Nearly 21 years ago, the paradigm for blood glucose (BG) control in hospitalized patients was challenged by a single-center, randomized controlled trial (RCT).1 Conducted in mechanically ventilated surgical ICU patients (63% following cardiovascular surgery, 87% without a prior diagnosis of diabetes), this trial used intravenous insulin to target euglycemia – BG 80–110 mg/dL (4·4–6·1 mmol/l) – and dramatic reductions in mortality and morbidity were achieved compared to outcomes of those in the control arm, in which intravenous insulin was administered only if BG exceeded 215 mg/dL (11·9 mmol/l), with a target range of 180–200 mg/dL (10·0–11·1 mmol/l).
The diabetes community took notice. The 2004 guidelines for glucose management in hospitalized patients published by the American Diabetes Association gave a Grade A recommendation to targeting BG levels of 80–110 mg/dL (4·4–6·1 mmol/l) in surgical ICU patients.1 However, subsequent RCT’s failed to reproduce the original findings,1 and a large multi-center RCT published in 2009 demonstrated higher 90-day mortality in patients (80% without diabetes) achieving the “tight control” BG target compared to those who were treated with an intermediate BG target of 140–180 mg/dL (7·8–10·0 mmol/l).1 Guideline groups quickly adopted this higher BG target for all critically ill patients.1
Explanations offered for the discordant results of these RCT’s include high rates of hypoglycemia (demonstrated to be independently associated with mortality in observational reports as well as RCT data); low time in targeted BG range; insufficiently frequent BG monitoring; and the “single center effect.”1 What has become clear more recently, however, is that the relationship between glucose control and mortality during ICU admission differs between patients with and without diabetes. Thus, whereas a higher mean BG level at the time of ICU admission is consistently associated with higher mortality in non-diabetic patients, this relationship does not appear to hold for patients with diabetes. Consequently, the benefit of “tight control” achieved by intensive insulin therapy in the RCT’s may be limited primarily to patients without diabetes.1
To shed light on this unexpected finding, recent studies have investigated whether preadmission glycemia, reflected by HbA1c level measured at the time of ICU admission, impacts the relationship between glycemic control in the ICU and mortality.2–5 Evaluation of a large, single-center ICU database of medical and surgical patients has demonstrated that higher mean BG during ICU admission was strongly associated with higher mortality for patients with a pre-admission HbA1c < 6·5% (48 mmol/mol) regardless of diabetes status, but lower mortality for patients with pre-admission HbA1c ≥ 8.0% (64 mmol/mol) (Figure 1).3 These findings may in part reflect the contribution to mortality outcomes made by ‘relative hypoglycemia’, defined as either a 30% reduction in BG level compared to preadmission glycemia,4 or any excursion into the 70–110 mg/dL (3·9–6·1 mmol/l) range for patients with HbA1c ≥ 8.0% (64 mmol/mol)5, among patients without documented hypoglycemia (<70 mg/dL or 3·9 mmol/l).
Figure 1.
