Abstract
Phospholipid signaling plays important roles in plant immune responses. Here, we focused on two phospholipase C3 (PLC3) orthologs in the Nicotiana benthamiana genome, NbPLC3-1 and NbPLC3-2. We generated NbPLC3-1 and NbPLC3-2-double-silenced plants (NbPLC3s-silenced plants). In NbPLC3s-silenced plants challenged with Ralstonia solanacearum 8107, induction of hypersensitive response (HR)-related cell death and bacterial population reduction was accelerated, and the expression level of Nbhin1, a HR marker gene, was enhanced. Furthermore, the expression levels of genes involved in salicylic acid and jasmonic acid signaling drastically increased, reactive oxygen species production was accelerated, and NbMEK2-induced HR-related cell death was also enhanced. Accelerated HR-related cell death was also observed by bacterial pathogens Pseudomonas cichorii, P. syringae, bacterial AvrA, oomycete INF1, and TMGMV-CP with L1 in NbPLC3s-silenced plants. Although HR-related cell death was accelerated, the bacterial population was not reduced in double NbPLC3s and NbCoi1-suppressed plants nor in NbPLC3s-silenced NahG plants. HR-related cell death acceleration and bacterial population reduction resulting from NbPLC3s-silencing were compromised by the concomitant suppression of either NbPLC3s and NbrbohB (respiratory oxidase homolog B) or NbPLC3s and NbMEK2 (mitogen activated protein kinase kinase 2). Thus, NbPLC3s may negatively regulate both HR-related cell death and disease resistance through MAP kinase- and reactive oxygen species-dependent signaling. Disease resistance was also regulated by NbPLC3s through jasmonic acid- and salicylic acid-dependent pathways.
Keywords: Hypersensitive response, jasmonic acid, MAP kinase, Nicotiana benthamiana, phosphatidylinositol-phospholipase C3, Ralstonia solanacearum, reactive oxygen species, salicylic acid, virus-induced gene silencing
In Nicotiana benthamiana, PLC3-1 and -2 complement each other during the hypersensitive response to negatively regulate and fine tune the response through several signaling pathways, thereby preventing disruption of the immune response.
Introduction
Plants have evolved sophisticated defense responses against phytopathogens, including preformed and active defenses (Thatcher et al., 2004). Preformed defenses include physical barriers, such as thick cell walls and wax layers (Ziv et al. 2018), and a diverse array of secondary metabolites, many of which have antifungal activities. Some of these compounds are constitutive, existing in healthy plants in their biologically active forms. Others, such as cyanogenic glycosides and glucosinolates, occur as inactive precursors, and are activated in response to tissue damage or pathogen attack (Osbourn, 1996). In addition to preformed defenses, plants have a variety of active defense mechanisms to protect themselves from microbial pathogen infection. These responses include stomatal closure (Panchal and Melotto, 2017), reactive oxygen species (ROS) burst (Baker and Orlandi, 1995), cell wall glycoprotein cross-linking (Bradly et al., 1992; Brisson et al., 1994), lignification (Vance et al., 1980), callose deposition (Voigt 2014), phytoalexin production (Ahuja et al. 2012; Jeandet 2015) and pathogenesis-related protein accumulation (Linthorst and van Loon, 2008). The most powerful defense response is the hypersensitive response (HR), which is accompanied by programmed cell death. HR is characterized by rapid, localized cell death triggered by an incompatible pathogen (Benoit and Dangl, 1997).
Plant defenses are initiated by the recognition of pathogen-derived molecules. A first example of pathogen recognition is the perception of pathogen-associated molecular patterns (PAMPs), leading to PAMP-triggered immunity (PTI). PAMPs have been recognized as generally conserved compounds, like chitin and glucan in fungi, and flagellins and lipopolysaccharides in bacteria. These PAMPs are recognized by plasma membrane-localized pattern-recognition receptors. PTI is induced by all invading pathogens (Bittel and Robatzek, 2007; Boller and He, 2009). The other mechanism of pathogen recognition is achieved through the recognition of pathogen effectors by resistance gene products (R proteins), followed by the induction of effector-triggered immunity (ETI; Jones and Dangl, 2006). PTI and ETI share signaling components that have distinct activation dynamics and amplitudes (Tsuda and Katagiri, 2010). Generally, PTI is characterized by broad-spectrum, transient and relatively mild immune responses without programmed cell death associated with the HR (Segonzac et al., 2011; Bigeard et al., 2014). In contrast, ETI is characterized by specific, sustainable, and robust immune responses with the HR (Tsuda and Katagiri, 2010).
After pathogen recognition, intracellular signal transduction cascades and transcriptional reprogramming are triggered during PTI and ETI. A ROS burst occurs rapidly through the respiratory burst oxidase homolog (RBOH), an NADPH oxidase that produces membrane-impermeable O2.–, and superoxide dismutase (SOD), which converts O2.– into H2O2 in the apoplast (Li et al., 2014; Podgórska et al., 2017). H2O2 enters the cytosol and induces an elevation of cytosolic Ca2+ (Yuan et al., 2017). Intracellular Ca2+ activates Ca2+-dependent protein kinases during the immune response in Nicotiana benthamiana and N. tabacum (Romeis et al., 2001). Ca2+ activates H+/K+ ion fluxes, which lead to extracellular alkalinization and the depolarization of the plasma membrane (Jeworutzki et al., 2010). Mitogen-activated protein kinase (MAPK) activation also occurs during immune signaling. MAPK activation leads to the phosphorylation of several transcription factors that regulate genes involved in ethylene, salicylic acid (SA) and jasmonic acid (JA) signaling, as well as antimicrobial compound production (Zhang and Klessig, 1998; Dahan et al., 2009; Nishad et al., 2020). This complex signaling network results in the establishment of PTI and/or ETI.
Phospholipid-based signaling cascades are another important component of intracellular signal transduction in plant immune responses. Phosphatidylinositol-specific phospholipase C (PI-PLC), a major component of phospholipid turnover, is an important lipid-hydrolyzing enzyme in both plants and animals. In plants, PLCs hydrolyze a specific substrate, phosphatidylinositol 4,5-bisphosphate, at the glycerophosphate ester linkages of membrane phospholipids, which leads to the generation of secondary messengers, such as diacylglycerol and inositol 1,4,5-trisphosphate (Singh et al., 2015). We previously screened for PI-PLC orthologs in the completed N. benthamiana genome sequence (https://solgenomics.net/), and identified 12 PI-PLCs (Kiba et al., 2020). Our objective is to clarify the roles of all 12 PLC orthologs in plant immunity. Our previous report showed that two NbPLC2 orthologs have important roles in pre- and post-invasion defenses, namely in PTI induction. During PTI induction, NbPLC2s can activate JA-mediated immune responses in plants, leading to the suppression of bacterial infections (Kiba et al., 2020). In contrast, one of the NbPLC1 orthologs, NbPLC1-2, might have important roles in suppression of defense responses in HR, via negative regulation of JA- and ROS-mediated signaling (Ueta et al., 2021). Thus, we hypothesized that there is role sharing between each PLC family member in plant defense regulation. In the present study, we focused on two PI-PLC3 orthologs, NbPLC3-1 and NbPLC3-2, from the remaining 10 PI-PLCs. We determined the effects of silencing NbPLC3-1, NbPLC3-2, individually and together, on immune responses in N. benthamiana. We also discuss the regulatory roles of NbPLC3s on immune responses in N. benthamiana.
Materials and methods
Biological and chemical materials
Nicotiana benthamiana and N. benthamiana NahG plants were cultivated in a growth room as described previously (Maimbo et al., 2007, 2010). Ralstonia solanacearum 8107 (Rs8107), Pseudomonas cichorii, and P. syringae pv. syringae were cultured in peptone yeast extract medium as described previously (Kiba et al., 2003). Agrobacterium tumefaciens was cultured in YEB medium (Maimbo et al., 2007). The bacterial populations of Rs8107, P. cichorii, and P. syringae pv. syringae were adjusted to 108 colony forming unit (CFU) ml–1. Primers and plasmids used in this study are shown in Supplementary Tables S1 and S2, respectively.
RNA isolation and cDNA synthesis
Total RNA was isolated from N. benthamiana leaves using a NucleoSpin RNA Plant kit (Macherey-Nagel, Düren, Germany). A 1-µg sample of total RNA was used as the template for reverse transcription using a PromeScript II 1st strand cDNA Synthesis kit (TaKaRa Bio Co., Ltd., Shiga, Japan), as described by Maimbo et al., (2007).
Isolation of full-length cDNA of NbPLC3-1 and NbPLC3-2
PCR amplification was performed with the primer combination PLC3-1S and PLC3-1A for NbPLC3-1, and the combination PLC3-2S and PLC3-2A for NbPLC3-2 (Supplementary Table S1), with 1 μg of cDNA from N. benthamiana. Cycling parameters were as follows: 30 cycles of 94 °C for 1 min, 55 °C for 2 min and 72 °C for 1 min. The full-length cDNAs were cloned into the pMD20 vector (TaKaRa Bio. Co., Ltd.) to generate pMD-NbPLC3-1 and pMD-NbPLC3-2.
