ABSTRACT
T cell immunity is crucial for long-term immunological memory, but the profile of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific memory T cells in individuals who recovered from COVID-19 (COVID-19-convalescent individuals) is not sufficiently assessed. In this study, the breadth and magnitude of SARS-CoV-2-specific T cell responses were determined in COVID-19-convalescent individuals in Japan. Memory T cells against SARS-CoV-2 were detected in all convalescent individuals, and those with more severe disease exhibited a broader T cell response relative to cases with mild symptoms. Comprehensive screening of T cell responses at the peptide level was conducted for spike (S) and nucleocapsid (N) proteins, and regions frequently targeted by T cells were identified. Multiple regions in S and N proteins were targeted by memory T cells, with median numbers of target regions of 13 and 4, respectively. A maximum of 47 regions were recognized by memory T cells for an individual. These data indicate that SARS-CoV-2-convalescent individuals maintain a substantial breadth of memory T cells for at least several months following infection. Broader SARS-CoV-2-specific CD4+ T cell responses, relative to CD8+ T cell responses, were observed for the S but not the N protein, suggesting that antigen presentation is different between viral proteins. The binding affinity of predicted CD8+ T cell epitopes to HLA class I molecules in these regions was preserved for the Delta variant and at 94 to 96% for SARS-CoV-2 Omicron subvariants, suggesting that the amino acid changes in these variants do not have a major impact on antigen presentation to SARS-CoV-2-specific CD8+ T cells.
IMPORTANCE RNA viruses, including SARS-CoV-2, evade host immune responses through mutations. As broader T cell responses against multiple viral proteins could minimize the impact of each single amino acid mutation, the breadth of memory T cells would be one essential parameter for effective protection. In this study, breadth of memory T cells to S and N proteins was assessed in COVID-19-convalescent individuals. While broad T cell responses were induced against both proteins, the ratio of N to S proteins for breadth of T cell responses was significantly higher in milder cases. The breadth of CD4+ and CD8+ T cell responses was also significantly different between S and N proteins, suggesting different contributions of N and S protein-specific T cells for COVID-19 control. Most CD8+ T cell epitopes in the immunodominant regions maintained their HLA binding to SARS-CoV-2 Omicron subvariants. Our study provides insights into understanding the protective efficacy of SARS-CoV-2-specific memory T cells against reinfection.
KEYWORDS: SARS-CoV-2, COVID-19, T cell immunity, variants
INTRODUCTION
Tcell immunity plays a critical role in controlling viral infection and immunological memory. Multiple studies have been conducted on T cell responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using blood samples from COVID-19 patients or individuals recovering from COVID-19 and suggest the importance of T cell responses in protective immunity to COVID-19 (1–6).
The quality of T cell immunity is generally represented by the frequency of specific T cells and their functions, e.g., cytokine production, cytotoxicity, and proliferative capacity, whereas the breadth of T cell responses is also a critical parameter. While neutralizing antibodies target a limited array of proteins that block virus entry, T cells can recognize peptides derived from multiple viral proteins and directly kill virus-infected cells. Following SARS-CoV-2 infection, T cell responses are induced to multiple targets across the virus proteome (5, 7–9).
A single amino acid substitution within an epitope is sufficient for complete evasion from a T cell response, and escape from T cell responses is associated with disease progression in infections caused by viruses with high mutation rates (10–13). SARS-CoV-2 variants have emerged continuously throughout the pandemic and most possess escape mutations from neutralizing antibodies (14–18), a major concern due to the reduction of efficacy of preexisting or vaccine-induced antibodies. However, the role of amino acid substitutions in SARS-CoV-2 variants in viral escape from memory T cells remains unclear. Therefore, it is crucial to identify individual regions that are recognized by T cells and to assess how amino acid mutations in these regions affect cognate T cell responses.
Although several studies have performed identification of SARS-CoV-2 immunodominant regions, most studies focused on epitopes presented by prevalent HLA molecules for a specific geographic region (7, 19–31), with a limited number of studies performing a comprehensive analysis (5, 9, 32). One assessment of SARS-CoV-2-specific T cells across the entire viral proteome using peripheral blood mononuclear cells (PBMCs) was performed in North America (9), with complete mapping of CD4+ T cell responses to spike (S), nucleocapsid (N), and membrane (M) proteins. However, analysis of CD8+ T cell responses focused on epitopes presented by common HLAs for that region.
Identification of antigenic regions eliciting T cell responses requires large numbers of PBMCs; hence, blood volume collection limitations pose a major challenge. Furthermore, virus-specific T cells contract following clearance of viral antigens, with a relatively small number maintained as memory against reinfection. This reduced memory T cell population in peripheral blood may underestimate a single peptide-specific response because of their low frequency or being masked by background responses in assays frequently used for such analyses, including enzyme-linked immunosorbent spot assay (ELISpot) and flow cytometry. In the present study, PBMCs from Japanese individuals who recovered from COVID-19 (COVID-19-convalescent individuals) were expanded with SARS-CoV-2 antigens in vitro to acquire higher numbers of SARS-CoV-2-specific T cells and increase the sensitivity of our assays. Comprehensive screening of T cell responses to S and N proteins was performed using in vitro-expanded PBMCs. Regions frequently recognized by memory T cells were identified, and the impact of amino acid mutations in these regions on T cell responses was assessed for SARS-CoV-2 variants.
RESULTS
Assessment of SARS-CoV-2-specific memory T cells with ex vivo PBMCs.
SARS-CoV-2-specific T cell responses were first measured with an interferon gamma (IFN-γ) ELISpot assay using PBMCs from 38 individuals who recovered from COVID-19 (Table 1). The distribution of HLA class I alleles was equivalent to that in the general Japanese population (33) (see Fig. S1 in the supplemental material). The maximum numbers of days post-symptom onset (DPSO) of blood sampling were 313 and 299 for the severe and mild cases, respectively.
TABLE 1.
Participant characteristics
| Parameter | Value for group |
P value | ||
|---|---|---|---|---|
| All | Severe | Mild | ||
| No. of patients | 38 | 10 | 28 | |
| Sex, no. (%) | ||||
| Male | 26 (68.4) | 6 (60) | 20 (71.4) | |
| Female | 12 (31.6) | 4 (40) | 8 (28.6) | |
| Age (yrs) | 45 (IQR, 32–53.5) | 44 (IQR, 37–52) | 47 (IQR, 29–54) | |
| DPSOa at sampling | 135 (IQR, 90–219) | 257 (IQR, 111.5–297) | 129.5 (IQR, 86.5–183) | 0.0314 |
Days post-symptom onset.
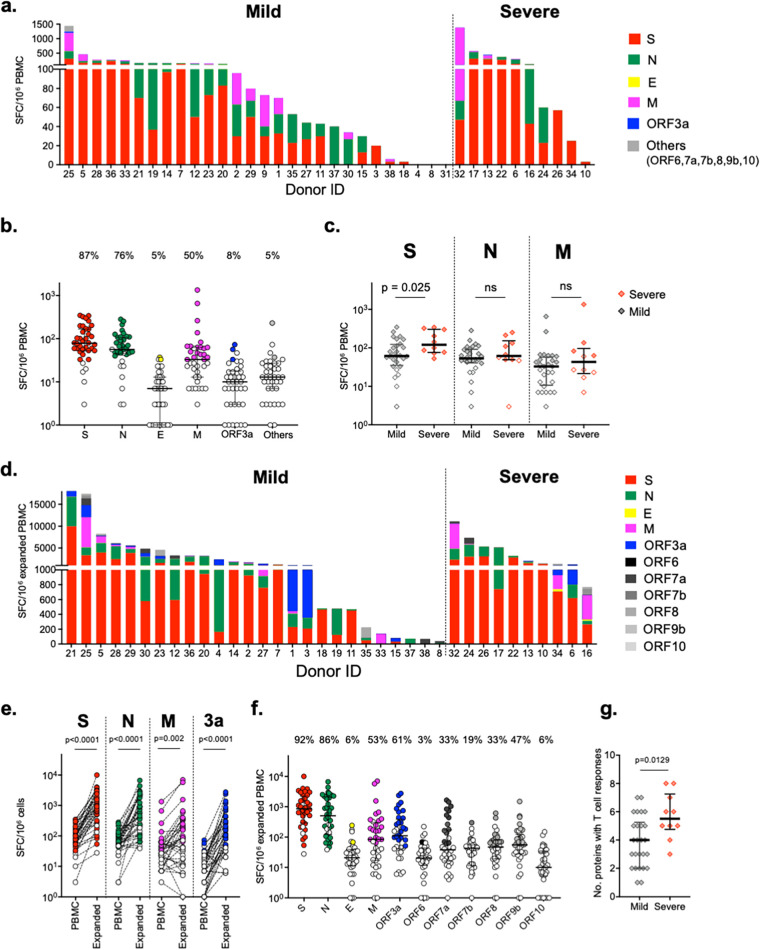
Due to cell number limitations, overlapping peptide (OLP) pools of selected SARS-CoV-2 proteins (ORF6, ORF7a, ORF7b, ORF8, ORF9b, and ORF10) were combined for the ELISpot assay. T cell responses against at least a single viral protein were detected in 35 of 38 (92%) individuals. The magnitude of T cell responses varied considerably between individuals (Fig. 1a). The highest frequency of T cell responses was to the S antigen (33/38 individuals [87%]), followed by the N (76%) and M (50%) proteins (Fig. 1b). The magnitude of T cell responses was highest to the S protein (median, 78 spot-forming cells [SFC]/million PBMCs; interquartile range [IQR], 52 to 168), followed by the N (median, 57 SFC/million PBMCs; IQR, 46 to 108) and M (median, 33 SFC/million PBMCs; IQR, 13 to 63) proteins. The magnitude of S protein-specific T cells, but not T cells specific to N or M protein, was significantly higher in individuals with severe symptoms (severe group) than those with mild symptoms (mild group) (P = 0.025) (Fig. 1c). The median number of proteins recognized was 2 for both the mild and severe groups (data not shown).
