gamma and tau subunits of DNA polymerase III (26). Allelic variants of dnaX may promote the mutator phenotype commonly observed in CF isolates (8, 10, 27), although we have not yet analyzed mutation rates with these isolates. Allelic variations were also observed in genes involved in motility (pilB, marked with “<”; pilQ, marked with “>”), long-chain fatty acid biosynthesis (accC, marked with “*”; accE, marked with “†”; accF, marked with “~”), and antibiotic resistance (oprD, marked with “$”; mexR, marked with “^”; phzB1, marked with “!”; vrf, marked with “–”) (Fig. S3) (28, 29).
CF isolates display antibiotic resistance without changes to associated genes.
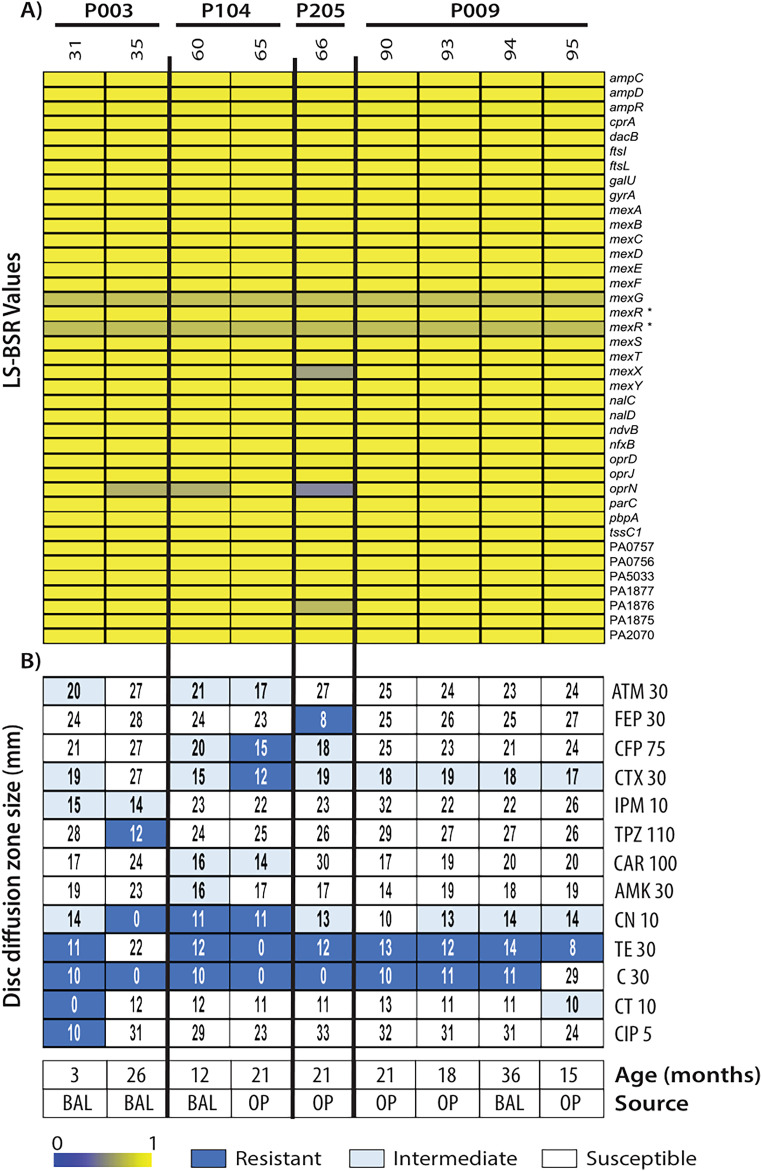
Antibiotic susceptibility profiles were determined by disc diffusion in our cohort of young CF isolates (30). We investigated whether changes in antibiotic susceptibility could be explained by changes in antibiotic resistance-associated genes. Only a subset of isolates was previously analyzed by disc diffusion at the time of isolation, and therefore the genomic content of only these isolates was analyzed (Fig. 4). Our analysis revealed little genomic variability in most of the genes previously associated with antibiotic resistance (31), including the mex and opr operon genes involved in antibiotic extrusion (32). At the nucleotide level, isolate 66 (from patient 205) had genetic divergence in mexX, oprN, and antibiotic-associated ABC transporter system gene PA1876 (defined by an LS-BSR score of <0.7) compared to all isolates in the cohort (33). The MexEF-OprN efflux pump has been associated with resistance to ciprofloxacin (34). However, this isolate was susceptible to ciprofloxacin (Fig. 4B [see CIP5]). Isolate 31, the only isolate with resistance to ciprofloxacin, had no detectable variation in the mexEF or oprN genes, suggesting mexEF-oprN gene regulation (or other gene involvement) dictates these antibiotic-resistant phenotypes. Isolates 35 and 60 also had genetic divergence in oprN, yet it was uncorrelated with changes to antibiotic susceptibility to any compound. However, there was no detectable genomic variation in mexT, a transcriptional regulator of the mexEF-oprN operon (Fig. 4A). In summary, our genomic analysis of antibiotic resistance-associated genes does not correlate with the observed phenotypic antibiotic susceptibility data.
FIG 4.
