Abstract
Epstein-Barr virus (EBV) infects and transforms primary B lymphocytes in vitro. Viral infection initiates the cell cycle entry of the resting B lymphocytes. The maintenance of proliferation in the infected cells is strictly dependent on functional EBNA2. We have recently developed a conditional immortalization system for EBV by rendering the function of EBNA2, and thus proliferation of the immortalized cells, dependent on estrogen. This cellular system was used to identify early events preceding induction of proliferation. We show that LMP1 and c-myc are directly activated by EBNA2, indicating that all cellular factors essential for induction of these genes by EBNA2 are present in the resting cells. In contrast, induction of the cell cycle regulators cyclin D2 and cdk4 are secondary events, which require de novo protein synthesis.
Transformation of primary B lymphocytes by Epstein-Barr virus (EBV) requires the concerted action of at least six viral gene products. The individual functions of these six genes cannot easily be analyzed during the infection and transformation process, since expression of these gene products is initiated consecutively over a period of 32 h, and the progression of the infected cells into the cell cycle is asynchronous (for reviews, see references 3 and 11).
EBV nuclear antigen 2 (EBNA2) is one of the first genes expressed postinfection and is absolutely required for viral transformation (2, 4). EBNA2 is a transactivator of viral genes (the latent membrane protein 1 [LMP1], LMP2A, and LMP2B genes), the viral C-promoter, and cellular genes (CD21, CD23, c-fgr, and BLR2) (for a review, see reference 11). EBNA2 carries a strong transactivation domain, which can interact with multiple constituents of the RNA polymerase II transcription complex, but does not bind to DNA directly (20–22). EBNA2 has to be recruited to the promoter of target genes by sequence-specific DNA binding proteins like recombination signal-binding protein Jκ (RBP-J). RBP-J binds to the conserved core sequence CGTGGGAA. This sequence motif has been identified in the 5′ untranslated regions of the viral Cp, LMP1 and LMP2, and the cellular CD21 and CD23 promoters (5, 12, 14, 24, 25). Binding of EBNA2 to these promoters via RBP-J is not sufficient to activate transcription and requires collaboration with factors like the Ets protein PU.1, POU domain proteins, activating transcription factor–c-Jun, or a component of the SNF-SWI complex, SNF5/Ini1 (7, 13, 18, 19, 23). In addition, EBNA2 can act as a repressor and efficiently suppress the expression of immunoglobulin M (IgM) (6).
In order to further define EBNA2 target genes, we have developed a model system to define EBNA2 target genes on the genetic background of an EBV-transformed cell line: a lymphoblastoid cell line conditional for EBNA2, designated ER/EB. This conditional EBNA2 was generated by fusing the open reading frame of EBNA2 to the gene fragment encoding the hormone-binding domain of the estrogen receptor (ER). The function of this ER-EBNA2 fusion protein is strictly dependent on estrogen (β-estradiol), and cell lines transformed by virus expressing ER-EBNA2 can grow only in the presence of estrogen (8). We have recently shown that ER-EBNA2 does not bind to RBP-J in the absence of estrogen and that binding is restored by estrogen addition (9).
Estrogen-deprived cultures of lymphoblastoid cell lines expressing ER-EBNA2 cease proliferation and acquire the phenotype of resting B lymphocytes but can reenter the cell cycle upon estrogen stimulation. Reentry into the cell cycle is accompanied by the consecutive expression of G1-phase cell cycle regulators and thus is reminiscent of serum stimulation of fibroblast proliferation, or the stimulation of primary B cells by EBV infection or CD40 ligation in the presence of interleukin 4. We have shown previously that upregulation of LMP1 and MYC protein was followed by upregulation of cyclin D2 and cyclin-dependent kinase 4 (cdk4) protein, suggesting that this set of genes might represent good candidate EBNA2 target genes (8).
In the absence of ligand, the steroid receptors are associated with heat shock proteins, which keep the steroid receptor in an inactive conformation, and this inhibition is released by ligand binding (1, 15). It is generally assumed that the same inhibitory complex is formed by steroid receptor fusion proteins and renders the fusion partner nonfunctional in the absence of ligand. Thus, the mechanism by which the hormone-binding domain of steroid receptors can regulate the function of the fusion partners in chimeric proteins does not require de novo protein synthesis.
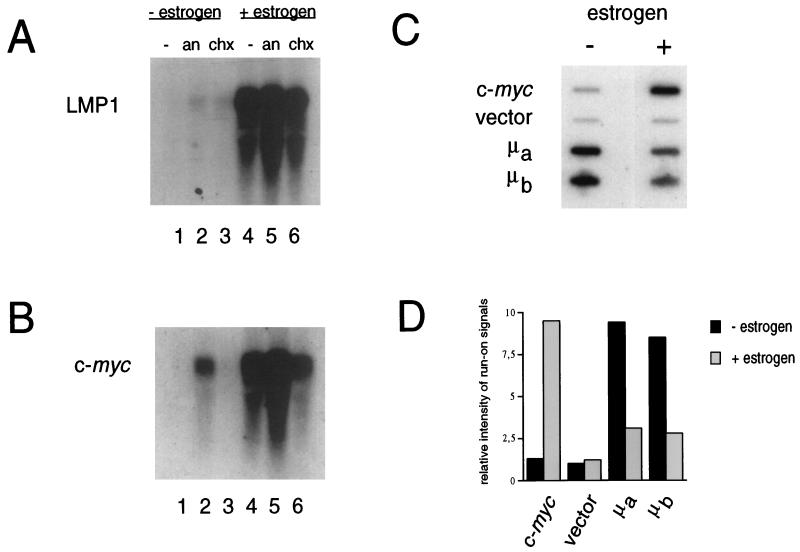
Since the objective of our study was to identify direct target genes of EBNA2, we reanalyzed induction of known EBNA2 target genes in the presence of the protein synthesis inhibitor anisomycin or cycloheximide. The abundance of LMP1 and c-myc RNA was increased upon estrogen stimulation in the absence of de novo protein synthesis (Fig. 1A and B, lanes 4 through 6).