Sequencing
Sequence analysis was performed using M4 and RV primers (Supplementary Table S1) with the reagents for a Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster, CA, USA) and an Applied Biosystems 3100 Avant Automated Sequencer (Applied Biosystems, Warrington, UK), in accordance with the manufacturer’s instructions. The sequence analysis was carried out using DNASIS (version 3.6; Hitachi, Yokohama, Japan) and the BLAST network service from the National Center for Biotechnology Information (Altschul et al., 1990).
Virus-induced gene silencing
Virus-induced gene silencing (VIGS) was performed as described previously (Mainbo et al., 2007). The plasmids used for VIGS experiments are listed in Supplementary Table S2. cDNA sequences used for VIGS experiments were selected using the SGN VIGS tool (Fernandez-Pozo et al., 2014, 2015). cDNA fragments for NbPLC3-1, NbPLC3-2, and the combined NbPLC3-1 + NbPLC3-2 sequences (NbPLC3s) were amplified with the primers listed in Supplementary Table S1 using N. benthamiana cDNA as the template (Supplementary Fig. S1). These cDNA fragments were independently sub-cloned into the TA cloning site of pMD20 (TaKaRa Bio. Co., Ltd.) to generate pMD-NbPLC3-1, pMD-NbPLC3-2 and pMDNbPLC3s. These plasmids were digested with SalI (TaKaRa Bio. Co., Ltd.) and ligated independently into SalI-digested pPVX201 (Baulcombe et al., 1995). VIGS was conducted with pPVX201 harboring NbPLC3-1 (pPVXPLC3-1), NbPLC3-2 (pPVXPLC3-2), or NbPLC3s (pPVXPLC3s), independently. The pPVX201 plasmid lacking any inserts was used as a control as described previously (Maimbo et al., 2007). These binary plasmids were transformed into A. tumefaciens strain GV3101, and inoculated into N. benthamiana and N. benthamiana NahG leaves. Three weeks after the initial A. tumefaciens inoculation, inoculations with A. tumefaciens carrying bacterial effector AvrA, oomycete elicitin, INF1, and coat protein from TMGMV with cognate L1 resistance protein, were performed on N. benthamiana leaves located three to four leaves above the Agrobacterium-inoculated leaf. The silencing efficiency levels were assessed using quantitative real-time PCR (qRT–PCR) assays (Kiba et al., 2020).
Bacterial population
The bacterial suspensions of R. solanacearum and A. tumefaciens were inoculated into N. benthamiana leaves using needleless syringes by the method described by Kiba et al. (2012). The bacterial populations were determined by plating on Hara–Ono plates (Maimbo et al., 2010).
Quantitative real-time PCR
Gene expression analysis was performed using qRT–PCR. Briefly, qRT–PCR was performed in 20 µl of reaction mixture, containing 1 µl of cDNA template, 10 pM of the respective primers (Supplementary Table S2) and THUNDERBIRD qPCR MIX (Toyobo Co. Osaka, Japan), on an Applied Biosystems (Thermo Fisher Scientific Inc., Tokyo, Japan) 7300 real-time PCR instrument. The cycling parameters were the same for all the primers: an initial 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 1 min. Melting curve runs were performed at the end of each reaction to verify the specificity of primers, by detecting the presence of a single amplification product. The relative quantification of gene expression was performed in accordance with the instructions for the Applied Biosystems 7300 real-time PCR system, using the comparative cycle threshold [Ct] method to calculate the Qty value. We used two genes for internal standards (NbUbe35 and NbNQO). When a pair of reference genes were used (NbUbe35/NbNQO), the geometric Cq mean and efficiency average were employed, as described previously (Pombo et al., 2018).
Agrobacterium-mediated transient expression
For agroinfiltration experiments, we used the binary vectors p35S-INF1, p35S-MEK2DD, p35S-AvrA, and p35S-TMGMV-CP, with p35S-L1 (Gupta et al., 2013). These binary plasmids were transformed independently into A. tumefaciens strain GV3101. The resulting strains were inoculated into N. benthamiana leaves as described previously (Maimbo et al., 2007).
Estimation of cell death
Cell death was determined by measuring electrolyte leakage by ion conductivity (Gupta et al., 2013) using a Twin Cord B-173 conductivity meter (HORIBA, Kyoto, Japan).
ROS measurements using 3,3-diaminobenzidine staining
To determine the production of H2O2, 3,3’-diaminobenzidine (DAB) staining was performed as described by Gupta et al. (2013). N. benthamiana leaves infected with bacteria were infiltrated with 1 mg ml−1 of DAB solution (pH 3.8) and incubated for 1 h in the dark at 25 °C. The leaf samples were boiled in a solution of ethanol: acetic acid: glycerol (3:1:1). The leaf samples were then decolorized in 2.5 g ml−1 chloral hydrate solution for at least 30 min until the chlorophyll were completely removed.
Immunoblot analysis
Leaf (~0.1 g) samples were homogenized in 200 μl SDS-PAGE sample buffer [50 mM Tris pH 7.9, 100 mM KCl, 1 mM EDTA, 20% (v/v) glycerol, and 1 mM DTT]. After centrifugation at 21 000 × g for 5 min, the clarified solution was used for protein analysis. Following separation of proteins using 10% SDS-PAGE, the appearance of phosphorylated MAPKs in the leaf extract was detected by western blotting using Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (197G2) rabbit monoclonal antibody (Cell Signaling Technology, MA, USA) according to the method described by Lee et al., (2018). The large subunit of ribulose-1,5-bisphosphate carboxylase in samples, shown in the Ponceau S-stained gel, was used to monitor equal loading of protein.
Statistical analysis
The statistical analysis was performed using t-tests (two-sided tests).
Results
Identification and characterization of PLC3s from N. benthamiana
On the basis of a phylogenetic analysis of the amino acid sequences of the PLCs, two PLC orthologs (Niben101Scf02280g02004.1 and Niben101Scf04093g00004.1) were classified into the same clade as PLC3 from Solanum lycopersicum (Kiba et al. 2020). Consequently, we designated them as NbPLC3-1 (Niben101Scf02280g02004.1) and NbPLC3-2 (Niben101Scf04093g00004.1). The deduced amino acid sequences of the full-length cDNAs of NbPLC3-1 and NbPLC3-2 contained complete PI-PLC-X, PI-PLC-Y and PI3K-C2 domains, which are required for enzymatic function of PLC3 from Solanum lycopersicum (Supplementary Fig. S2A, B).
Effects of NbPLC3-1 and NbPLC3-2 silencing on the hypersensitive response towards R. solanacearum
To investigate the roles of NbPLC3-1 and NbPLC3-2 in plant immunity, VIGS of these genes was performed. We generated constructs for silencing NbPLC3-1 (PLC3-1-VIGS), NbPLC3-2 (PLC3-2-VIGS) and both PLC3-1 and PLC3-2 (PLC3s-VIGS). We estimated the suppression levels of NbPLC3-1 and NbPLC3-2 using qRT–PCR. Both NbPLC3-1 and NbPLC3-2 expression levels were reduced in the plants inoculated with Agrobacterium carrying the NbPLC3s-VIGS construct. In addition, the specific suppression of NbPLC3-1 and NbPLC3-2 expression was observed in the plants inoculated with Agrobacterium carrying NbPLC3-1-VIGS and NbPLC3-2-VIGS constructs, respectively (Supplementary Fig. S3A). In contrast, we could not observe significant suppression of expression of NbPLC1-2 (Ueta et al. 2021), NbPLC2-1, and NbPLC2-2 (Kiba et al., 2020) closely related to NbPLC3s (Supplementary Fig. S3B).
We analyzed the function of NbPLC3-1 and NbPLC3-2 in immune responses using an N. benthamiana–R. solanacearum interaction model. We used Rs8107, which is an incompatible pathogen that induces the HR in N. benthamiana. The bacterial population was significantly reduced (P<0.05) in the NbPLC3s-silenced plants 18 h and 24 h after inoculation, compared with empty vector control plants (Fig. 1A). Induced cell death appeared in the control plants 18 h and 24 h after inoculation, whereas cell death acceleration was observed in NbPLC3s-silenced plants (Fig. 1B). The expression of the HR marker gene Nbhin1 was also up-regulated in NbPLC3s plants (Fig. 1C). Thus, NbPLC3s might play important roles in the negative regulation of HR against Rs8107. In contrast, no significant changes in cell death induction or bacterial population were observed in individual NbPLC3-1- or NbPLC3-2-silenced plants (Supplementary Fig. S4A, B). Therefore, we used NbPLC3s-silenced plants for the functional analysis of NbPLC3s in the suppression of HR. We estimated HR-related cell death induction using P. cichorii, P. syringae pv. syringae, the Agrobacterium-mediated transient expression of R. solanacearum effector AvrA, the oomycete elicitin INF1, and TMGMV-CP with L1 (Huitema et al., 2005; Poueymiro et al., 2009; Gupta et al. 2013). Similar to the HR induction caused by Rs8107, an acceleration of HR-related cell death was observed in NbPLC3s-silenced plants in response to inoculation with P. cichorii, P. syringae, as well as transient expression of AvrA, INF1 and TMGMV-CP with L1 (Supplementary Fig. S5).
Fig. 1.