FIG 1.
Magnitude of SARS-CoV-2-specific memory T cells in COVID-19-convalescent individuals. T cell responses against SARS-CoV-2 proteins in PBMCs (a to c) and in vitro expanded PBMCs (d to g) were measured by the IFN-γ ELISpot assay. Bars show the total T cell responses specific to all SARS-CoV-2 proteins tested for each individual. (a and d) Corrected (minus the negative control) SFC/106 PBMCs. (b and f) Magnitude (SFC/106 PBMCs) and frequency (percent) of T cell responses against each protein. (c) Magnitude (SFC/106 PBMCs) of T cell responses specific for the indicated proteins between groups with mild and severe COVID-19. (e) Magnitude of T cell-specific responses to SARS-CoV-2 proteins before (PBMC) and after (Expanded) in vitro expansion. (g) Number of proteins recognized by memory T cells for the 11 SARS-CoV-2 proteins tested in the mild and the severe groups. Negative responses are shown as open circles in panels b, c, e, and f. Differences between groups were determined using the paired t test (ex vivo versus expanded PBMCs) and the Mann-Whitney test. ns, not significant.
Assessment of SARS-CoV-2-specific memory T cells with in vitro-expanded PBMCs.
PBMCs were cultured with SARS-CoV-2 antigens in the presence of interleukin 2 (IL-2) for 2 weeks to expand SARS-CoV-2-specific T cells. The average total number of expanded PBMCs increased about 9-fold after 2 weeks of culture (data not shown). T cell responses against all tested SARS-CoV-2 proteins were also assessed using expanded PBMCs (Fig. 1d). The magnitude of S (median, 864 SFC/million PBMCs; IQR, 289 to 2,279) and N (median, 505 SFC/million PBMCs; IQR, 124 to 2,130) protein-specific T cells after expansion was significantly correlated with that of ex vivo PBMCs (Fig. S2), and both were significantly higher (approximately 10-fold) than that of PBMCs (P < 0.0001) (Fig. 1e and f). T cells specific for other SARS-CoV-2 proteins were also detected in the expanded PBMCs, but with considerably higher frequencies than ex vivo PBMCs. Interestingly, while the frequency of T cell responses against E and M proteins remained similar following in vitro PBMC expansion, T cell responses to ORF3a protein were detected in 22 individuals (61%), compared to only ex vivo PBMCs from 3 individuals (8%) (Fig. 1e). These data suggest that the overall sensitivity of detection of T cell response is higher when expanded PBMCs are used in this assay. Each donor had T cell responses to multiple viral proteins in the expanded PBMCs, with a median of 4.5 proteins per individual (Fig. 1d). These results indicate that memory T cells against SARS-CoV-2 proteins possess proliferative capacity to antigen stimulation and are maintained in individuals who have recovered from COVID-19 for at least several months. Although no significant difference was found in the magnitude of T cell responses against each protein between COVID-19 severity (data not shown), unlike the finding with ex vivo PBMCs, the number of proteins recognized by T cells was significantly higher in the severe group than in the mild group (Fig. 1g).
Comprehensive identification of T cell target regions in S and N proteins.
To evaluate the breadth of SARS-CoV-2-specific memory T cells, target peptides in S and N proteins were screened with two-dimensional peptide matrices using the ELISpot assay (Fig. S3).
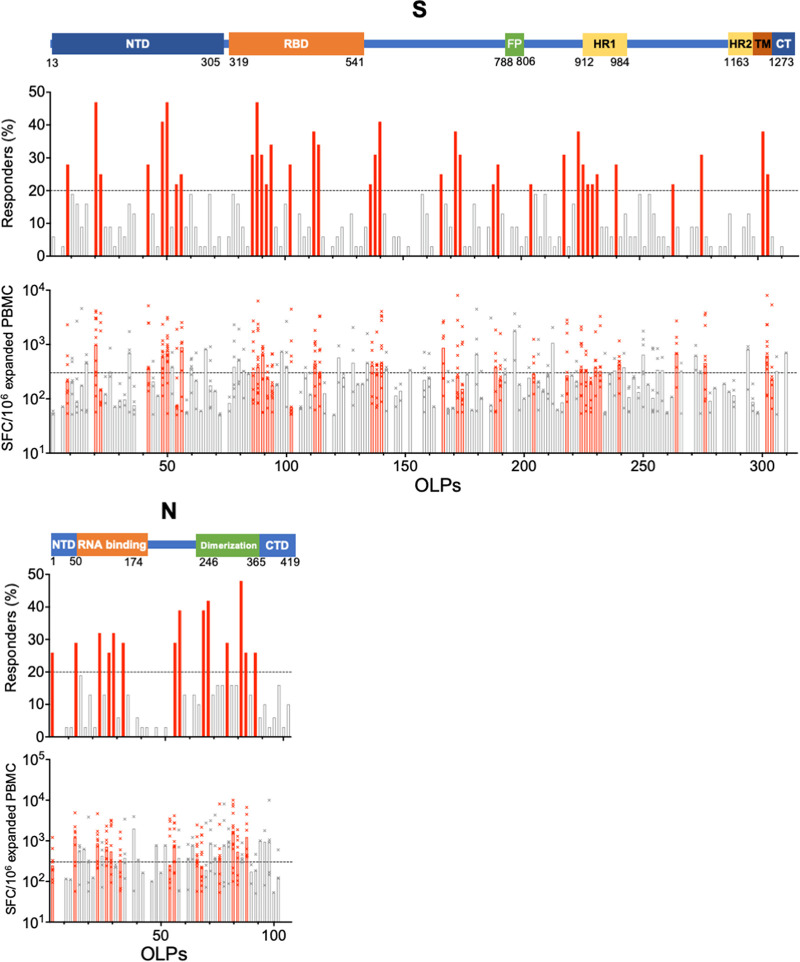
T cell target peptides were successfully identified from 32 and 31 convalescent individuals for S and N proteins, respectively, using expanded PBMCs (Fig. 2). T cell target peptides were widely distributed across each protein, with 133/157 (84.7%) and 46/51 (90.2%) OLP pairs recognized by at least one individual for S and N proteins, respectively (Fig. 2). Several peptides were commonly recognized across individuals. Thirty-six and 14 OLP pairs representing 22.7% and 27.5% of S and N proteins, respectively, were recognized by T cells in more than 20% of individuals. These peptide regions were defined as frequent T cell target regions (Fig. 2 and 3).
FIG 2.