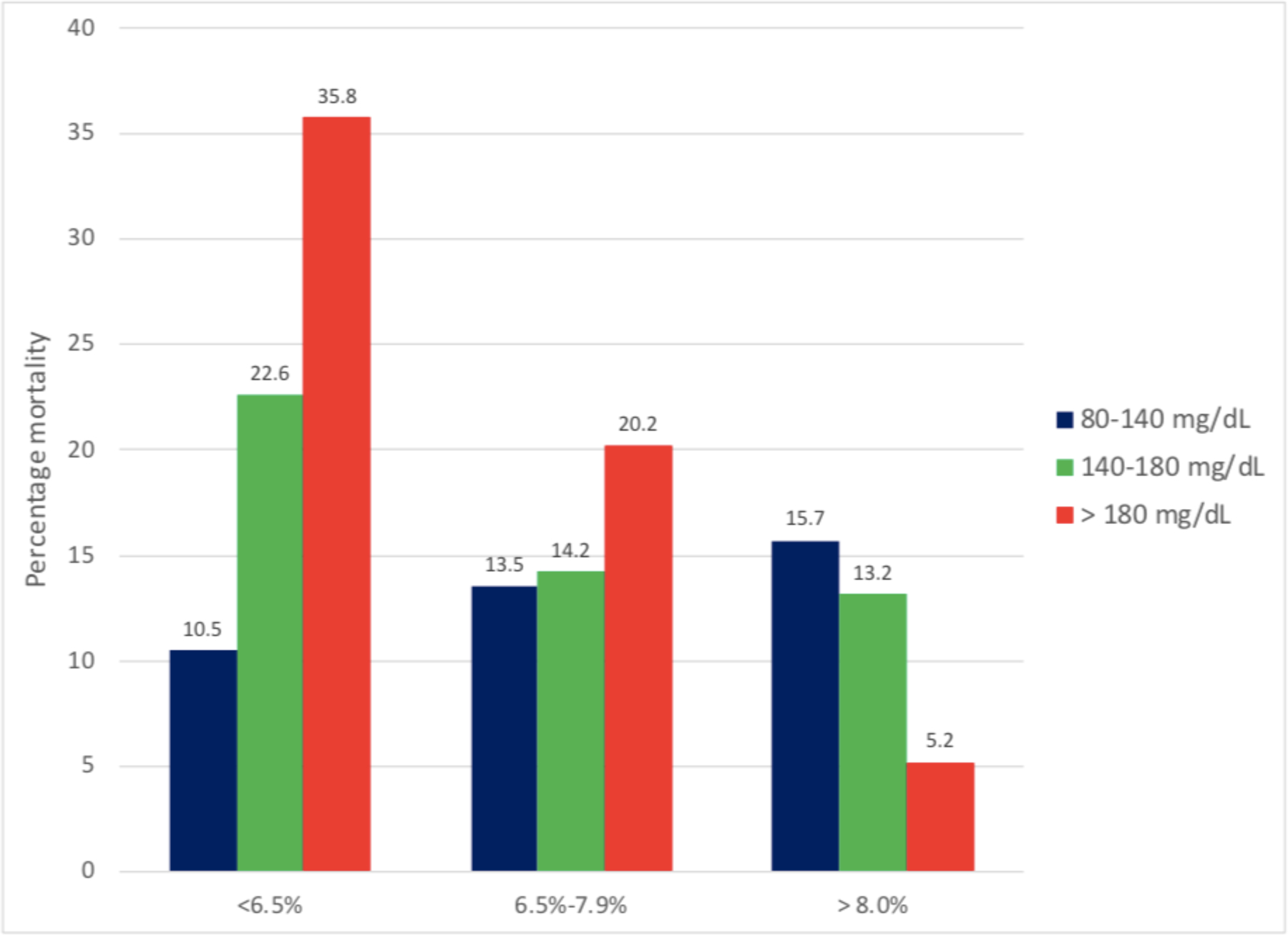
Relationship between mean BG (mg/dL) and mortality, stratified by HgbA1c level
For patients with HgbA1c < 6.5% higher mean BG is strongly associated with increased mortality.
For patients with HgbA1c ≥ 8.0% the opposite relationship is observed.
The pathophysiologic basis for the difference in outcomes associated with different levels of antecedent hyperglycemia is unclear and may involve multiple mechanisms. One possibility is that in patients with long-standing hyperglycemia prior to admission, processing of sensory input may be altered such that the circulating glucose level is perceived by the brain to be lower than it truly is. Indeed, this type of defect may contribute the development and maintenance of hyperglycemia in some patients with Type 2 diabetes.6 Consistent with this possibility is evidence that the glycemic threshold for triggering so-called “counter-regulatory responses” to a falling blood glucose level is elevated by as much as 40–50% in patients with well controlled Type 2 diabetes compared to controls.7 In such individuals, rapid normalization of the BG level can be anticipated to create the false perception of hypoglycemia, resulting in autonomic and adrenomedullary activation that can worsen clinical outcomes.