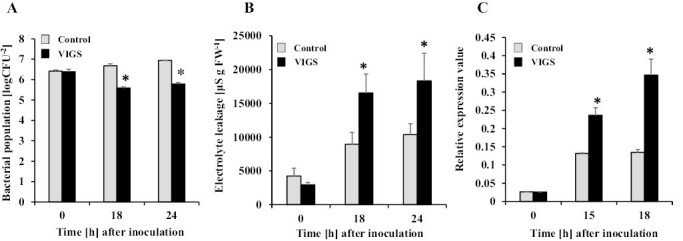
Responses of NbPLC3s-silenced plants to Ralstonia solanacearum. Empty vector control and NbPLC3s-silenced plant leaves were infiltrated with R. solanacearum strain 8107. (A) The bacterial populations of R. solanacearum 8107 in control (ray bar) and NbPLC3s-silenced (VIGS; black bar) plants were determined by plating at 18 and 24 h. Values are means ±SD of five replicate experiments. (B) Cell death induced by R. solanacearum was determined by measuring electrolyte leakage. Values are means ± SD (n=5). (C) Total RNA was isolated from R. solanacearum 8107-inoculated plants, and the expression levels of Nbhin1 transcripts relative to the absolute non-treated control were normalized to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test).
Silencing of NbPLC3s activates jasmonic acid and salicylic acid-dependent signaling in response to R. solanacearum
We previously reported that the HR in response to R. solanacearum is at least partially regulated by JA and SA signaling (Kiba et al., 2003; Gupta et al., 2013). Thus, we investigated the influence of NbPLC3 silencing on JA and SA signaling. Total RNA was extracted from NbPLC3s-silenced and control plants at 0, 18, and 24 h after inoculation with Rs8107. As shown in Fig. 2A, the expression level of PR-1, a marker gene for the SA signaling pathway, was significantly increased (P<0.05) in NbPLC3s-silenced plants, compared with empty vector control plants challenged with Rs8107. In addition, the expression of NbICS1, encoding the SA biosynthetic enzyme isochorismate synthase 1, was also up-regulated in NbPLC3s-silenced plants. Thus, NbPLC3s may be involved in the SA-mediated defense signaling cascade against R. solanacearum.
Fig. 2.
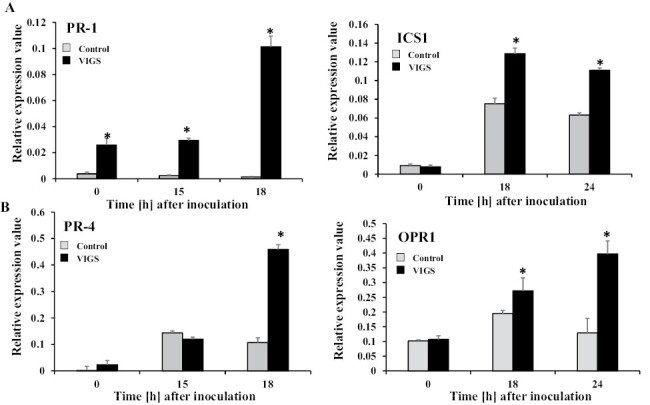
Up-regulation of jasmonic acid- and salicylic acid signaling pathways during accelerated HR in NbPLC3s-silenced plants. Empty vector control and NbPLC3s-silenced plant leaves were infiltrated with R. solanacearum strain 8107. Total RNA was isolated from control and NbPLC3s-silenced plants at 0, 15, 18, or 24 h following inoculation with R. solanacearum 8107. Expression values of (A) salicylic acid-related PR-1 and ICS1, and (B) jasmonic acid-related PR-4 and OPR1 are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test).
Next, we investigated the effects of NbPLC3 silencing on JA signaling. The expression of PR-4, a marker gene for the JA signaling pathway, was significantly up-regulated (P<0.05) in NbPLC3s-silenced plants challenged with Rs8107, compared with control plants. The expression of NbOPR1, encoding the JA biosynthetic enzyme 12-oxophytodienoate reductase 1, was also increased in NbPLC3s-silenced plants (Fig. 2B). Thus, NbPLC3s may be involved in JA-mediated immune responses against R. solanacearum.
Silencing of NbPLC3s activates SA-dependent disease resistance against Ralstonia solanacearum
To further analyze the involvement of SA in the accelerated HR caused by silencing NbPLC3s, we used plants expressing a SA-degrading enzyme (NahG plants). The enhanced disease resistance phenotype was not observed in NbPLC3s-silenced NahG plants, because no significant changes in bacterial growth were observed in either NahG or NbPLC3s-silenced NahG plants (Fig. 3A). An acceleration in HR-related cell death in response to Rs8107 infiltration was observed in NbPLC3s-silenced NahG plants compared with empty vector control NahG plants (Fig. 3B). The expression of the HR marker Nbhin1 was also up-regulated in NbPLC3s-silenced NahG plants compared with NahG control plants after inoculation with Rs8107 (Fig. 3C). Thus, NbPLC3s may regulate resistance induction through SA-mediated signaling against R. solanacearum. The acceleration of HR-related cell death by NbPLC3s-silencing may not be related to SA-dependent signaling, since NahG plants could not accumulate SA.
Fig. 3.

Role of the salicylic acid pathway in accelerated HR in NbPLC3s-silenced plants. NahG and NbPLC3s-silenced NahG (NahG-VIGS) plants were infiltrated with R. solanacearum strain 8107. (A) Bacterial populations were determined by plating at 0, 18, or 24 h following inoculation. Values are means ±SD of four replicate experiments. (B) NahG and NahG-VIGS plants were infiltrated with R. solanacearum 8107. Cell death induction was determined by measuring electrolyte leakage. Values are means ±SD of four replicate experiments. Asterisks denote values significantly different from those of empty vector control plants (C) Total RNA was isolated from NahG and NahG-VIGS plants at 0, 15, and 18 h after inoculation with R. solanacearum 8107. Expression values of Nbhin1 are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of empty vector control plants (*; P<0.05, t-test).
Silencing of NbPLC3s activates JA-dependent defenses against R. solanacearum
Because NbPLC3s-silenced plants showed the rapid activation of JA-dependent PR-4 expression, as well as JA biosynthetic NbOPR1 expression in response to Rs8107 infection, we further analyzed the roles of the JA pathway in accelerated HR caused by silencing NbPLC3. We generated silenced plants for NbPLC3s as well as NbCoi1 (NbPLC3s:Coi1-silenced plants), which encodes an F-box protein required for JA signaling (Zhai et al., 2015). The enhanced disease resistance phenotype was weaker in NbPLC3s:Coi1-silenced plants compared with NbPLC3s-silenced plants, because there was more bacterial growth in NbPLC3s:Coi1-silenced plants than in NbPLC3s-silenced plants, and the bacterial population recovered to that seen in empty vector control plants (Fig. 4A). An acceleration in HR-related cell death in response to Rs8107 infiltration was observed in NbPLC3s:Coi1-silenced plants, similar to that seen in NbPLC3s-silenced plants (Fig. 4B). The expression of Nbhin1 also increased in both NbPLC3s- and NbPLC3s:Coi1-silenced plants (Fig. 4C). There was no change in bacterial population and cell death induction when only NbCoi1 was silenced, compared with control plants (Supplementary Fig. S6). Although the acceleration of HR-related cell death by NbPLC3s-silencing may not be related to JA-dependent signaling, the enhanced disease resistance may be regulated through JA-mediated signaling against R. solanacearum in NbPLC3s-silenced plants (Fig. 4).
Fig. 4.
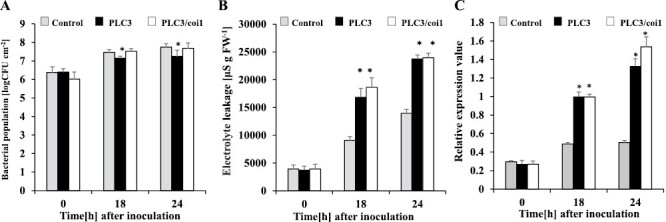
Role of the jasmonic acid pathway in accelerated HR in NbPLC3s-silenced plants. Empty vector control, NbPLC3s (PLC3s) and NbPLC3s:NbCoi1 double-knockdown (PLC3/coi1) plant leaves were infiltrated with R. solanacearum strain 8107. (A) Bacterial populations were determined in control, PLC3s, and PLC3/coi1 plants by plating at 0, 18, and 24 h following inoculation. Values are means ±SD of four replicate experiments. Asterisks show significant differences (P<0.05) between the control, NbPLC3s and double-knockdown plants. (B) Cell death induction was determined by measuring the ion conductivity levels in control, PLC3s, and PLC3s/coi1 plants. Values are means ±SD of four replicate experiments. (C) Total RNA was isolated from control, PLC3s, and PLC3s/coi1 plants at 0, 18, and 24 h after inoculation with R. solanacearum 8107. Expression values of Nbhin1 are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test).