LS-BSR analysis of genes associated with antibiotic resistance and disc diffusion data. LS-BSR analysis of genes associated with antibiotic resistance (A) was coupled with disc diffusion data for several antibiotics (B): aztreonam (ATM 30), cefepime (FEP 30), cefoperazone (CFP 30), cefotaxime (CTX 30), imipenem (IPM 10), piperacillin-tazobactam (TPZ 110), carbenicillin (CAR 100), amikacin (AMK 30), gentamicin (CN 10), tetracycline (TE 30), chloramphenicol (C 30), colistin (CT 10), and ciprofloxacin (CIP 5). The numbers following the antibiotic abbreviations correspond to the antibiotic disc concentrations in micrograms. For reference, the patient age at the time of isolation and the isolation source are included. “*” indicates allelic variation.
PQS production in CF isolates cannot be explained by genetic variation.
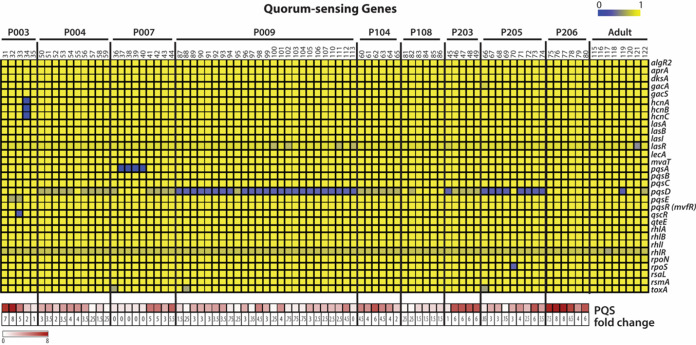
Loss of quorum sensing (QS) is a hallmark of P. aeruginosa adaptation within the CF airway (35–37). Pseudomonas quinolone signal (PQS) is one of the many QS molecules in P. aeruginosa involved in pathogenesis, and its synthesis is dependent on the pqsABCDE operon (38). In the CF airway, PQS downregulates host innate immune responses and is secreted in various amounts by P. aeruginosa isolates (39). Previously, PQS secretion was quantified for the CF isolates from young children by a liquid chromatography-mass spectrometry (LC-MS) method (40). The PQS secretion of the isolates was compared to that of a PQS-null mutant isolate to calculate a fold change over the reference null isolate. We examined QS genes using LS-BSR analysis and visualized the sequence identity scores as a heat map (Fig. 5). Genetic variation in PQS biosynthetic genes pqsA, pqsD, and pqsE was observed (Fig. S4). We additionally examined lasR and rhlR, transcriptional activators of virulence-associated genes, including QS genes, which are often mutated in CF isolates of P. aeruginosa (1, 8). Among the young child CF isolates, lasR had observable, but low genetic variability (LS-BSR score ratio of >0.6) in only four isolates from patient 009 (isolates 100, 102, 111, and 113), suggesting that lasR mutation is not a hallmark of early stage adaptation. The genetic sequences of rhlR and its associated regulator rhlI were consistent across all isolates. In summary, limited genetic variability was observed in QS-related genes in CF isolates from young children, which supports loss of QS as an adaptation associated with long-term chronic infection in older patients.
FIG 5.
LS-BSR analysis of QS-related genes. The top array shows results from LS-BSR analysis of quorum sensing (QS)-related genes from CF isolates of P. aeruginosa (top array). QS genes were identified in a literature search. The bottom array indicates PQS production, as previously assayed from passage 2 isolates. Fold change is respective to a P. aeruginosa mutant that does not express PQS.
Analysis of lipid A-related genes reveals limited genetic variation.
Lipid A is structurally altered during the transition from acute to chronic P. aeruginosa infection in CF patient airways (14, 41–43). Notably, a hexa-acylated lipid A structure results from activation of acyltransferase enzyme, PagP, a structure specifically associated with CF isolates (14, 42). Thus, the mechanism behind PagP overactivation is of interest as a cause has yet to be determined.
We examined all newly sequenced isolates to determine genetic variation in lipid A biosynthesis and modification genes using LS-BSR analysis (Fig. S5). We observed no nucleotide sequence divergence in the non-CF clinical isolates, and minimal divergence was observed in only one gene (arnF, required for aminoarabinose modification of lipid A) in environmental and non-CF bronchiectasis isolates. Interestingly, two divergent sequences were identified in arnF, indicating additional allelic variation (Fig. S5 [noted with *]). CF isolates showed little variation. Variations in pagL and pagP were seen in only two isolates (122 and 119, respectively).
To determine whether nucleotide-level variation is responsible for aberrant PagP activation, the canonical pagP sequence from Pseudomonas aeruginosa PAO1 (GenBank accession no. NC_002516.2) was used to examine our sequenced isolates using BLAST. The identified pagP sequences from all isolates were aligned, and sequence logos were generated to identify common SNPs (Fig. S6). The translated amino acid sequences of pagP associated with these SNPs revealed no divergence in the PagP active site residues (44, 45).