Distribution of T cell-target regions in spike (S) and nucleocapsid (N) proteins. Frequency of responders (upper graphs) and magnitude of T cells response (lower graphs) to individual overlapping peptide (OLP) pairs in SARS-CoV-2 S and N proteins are shown. The OLP pairs that recognized more than 20% of tested individuals are shown as red bars. For graphs showing frequency of responders, the black line at the y axis indicates the cutoff for defining regions frequently targeted by T cells (20%). For graphs showing the magnitude of the ELISpot response, the black line on the y axis shows the cutoff for samples selected for ICS (≥300 SFC/million expanded PBMCs). NTD, N-terminal domain; RBD, receptor binding domain; FP, fusion peptide; CT, C terminus; CTD, C-terminal domain.
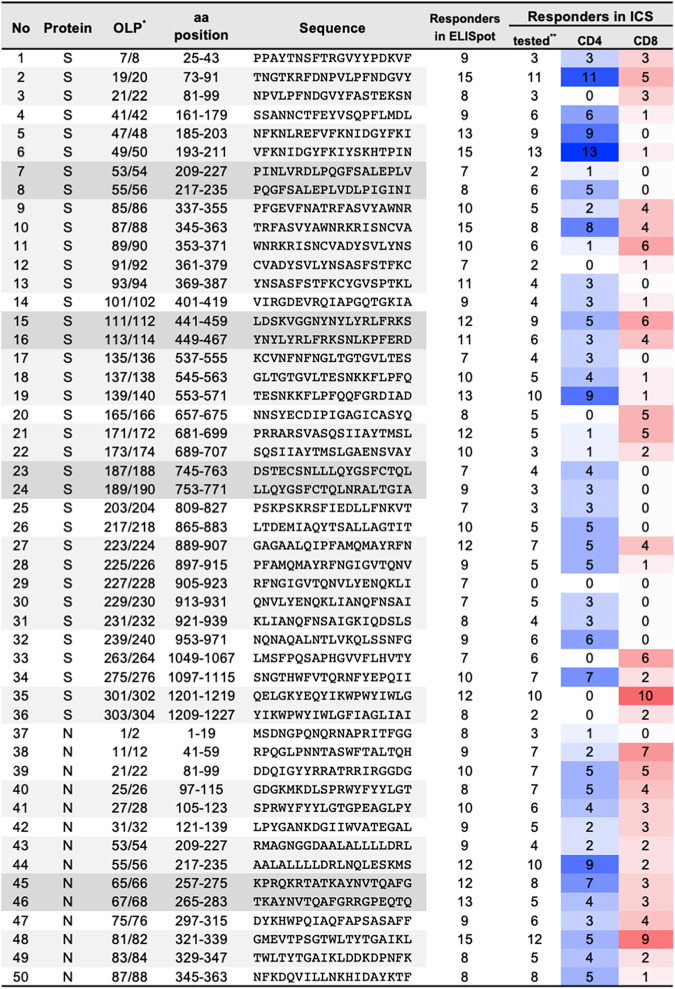
FIG 3.
Frequent T cell target regions in spike and nucleocapsid proteins. aa, amino acid. *, overlapped peptide pairs are shown by shading. **, the numbers of the samples for which ICS was performed (the samples with ≥300 SFC/expanded PBMCs in the ELISpot assay) are shown. The frequencies of the responders with CD4+ and CD8+ T cell responses are shown as the intensities of blue and red, respectively.
Evaluation of the breadth of T cell responses in S and N proteins.
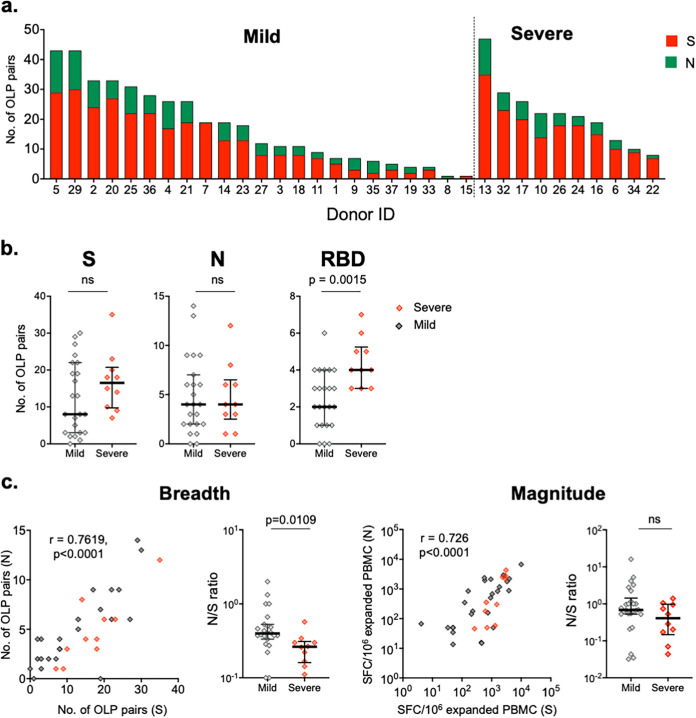
Measurement of the breadth of T cell responses against S and N proteins in COVID-19-convalescent individuals was performed as follows. Positive T cell responses in adjacent OLP pairs were counted as 1 (the minimum possible number), and three consecutive OLP pairs showing positive responses were scored as 2. The median breadths of T cell responses against S and N proteins were 13 (IQR, 6 to 21) and 4 (IQR, 2 to 6.5), respectively (Fig. 4a). A maximum of 47 peptides was recognized by expanded PBMCs from an individual (donor 13). The breadth of T cell responses correlated with the magnitude of the expanded PBMCs (P ≤ 0.0002) (Fig. S4). These data indicate that broad T cell responses were induced against both S and N proteins and maintained for up to 313 days in one individual, the maximum DPSO assessed for the study, although breadth varied considerably among the study cohort.
FIG 4.
Breadth of SARS-CoV-2-specific memory T cells. (a) Number of overlapping peptide (OLP) pairs that were recognized in S and N proteins by PBMCs expanded in vitro from COVID-19-convalescent individuals. (b) Comparison of the breadth of memory T cells in whole S and N proteins and the RBD between the mild and severe disease groups. (c) Correlation of the breadth and magnitude of memory T cells between S and N proteins. The ratio of N protein-specific to S protein-specific T cell responses (N/S) was calculated for both the breadth and the magnitude of the T cell response. Differences between groups were determined using the Mann-Whitney test. Correlations were performed using Spearman’s test.
The median breadth of T cell responses against the S protein was higher in the severe group (16) than in the mild group (8), and the difference was significant when T cell responses against the receptor binding domain (RBD) were assessed (median, 4 for the severe group versus 2 for the mild group) (Fig. 4b). In contrast, the median breadths of T cell responses against the N protein were equivalent between groups (4 for both groups).
Significant correlations (P < 0.01) were observed for both the breadth and magnitude for S and N protein-specific T cell responses (Fig. 4c). The ratio of N to S proteins (N/S proteins) for breadth, but not magnitude, was significantly higher in the mild group.
Distribution of CD4+ and CD8+ T cell responses in regions frequently targeted by T cells.
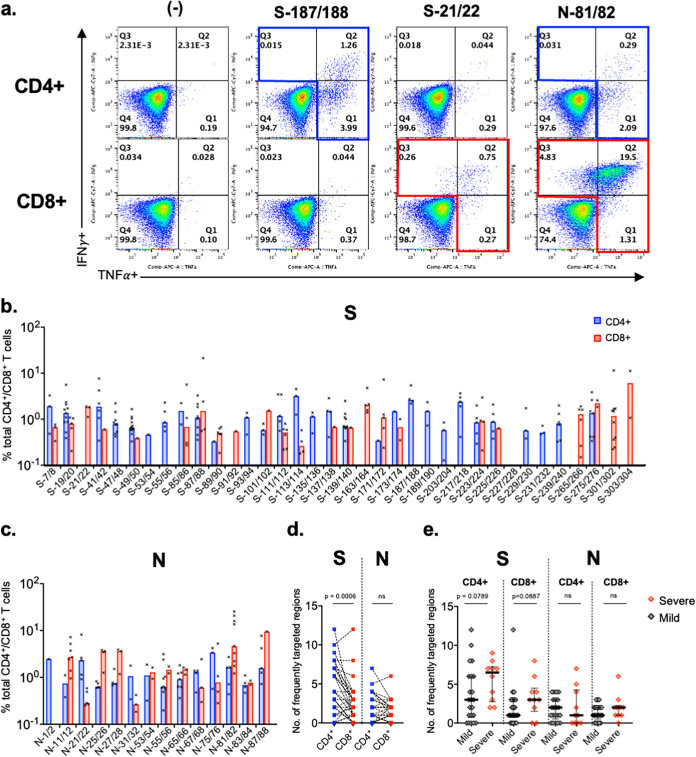
Intracellular cytokine staining (ICS), using expanded PBMCs, was performed to elucidate the distribution of CD4+ or CD8+ T cell responses in SARS-CoV-2 regions frequently targeted by T cells (>20% of individuals assessed). Expanded PBMCs were assessed if responses of >300 SFC/million cells to OLP pairs were observed (Fig. 5). Of the 36 and 14 frequently targeted regions, positive ICS results were obtained for more than 3 individuals in 32 and all regions in S and N proteins, respectively (Fig. 2 and Fig. 5b and c). Eleven (34%) and 4 (13%) regions were recognized by CD4+ and CD8+ T cells, respectively, and 17 regions (53%) were recognized by both T cell subsets for the S protein, indicating that CD4+ T cell responses were dominant for the S protein (Fig. 5d). However, for the N protein, both CD4+ and CD8+ T cell responses were detected against 13 of 14 OLP pairs (93%) (Fig. 5c), showing that the two subsets equally contributed to T cell responses (Fig. 5d).