Silencing of NbPLC3s activates reactive oxygen species burst in response to R. solanacearum
Plants undergoing HR usually produce ROS (Dumnovic et al., 2021). Therefore, we evaluated ROS production in control and NbPLC3s-silenced plants in response to Rs8107 infection. The staining of H2O2 with DAB revealed more brown patches in NbPLC3s-silenced plants, which were comparable to those on control plants infected with Rs8107. Increased DAB staining was observed in NbPLC3s-silenced plants in response to Rs8107 infection (Fig. 5A). ROS levels are regulated by the balance between ROS production and elimination (Dumnovic et al., 2021). Subsequently, we analyzed the expression pattern of a gene encoding a major ROS-producing enzyme, NbrbohB, which is a membrane-bound NADPH oxidase (Yoshioka et al., 2003). NbrbohB expression level increased at 15 h after the inoculation of control plants with Rs8107, and significantly increased (P<0.05) in NbPLC3s-silenced plants challenged with Rs8107, compared with empty vector control plants (Fig. 5B). Next, we investigated the effects of NbPLC3s-silencing on the expression levels of genes encoding the ROS scavenging enzymes ascorbate peroxidase (NbAPX) and superoxide dismutase (NbSOD). Although the expression of NbSOD was up-regulated in both control and NbPLC3s-silenced plants at 15 h after inoculation with Rs8107, a significant reduction (P<0.05) in NbSOD expression was observed in NbPLC3s-silenced plants compared with empty vector control plants (Fig. 5C). In addition, the expression level of NbAPX was significantly reduced (P<0.05) in NbPLC3s-silenced plants 0–15 h after inoculation with Rs8107, compared with control plants (Fig. 5D). Thus, NbPLC3s may be involved in the ROS-mediated defense signaling cascade against R. solanacearum. In addition, the hyper-accumulation of ROS in NbPLC3s-silenced plants may result from both the induced expression of a ROS biosynthetic enzyme, and reduced expression of ROS scavenging enzymes.
Fig. 5.
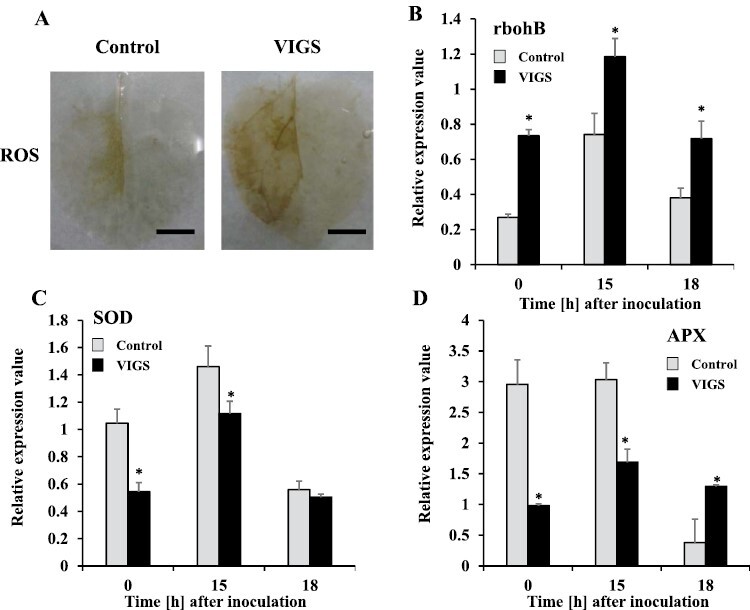
Hyper-production of reactive oxygen species during accelerated HR in NbPLC3s-silenced plants. Empty vector control and NbPLC3s-silenced plant leaves were infiltrated with R. solanacearum strain 8107. (A) ROS production visualized 18 h after inoculation as assessed by DAB staining. These experiments were repeated with 10 biological replicates, and representative results are shown. Scale bars=1 cm. (B–D) Total RNA was isolated from control and NbPLC3s-silenced plants at 0, 15, and 18 h after inoculation with R. solanacearum 8107. Expression values of rbohB, APX1 and SOD are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test).
Silencing of NbPLC3s activates ROS-dependent defense signaling in response to R. solanacearum
Increased ROS accumulation and elevated levels of NbrbohB expression were observed in NbPLC3s-silenced plants (Fig. 5A, B). To determine whether the increased HR was caused by NbrbohB-dependent accumulation of ROS, we generated plants in which both NbPLC3s and NbrbohB were silenced (NbPLC3s:rbohB plants). The enhanced disease resistance phenotype was weaker in NbPLC3s:rbohB-silenced plants compared with NbPLC3s-silenced plants, because the bacterial population recovered to that seen in empty vector control plants (Fig. 6A). There was no change in bacterial population and cell death induction in only NbrbohB-silenced plants when compared with control plants (Supplementary Fig. S6). Intriguingly, the HR-related cell death phenotype, as well as enhanced Nbhin1 expression, in response to Rs8107 infiltration, were also compromised in NbPLC3s:rbohB plants (Fig. 6B, C). In NbPLC3s:rbohB plants, elevated ROS production was compromised in response to Rs8107 infection, and ROS accumulation returned to approximately that seen in empty vector control plants (Fig. 6D). These results indicate that elevated ROS production through NbrbohB is involved in both the acceleration of HR-related cell death induction and enhanced disease resistance in NbPLC3s-silenced plants.
Fig. 6.
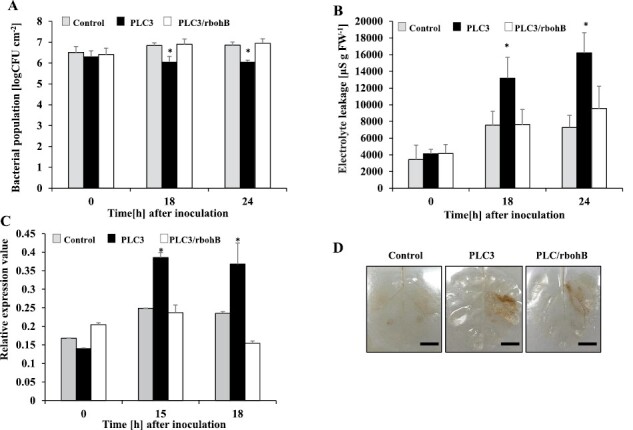
Role of reactive oxygen species in accelerated HR in NbPLC3s-silenced plants. Empty vector control, NbPLC3s (PLC3), and NbPLC3s:NbrbohB double-knockdown (PLC3/rbohB) plant leaves were infiltrated with R. solanacearum 8107. (A) Bacterial populations were determined in control, PLC3, and PLC3/rbohB plants by plating at 0, 18, and 24 h following inoculation. Values are means ±SD of four replicate experiments. Asterisks show significant differences among control, PLC3, and PLC3/rbohB plants (P<0.05, t-test). (B) Cell death induction was determined at 0, 18, and 24 h by measuring the ion conductivity levels in the control, PLC3s, and PLC3s/rbohB plants. Values are means ±SD of four replicate experiments. Asterisks show significant differences among control, PLC3, and PLC3/rbohB plants (P<0.05, t-test). (C) Total RNA was isolated from control, NbPLC3s (PLC3), and NbPLC3s:NbrbohB double-knockdown (PLC3/rbohB) plants at 0, 15, and 18 h after inoculation with R. solanacearum 8107. Expression values of Nbhin1 are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test). (D) ROS production was seen 18 h after inoculation as assessed by DAB staining. These experiments were repeated with 10 biological replicates, and representative results are shown. Scale bars=1 cm.
Silencing of NbPLC3s activates MAPK-dependent defense signaling in response to R. solanacearum
MAPK cascades are sufficient to induce the HR in N. benthamiana (Yoshioka et al., 2009). To evaluate the roles of the MAPK cascade in accelerating the HR in NbPLC3s-silenced plants, we used Agrobacterium carrying a constitutively active form of MAPK kinase 2 (NbMEK2DD; Katou et al., 2003). The induction of HR-related cell death was observed in both control and NbPLC3s-silenced plants at 48 h and 72 h after inoculation. A strong induction of HR-related cell death was observed in NbPLC3s-silenced plants at 72 h after inoculation with A. tumefaciens expressing NbMEK2DD, compared with control plants (Fig. 7A). The induction of Nbhin1 expression was significantly accelerated (P<0.05) in NbPLC3s-silenced plants in comparison with control plants at 48 h and 72 h after inoculation (Fig. 7B). To investigate how NbPLC3s-silencing affected the MAPK cascade, we analyzed activation of MAPK by immunoblot analysis. We observed phosphorylated MAPK as immunoreactive bands similar in size to SA-induced protein kinase (SIPK: 48 kDa) and wound-inducible protein kinase (WIPK: 46 kDa) by Agrobacterium-mediated expression of NbMEK2DD. Phosphorylated MAPKs were slightly increased even at the 0 h time point in NbPLC3s-silenced plants in comparison with control plants, and increased at 24 h and 48 h after expression of NbMEK2DD (Fig. 7C). Phosphorylated MAPKs were also observed in both control and NbPLC3s-silenced plants in response to Rs1807 inoculation. Increased phosphorylated MAPKs were observed at 9 h and 12 h after inoculation with Rs8107 in NbPLC3s-silenced plants in comparison with control plants (Fig. 8A). Expression analysis of NbMEK2 showed that enhanced expression was observed at 0 h and 15 h after inoculation with Rs8107 in NbPLC3s-silenced plants in comparison with control plants (Fig. 8B). The expression of SIPK was enhanced at 0 h and 18 h after inoculation with Rs8107 (Fig. 8C). Transcriptional activation of WIPK was also observed at 0 h and 18 h after inoculation with Rs8107 (Fig. 8D). Therefore, MAPK signaling cascade appears to be enhanced by NbPLC3s-silencing.