Given their inherent limitations, recent findings from observational data2–5 should be considered hypothesis-generating. For example, while these studies include consecutive series of patients and adjustment for age, severity of illness and comorbidities,3–5 they neither distinguish between iatrogenic or spontaneous hypoglycemia nor identify specific causes of death, such as arrhythmia or insulin-associated hypokalemia. Hypoglycemia has been identified as a significant contributor to increased mortality in patients with type 1 diabetes, and there is no doubt that it can be fatal, whether a patient has diabetes or not.8 Whether hypoglycemic events are more common among insulin-treated ICU patients with diabetes – especially, those with a high pre-admission HbA1c level – is therefore an important unanswered question. Another potential limitation involves reliance on HbA1c as a surrogate for antecedent glycemia, which can be impacted by variables including race, hemoglobin level and hematologic disorders, mechanical heart valves, renal failure and hypothyroidism.9
Despite these limitations, we believe that available data call into question the “one size fits all” approach to BG control in the critically ill, and that pre-admission HbA1c levels will prove valuable in identifying patient-specific glucose targets in the ICU. However, the appropriate target for diabetes patients remains uncertain, despite a recently published multi-center trial undertaken to assess the benefit of individualized BG targets based on HbA1c10. This trial was discontinued both because the interventional arm sustained higher rates of hypoglycemia than did the patients in the conventional-treatment arm, and because the time-weighted mean BG values differed only slightly between the two study groups.
Based on these considerations, studies are needed to assess the risks and benefits of a “personalized” approach to glucose control based on glycemic targets that incorporate HbA1c or other measures of pre-admission glycemia. For meaningful conclusions to be drawn, such studies must ensure both that hypoglycemic events are kept to a minimum (through the use of continuous glucose monitoring during hospitalization) and that adequate “glycemic separation” is achieved between study groups. Only with the benefit of this information will we know whether glycemic targets for critically ill patients with diabetes should be established using an individualized approach.
REFERENCES
- 1.Krinsley JS, Preiser JC. Is it time to abandon glucose control in critically ill patients? Curr Opin in Crit Care 2019; 25: 299–306. [DOI] [PubMed] [Google Scholar]
- 2.Roberts G, Quinn SJ, Valentine N, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endo Met. 2015; 100: 4490–97. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS, Rule P, Pappy L, et al. The interaction of acute and chronic glycemia on the relationship of hyperglycemia, hypoglycemia and glucose variability to mortality in the critically ill. Crit Care Med. 2020; 48: 1744–51. [DOI] [PubMed] [Google Scholar]
- 4.Kwan TN, Zwakman-Hessels L, Marhoon N, et al. Relative hypoglycemia in diabetic patients with critical illness. Crit Care Med. 48; E233–E240. [DOI] [PubMed] [Google Scholar]
- 5.Krinsley JS, Rule PR, Brownlee M, Roberts G, Preiser JC, Chaudry S, Heluey-Rodrigues C, Dionne K, Umpierrez GE, Hirsch IB. Relative hypoglycemia and lower hemoglobin A1c-adjusted time in band (HA-TIB) are strongly associated with increased mortality in critically ill patients. Crit Care Med. Published online February 1, 2022. [DOI] [PubMed] [Google Scholar]
- 6.Mirzadeh Z, Faber CL, Schwartz MW. CNS Control of Glucose Homeostasis: A Therapeutic Target for Type 2 Diabetes? Annu Rev Pharmacol Toxicol. 2022; 62: 55–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakera AJ, Hurst PS, Spyer G, et al. Molecular reductions in glucokinase activity increase counter-regulatory responses to hypoglycemia in mice and humans with diabetes. Mol Metab. 2018; 17: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care. 2012; 35; 1814–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch IB, Brownlee M. Beyond Hemoglobin A1c – Need for additional markers of risk for diabetic microvascular complications. JAMA 2010; 303: 2291–92. [DOI] [PubMed] [Google Scholar]
- 10.Bohé J, Abidi H, Brunot V, et al. Individualised versus conventional glucose control in critically-ill patients: the CONTROLING study-a randomized clinical trial. Int Care Med. 2021; 47: 1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