FIG 5.
CD4+ and CD8+ T cell responses against regions of SARS-CoV-2 spike and nucleocapsid proteins frequently targeted by T cells. (a) Representative flow cytometry plots of intracellular cytokine staining (ICS) from a single individual. The T cell response against each OLP pair was defined as positive when the frequency of IFN-γ+ TNF-α+ T cells was >0.1%. (b and c) CD4+ and CD8+ T cell responses for frequently targeted SARS-CoV-2 S and N regions. ICS was performed on OLP pairs using in vitro-expanded PBMCs with results of >300 SFC/million cells in the ELISpot assay. The bars show the median response. (d and e) Number of SARS-CoV-2 S and N regions targeted by CD4+ and CD8+ T cells for each individual assessed. Paired t test (d) and Mann-Whitney U test (e) were used for comparisons.
The breadths of CD4+ and CD8+ T cell responses against frequently targeted regions were compared between the severe and the mild groups, and those with severe symptoms showed a trend for broader CD4+ and CD8+ T cell responses against the S protein but not the N protein (Fig. 5e).
Impact of amino acid mutations in SARS-CoV-2 variants on HLA binding in putative CD8+ T cell epitopes in regions frequently targeted by T cells.
Several SARS-CoV-2 variants have shown neutralization escape compared to the wild-type virus (Wuhan strain) (14, 15, 17, 34). Importantly, amino acid mutations that resulted in decreased neutralizing antibody sensitivity accumulated in Omicron subvariants (16, 18, 35–38), indicating that viruses that escaped from immune pressure were selected as the pandemic progressed. Therefore, we assessed how amino acid mutations in SARS-CoV-2 variants affect T cell immune responses. First, the number of amino acid changes in the S and N proteins was quantified for Delta (B.1.617.2) and Omicron subvariants (B.1.1.529, BA.1, BA.2, BA.4, BA.5, BQ.1, and XBB) in regions frequently targeted by T cells (39). While amino acid mutations in these regions were only 2 in the S protein and none in the N protein for the Delta variant, there were multiple mutations in Omicron subvariants, particularly in the S protein, where the number of amino acid mutations ranged from 10 to 17 (Table S1).
The impact of amino acid mutations on CD8+ T cell responses was further investigated using the epitope prediction tool NetMHCpan-4.1 (40). Epitopes presented by HLA class I alleles with a prevalence greater than 1% in the Japanese population (Fig. S1) were predicted for SARS-CoV-2 S and N protein regions frequently targeted by CD8+ T cells (Fig. 3). Epitope prediction was performed on 10 HLA-A, 18 HLA-B, and 11 HLA-C alleles, for which the coverages of each locus in the Japanese population are 96.85%, 93.69%, and 96.45%, respectively (33). There were 302 and 169 predicted epitopes in S and N proteins targeted by CD8+ T cells, respectively (Table 2 and Tables S2 and S3). There was no predicted epitope which diminished HLA binding for the Delta variant (Table 2). Although 23 to 62 (8 to 21%) of the 302 predicted epitopes in the S protein had amino acid mutations, 13 to 18 (4 to 6%) of them reduced or lost their HLA binding affinity for the Omicron subvariants assessed (Table 2). A single amino acid mutation occurring in frequently targeted CD8+ T cell regions in the N protein for the Omicron XBB subvariant caused a reduction or loss of HLA binding in 8 of 169 (5%) predicted epitopes.
TABLE 2.
Amino acid mutations located in predicted CD8+ T cell epitopes for regions frequently targeted by T cells in SARS-CoV-2 variants
| Protein | No. of predicted epitopes | Parameter | No. (%) in variant |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta, B.1.617.2 | Omicron |
|||||||||
| B.1.1.529 | BA.1 | BA.2 | BA.4 | BA.5 | BQ.1 | XBB | ||||
| S | 302 | Amino acid mutations | 2 | 8 | 8 | 9 | 10 | 10 | 12 | 15 |
| Epitopes with amino acid mutation | 8 (3) | 23 (8) | 23 (8) | 20 (7) | 25 (8) | 25 (8) | 35 (12) | 62 (21) | ||
| Epitopes with less/no HLA bindinga | 0 (0) | 13 (4) | 13 (4) | 13 (4) | 13 (4) | 13 (4) | 16 (5) | 18 (6) | ||
| N | 169 | Amino acid mutations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Epitopes with amino acid mutation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (8) | ||
| Epitopes with less/no HLA bindinga | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (5) | ||
Epitopes with percent rank of ≥0.5.
To further define epitopes by CD8+ T cells, HLA class I genotypes of individuals with positive responses and HLA restriction of predicted epitopes were assessed for each region targeted by CD8+ T cells. Epitopes restricted by HLA which shared by any of the responders were selected. The selected epitopes are shown (yellow highlight) in Table S2. Thirty (23%) of 129 selected epitopes possessed amino acid mutations, and only 6/129 (3%) of the mutated epitopes showed diminished HLA binding (Table S4).
These data suggest that amino acid mutations in S and N proteins of Omicron subvariants had minimal impact on the presentation of CD8+ T cell epitopes.
DISCUSSION
In this study, memory T cells for SARS-CoV-2 were characterized in COVID-19-convalescent individuals. In vitro expansion of PBMCs with SARS-CoV-2 peptides enabled assessment of SARS-CoV-2-specific memory T cells with high resolution and sensitivity. Antigen-specific T cells were selectively expanded during the culture period, resulting in enhanced detection of low-level T cell responses. T cell responses that are unlikely to be detected in ex vivo PBMCs were detected in the expanded PBMCs with much higher frequency in the present study for most proteins assessed. The possibility that in vitro PBMC culture could expand T cells cross-reactive with SARS-CoV-2 antigens was explored using PBMCs obtained prior to the pandemic from 8 healthy individuals and analyzed under identical conditions. SARS-CoV-2-specific T cell responses were not detected in ex vivo PBMCs. However, low-level T cell responses were detected only against the S protein using expanded PBMCs from 3 individuals (data not shown). These data suggest that SARS-CoV-2-specific T cell responses detected in expanded PBMCs from convalescent individuals were induced due to SARS-CoV-2 infection and not preexisting cross-reactive T cells or de novo induction of SARS-CoV-2-specific T cells. While the magnitude of T cell responses in expanded PBMCs may be biased due to differences in proliferation of each T cell clone during culture, in vitro expansion of antigen-specific T cells for comprehensive T cell analysis is often a necessary approach in clinical studies where blood volumes are extremely limited.
The breadth of T cell responses, which is a critical parameter in the quality of T cell immunity, was evaluated in S and N proteins, and memory T cells that recognize multiple regions across these proteins were maintained in convalescent individuals in the present study. Since robust T cell responses outside SARS-CoV-2 S and N antigens occur (5, 8, 9, 20, 41), the breadth of SARS-CoV-2-specific T cells recognizing the entire viral proteome in each individual could be considerably greater in COVID-19-convalescent individuals.