Fig. 7.

Relation of the MAPK pathway in accelerated HR cell death in NbPLC3s-silenced plants. Empty vector control and NbPLC3s-silenced plant leaves were infiltrated with Agrobacterium tumefaciens harboring 35S-MEK2DD. (A) Cell death induction was determined by measuring the ion conductivity levels in control and NbPLC3s-silenced (PLC3s) plants. Values are means ±SD of four replicate experiments. (B) Total RNA was isolated from control and PLC3s plants at 0, 48, and 72 h after inoculation with A. tumefaciens harboring 35S-MEK2DD. Expression values of Nbhin1 are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ± SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test). (C) MAPK activation in control and VIGS plants was analyzed by immunoblots with anti-P-p44/42 MAPK (T202/Y204) antibodies at 0, 24, and 48 h after inoculation. Protein loading was monitored by Ponceau S staining of the bands corresponding to ribulose-1,5-bisphosphate carboxylase large subunit.
Fig. 8.
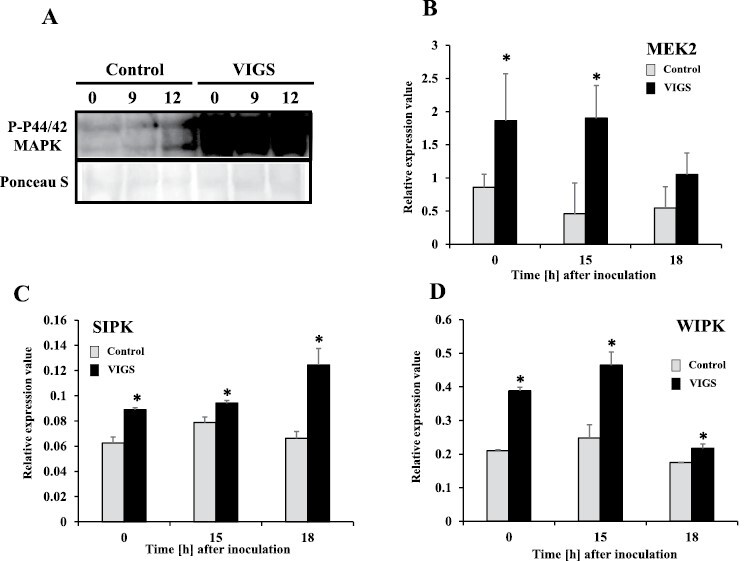
Hyper-induction of MAP kinase cascade during the accelerated HR in NbPLC3s-silenced plants. Empty vector control and NbPLC3s-silenced plant leaves were infiltrated with R. solanacearum strain 8107. (A) MAPK activation was analyzed by immunoblots with anti-P-p44/42 MAPK (T202/Y204) antibody 0, 9, and 12 h after inoculation. (B–D) Total RNA was isolated from control and NbPLC3s-silenced plants at 0, 15, and 18 h after inoculation with R. solanacearum 8107. Expression values of MEK2, SIPK and WIPK are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test).
To determine whether the acceleration of the HR by Rs8107 in NbPLC3s-silenced plants involved the MAPK cascade, we generated plants in which both NbPLC3s and NbMEK2 were silenced (NbPLC3s:MEK2 plants). The enhanced disease resistance phenotype was compromised in NbPLC3s:MEK2 plants compared with NbPLC3s-silenced plants, because the bacterial population recovered to numbers seen in control plants (Fig. 9A). Both the acceleration of HR-related cell death and Nbhin1 expression in response to Rs8107 were compromised in the NbPLC3s:MEK2 plants (Fig. 9B, C). We observed no significant change in bacterial population and cell death induction in single NbMEK2-silenced plants when compared with control plants (Supplementary Fig. S6). Therefore, the MAPK cascade might be involved in both acceleration of HR-related cell death induction and disease resistance in NbPLC3s-silenced plants.
Fig. 9.

Role of the MAPK pathway in accelerated HR in NbPLC3s-silenced plants. Empty vector control, NbPLC3s-silenced (PLC3s), and NbPLC3s:NbMEK2 double-knockdown (PLC3/MEK2) plant leaves were infiltrated with R. solanacearum strain 8107. (A) Bacterial populations were determined in control, PLC3s, and PLC3/MEK2 plants by plating at 0, 18, and 24 h after inoculation. Values are means ±SD of four replicate experiments. Asterisks indicate significant differences among control, PLC3s, and PLC3/MEK2 plants (P<0.05, t-test). (B) Cell death induction was determined by measuring the ion conductivity levels in control, PLC3s, and PLC3s/MEK2 plants. Values are means ±SD of four replicate experiments. (D) Total RNA was isolated from control, PLC3s, and PLC3/MEK2 plants at 0, 15, and 18 h after inoculation with R. solanacearum 8107. Expression values of Nbhin1 are shown as relative expression after normalization to internal standard genes (NbUbe35/NbNQO). Values represent means ±SD from triplicate experiments. Asterisks denote values significantly different from those of control plants (*; P<0.05, t-test).
Discussion
Plant PI-PLCs have been implicated in a number of cellular processes and signal transduction events (Pokotylo et al., 2014). Nine PI-PLCs, PI-PLC1–9, have been identified in Arabidopsis thaliana (Tasma et al. 2008). Recently, PI-PLCs have been identified in the tomato genome and classified into seven groups (Abd-El-Haliem et al., 2016). We previously found 12 PI-PLC orthologs, classified into seven groups, in N. benthamiana (Kiba et al., 2020). In this research, we analyzed two PI-PLC3 orthologs, NbPLC3-1 (Niben101Scf02280g02004.1) and NbPLC3-2 (Niben101Scf04093g00004.1; Supplementary Fig. S2A). The deduced amino acid sequences of the full-length cDNAs of NbPLC3-1 and NbPLC3-2 contained complete PI-PLC-X, PI-PLC-Y, and PI3K-C2 domains, which are required for enzymatic activity, suggesting that both NbPLC3-1 and NbPLC3-2 encode an enzymatically active form of PI-PLC3 (Supplementary Fig. S2B, C). PLCs convert phosphatidylinositol 4,5-biphosphate (PIP2) into inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG; Don et al., 2012). DAG is rapidly converted to phosphatidic acid (PA) in plant cells. PA might act as an intracellular second messenger to activate downstream signaling enzymes such as RboH and PDK (Putta et al., 2016). PA is a key lipid signaling molecule, and is involved in stress responses, in metabolism, and in development (Testerlink et al., 2011). Another PLC product, IP3, participates in the response to various abiotic stresses, gravitropism, phototropism, and auxin transport (Salinas-Mondragon et al., 2010; Zhang et al., 2011). Further analysis is required to understand enzymatic function, substrates, products, and downstream signaling related to NbPLC3s.
PI-PLCs affect biotic stress responses, including plant immunity (Canonne et al., 2011). Both the flagellin-triggered response and the internalization of the corresponding receptor, FLS2, of A. thaliana, are suppressed by the inhibition of PI-PLC activities (Abd-El-Haliem et al., 2016). PI-PLC2-silenced Arabidopsis plants are susceptible to the type III secretion system-deficient bacterial strain of Pseudomonas syringae pv. tomato DC3000 (hrcC-), indicating the role of PI-PLC2 in stomatal pre-invasive defenses (D’Ambrosio et al., 2017). Our previous report also showed that PI-PLC2 orthologs, NbPLC2s, may have important roles in pre- and post-invasive defenses, namely in the induction of PTI in N. benthamiana (Kiba et al., 2020). Thus, PLCs may play important roles in PTI induction. Several plant PI-PLC families are required for HR-mediated immune responses, including ETI (Canonne et al., 2011). Treatment with fungal xylanase leads to the rapid induction of PI-PLC activity and consequent HR in tomato suspension cells, and induction of HR is required to increase SlPLC5 expression (Raho et al., 2011). SlPLC2 is reportedly required for the HR because HR-related Hsr203J expression is suppressed in SlPLC2-silenced tomato plants (Gonorazky et al., 2014). The silencing of SlPLC6 results in HR reduction and increased colonization by Cladosporium fulvum in Cf-4 tomato plants. Avr4-induced HR is also compromised in SlPLC4-silenced Cf-4-carrying tomato. In addition, the heterologous expression of SlPLC4 results in an accelerated Avr4/Cf-4-induced HR in N. benthamiana (Abd-El-Haliem et al., 2016). Thus, HR and/or ETI induction are dependent on the functions of PI-PLC members. Intriguingly, our data showed that the silencing of NbPLC3s accelerated HR induction after inoculation with bacterial pathogens Rs8107 and P. cichorii, P. syringae pv. phaseolicola, and the transient expression of the bacterial effector AvrA and oomycete effector INF1 (Fig. 1; Supplementary Fig. S5). Accelerated HR was also observed by the concomitant expression of TMGMV-CP with L1 (Fig. 1; Supplementary Fig. S5). Thus, NbPLC3s may play important roles in the negative regulation of HR and/or ETI. Intriguingly, no changes in HR induction were observed in NbPLC3-1- or NbPLC3-2-individually silenced plants (Supplementary Fig. S4). Therefore, NbPLC3-1 and NbPLC3-2 may cooperatively regulate HR and/or induction. It is also possible that the activity of just one NbPLC3 is not sufficient to suppress the HR. In addition, the expression of NbPLC3-1 and NbPLC3-2 in NbPLC3-2-silenced and NbPLC3-1-silenced leaves, respectively, was significantly higher than the controls (Supplementary Fig. S3A). Therefore, NbPLC3-1 and NbPLC3-2 may mutually complement each other at the transcriptional level during the development of HR.