The lipid A structure of each isolate was investigated using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) to determine whether the observed SNP-level differences impact PagP function. Lipid A mass spectra were obtained from the same culture that was used for sequencing (passage 4 for all CF isolates). The expected m/z in negative-ion mode for hexa-acylated lipid A resulting from PagP activity is m/z = 1,684 (m/z = 1,700 if the lipid A is doubly hydroxylated, m/z = 1,668 if there is no hydroxylation, or m/z = 1,604 for monophosphorylation) (Fig. S7 and Table S3); many CF isolates (64 total) did not have detectable PagP-specific hexa-acylated lipid A. These data suggest that the observed SNP-level variation was insufficient to induce lasting activation of PagP outside the context of the CF lung. We did not observe any genetic divergence in phoP or phoQ (regulators of pagP expression) (14). Alternatively, the pagP promoter region may be altered; however, the suspected loss of the hexa-acylated lipid A phenotype after multiple passages suggests that this likely has no significant influence on PagP activity.
DISCUSSION
While several studies have described genetic adaptations of P. aeruginosa during infection of CF patient airways (4, 9–11, 46), the studies of patient populations in the United States lack longitudinal and cross-sectional isolates from infants and young children. Here, we provide the first multipatient, longitudinal sequencing study of P. aeruginosa isolates from young children. Whole-genome sequencing of 81 isolates from nine different patients, all aged 3 years or younger, provides a rich resource for the Pseudomonas and CF research communities. Eight additional isolates from adults with long-term P. aeruginosa infection were also analyzed, in addition to isolates from non-CF clinical presentations and various environmental contexts. The average genome size of the combined CF isolates was slightly higher than the average of other groups of isolates (6.609 Mb versus 6.545 Mb), including those isolated from the environment and laboratory-adapted PAO1 isolates. However, the genome size was comparable to that of non-CF clinical acute isolates, suggesting that disease-causing isolates of P. aeruginosa may undergo genetic variation to gain advantage within their infectious niche. P. aeruginosa has a highly plastic genome that is amenable to many genetic adaptations in these niches (47).
Analysis of all available whole-genome sequences of P. aeruginosa combined with our newly sequenced isolates revealed a distribution of our isolates throughout the P. aeruginosa genomic landscape. CF isolates from individual patient’s cluster together, but isolates from the environment and non-CF infections were dispersed among CF isolates. This observation further supports the notion that patients acquire P. aeruginosa from environmental reservoirs, and the divergence between environmental and CF-adapted isolates is not as significant as one might expect (19).
Of particular interest to membrane biologists is the structural alteration of lipid A during the transition from early to chronic infection. The emergence of hexa-acylated structure resulting from palmitoyl addition by the enzyme PagP is a well-documented adaptation in CF patient airways (14, 41, 44). Here, we investigated whether genetic changes in the P. aeruginosa isolates in our study could explain this lipid A phenotype. We demonstrate that while SNPs were present in various P. aeruginosa CF isolates, they did not correlate with phenotypic changes observed in lipid A structure when analyzed via mass spectrometry. Future studies using more sensitive methods such as gas chromatography to quantitatively analyze palmitoylated lipid A may be useful to complement the mass spectrometry data presented here. Lipid A was analyzed from the fourth passage of these isolates, suggesting that the hexa-acylated lipid A structure phenotype was lost or suppressed once cultured outside the selective pressures of the CF airway. It is likely that environmental conditions in the CF lung (e.g., oxygen concentration gradient, nutrient availability) trigger altered regulation of the PagP enzyme, potentially through the PhoP/Q two-component regulatory system (48, 49). Future transcriptional and proteomic studies of low-passage-number isolates from early infections may define the mechanism underlying PagP activation.
To date, the influence of geography at the genetic level has not been investigated within CF isolates of P. aeruginosa in the United States. While geography and climate are thought to influence acquisition of P. aeruginosa and patient prognosis once infected (22, 50), it was unknown whether geography would specifically influence colonization and adaptation. Here, we demonstrate that geography does not significantly influence gene-level or genome-scale alterations and that the selective pressures from the individual patient themselves appear to be a greater influence than geography. Within the United States, only 51 genes of 9,663 (~0.5%) were identified as being potentially region specific among three patient populations from the represented distinct regions. Of these genes, many were present in isolates from specific patients and were not distributed across all isolates from the region. This pattern suggests that geographic location has little impact on genetic adaptation. We also observed a similar trend when analyzing our 89 CF isolates versus 474 CF isolates from Danish patients (8). Isolates from different countries did not cluster separately, indicating their level of genetic similarity was not significantly influenced by country of origin; rather, clustering of isolates from the same patient was again observed.
In sum, our data suggest that region of residence does not meaningfully influence genetic adaptation of P. aeruginosa, both during early infection and at the stage of chronic infection. Taken together, our data provide the first genetic-level analysis of the hexa-acylated lipid A phenotype and influence of patient region of residence on the genome. Furthermore, even though CFTR modulator therapies (e.g., ETI) have revolutionized CF patient care, the microbiology of the CF airway does not change as drastically as anticipated (51–53). In this study, our analysis of archived young patient P. aeruginosa isolates offers a glimpse into early P. aeruginosa infection without cftr-directed or antimicrobial therapies. This work represents a significant data set for the Pseudomonas and CF research communities that will be foundational and serve as a baseline for future studies in the post-ETI era.
MATERIALS AND METHODS
Bacterial isolates.