Tarke et al. performed comprehensive SARS-CoV-2 T cell epitope mapping using clinical samples collected in the United States and defined the immunodominant regions in S, N, and M proteins (9). Peptides frequently targeted in the present study only partially overlapped with immunodominant regions in the study by Tarke et al. (9), which may be due to different HLA allele frequency distributions between U.S. and Japanese populations. The strongest CD8+ T cell response observed in this study was against 301/302 OLP pairs (QELGKYEQYIKWPWYIWLG) located in the C terminus of S protein with the highest frequency. All 10 individuals with CD8+ T cell responses against this OLP pair possessed HLA-A*24:02. HLA- A*24:02 is the most prevalent HLA in the Japanese population (allele frequency, 0.361, compared to 0.065 in the North American study by Tarke et al. [9]). S protein peptide pair 301/302 has 5 predicted epitopes, of which 4 are HLA-A*24:02 restricted: KYEQYIKW, QYIKWPWYI, QYIKWPWYIW, and QYIKWPWYWL. Epitope QYIKWPWYI has been reported as an immunodominant HLA-A*24:02-restricted epitope (42, 43). Considering the different global distribution of HLA genotypes, evaluation of T cell responses should be assessed in each population based on genetic background.
Individuals with severe symptoms maintained T cell responses against multiple SARS-CoV-2 antigens following recovery, as previously described (5, 44–46). Patients with severe COVID-19 have higher viral loads and longer durations of virus shedding than those with mild symptoms (47, 48), so exposure to higher viral antigen for longer periods in severe cases may elicit stronger T cell responses. Notably, the breadth and magnitude of S protein-specific, but not N protein-specific, T cells were predominant in the severe group. The relative ratio of N to S protein in the breadth of the specific memory T cell responses was higher in individuals with mild symptoms, suggesting different contributions of S protein- and N protein-specific T cells in COVID-19 control. The relatively higher breadth of N to S protein-specific T cells may be associated with milder disease symptoms.
Since February 2022, the Omicron variant has represented over 98% of publicly available SARS-CoV-2 sequences. New Omicron subvariants are continuously emerging with accumulating amino acid mutations (38, 49, 50). These emerging viruses have shown decreased susceptibility to neutralization (18, 38, 50, 51), implying that escape from neutralizing antibodies is a driving force for viral evolution. However, the binding affinity to HLA class I molecules was preserved in 94 to 96% of CD8+ T cell epitopes located in regions of S and N proteins frequently targeted by T cells in our cohort, suggesting that amino acid mutations in the present SARS-CoV-2 variants have no serious impact on T cell immunity, similar to previous reports (34, 52–55). Limitations of our study include not having data on HLA-restricted epitopes for T cell responses in these frequently targeted regions and that T cell responses against mutant peptides were not assessed. In order to reveal the impact of amino acid changes in SARS-CoV-2 variants on T cell responses, more detailed analyses are required.
Some studies support the notion that escape mutations from T cell responses are accumulating in emerging SARS-CoV-2 variants (54, 56, 57). Amino acid changes in T cell target peptides should continue to be carefully monitored in emerging viruses. Identification of T cell target regions at the individual level and evaluation of immunodominant regions at the population level are essential for an accurate understanding of the impact of amino acid mutations on SARS-CoV-2 transmission and pathogenesis.
MATERIALS AND METHODS
Study population.
Volunteers over the age of 18 years who recovered from COVID-19 were recruited via the Internet. All applicants received an in-person explanation, and informed consent was obtained from all participants prior to the conduct of study procedures. Thirty-eight convalescent individuals, who were infected between March 2020 and January 2021, participated in the study. All participants were Japanese. SARS-CoV-2 infection was confirmed by PCR-based testing at hospitals or clinics. Characteristics of participants are shown in Table 1. Individuals were divided into two groups, severe and mild, based on pneumonia diagnosed by chest computed tomography or X-ray during COVID-19 (Table 1). Three participants in the severe group received oxygen. This study was approved by ethics committees of the Japan Conference of Clinical Research (no. 384) and the National Institute of Infectious Diseases (no. 1192).
Human leukocyte antigen genotyping.
Genomic DNA was extracted from PBMCs using the QIAamp DNA blood minikit (Qiagen). HLA genotyping was performed with the AlloSeq Tx17 kit (CareDx) following the manufacturer’s instructions. Next-generation sequencing (NGS) libraries were sequenced by the MiSeq (Illumina) instrument. Bioinformatics analysis was done with the bioinformatics pipeline AlloSeq Assign (CareDx).
Peptides.
Pools of 15-mer peptides (JPT Peptide Technologies), overlapping by 11 amino acids and spanning the full proteome of SARS-CoV-2, with the exception of ORF1ab, were used as SARS-CoV-2 antigens. Overlapping peptide (OLP) pools of S, N, M, and envelope (E) proteins, ORF3a, ORF6, ORF7a, ORF7b, ORF8, ORF9b, and ORF10 with amino acid sequences representing wild-type SARS-CoV-2 (Wuhan strain) were composed of 315, 102, 53, 16, 66, 13, 28, 8, 28, 22, and 7 OLPs, respectively.
Additionally, OLPs included in the S and N protein OLP pools were synthesized individually (Eurofin Genomics) to facilitate comprehensive screening as described previously (58). All 315 and 102 OLPs covering the entire S and N proteins, respectively, were included in a peptide matrix for each protein. To reduce the number of PBMCs required for analysis, adjacent OLPs were paired (see Fig. S3 in the supplemental material), resulting in 157 and 51 OLP pairs for the S and N protein matrices, respectively. Each OLP pair was represented in two different pools, allowing identification of the respective peptides in two corresponding pools, with maxima of 26 peptides for the S protein and 16 for the N protein pools.
In vitro expansion of PBMCs.
PBMCs were stimulated with 100 ng/mL of OLP pools of SARS-CoV-2 proteins and cultured for 2 weeks in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM glutamine with 50 U/mL of IL-2 (Miltenyi Biotec). Expanded PBMCs were cryopreserved prior to use.
IFN-γ ELISpot assay.
The interferon gamma (IFN-γ) ELISpot assay was performed on cryopreserved samples as previously described (58). One hundred thousand ex vivo or expanded PBMCs were cultured overnight with peptides. The final concentration of each peptide for the ELISpot assay with OLP pools for each protein was 100 ng/mL.
Two-dimensional peptide matrices were designed for screening of T cell target peptides by the ELISpot assay using expanded PBMCs. The final concentration of each peptide was 2 μg/mL. All peptides located at the intersection of positive row and column wells in the matrices were tested individually to confirm responses.
Thresholds for positive responses were determined as at least 30 and 50 spot-forming cells (SFC)/106 cells for PBMCs and expanded PBMCs, respectively, and/or responses exceeding two times the mean negative (medium-only) wells, whichever was greater. Results are reported as SFC/106 PBMCs.
ICS.
Intracellular cytokine staining (ICS) was performed as previously described (58). Expanded PBMCs from each participant were stimulated with the reactive OLP pairs at 10 μg/mL for 6 h in the presence of anti-CD28, anti-CD49d, and an inhibitor of the Golgi transporter.
The following antibodies and reagents were used for ICS: anti-CD4-fluorescein isothiocyanate (FITC), anti-CD8-peridinin chlorophyll protein (PerCP), anti-CD3-pacific blue, anti-tumor necrosis factor alpha (TNF-α)–allophycocyanin (APC), anti-IFN-γ–APC–Cy7 (BioLegend), anti-CD28, anti-CD49d, GolgiStop (BD Biosciences), and LIVE/DEAD fixable aqua dead cell stain kit (Thermo Scientific). Flow data were acquired using a FACS Canto II (BD Biosciences) and analyzed using FlowJo software version 10.8.1 (Tree Star). T cell responses were defined as positive if the frequency of both IFN-γ- and TNF-positive cells was more than 0.1% in total CD3+ CD4+ or CD3+ CD8+ T cells.
T cell epitope prediction.
T cell epitope prediction was performed using the NetMHCpan-4.1 web server (40) for HLA-A, -B, and -C, for allele frequencies greater than 1% in the Japanese population. Peptides with a percent rank of <0.5 were defined as predicted epitopes following the recommendation of the software developer.
Statistical analysis.
GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. The paired t test was used for comparisons between T cell subset responses, and the Mann-Whitney test was used for all other comparisons. Correlations were performed with Spearman’s rank correlation.
ACKNOWLEDGMENTS
We are grateful to the study participants.
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP19fk0108104 and JP20he0622022 and was partly supported by JSPS KAKENHI grant number JP20K20498.