In plants, JA and SA are required for defense responses against R. solanacearum. Extracellular polysaccharides from R. solanacearum trigger the expression of genes involved in SA-mediated defenses in resistant tomato plants (Milling et al., 2011). The RipB effector strongly induces the production of SA-related defensive genes and the HR in N. benthamiana (Nakano and Mukaihara, 2019). The RipE1 effector induces the expression of JA-responsive genes and JA biosynthesis (Sang et al., 2020). Our previous report also showed an induction of both SA- and JA-mediated responses in response to Rs8107 in N. tabacum (Kiba et al., 2003, 2014). Therefore, drastic changes in the JA and SA signaling pathways may be required for immune responses against R. solanacearum. In fact, the overexpression of NtWRKY50 enhances bacterial resistance, which correlates with enhanced SA and JA signaling-related gene expression levels (Liu et al., 2017). A JA-dependent signaling pathway is required for Pythium oligandrum-induced resistance against R. solanacearum in tomato (Hase et al., 2008). SA induces disease resistance against R. solanacearum in tomato as a result of cell wall strengthening, through the deposition of lignin and the induction of defense-related enzymes (Mandal et al., 2013). The SA signaling pathway also plays a significant role in defense against R. solanacearum in several Solanum lines (Baichoo and Jaufeerally-Fakim, 2017). In this study, we showed an enhancement in disease resistance accompanied by the up-regulation of both JA and SA signaling in NbPLC3s-silenced plants (Figs 2–4). Therefore, NbPLC3s may negatively regulate disease resistance through these plant hormone signaling pathways during the HR against R. solanacearum. Inversely, cell death acceleration may not be regulated through JA and SA signaling by NbPLC3s (Figs 3, 4). These results provide novel insights into the regulatory roles of PI-PLC3s in phytohormone signaling during the HR.
ROS generation has been implicated in HR induction (Gechev and Hille 2005; Asai and Yoshioka, 2009; Yoshioka et al., 2009). In plants, NADPH oxidases, designated as RBOHs, have been identified as being encoded by genes related to mammalian gp91phox in various plants (Groom et al., 1996; Torres et al., 2002; Yoshioka et al., 2003). The silencing of NbrbohB compromises ROS generation during the HR, and NbrbohB-dependent ROS production has a significant role in HR induction (Yoshioka et al., 2003). In contrast, aerobic organisms have evolved antioxidant defenses that include catalases, peroxidases, and SODs, to minimize the damaging effects of ROS (Dumanovic et al., 2021). Plant tissue antioxidants counterbalance the damaging effects of ROS (Dey et al., 2020). However, if a severe infection occurs and the antioxidants fail to scavenge the over-produced ROS, then cell death occurs. Consequently, an elevation in the antioxidant potential of a plant should enhance its tolerance to the development of cell death caused by pathogens (Barna et al., 1993). In the present study, the acceleration of HR may have been due to the activation of NbrbohB-dependent ROS production, because HR-related cell death acceleration and enhanced disease resistance were compromised in the NbPLC3s and NbrbohB VIGS lines (Figs 5,6). In addition, reductions in NbAPX and NbSOD expression were also observed in NbPLC3s-silenced plants, indicating that the suppression of antioxidant enzymes might be involved in ROS hyper-production, leading to the accelerated HR in NbPLC3s-silenced plants (Fig. 5). Therefore, NbPLC3s may have dual regulatory roles in ROS generation, by affecting both ROS-generating and ROS scavenging systems during the HR. These results provide novel insights into the regulatory roles of PLC3 in the counterbalancing of ROS during the HR.
In addition to ROS signaling, members of the MAPK family, SIPK, WIPK, and NTF6 are involved in HR induction in response to PAMPs, INF1, and hyphal wall components from Phytophthora infestans in N. benthamiana (Yoshioka et al., 2003; Asai et al., 2008). Both WIPK and SIPK are also required to induce the N-gene-mediated HR to tobacco mosaic virus (Jin et al., 2003). LeMKK2, LeMKK3, and LeMPK3 are required for Pto-mediated HR against P. syringae pv. tomato carrying AvrPto (Ekengren et al., 2003). Our present results showed that HR-related cell death induction by NbMEK2DD expression was accelerated significantly in the NbPLC3s-silenced plants (Fig. 7). Activation of WIPK and SIPK was also enhanced in the NbPLC3s-silenced plants. In addition, transcriptional activation of MEK2, SIPK, and WIPK was observed in the NbPLC3s-silenced plants (Figs 7, 8). Furthermore, co-silencing of NbPLC3s and NbMEK2 compromised both accelerated HR-related cell death and bacterial population reduction in NbPLC3s-silenced plants inoculated with Rs8107 (Fig. 9). Therefore, the MAPK cascade might also participate in accelerated HR through NbPLC3s-silencing, and could be up-regulated in NbPLC3s-silenced plants at the transcriptional and post-translational level. Further analyses of the relationship between PLC3 and the MAPK cascade need to be performed to clarify the complex signaling interaction between phospholipids and MAPKs.
Plants have evolved sophisticated immune regulatory systems controlled by complex mechanisms (Tsuda and Katagiri, 2010). Based on the results with coi1, rbohB, MEK2-silenced plants, and NahG plants, we also suggest that these signaling components might regulate immune responses in a complex manner, and cannot drastically affect HR induction during the Rs8107 and N. benthamiana interaction by a single signaling cascade (Supplementary Fig. S6). The immune regulatory switching mechanisms might turn on immune responses after pathogen attacks. The switch follows with a deactivating ‘off’ signal to avoid self-inflicted damage to the plant (Lu et al., 2018; Mengarelli et al., 2021). Our previous report showed that other PLC orthologs, NbPLC2s, contribute to the induction of pre- and post-invasive PTI responses in plants (Kiba et al., 2020). Conversely, our present findings suggest that NbPLC3s negatively regulate plant immune responses, by suppression of HR. Therefore, the PLC family might switch the immune responses ‘on’ and ‘off’ during pathogen attack, which allows a cost-efficient way to prevent undesirable immune responses. Further studies are necessary to clarify the complex NbPLC-mediated signaling networks involved in switching plant immune signaling cascades ‘on’ and ‘off’.
From our findings, we conclude that while undergoing HR induction, NbPLC3s may be suppressing JA- and SA-mediated signaling as part of disease resistance suppression. In addition, NbPLC3s may down-regulate both HR-related cell death and disease resistance through the MAPK cascade and ROS production pathway in N. benthamiana. Further studies are necessary to clarify the complex NbPLC3-mediated signaling networks that act through MAPK, ROS, JA, and SA to characterize the phospholipid turnover involved in the HR signaling cascade. In summary, NbPLC3s act as negative HR regulators and fine tune the HR to prevent immune disruption, which has undesirable developmental effects.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Nucleotide sequences of NbPLC3-1 and NbPLC3-2.
Fig. S2. Characterization of phosphatidylinositol-specific phospholipase C3 (PI-PLC3) in Nicotiana benthamiana.
Fig. S3. Estimation of expression value of NbPLC3-1, NbPLC3-2, NbPLC1-2, NbPLC2-1, NbPLC2-2, Nbcoi1, NbrbohB, and NbMEK2.
Fig. S4. Effects of NbPLC3-1- and NbPLC3-2-specific silencing on bacterial population and hypersensitive cell death induction by incompatible Ralstonia solanacearum 8107.
Fig. S5. Effects of NbPLC3s-silencing on HR induction by Pseudomonas cichorii, Pseudomonas syringae pv. phaseolicola, AvrA, INF1, and TMGMV-CP with L1.
Fig. S6. Bacterial population and hypersensitive cell death induction by incompatible Ralstonia solanacearum 8107 in Nbcoi1, NbrbohB and NbMEK2-silenced plants and NahG plants.
Table S1. Primers used in this study.
Table S2. List of plasmids used in this study.
Acknowledgements
The authors thank S. Genin (INRA, France) for providing p35S-PopP1 and p35S-AvrA, and Prof. D. Baulcombe (University of Cambridge, UK) for the pPVX201 vector. We also thank Dr H. Yoshioka (Nagoya University, Japan) for providing p35S-INF1 and p35S-MEKDD. We thank to L. Benyon for editing a draft of this manuscript.
Glossary
Abbreviations
- CFU
colony forming unit
- DAB
3,3’-diaminobenzidine
- ETI
effector-triggered immunity
- HR
hypersensitive response
- JA
jasmonic acid
- MAPK
mitogen-activated protein kinase
- PA
phosphatidic acid
- PAMPs
pathogen-associated molecular patterns
- PLC
phospholipase C
- PTI
pathogen-associated molecular pattern-triggered immunity; ROS; reactive oxygen species
- SA
salicylic acid
- VIGS
virus-induced gene silencing.