Isolates were selected from a bank of P. aeruginosa isolates collected by Jane Burns, Margaret Rosenfeld, and Bonnie Ramsey (12, 13) and from the Ernst lab isolate bank (see Table S1 in the supplemental material). This study did not constitute human subjects research (as determined by the University of Maryland, Baltimore IRB [IRB no. HP-00099314]), as all patient data were deidentified prior to the strains being received in the Ernst laboratory. P. aeruginosa strains were isolated from various sources, including the following (Table S1): environmental isolates (n = 15), PAO1 laboratory sublines (n = 9, from Chandler et al. [18]), isolates from subjects with non-CF bronchiectasis (n = 5), CF young child (≤3 years old) isolates (n = 81), CF adult isolates (n = 10), and extrapulmonary, acute disease isolates (n = 10). Within the cohort of CF young children, isolates were obtained from subjects in three geographical regions: Seattle, WA (location 1), Houston, TX (location 2), and Cleveland, OH (location 3) (Table 2). All longitudinal CF isolates were obtained from subjects 3 years or younger; the earliest isolate was collected at 3 months of age. Isolates were collected by bronchial alveolar lavage (BAL) or oropharyngeal swab (OP). Patients are often infected with multiple isolates of P. aeruginosa (10, 54–56); therefore, multiple isolates (determined by colony morphology) from each subject were collected at one time point (Fig. 1).
The CF “adult” isolates were from subjects with chronic P. aeruginosa infection (average patient age, 15.49 ± 6.55 years) and were used to contrast the “early” isolates. Isolates from patients with non-CF bronchiectasis were collected to identify CF-specific influences of adaptation. All isolates were maintained in 20% glycerol stocks at −80°C prior to sequencing. For sequencing, all isolates were grown aerobically, and genomic DNA was isolated and used for whole-genome sequencing. During sequencing and analysis, three isolates (ID no. 19, 114, and 123) were removed as they were determined not to be P. aeruginosa based on quality checks of the data, contamination, or aberrant genome size of GC% and were therefore excluded from all subsequent analyses. Sample ID no. 108 and 109 were also excluded from analysis prior to sequencing due to missing culture stocks. Large-scale BLAST score ratio (LS-BSR) analysis was used to query the gene content in the total collection of genes (15).
For a complete list of isolates used in this study, see Table S1. All CF isolates were acquired during previous studies (12, 13) and have been maintained at −80°C. The cultures used for sequencing were passage 4.
Preparation of genomic DNA.
Genomic DNA was isolated from aerobic culture grown at 37°C in lysogeny broth (LB) supplemented with 1 mM MgCl2 using a GenElute bacterial genomic DNA kit (Sigma-Aldrich, St. Louis, MO). The kit instructions were followed with the following exception: all DNA was eluted in 400 mL of ultrapure diethylpyrocarbonate-treated water (Thermo Fisher Scientific, Waltham, MA). The concentrations of all DNA preparations were determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). All preparations were stored at −20°C prior to sequencing.
Whole-genome sequencing.
The genomes of all isolates analyzed in this study were sequenced as previously described (18). The sequencing libraries were generated with the Kapa HyperPrep kit (catalog no. KK8504) and sequenced on the Illumina HiSeq 4000 using a 2 × 150-bp paired-end kit. All software was used with default values. Raw sequencing reads were filtered to remove contaminating phiX reads using BBDuk, one of the BBTools software suite (sourceforge.net/projects/bbmap/). The raw reads were also filtered to remove contaminating Illumina adaptor sequences and quality trimmed using Trimmomatic v.0.36 (57). The resulting filtered reads were assembled using SPAdes v.3.13.0 (58). The resulting assemblies were then filtered to contain only contigs longer than 500 bp with a k-mer coverage of ≥5×. Genomes containing more than 500 contigs or an aberrant GC content were removed from further analysis.
LS-BSR analysis.
The genomes of all 128 isolates or just the CF isolates (91 total) were compared using a large-scale BLAST score ratio (LS-BSR) as previously described (15, 59). The predicted protein-encoding genes of each genome that had ≥ 80% nucleotide sequence identity to each other were assigned to gene clusters using the UCLUST algorithm (60). Subsequently, representative sequences of each gene cluster were compared to the sequence of each genome using tblastn (61). Composition-based adjustment was turned off and the resultant tblastn scores were used to generate a BLAST score ratio (BSR) value indicating the detection of each gene cluster in each of the genomes. The BSR value was determined by dividing the score of a gene compared to a sequenced genome by the score of the gene compared to its own sequence. Heat maps of the LS-BSR values of the predicted genes were generated using the multiple-alignment viewing software MeV (62). The gene lists used in LS-BSR analysis and raw values generated for heat maps are listed in Tables S4 and S5.
Phylogenomic analysis.