Footnotes
Supplemental material is available online only.
Contributor Information
Ai Kawana-Tachikawa, Email: aiktachi@niid.go.jp.
Takamasa Ueno, Kumamoto Daigaku.
REFERENCES
- 1.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, Kato Y, Crotty EG, Kim C, Rawlings SA, Mateus J, Tse LPV, Frazier A, Baric R, Peters B, Greenbaum J, Ollmann Saphire E, Smith DM, Sette A, Crotty S. 2020. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, Zhuang Y, Tham CYL, Chia A, Smith GJD, Young B, Kalimuddin S, Low JGH, Lye D, Wang LF, Bertoletti A. 2021. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep 34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarke A, Potesta M, Varchetta S, Fenoglio D, Iannetta M, Sarmati L, Mele D, Dentone C, Bassetti M, Montesano C, Mondelli MU, Filaci G, Grifoni A, Sette A. 2022. Early and polyantigenic CD4 T cell responses correlate with mild disease in acute COVID-19 donors. Int J Mol Sci 23:7155. doi: 10.3390/ijms23137155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Stralin K, Gorin JB, Olsson A, Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, Wullimann DJ, Kammann T, Emgard J, Parrot T, Folkesson E, Karolinska C-SG, Rooyackers O, Eriksson LI, Henter JI, Sonnerborg A, Allander T, Albert J, Nielsen M, Klingstrom J, Gredmark-Russ S, Bjorkstrom NK, Sandberg JK, Price DA, Ljunggren HG, Aleman S, Buggert M, Karolinska COVID-19 Study Group . 2020. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, Dejnirattisai W, Rostron T, Supasa P, Liu C, Lopez-Camacho C, Slon-Campos J, Zhao Y, Stuart DI, Paesen GC, Grimes JM, Antson AA, Bayfield OW, Hawkins D, Ker DS, Wang B, Turtle L, Subramaniam K, Thomson P, Zhang P, Dold C, Ratcliff J, Simmonds P, de Silva T, Sopp P, Wellington D, Rajapaksa U, Chen YL, Salio M, Napolitani G, Paes W, Borrow P, Kessler BM, Fry JW, Schwabe NF, Semple MG, Baillie JK, Moore SC, Openshaw PJM, Ansari MA, Dunachie S, Barnes E, Frater J, Kerr G, Goulder P, ISARIC4C Investigators , et al. 2020. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallajosyula V, Ganjavi C, Chakraborty S, McSween AM, Pavlovitch-Bedzyk AJ, Wilhelmy J, Nau A, Manohar M, Nadeau KC, Davis MM. 2021. CD8(+) T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci Immunol 6:eabg5669. doi: 10.1126/sciimmunol.abg5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferretti AP, Kula T, Wang Y, Nguyen DMV, Weinheimer A, Dunlap GS, Xu Q, Nabilsi N, Perullo CR, Cristofaro AW, Whitton HJ, Virbasius A, Olivier KJ, Jr, Buckner LR, Alistar AT, Whitman ED, Bertino SA, Chattopadhyay S, MacBeath G. 2020. Unbiased screens show CD8(+) T cells of COVID-19 patients recognize shared epitopes in SARS-CoV-2 that largely reside outside the spike protein. Immunity 53:1095–1107.e3. doi: 10.1016/j.immuni.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A. 2020. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarke A, Sidney J, Kidd CK, Dan JM, Ramirez SI, Yu ED, Mateus J, da Silva Antunes R, Moore E, Rubiro P, Methot N, Phillips E, Mallal S, Frazier A, Rawlings SA, Greenbaum JA, Peters B, Smith DM, Crotty S, Weiskopf D, Grifoni A, Sette A. 2021. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep Med 2:100204. doi: 10.1016/j.xcrm.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katoh J, Kawana-Tachikawa A, Shimizu A, Zhu D, Han C, Nakamura H, Koga M, Kikuchi T, Adachi E, Koibuchi T, Gao GF, Brumme ZL, Iwamoto A. 2016. Rapid HIV-1 disease progression in individuals infected with a virus adapted to its host population. PLoS One 11:e0150397. doi: 10.1371/journal.pone.0150397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammaranond P, van Bockel DJ, Petoumenos K, McMurchie M, Finlayson R, Middleton MG, Davenport MP, Venturi V, Suzuki K, Gelgor L, Kaldor JM, Cooper DA, Kelleher AD. 2011. HIV immune escape at an immunodominant epitope in HLA-B*27-positive individuals predicts viral load outcome. J Immunol 186:479–488. doi: 10.4049/jimmunol.0903227. [DOI] [PubMed] [Google Scholar]
- 12.Erickson AL, Kimura Y, Igarashi S, Eichelberger J, Houghton M, Sidney J, McKinney D, Sette A, Hughes AL, Walker CM. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883–895. doi: 10.1016/s1074-7613(01)00245-x. [DOI] [PubMed] [Google Scholar]
- 13.Feeney ME, Tang Y, Roosevelt KA, Leslie AJ, McIntosh K, Karthas N, Walker BD, Goulder PJ. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J Virol 78:8927–8930. doi: 10.1128/JVI.78.16.8927-8930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Pere H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Loriere E, Rey FA, Schwartz O. 2021. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 15.Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, Schmitz KS, Rijsbergen LC, van Osch JAT, Dijkhuizen E, Smits G, Comvalius A, van Mourik D, Caniels TG, van Gils MJ, Sanders RW, Oude Munnink BB, Molenkamp R, de Jager HJ, Haagmans BL, de Swart RL, Koopmans MPG, van Binnendijk RS, de Vries RD, GeurtsvanKessel CH. 2021. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 6:eabj1750. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Valtanen P, Hope CM, Masavuli MG, Yeow AEL, Balachandran H, Mekonnen ZA, Al-Delfi Z, Abayasingam A, Agapiou D, Stella AO, Aggarwal A, Bouras G, Gummow J, Ferguson C, O'Connor S, McCartney EM, Lynn DJ, Maddern G, Gowans EJ, Reddi BAJ, Shaw D, Kok-Lim C, Beard MR, Weiskopf D, Sette A, Turville SG, Bull RA, Barry SC, Grubor-Bauk B. 2022. SARS-CoV-2 Omicron variant escapes neutralizing antibodies and T cell responses more efficiently than other variants in mild COVID-19 convalescents. Cell Rep Med 3:100651. doi: 10.1016/j.xcrm.2022.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, Muecksch F, Rutkowska M, Hoffmann HH, Michailidis E, Gaebler C, Agudelo M, Cho A, Wang Z, Gazumyan A, Cipolla M, Luchsinger L, Hillyer CD, Caskey M, Robbiani DF, Rice CM, Nussenzweig MC, Hatziioannou T, Bieniasz PD. 2020. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife 9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, Bowen AD, Liu M, Wang M, Yu J, Valdez R, Lauring AS, Sheng Z, Wang HH, Gordon A, Liu L, Ho DD. 2023. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186:279–286.e8. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kared H, Redd AD, Bloch EM, Bonny TS, Sumatoh H, Kairi F, Carbajo D, Abel B, Newell EW, Bettinotti MP, Benner SE, Patel EU, Littlefield K, Laeyendecker O, Shoham S, Sullivan D, Casadevall A, Pekosz A, Nardin A, Fehlings M, Tobian AA, Quinn TC. 2021. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J Clin Invest 131:e145476. doi: 10.1172/JCI145476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelde A, Bilich T, Heitmann JS, Maringer Y, Salih HR, Roerden M, Lübke M, Bauer J, Rieth J, Wacker M, Peter A, Hörber S, Traenkle B, Kaiser PD, Rothbauer U, Becker M, Junker D, Krause G, Strengert M, Schneiderhan-Marra N, Templin MF, Joos TO, Kowalewski DJ, Stos-Zweifel V, Fehr M, Rabsteyn A, Mirakaj V, Karbach J, Jäger E, Graf M, Gruber L-C, Rachfalski D, Preuß B, Hagelstein I, Märklin M, Bakchoul T, Gouttefangeas C, Kohlbacher O, Klein R, Stevanović S, Rammensee H-G, Walz JS. 2021. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol 22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 21.Bilich T, Nelde A, Heitmann JS, Maringer Y, Roerden M, Bauer J, Rieth J, Wacker M, Peter A, Horber S, Rachfalski D, Marklin M, Stevanovic S, Rammensee HG, Salih HR, Walz JS. 2021. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci Transl Med 13:eabf7517. doi: 10.1126/scitranslmed.abf7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee E, Sandgren K, Duette G, Stylianou VV, Khanna R, Eden JS, Blyth E, Gottlieb D, Cunningham AL, Palmer S. 2021. Identification of SARS-CoV-2 nucleocapsid and spike T-cell epitopes for assessing T-cell immunity. J Virol 95:e02002-20. doi: 10.1128/JVI.02002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis JM, Leistritz-Edwards D, Dunn A, Tarr C, Lehman J, Dempsey C, Hamel A, Rayon V, Liu G, Wang Y, Wille M, Durkin M, Hadley K, Sheena A, Roscoe B, Ng M, Rockwell G, Manto M, Gienger E, Nickerson J, MGH COVID-19 Collection and Processing Team, Moarefi A, Noble M, Malia T, Bardwell PD, Gordon W, Swain J, Skoberne M, Sauer K, Harris T, Goldrath AW, Shalek AK, Coyle AJ, Benoist C, Pregibon DC. 2022. Allelic variation in class I HLA determines CD8(+) T cell repertoire shape and cross-reactive memory responses to SARS-CoV-2. Sci Immunol 7:eabk3070. doi: 10.1126/sciimmunol.abk3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, Sagar Daul F, Salvat Lago M, Decker A, Luxenburger H, Binder B, Bettinger D, Sogukpinar O, Rieg S, Panning M, Huzly D, Schwemmle M, Kochs G, Waller CF, Nieters A, Duerschmied D, Emmerich F, Mei HE, Schulz AR, Llewellyn-Lacey S, Price DA, Boettler T, Bengsch B, Thimme R, Hofmann M, Neumann-Haefelin C. 2021. Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat Med 27:78–85. doi: 10.1038/s41591-020-01143-2. [DOI] [PubMed] [Google Scholar]
- 25.van den Dijssel J, Hagen RR, de Jongh R, Steenhuis M, Rispens T, Geerdes DM, Mok JY, Kragten AH, Duurland MC, Verstegen NJ, van Ham SM, van Esch WJ, van Gisbergen KP, Hombrink P, Ten Brinke A, van de Sandt CE. 2022. Parallel detection of SARS-CoV-2 epitopes reveals dynamic immunodominance profiles of CD8(+) T memory cells in convalescent COVID-19 donors. Clin Transl Immunology 11:e1423. doi: 10.1002/cti2.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson RW, Chen Y, Venezia OL, Majerus RM, Shin DS, MGH COVID-19 Collection & Processing Team, Carrington MN, Yu XG, Wesemann DR, Moon JJ, Luster AD. 2022. SARS-CoV-2 epitope-specific CD4(+) memory T cell responses across COVID-19 disease severity and antibody durability. Sci Immunol 7:eabl9464. doi: 10.1126/sciimmunol.abl9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin X, Ding Y, Sun S, Wang X, Zhou Z, Liu X, Li M, Chen X, Shen A, Wu Y, Liu B, Zhang J, Li J, Yang Y, Qiu H, Shen C, He Y, Zhao G. 2021. Screening HLA-A-restricted T cell epitopes of SARS-CoV-2 and the induction of CD8(+) T cell responses in HLA-A transgenic mice. Cell Mol Immunol 18:2588–2608. doi: 10.1038/s41423-021-00784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C, Shen M, Han X, Chen Q, Li L, Chen S, Zhang J, Gao F, Wang W, Wang Y, Li T, Li S, Huang J, Wang J, Zhu J, Chen D, Wu Q, Tao K, Pang D, Jin A. 2022. Identification of cross-reactive CD8(+) T cell receptors with high functional avidity to a SARS-CoV-2 immunodominant epitope and its natural mutant variants. Genes Dis 9:216–229. doi: 10.1016/j.gendis.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Deng S, Ren L, Zheng P, Hu X, Jin T, Tan X. 2021. Profiling CD8(+) T cell epitopes of COVID-19 convalescents reveals reduced cellular immune responses to SARS-CoV-2 variants. Cell Rep 36:109708. doi: 10.1016/j.celrep.2021.109708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner KI, Mateyka LM, Jarosch S, Grass V, Weber S, Schober K, Hammel M, Burrell T, Kalali B, Poppert H, Beyer H, Schambeck S, Holdenrieder S, Strotges-Achatz A, Haselmann V, Neumaier M, Erber J, Priller A, Yazici S, Roggendorf H, Odendahl M, Tonn T, Dick A, Witter K, Mijocevic H, Protzer U, Knolle PA, Pichlmair A, Crowell CS, Gerhard M, D’Ippolito E, Busch DH. 2022. Recruitment of highly cytotoxic CD8(+) T cell receptors in mild SARS-CoV-2 infection. Cell Rep 38:110214. doi: 10.1016/j.celrep.2021.110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Titov A, Shaykhutdinova R, Shcherbakova OV, Serdyuk YV, Sheetikov SA, Zornikova KV, Maleeva AV, Khmelevskaya A, Dianov DV, Shakirova NT, Malko DB, Shkurnikov M, Nersisyan S, Tonevitsky A, Khamaganova E, Ershov AV, Osipova EY, Nikolaev RV, Pershin DE, Vedmedskia VA, Maschan M, Ginanova VR, Efimov GA. 2022. Immunogenic epitope panel for accurate detection of non-cross-reactive T cell response to SARS-CoV-2. JCI Insight 7:e157699. doi: 10.1172/jci.insight.157699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Wang L, Schank M, Dang X, Lu Z, Cao D, Khanal S, Nguyen LN, Nguyen LNT, Zhang J, Zhang Y, Adkins JL, Baird EM, Wu XY, Ning S, Gazzar ME, Moorman JP, Yao ZQ. 2021. SARS-CoV-2 specific memory T cell epitopes identified in COVID-19-recovered subjects. Virus Res 304:198508. doi: 10.1016/j.virusres.2021.198508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda N, Kojima H, Nishikawa M, Hayashi K, Futagami T, Tsujino T, Kusunoki Y, Fujii N, Suegami S, Miyazaki Y, Middleton D, Tanaka H, Saji H. 2015. Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens 85:252–259. doi: 10.1111/tan.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L, Wang G, Wang Y, Zhang Q, Ren L, Gu X, Huang T, Zhong J, Wang Y, Wang X, Huang L, Xu L, Wang C, Chen L, Xiao X, Peng Y, Knight JC, Dong T, Cao B, Wang J. 2022. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study. Lancet Microbe 3:e348–e356. doi: 10.1016/S2666-5247(22)00036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang H, Nian X, Wu J, Liu D, Feng L, Lu J, Peng Y, Zhou Z, Deng T, Liu J, Ji D, Qiu R, Lin L, Zeng Y, Xia F, Hu Y, Li T, Duan K, Li X, Wang Z, Zhang Y, Zhang H, Zhu C, Wang S, Wu X, Wang X, Li Y, Huang S, Mao M, Guo H, Yang Y, Jia R, Xufang J, Wang X, Liang S, Qiu Z, Zhang J, Ding Y, Li C, Zhang J, Fu D, He Y, Zhou D, Li C, Zhang J, Yu D, Yang XM. 2022. COVID-19 vaccination boosts the potency and breadth of the immune response against SARS-CoV-2 among recovered patients in Wuhan. Cell Discov 8:131. doi: 10.1038/s41421-022-00496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann M, Kruger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, Lier M, Dopfer-Jablonka A, Jack HM, Behrens GMN, Pohlmann S. 2022. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, Scherbeijn S, Gommers L, Sablerolles RSG, Nieuwkoop NN, Rijsbergen LC, van Dijk LLA, de Wilde J, Alblas K, Breugem TI, Rijnders BJA, de Jager H, Weiskopf D, van der Kuy PHM, Sette A, Koopmans MPG, Grifoni A, Haagmans BL, de Vries RD. 2022. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol 7:eabo2202. doi: 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y, Yisimayi A, Jian F, Song W, Xiao T, Wang L, Du S, Wang J, Li Q, Chen X, Yu Y, Wang P, Zhang Z, Liu P, An R, Hao X, Wang Y, Wang J, Feng R, Sun H, Zhao L, Zhang W, Zhao D, Zheng J, Yu L, Li C, Zhang N, Wang R, Niu X, Yang S, Song X, Chai Y, Hu Y, Shi Y, Zheng L, Li Z, Gu Q, Shao F, Huang W, Jin R, Shen Z, Wang Y, Wang X, Xiao J, Xie XS. 2022. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Library of Medicine. 2023. SARS-CoV-2 variants overview. https://www.ncbi.nlm.nih.gov/activ.