Contributor Information
Shiori Takasato, Laboratory of Plant Pathology and Biotechnology, Faculty of Agriculture and Marine Science Kochi University, Nankoku, Kochi 783-8502, Japan.
Takuya Bando, Laboratory of Plant Pathology and Biotechnology, Faculty of Agriculture and Marine Science Kochi University, Nankoku, Kochi 783-8502, Japan.
Kouhei Ohnishi, Laboratory of Defense in Plant–Pathogen Interactions, Research Institute of Molecular Genetics, Kochi University, Nankoku, Kochi 783-8502, Japan.
Masayuki Tsuzuki, Laboratory of Plant Pathology and Biotechnology, Faculty of Agriculture and Marine Science Kochi University, Nankoku, Kochi 783-8502, Japan.
Yasufumi Hikichi, Laboratory of Plant Pathology and Biotechnology, Faculty of Agriculture and Marine Science Kochi University, Nankoku, Kochi 783-8502, Japan.
Akinori Kiba, Laboratory of Plant Pathology and Biotechnology, Faculty of Agriculture and Marine Science Kochi University, Nankoku, Kochi 783-8502, Japan.
Monica Höfte, University of Ghent, Belgium.
Author contributions
AK, KO, MT, and YH designed the research; AK, ST, and TB performed the research; AK analyzed the data and wrote the article.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by a Cabinet Office Grant-in-Aid, the Advanced Next-Generation Greenhouse Horticulture by the Internet of Plants (IoP), Japan. AK is also grateful for financial support from Grants-in-Aid for Scientific Research (24580066) from the Ministry of Education, Science, Sports, and Culture, Japan, the Asahi Glass Foundation, the Agricultural Chemical Research Foundation and the Sapporo Bioscience Foundation.
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online.
References
- Abd-El-Haliem, AM, Vossen,JH, van Zeijl, A, Dezhsetan, S, Testerink, C, Seidl, MF, Beck M, Strutt J, Robatzek S, Joosten MHA.. 2016. Biochemical characterization of the tomato phosphatidylinositol-specific phospholipase C (PI-PLC) family and its role in plant immunity. Biochimica Biophysica Acta 1861, 1365–1378. [DOI] [PubMed] [Google Scholar]
- Ahuja I, Kissen R, Bones AM.. 2012. Phytoalexins in defense against pathogens. Trends in Plant Sciences 17, 73–90. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Liptman DJ.. 1990. Basic local alignment search tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Asai S, Ohta K, Yoshioka H.. 2008. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. The Plant Cell 20, 1390–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai S, Yoshioka H.. 2009. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis cinerea in Nicotiana benthamiana. Molecular Plant-Microbe Interactions 22, 619–629. [DOI] [PubMed] [Google Scholar]
- Baichoo Z, Jaufeerally-Fakim Y.. 2017. Ralstonia solanacearum upregulates marker genes of the salicylic acid and ethylene signaling pathways but not those of the jasmonic acid pathway in leaflets of Solanum lines during early stage of infection. European Journal of Plant Pathology 147, 615–625. [Google Scholar]
- Baker CG, Orlandi EW.. 1995. Active oxygen in plant pathogenesis. Annual Review of Phytopathology 33, 299–321. [DOI] [PubMed] [Google Scholar]
- Barna B, Adam A, Kiraly Z.. 1993. Juvenility and resistance of a superoxide-tolerant plant to disease and other stresses. Naturwissenshaften 80, 420–422. [Google Scholar]
- Baulcombe DC, Chapman S, Cruz SS.. 1995. Jellyfish green fluorescent protein as a reporter for virus infections. The Plant Journal 7, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Dangl JL.. 1997. The hypersensitive response and the induction of cell death in plants. Cell Death and Differentiation 4, 671–683. [DOI] [PubMed] [Google Scholar]
- Bigeard J, Colcombet J, Hirt H.. 2014. Signaling mechanisms in pattern-triggered immunity (PTI). Molecular Plant 8, 521–539. [DOI] [PubMed] [Google Scholar]
- Bittel P, Robatzek S.. 2007. Microbe-associated molecular patterns (MAMPs) probe plant immunity. Current Opinion in Plant Biology 10, 335–341. [DOI] [PubMed] [Google Scholar]
- Boller T, He SY.. 2009. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradly D, Kjellbom P, Lamb CJ.. 1992. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel rapid defense response. Cell 70, 21–30. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb CJ.. 1994. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. The Plant Cell 6, 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonne J, Froidure-Nicolas S, Rivas S.. 2011. Phospholipases in action during plant defense signaling. Plant Signaling & Behavior 6, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan J, Pichereaux C, Rossignol M, Blanc S, Wendehenne D, Pugin A, Bourque S.. 2009. Activation of a nuclear-localized SIPK in tobacco cells challenged by cryptogein, an elicitor of plant defence reactions. Biochemical Journal 418, 191–200. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio JM, Couto D, Fabro G, Scuffi D, Lamattina L, Munnik T, Andersson MX, Álvarez ME, Zipfel C, Laxalt AM.. 2017. Phospholipase C2 Affects MAMP-triggered immunity by modulating ROS production. Plant Physiology 175, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey N, Roy UK, Aditya M, Bhattacharjee S.. 2020. Defensive strategies of ROS in programmed cell death associated with hypertensive response in plant pathogenesis. Annals of Systems Biology 3, 001–009. [Google Scholar]
- Dumanovic´ J, Nepovimova E, Natic M, Kuc K, Jacevic V.. 2021. The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Frontiers in Plant Science. doi: 10.3389/fpls.2020.552969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB.. 2003. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. The Plant Journal 36, 905–917. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pozo N, Menda N, Edwards JDJ, et al. 2014. The Sol Genomics Network (SGN)––from genotype to phenotype to breeding. Nucleic Acids Research 43, doi: 10.1093/nar/gku1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pozo N, Rosli HG, Martin GB, Mueller LA.. 2015. The SGN VIGS tool: user-friendly software to design Virus-Induced Gene Silencing (VIGS) constructs for functional genomics. Molecular Plant 8, 486–488. [DOI] [PubMed] [Google Scholar]
- Gechev TS, Hille J.. 2005. Hydrogen peroxide as a signal controlling plant programmed cell death. Journal of Cell Science 168, 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonorazky G, Ramireza L, Abd-El-Haliemb A, Vossenc JH, Lamattinaa L, ten Have A, Joosten MHA, Laxalt A.. 2014. The tomato phosphatidylinositol-phospholipase C2 (SlPLC2) is required for defense gene induction by the fungal elicitor xylanase. Journal of Plant Physiology 171, 959–965. [DOI] [PubMed] [Google Scholar]
- Groom QJ, Torres M, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG.. 1996. RbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. The Plant Journal 10, 515–522. [DOI] [PubMed] [Google Scholar]
- Gupta M, Yoshioka H, Ohinishi K, Mizumoto H, Hikichi Y, Kiba A.. 2013. A translationally controlled tumor protein negatively regulates the hypersensitive response in Nicotiana benthamiana. Plant Cell Physiology 54, 1403–1414. [DOI] [PubMed] [Google Scholar]
- Hase S, Takahashi S, Takenaka S, Nakaho K, Arie T, Seo S, Ohashi Y, Takahashi H.. 2008. Involvement of jasmonic acid signalling in bacterial wilt disease resistance induced by biocontrol agent Pythium oligandrum in tomato. Plant Pathology 57, 870–876. [Google Scholar]
- Huitema E, Vleeshouwers VGAA, Cakir C, Kamoun S, Govers F.. 2005. Differences in intensity and specificity of hypersensitive response induction in Nicotiana spp. by INF1, INF2A, and INF2B of Phytophthora infestans. Molecular Plant-Microbe Interactions 18, 183–193. [DOI] [PubMed] [Google Scholar]
- Jeandet P. 2015. Phytoalexins: current progress and future prospects. Molecules 20, 2770–2774. [Google Scholar]
- Jeworutzki E, Roelfsema MR, Anschutz U, Krol E, Elzenga JT, Felix G, Boller T, Hedrich R, Becker D.. 2010. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca-associated opening of plasma membrane anion channels. The Plant Journal 62, 367–378. [DOI] [PubMed] [Google Scholar]
- Jin H, Liu Y, Yang KY, Kim CY, Baker B, Zhang S.. 2003. Function of mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. The Plant Journal 33, 719–731. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL.. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Katou S, Yamamoto A, Yoshioka H, Kawakita K, Doke N.. 2003. Functional analysis of potato mitogen-activated protein kinase kinase, StMEK1. Journal of General Plant Pathology 69, 161–168. [Google Scholar]
- Kiba A, Galis I, Hojo Y, Ohnishi K, Yoshioka H, Hikichi Y.. 2014. SEC14 phospholipid transfer protein is involved in lipid signaling-mediated plant immune responses in Nicotiana benthamiana. PLoS One 9, e98150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba A, Nakano M, Hosokawa M, Galis I, Nakatani H, Shinya T, Ohnishi K, Hikichi Y.. 2020. Phosphatidylinositol-phospholipase C2 regulates pattern-triggered immunity in Nicotiana benthamiana. Journal of Experimental Botany 71, 5027–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba A, Nakano M, Vincent-Pope P, Takahashi H, Sawasaki T, Endo Y, Ohnishi K, Yoshioka H, Hikichi Y.. 2012. A novel Sec14 phospholipid transfer protein from Nicotiana benthamiana is up-regulated in response to Ralstonia solanacearum infection, pathogen associated molecular patterns and effector molecules and involved in plant immunity. Journal of Plant Physiology 169, 1017–1022. [DOI] [PubMed] [Google Scholar]