The genomes of our isolates were compared to previously sequenced complete Pseudomonas aeruginosa genomes available from GenBank before 1 January 2018 (all accession numbers are listed in Fig. 2) or to 474 draft whole-genome sequences of P. aeruginosa CF isolates from a prior study (8); the study authors, Søren Molin and Helle Johansen, kindly shared their assembled genome data with us. Comparison was conducted using an in silico genotyper (ISG) (63). Single nucleotide polymorphisms (SNPs) were detected relative to the completed genome sequence of reference P. aeruginosa isolate PAO1 (GenBank accession no. NC_002516.2) using the ISG, which uses the NUCmer (v.3.22) program (64) for SNP detection. SNP sites that were identified in all analyzed genomes were concatenated and used to construct a maximum likelihood phylogeny using RAxML (v.7.2.8) (65). The phylogeny was constructed using the general time-reversible (GTR) model of nucleotide substitution with the GAMMA model of rate heterogeneity and 100 bootstrap replicates. All phylogenies were visualized using the FigTree (v.1.4.2) program (http://tree.bio.ed.ac.uk/software/figree/).
Disc diffusion and PQS analysis.
Disc diffusion and PQS analysis were conducted as a part of the original study during which the CF isolates were collected (12, 13).
Multiple alignment and sequence logos.
pqsA, pqsD, and pagP sequences in individual genomes were identified by BLAST using the associated P. aeruginosa PAO1 sequence (downloaded from https://pseudomonas.com) in Geneious Pro software (https://www.geneious.com/). Sequences were aligned using ClustalOmega (https://www.ebi.ac.uk/Tools/msa/clustalo/). For pagP analysis, the Pearson/FASTA output was downloaded after ClustalOmega processing. AliView version 1.25 (http://www.ormbunkar.se/aliview/) was used to view the alignments and select the regions of interest. Sequence logos were generated using WebLogo (https://weblogo.berkeley.edu/logo.cgi) (66).
Small-scale lipid A isolation and MALDI-TOF-MS analysis.
Lipid A was isolated from whole cells using an isobutyric acid-ammonium hydroxide-based extraction procedure as previously described (67). Cells from ~3 mL of culture left over after genomic DNA isolation (for sequencing) were centrifuged, and the supernatant was removed. Cell pellets were resuspended in 400 mL of 70% isobutyric acid and 1 M ammonium hydroxide at 5:3 (vol/vol). Samples were incubated at 100°C for 1 h, cooled on ice, and centrifuged for 5 min at 8,000 × g. Supernatants were transferred to a new tube and diluted 1:1 (vol/vol) with endotoxin-free water. Samples were flash-frozen on dry ice and lyophilized overnight. The dried material was washed twice with 1 mL of methanol, and lipid A was extracted in 100 mL of a mixture of chloroform-methanol-water (3:1:0.25 [vol/vol/vol]). One microliter of the extract was spotted onto a stainless steel MALDI target plate, followed by 1 mL of norharmane matrix (Sigma, St. Louis, MO) at a concentration of 10 mg/mL in 2:1 chloroform-methanol (vol/vol). All spots were allowed to air dry before MALDI-TOF MS analysis.
Lipid A was analyzed in negative-ion mode with reflectron mode on a Bruker microFlex (Bruker Daltonics, Billerica, MA) matrix-assisted laser desorption ionization–time-of-flight mass spectrometer. Data were acquired in negative-ion mode. The instrument was mass calibrated with an electrospray tuning mix (Agilent, Palo Alto, CA). Data were acquired with flexControl software and processed with flexAnalysis (v.3.4; Bruker Daltonics, Billerica, MA). All spectra were baseline smoothed before publication. The resultant spectra were used to estimate the lipid A structures present in each isolate based on their predicted structures and associated molecular weights.
Gene overlap analysis.
To determine genes shared between sets of isolates, the online software Venny (v.2.1.0) was used (http://bioinfogp.cnb.csic.es/tools/venny/).
Data availability.
All sequence data and genome assemblies generated in this study have been submitted to GenBank under BioProject no. PRJNA607994 (this study) and PRJNA490649 (18). The individual assembly accession numbers and Illumina sequence read accession numbers are listed in Table S1.
ACKNOWLEDGMENTS
We thank Francesca Gardner for review and critique of the manuscript.
This work was supported in part by NIH grants U19AI110820 (D.A.R.), R01AI104895 (R.K.E.), R01AI147314 (R.K.E.), and T32AI162579 (C.E.H.) and Cystic Fibrosis Foundation grant Ernst18G0 (C.E.C., C.E.H., and R.K.E.).
C.E.C., C.E.H., T.H.H., D.A.R., and R.K.E. designed research and contributed to experimental design. C.E.C., C.E.H., T.H.H., and D.A.R. performed experiments. C.E.C., C.E.H., T.H.H., D.A.R., and R.K.E. analyzed data. C.E.C., C.E.H., and D.A.R. wrote the manuscript.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Robert K. Ernst, Email: rkernst@umaryland.edu.
Ayush Kumar, University of Manitoba.
REFERENCES
- 1.Elborn JS. 2016. Cystic fibrosis. Lancet 388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 2.Boucher RC. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC. 2004. Relationship of airway epithelial ion transport to chronic bronchitis. Proc Am Thorac Soc 1:66–70. doi: 10.1513/pats.2306018. [DOI] [PubMed] [Google Scholar]
- 4.Winstanley C, O'Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittar F, Richet H, Dubus J-C, Reynaud-Gaubert M, Stremler N, Sarles J, Raoult D, Rolain J-M. 2008. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One 3:e2908. doi: 10.1371/journal.pone.0002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation. 2021. Cystic Fibrosis Foundation Patient Registry 2021 annual data report. https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf.