- 40.Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. 2020. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res 48:W449–W454. doi: 10.1093/nar/gkaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saini SK, Hersby DS, Tamhane T, Povlsen HR, Amaya Hernandez SP, Nielsen M, Gang AO, Hadrup SR. 2021. SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8(+) T cell activation in COVID-19 patients. Sci Immunol 6:eabf7550. doi: 10.1126/sciimmunol.abf7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grifoni A, Sidney J, Vita R, Peters B, Crotty S, Weiskopf D, Sette A. 2021. SARS-CoV-2 human T cell epitopes: adaptive immune response against COVID-19. Cell Host Microbe 29:1076–1092. doi: 10.1016/j.chom.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuse N, Zhang Y, Chikata T, Nguyen HT, Oka S, Gatanaga H, Takiguchi M. 2022. Long-term memory CD8(+) T cells specific for SARS-CoV-2 in individuals who received the BNT162b2 mRNA vaccine. Nat Commun 13:5251. doi: 10.1038/s41467-022-32989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu KK, Fischinger S, Smith MT, Atyeo C, Cizmeci D, Wolf CR, Layton ED, Logue JK, Aguilar MS, Shuey K, Loos C, Yu J, Franko N, Choi RY, Wald A, Barouch DH, Koelle DM, Lauffenburger D, Chu HY, Alter G, Seshadri C. 2021. Comorbid illnesses are associated with altered adaptive immune responses to SARS-CoV-2. JCI Insight 6:e146242. doi: 10.1172/jci.insight.146242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuo J, Dowell AC, Pearce H, Verma K, Long HM, Begum J, Aiano F, Amin-Chowdhury Z, Hoschler K, Brooks T, Taylor S, Hewson J, Hallis B, Stapley L, Borrow R, Linley E, Ahmad S, Parker B, Horsley A, Amirthalingam G, Brown K, Ramsay ME, Ladhani S, Moss P. 2021. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat Immunol 22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J, Law R, Korosec CS, Zhou C, Koh WH, Ghaemi MS, Samaan P, Ooi HK, Matveev V, Yue F, Gingras AC, Estacio A, Buchholz M, Cheatley PL, Mohammadi A, Kaul R, Pavinski K, Mubareka S, McGeer AJ, Leis JA, Heffernan JM, Ostrowski M. 2022. Longitudinal assessment of SARS-CoV-2-specific T cell cytokine-producing responses for 1 year reveals persistence of multicytokine proliferative responses, with greater immunity associated with disease severity. J Virol 96:e00509-22. doi: 10.1128/jvi.00509-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. 2020. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. 2020. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kemp SA, Collier DA, Datir RP, Ferreira I, Gayed S, Jahun A, Hosmillo M, Rees-Spear C, Mlcochova P, Lumb IU, Roberts DJ, Chandra A, Temperton N, CITIID-NIHR BioResource COVID-19 Collaboration, COVID-19 Genomics UK (COG-UK) Consortium, Sharrocks K, Blane E, Modis Y, Leigh KE, Briggs JAG, van Gils MJ, Smith KGC, Bradley JR, Smith C, Doffinger R, Ceron-Gutierrez L, Barcenas-Morales G, Pollock DD, Goldstein RA, Smielewska A, Skittrall JP, Gouliouris T, Goodfellow IG, Gkrania-Klotsas E, Illingworth CJR, McCoy LE, Gupta RK. 2021. SARS-CoV-2 evolution during treatment of chronic infection. Nature 592:277–282. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Y, Jian F, Wang J, Yu Y, Song W, Yisimayi A, Wang J, An R, Chen X, Zhang N, Wang Y, Wang P, Zhao L, Sun H, Yu L, Yang S, Niu X, Xiao T, Gu Q, Shao F, Hao X, Xu Y, Jin R, Shen Z, Wang Y, Xie XS. 2023. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 614:521–529. doi: 10.1038/s41586-022-05644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, Wang J, Wang Y, Niu X, Yang S, Liang H, Sun H, Li T, Yu Y, Cui Q, Liu S, Yang X, Du S, Zhang Z, Hao X, Shao F, Jin R, Wang X, Xiao J, Wang Y, Xie XS. 2022. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Redd AD, Nardin A, Kared H, Bloch EM, Abel B, Pekosz A, Laeyendecker O, Fehlings M, Quinn TC, Tobian AAR. 2022. Minimal crossover between mutations associated with Omicron variant of SARS-CoV-2 and CD8(+) T-cell epitopes identified in COVID-19 convalescent individuals. mBio 13:e03617-21. doi: 10.1128/mbio.03617-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panikkar A, Lineburg KE, Raju J, Chew KY, Ambalathingal GR, Rehan S, Swaminathan S, Crooks P, Le Texier L, Beagley L, Best S, Solomon M, Matthews KK, Srihari S, Neller MA, Short KR, Khanna R, Smith C. 2022. SARS-CoV-2-specific T cells generated for adoptive immunotherapy are capable of recognizing multiple SARS-CoV-2 variants. PLoS Pathog 18:e1010339. doi: 10.1371/journal.ppat.1010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naranbhai V, Nathan A, Kaseke C, Berrios C, Khatri A, Choi S, Getz MA, Tano-Menka R, Ofoman O, Gayton A, Senjobe F, Zhao Z, St Denis KJ, Lam EC, Carrington M, Garcia-Beltran WF, Balazs AB, Walker BD, Iafrate AJ, Gaiha GD. 2022. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell 185:1041–1051.e6. doi: 10.1016/j.cell.2022.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang-Meli J, Luxenburger H, Wild K, Karl V, Oberhardt V, Salimi Alizei E, Graeser A, Reinscheid M, Roehlen N, Reeg DB, Giese S, Ciminski K, Gotz V, August D, Rieg S, Waller CF, Wengenmayer T, Staudacher D, Huzly D, Bengsch B, Kochs G, Schwemmle M, Emmerich F, Boettler T, Thimme R, Hofmann M, Neumann-Haefelin C. 2022. SARS-CoV-2-specific T-cell epitope repertoire in convalescent and mRNA-vaccinated individuals. Nat Microbiol 7:675–679. doi: 10.1038/s41564-022-01106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, Consortium C-GU, Peacock SJ, Barclay WS, de Silva TI, Towers GJ, Robertson DL, COVID-19 Genomics UK Consortium . 2023. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol 21:162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kombe Kombe AJ, Biteghe FAN, Ndoutoume ZN, Jin T. 2022. CD8(+) T-cell immune escape by SARS-CoV-2 variants of concern. Front Immunol 13:962079. doi: 10.3389/fimmu.2022.962079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawana-Tachikawa A, Llibre JM, Bravo I, Escrig R, Mothe B, Puig J, Puertas MC, Martinez-Picado J, Blanco J, Manzardo C, Miro JM, Iwamoto A, Pozniak AL, Gatell JM, Clotet B, Brander C, MARAVIBOOST Investigators . 2014. Effect of maraviroc intensification on HIV-1-specific T cell immunity in recently HIV-1-infected individuals. PLoS One 9:e87334. doi: 10.1371/journal.pone.0087334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Download spectrum.02143-23-s0001.tif, TIF file, 6.4 MB (6.4MB, tif)
Fig. S2. Download spectrum.02143-23-s0002.tif, TIF file, 6.4 MB (6.4MB, tif)
Fig. S3. Download spectrum.02143-23-s0003.tif, TIF file, 6.4 MB (6.4MB, tif)
Fig. S4. Download spectrum.02143-23-s0004.tif, TIF file, 6.4 MB (6.4MB, tif)
Table S1. Download spectrum.02143-23-s0005.xlsx, XLSX file, 0.01 MB (9.7KB, xlsx)
Table S2. Download spectrum.02143-23-s0006.xlsx, XLSX file, 0.02 MB (25.2KB, xlsx)
Table S3. Download spectrum.02143-23-s0007.xlsx, XLSX file, 0.02 MB (18.2KB, xlsx)
Table S4. Download spectrum.02143-23-s0008.xlsx, XLSX file, 0.01 MB (14.5KB, xlsx)