- Kiba A, Tomiyama H, Takahashi H, Hamada H, Ohnishi K, Okuno T, Hikichi Y.. 2003. Induction of resistance and expression of defense-related genes in tobacco leaves infiltrated with Ralstonia solanacearum. Plant and Cell Physiology 44, 287–295. [DOI] [PubMed] [Google Scholar]
- Lee CC, Wu YJ, Hsueh CH, Huang YT, Hsu YH, Meng M.. 2018. Mitogen-activated protein kinase phosphatase 1 reduces the replication efficiency of Bamboo mosaic virus in Nicotiana benthamiana. Molecular Plant Pathology 19, 2319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ma X, Chiang YHH, et al. 2014. Proline isomerization of the immune receptor-interacting protein RIN4 by a cyclophilin inhibits effector-triggered immunity in Arabidopsis. Cell Host and Microbe 16, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst HJM, VanLoon LC.. 2008. Pathogenesis-related proteins of plants. Critical Reviews in Plant Science 10, 123–150. [Google Scholar]
- Liu Q, Liu Y, Tang Y, Chen J, Ding W.. 2017. Overexpression of NtWRKY50 increases resistance to Ralstonia solanacearum and alters salicylic acid and jasmonic acid production in tobacco. Frontiers in Plant Science 8, 1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Truman W, Liu X, Bethke G, Zhou M, Myers CL, Katagiri F, Glazebrook J.. 2018. Different Modes of negative regulation of plant immunity by calmodulin-related genes. Plant Physiology 176, 3046–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimbo M, Ohnishi K, Hikichi Y, Yoshioka H, Kiba A.. 2007. Induction of a small heat shock protein and its functional roles in Nicotiana Plants in the defense response against Ralstonia solanacearum. Plant Physiology 145, 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimbo M, Ohnishi K, Hikichi Y, Yoshioka H, Kiba A.. 2010. S glycoprotein-like protein regulates defense responses in Nicotiana plants against Ralstonia solanacearum. Plant Physiology 152, 2023–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Kar I, Mukherjee AK, Acharya P.. 2013. Elicitor-induced defense responses in Solanum lycopersicum against Ralstonia solanacearum. Scientific World Journal Article ID 561056, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengarelli DA, Tewes LR, Balazadeh S, Zanor MI.. 2021. FITNESS acts as a negative regulator of immunity and influences the plant reproductive output after Pseudomonas syringae infection. Frontiers in Plant Science. doi: 10.3389/fpls.2021.606791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milling A, Babujee L, Allen C.. 2011. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One 6, e15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Mukaihara T.. 2019. The type III effector RipB from Ralstonia solanacearum RS1000 acts as a major avirulence factor in Nicotiana benthamiana and other Nicotiana species. Molecular Plant Pathology 20, 1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishad R, Ahmed T, Rahman VJ, Kareem A.. 2020. Modulation of plant defense system in response to microbial interactions. Frontiers in Microbiology 11, 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn AE. 1996. Preformed antimicrobial compounds and plant defense against funga1 attack. The Plant Cell 8, 1821–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S, Melotto M.. 2017. Stomate-based defense and environmental cues. Plant Signaling & Behavior 12, doi: 10.1080/15592324.2017.1362517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórska A, Burian M, Szal B.. 2017. Extra-cellular but extra-ordinarily important for cells: apoplastic reactive oxygen species metabolism. Frontiers in Plant Science 8, 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokotylo I, Kolesnikov Y, Kravets V, Zachowski A, Ruelland E.. 2014. Plant phosphoinositide -dependent phospholipases C: variations around a canonical theme. Biochimie 96, 144–157. [DOI] [PubMed] [Google Scholar]
- Pombo MA, Ramos RN, Zheng Y, Fei Z, Martin GB, Rosli HG.. 2018. Transcriptome-based identification and validation of reference genes for plant-bacteria interaction studies using Nicotiana benthamiana. Scientific Reports 9, 1632. doi: 10.1038/s41598-018-38247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poueymiro M, Cunnac S, Barberis P, Deslandes L, Peeters N, Cazale-Noel AC, Boucher C, Genin S.. 2009. Two type III secretion system effectors from Ralstonia solanacearum GMI1000 determine host-range specificity on tobacco. Molecular Plant-Microbe Interactions 22, 538–550. [DOI] [PubMed] [Google Scholar]
- Putta P, Rankenberg J, Korver RA, van Wijk R, Munnik T, Testerink C, Kooijman EE.. 2016. Phosphatidic acid binding proteins display differential binding as a function of membrane curvature stress and chemical properties. Biochimica et Biophysica Acta 1858, 2709–2716. [DOI] [PubMed] [Google Scholar]
- Raho N, Ramirez L, Lanteri ML, Gonorazky G, Lamattina L, ten Have A, Laxalt AM.. 2011. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. Journal of Plant Physiology 168, 534–539. [DOI] [PubMed] [Google Scholar]
- Romeis T, Ludwig AA, Martin R, Jones JDG.. 2001. Calcium-dependent protein kinases play an essential role in a plant defence response. The EMBO Journal 20, 5556–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Mondragon RE, Kajla JD, Perera IY, Brown CS, Sederoff HW.. 2010. Role of inositol 1,4,5-triphosphate signalling in gravitropic and phototropic gene expression. Plant Cell and Environment 33, 2041–2055. doi: 10.1111/j.1365-3040.2010.02204.x. [DOI] [PubMed] [Google Scholar]
- Sang Y, Yu W, Zhuang H, Wei Y, Derevnina L, Yu G, Luo J, Macho AP.. 2020. Intra-strain elicitation and suppression of plant immunity by Ralstonia solanacearum type-III effectors in Nicotiana benthamiana. Plant Communications 1, 100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen PJ.. 2011. Hierarchy and roles of PAMP-induced responses in Nicotiana benthamiana. Plant Physiology 156, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Bhatnagar N, Pandey A, Pandey GK.. 2015. Plant phospholipase C family: regulation and functional role in lipid signaling. Cell Calcium 58, 139–146. [DOI] [PubMed] [Google Scholar]
- Tasma IM, Brendel V, Whitham SA, Bhattacharyya MK.. 2008. Expression and evolution of the phosphoinositide-specific phospholipase C gene family in Arabidopsis thaliana. Plant Physiology and Biochemistry 46, 627–637. [DOI] [PubMed] [Google Scholar]
- Testerink C, Munnik T.. 2011. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. Journal of Experimental Botany 62, 2349–2361. doi: 10.1093/jxb/err079. [DOI] [PubMed] [Google Scholar]
- Thatcher LF, Anderson JP, Singh KB.. 2004. Plant defence responses: what have we learnt from Arabidopsis? Functional Plant Biology 32, 1–19. doi: 10.1071/FP04135. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD.. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA 99, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F.. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current Opinion in Plant Biology 13, 459–465. [DOI] [PubMed] [Google Scholar]
- Ueta Y, Mizutani Y, Ohnishi K, Hikichi Y, Kibna A.. 2021. Phosphatidylinositol-phospholipase C1 negatively regulates the hypersensitive response in Nicotiana benthamiana. Physiological and Molecular Plant Pathology 116, 101724. [Google Scholar]
- Vance CP, Kink IK, Sherwood RT.. 1980. Lignification as a mechanism of disease resistance. Annual Review of Phytopathology 18, 259–288. [Google Scholar]
- Voigt CA. 2014. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Frontiers in Plant Science 5, doi: 10.3389/fpls.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Asai S, Yoshioka M, Kobayashi M.. 2009. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Molecular Cells 28, 321–329. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N.. 2003. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. The Plant Cell 15, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Jauregui E, Du L, Tanaka K, Poovaiah B.. 2017. Calcium signatures and signaling events orchestrate plant–microbe interactions. Current Opinion in Plant Biology 38, 173–183. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Zhang X, Wu F, Feng H, Deng L, Xu L, Zhang M, Wang Q, Li C.. 2015. Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering time in Arabidopsis. Plant Cell 27, 2814–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF.. 1998. The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proceedings of the National Academy of Sciences, USA 95, 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Vanneste S, Brewer PB, et al. 2011. Inositol trisphosphate-induced Ca2+ signaling modulates auxin transport and PIN polarity. Developmental Cell 20, 855–866. doi: 10.1016/j.devcel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Ziv C, Zhao Z, Gao YG, Xia Y.. 2018. Multifunctional roles of plant cuticle during plant-pathogen interactions. Frontiers in Plant Science 9, doi: 10.3389/fpls.2018.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary data published online.