- 7.Hoiby N. 2007. Pseudomonas aeruginosa infection in cystic fibrosis. APMIS 115:621–628. doi: 10.1111/j.1600-0463.2007.apm_684a.x. [DOI] [PubMed] [Google Scholar]
- 8.Marvig RL, Sommer LM, Molin S, Johansen HK. 2015. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet 47:57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 9.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, Molin S. 2012. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol 10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 10.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mena A, Smith EE, Burns JL, Speert DP, Moskowitz SM, Perez JL, Oliver A. 2008. Genetic adaptation of Pseudomonas aeruginosa to the airways of cystic fibrosis patients is catalyzed by hypermutation. J Bacteriol 190:7910–7917. doi: 10.1128/JB.01147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, Ramsey BW. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 183:444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 14.Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 15.Sahl JW, Caporaso JG, Rasko DA, Keim P. 2014. The large-scale BLAST score ratio (LS-BSR) pipeline: a method to rapidly compare genetic content between bacterial genomes. PeerJ 2:e332. doi: 10.7717/peerj.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freschi L, Vincent AT, Jeukens J, Emond-Rheault J-G, Kukavica-Ibrulj I, Dupont M-J, Charette SJ, Boyle B, Levesque RC. 2019. The Pseudomonas aeruginosa pan-genome provides new insights on its population structure, horizontal gene transfer, and pathogenicity. Genome Biol Evol 11:109–120. doi: 10.1093/gbe/evy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosquera-Rendón J, Rada-Bravo AM, Cárdenas-Brito S, Corredor M, Restrepo-Pineda E, Benítez-Páez A. 2016. Pangenome-wide and molecular evolution analyses of the Pseudomonas aeruginosa species. BMC Genomics 17:45. doi: 10.1186/s12864-016-2364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandler CE, Horspool AM, Hill PJ. 2019. Genomic and phenotypic diversity among ten laboratory isolates of Pseudomonas aeruginosa PAO1. J Bacteriol 201:e00595-18. doi: 10.1128/JB.00595-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosso-Becerra MV, Croda-García G, Merino E, Servín-González L, Mojica-Espinosa R, Soberón-Chávez G. 2014. Regulation of Pseudomonas aeruginosa virulence factors by two novel RNA thermometers. Proc Natl Acad Sci USA 111:15562–15567. doi: 10.1073/pnas.1402536111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan SC, Skoric B, Ramsay KA, Carzino R, Gibson A-M, Hart E, Harrison J, Bell SC, Kidd TJ, Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) . 2013. Geographical differences in first acquisition of Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc 10:108–114. doi: 10.1513/AnnalsATS.201209-077OC. [DOI] [PubMed] [Google Scholar]
- 21.Psoter KJ, Rosenfeld M, de Roos AJ, Mayer JD, Wakefield J. 2014. Differential geographical risk of initial Pseudomonas aeruginosa acquisition in young US children with cystic fibrosis. Am J Epidemiol 179:1503–1513. doi: 10.1093/aje/kwu077. [DOI] [PubMed] [Google Scholar]
- 22.Warrier R, Skoric B, Vidmar S, Carzino R, Ranganathan S. 2019. The role of geographical location and climate on recurrent Pseudomonas infection in young children with cystic fibrosis. J Cyst Fibros 18:817–822. doi: 10.1016/j.jcf.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Wee BA, Tai AS, Sherrard LJ, Ben Zakour NL, Hanks KR, Kidd TJ, Ramsay KA, Lamont I, Whiley DM, Bell SC, Beatson SA. 2018. Whole genome sequencing reveals the emergence of a Pseudomonas aeruginosa shared strain sub-lineage among patients treated within a single cystic fibrosis centre. BMC Genomics 19:644. doi: 10.1186/s12864-018-5018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datar R, Coello Pelegrin A, Orenga S, Chalansonnet V, Mirande C, Dombrecht J, Perry JD, Perry A, Goossens H, van Belkum A. 2021. Phenotypic and genomic variability of serial peri-lung transplantation Pseudomonas aeruginosa isolates from cystic fibrosis patients. Front Microbiol 12:604555. doi: 10.3389/fmicb.2021.604555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherrard LJ, Tai AS, Wee BA, Ramsay KA, Kidd TJ, Ben Zakour NL, Whiley DM, Beatson SA, Bell SC. 2017. Within-host whole genome analysis of an antibiotic resistant Pseudomonas aeruginosa strain sub-type in cystic fibrosis. PLoS One 12:e0172179. doi: 10.1371/journal.pone.0172179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis TC, Beaudry AA, Bullard JM, Janjic N, McHenry CS. 2005. Reconstitution of a minimal DNA replicase from Pseudomonas aeruginosa and stimulation by non-cognate auxiliary factors. J Biol Chem 280:7890–7900. doi: 10.1074/jbc.M412263200. [DOI] [PubMed] [Google Scholar]
- 27.Bianconi I, Jeukens J, Freschi L, Alcalá-Franco B, Facchini M, Boyle B, Molinaro A, Kukavica-Ibrulj I, Tümmler B, Levesque RC, Bragonzi A. 2015. Comparative genomics and biological characterization of sequential Pseudomonas aeruginosa isolates from persistent airways infection. BMC Genomics 16:1105. doi: 10.1186/s12864-015-2276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumas JL, Delden C, Perron K, Köhler T. 2006. Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol Lett 254:217–225. doi: 10.1111/j.1574-6968.2005.00008.x. [DOI] [PubMed] [Google Scholar]
- 29.Martin C, Ichou MA, Massicot P, Goudeau A, Quentin R. 1995. Genetic diversity of Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis revealed by restriction fragment length polymorphism of the rRNA gene region. J Clin Microbiol 33:1461–1466. doi: 10.1128/jcm.33.6.1461-1466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorgensen JH, Turnidge JD. 2007. Susceptibility test methods: dilution and disk diffusion methods, 1152–1172. In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC. [Google Scholar]
- 31.Kos VN, Déraspe M, McLaughlin RE, Whiteaker JD, Roy PH, Alm RA, Corbeil J, Gardner H. 2015. The resistome of Pseudomonas aeruginosa in relationship to phenotypic susceptibility. Antimicrob Agents Chemother 59:427–436. doi: 10.1128/AAC.03954-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneyama H, Ocaktan A, Tsuda M, Nakae T. 1997. The role of mex-gene products in antibiotic extrusion in Pseudomonas aeruginosa. Biochem Biophys Res Commun 233:611–618. doi: 10.1006/bbrc.1997.6506. [DOI] [PubMed] [Google Scholar]
- 33.Poudyal B, Sauer K. 2018. The PA3177 gene encodes an active diguanylate cyclase that contributes to biofilm antimicrobial tolerance but not biofilm formation by Pseudomonas aeruginosa. Antimicrob Agents Chemother 62:e01049-18. doi: 10.1128/AAC.01049-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llanes C, Köhler T, Patry I, Dehecq B, van Delden C, Plésiat P. 2011. Role of the MexEF-OprN efflux system in low-level resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob Agents Chemother 55:5676–5684. doi: 10.1128/AAC.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciofu O, Mandsberg LF, Bjarnsholt T, Wassermann T, Høiby N. 2010. Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology (Reading) 156:1108–1119. doi: 10.1099/mic.0.033993-0. [DOI] [PubMed] [Google Scholar]
- 36.Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK, Rybtke MT, Tolker-Nielsen T, Givskov M, Høiby N, Ciofu O, Scandinavian Cystic Fibrosis Study Consortium . 2010. Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One 5:e10115. doi: 10.1371/journal.pone.0010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman LR, Kulasekara HD, Emerson J, Houston LS, Burns JL, Ramsey BW, Miller SI. 2009. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros 8:66–70. doi: 10.1016/j.jcf.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bredenbruch F, Nimtz M, Wray V, Morr M, Müller R, Häussler S. 2005. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J Bacteriol 187:3630–3635. doi: 10.1128/JB.187.11.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim K, Kim YU, Koh BH, Hwang SS, Kim S-H, Lépine F, Cho Y-H, Lee GR. 2010. HHQ and PQS, two Pseudomonas aeruginosa quorum-sensing molecules, down-regulate the innate immune responses through the nuclear factor-κB pathway. Immunology 129:578–588. doi: 10.1111/j.1365-2567.2009.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr HL, Halliday N, Cámara M, Barrett DA, Williams P, Forrester DL, Simms R, Smyth AR, Honeybourne D, Whitehouse JL, Nash EF, Dewar J, Clayton A, Knox AJ, Fogarty AW. 2015. Pseudomonas aeruginosa quorum sensing molecules correlate with clinical status in cystic fibrosis. Eur Respir J 46:1046–1054. doi: 10.1183/09031936.00225214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst RK, Hajjar AM, Tsai JH, Moskowitz SM, Wilson CB, Miller SI. 2003. Pseudomonas aeruginosa lipid A diversity and its recognition by Toll-like receptor 4. J Endotoxin Res 9:395–400. doi: 10.1179/096805103225002764. [DOI] [PubMed] [Google Scholar]
- 42.Ernst RK, Moskowitz SM, Emerson JC, Kraig GM, Adams KN, Harvey MD, Ramsey B, Speert DP, Burns JL, Miller SI. 2007. Unique lipid A modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J Infect Dis 196:1088–1092. doi: 10.1086/521367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cigana C, Curcurù L, Leone MR, Ieranò T, Lorè NI, Bianconi I, Silipo A, Cozzolino F, Lanzetta R, Molinaro A, Bernardini ML, Bragonzi A. 2009. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One 4:e8439. doi: 10.1371/journal.pone.0008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thaipisuttikul I, Hittle LE, Chandra R, Zangari D, Dixon CL, Garrett TA, Rasko DA, Dasgupta N, Moskowitz SM, Malmström L, Goodlett DR, Miller SI, Bishop RE, Ernst RK. 2014. A divergent Pseudomonas aeruginosa palmitoyltransferase essential for cystic fibrosis-specific lipid A. Mol Microbiol 91:158–174. doi: 10.1111/mmi.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop RE. 2005. The lipid A palmitoyltransferase PagP: molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol 57:900–912. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 46.Rossi E, La Rosa R, Bartell JA, Marvig RL, Haagensen JAJ, Sommer LM, Molin S, Johansen HK. 2021. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat Rev Microbiol 19:331–342. doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 47.Shen K, Sayeed S, Antalis P, Gladitz J, Ahmed A, Dice B, Janto B, Dopico R, Keefe R, Hayes J, Johnson S, Yu S, Ehrlich N, Jocz J, Kropp L, Wong R, Wadowsky RM, Slifkin M, Preston RA, Erdos G, Post JC, Ehrlich GD, Hu FZ. 2006. Extensive genomic plasticity in Pseudomonas aeruginosa revealed by identification and distribution studies of novel genes among clinical isolates. Infect Immun 74:5272–5283. doi: 10.1128/IAI.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moskowitz SM, Ernst RK. 2010. The role of Pseudomonas lipopolysaccharide in cystic fibrosis airway infection. Subcell Biochem 53:241–253. doi: 10.1007/978-90-481-9078-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McPhee JB, Bains M, Winsor G, Lewenza S, Kwasnicka A, Brazas MD, Brinkman FSL, Hancock REW. 2006. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J Bacteriol 188:3995–4006. doi: 10.1128/JB.00053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsay K, Stockwell R, Bell S, Kidd T. 2016. Infection in cystic fibrosis: impact of the environment and climate. Expert Rev Respir Med 10:505–519. doi: 10.1586/17476348.2016.1162715. [DOI] [PubMed] [Google Scholar]
- 51.Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, Konstan MW, Sawicki GS, Sewall A, Nyangoma S, Elbert A, Marshall BC, Bilton D. 2018. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 73:731–740. doi: 10.1136/thoraxjnl-2017-210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruce JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. 2017. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, Rowe SM, Sagel SD. 2020. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann Am Thorac Soc 17:212–220. doi: 10.1513/AnnalsATS.201907-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobrindt U, Hacker JH, Svanborg C. 2013. Between pathogenicity and commensalism. Curr Top Microbiol 358:v–vii. doi: 10.1007/978-3-642-36560-7. [DOI] [PubMed] [Google Scholar]
- 55.Workentine ML, Sibley CD, Glezerson B, Purighalla S, Norgaard-Gron JC, Parkins MD, Rabin HR, Surette MG. 2013. Phenotypic heterogeneity of Pseudomonas aeruginosa populations in a cystic fibrosis patient. PLoS One 8:e60225. doi: 10.1371/journal.pone.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mowat E, Paterson S, Fothergill JL, Wright EA, Ledson MJ, Walshaw MJ, Brockhurst MA, Winstanley C. 2011. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am J Respir Crit Care Med 183:1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- 57.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nurk S, Bankevich A, Antipov D, Gurevich AA, Korobeynikov A, Lapidus A, Prjibelski AD, Pyshkin A, Sirotkin A, Sirotkin Y, Stepanauskas R, Clingenpeel SR, Woyke T, McLean JS, Lasken R, Tesler G, Alekseyev MA, Pevzner PA. 2013. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol 20:714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hazen TH, Donnenberg MS, Panchalingam S, Antonio M, Hossain A, Mandomando I, Ochieng JB, Ramamurthy T, Tamboura B, Qureshi S, Quadri F, Zaidi A, Kotloff KL, Levine MM, Barry EM, Kaper JB, Rasko DA, Nataro JP. 2016. Genomic diversity of EPEC associated with clinical presentations of differing severity. Nat Microbiol 1:15014. doi: 10.1038/nmicrobiol.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 61.Gertz EM, Yu YK, Agarwala R, Schäffer AA, Altschul SF. 2006. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol 4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. 2006. TM4 microarray software suite. Methods Enzymol 411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 63.Hazen TH, Kaper JB, Nataro JP, Rasko DA. 2015. Comparative genomics provides insight into the diversity of the attaching and effacing Escherichia coli virulence plasmids. Infect Immun 83:4103–4117. doi: 10.1128/IAI.00769-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delcher AL, Salzberg SL, Phillippy AM. 2003. Using MUMmer to identify similar regions in large sequence sets. Curr Protoc Bioinformatics Chapter 10:Unit 10.3. doi: 10.1002/0471250953.bi1003s00. [DOI] [PubMed] [Google Scholar]
- 65.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 66.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.el Hamidi A, Tirsoaga A, Novikov A, Hussein A, Caroff M. 2005. Microextraction of bacterial lipid A: easy and rapid method for mass spectrometric characterization. J Lipid Res 46:1773–1778. doi: 10.1194/jlr.D500014-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.01556-23-s0001.pdf, PDF file, 3.0 MB (3MB, pdf)
Supplemental material. Download spectrum.01556-23-s0002.xlsx, XLSX file, 0.04 MB (36.1KB, xlsx)
Supplemental material. Download spectrum.01556-23-s0003.xlsx, XLSX file, 0.02 MB (19.8KB, xlsx)
Supplemental material. Download spectrum.01556-23-s0004.xlsx, XLSX file, 0.1 MB (115.1KB, xlsx)
Data Availability Statement
All sequence data and genome assemblies generated in this study have been submitted to GenBank under BioProject no. PRJNA607994 (this study) and PRJNA490649 (18). The individual assembly accession numbers and Illumina sequence read accession numbers are listed in Table S1.