FIG. 1.
(A and B) EBNA2 enhances c-myc and LMP1 RNA expression in the absence of de novo protein synthesis. ER/EB2-5 estrogen-deprived cell cultures were treated with 10 μM anisomycin (an) or 50 μg of cycloheximide (chx)/ml in the presence or absence of estrogen for 6 h. Protein synthesis inhibitors were added to the medium 1 h prior to estrogen stimulation in order to establish the translational block before activation of the cells. Total RNA (10 μg/lane) from each culture was isolated and analyzed for c-myc and LMP1 RNA expression. (C and D) Induction of high c-myc RNA levels occurs at the transcriptional level. (C) Nuclei were prepared from cells cultured in the presence (+) or absence (−) of estrogen for 6 h. Nuclear run-on RNAs were hybridized to c-myc and Ig-μ gene probes or vector DNA. (D) Hybridization signals were quantified with a Fuji BAS 100 scanner.
While anisomycin weakly stabilized c-myc transcripts independently of EBNA2 induction (Fig. 1B, lane 2), induction of both RNAs was strictly dependent on estrogen stimulation in the presence of cycloheximide (Fig. 1A and B, lanes 6). This EBNA2 requirement defined LMP1 and c-myc as direct EBNA2 target genes.
In order to further elucidate the mechanism by which EBNA2 induces c-myc RNA, we performed run-on assays of c-myc transcription. These transcription studies in isolated nuclei showed that transcription of the first exon of the c-myc gene was enhanced sevenfold in the presence of estrogen. We have recently reported that EBNA2 interferes with immunoglobulin (Ig) μ gene transcription in Burkitt’s lymphoma cells (6). Here, we repeated our former experiments in order to show that the increase in c-myc transcription in ER/EB cells paralleled the decrease in Ig-μ gene transcription. Thus the increase was indeed specific for the c-myc gene (Fig. 1C and D). These data clearly demonstrate that transcription of the c-myc gene is activated by EBNA2. In the absence of functional EBNA2, transcription of the c-myc gene was not significantly increased compared to that of the vector control.
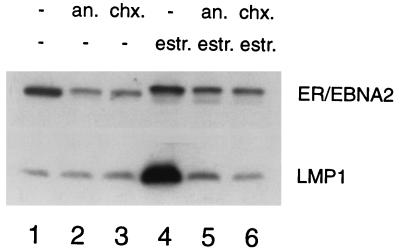
Blocking of protein synthesis was controlled by Western blot analysis of LMP1 protein, a well-characterized EBNA2 target gene. As expected, LMP1 protein was induced by EBNA2 (Fig. 2, lane 4), and both inhibitors (Fig. 2, lanes 5 and 6) efficiently abolished induction.
FIG. 2.
Protein synthesis is efficiently blocked by anisomycin and cycloheximide in estrogen-induced ER/EB2-5 cells. ER/EB2-5 cells were estrogen starved for 4 days and were then restimulated with estrogen (estr.) for 6 h in the presence or absence of 10 μM anisomycin (an) or 50 μg of cycloheximide (chx)/ml. Protein extracts (50 μg/lane) were subjected to Western blot analysis for ER-EBNA2 and LMP1 expression. Note that estrogen, in the absence of protein synthesis, induces a shift in the mobility of the ER-EBNA2 protein, which is due to phosphorylation (10).
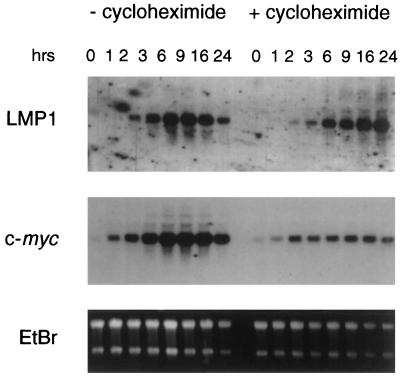
The time course of LMP1 RNA expression was compared to the expression of the c-myc oncogene in the absence and presence of cycloheximide (Fig. 3). Transcripts of both genes could be detected within the first 2 h postinduction. This induction could also be observed in the presence of cycloheximide, confirming that EBNA2 can enhance expression of both genes in the absence of de novo protein synthesis.
FIG. 3.
c-myc and LMP1 are direct EBNA2 target genes. ER/EB2-5 estrogen-deprived cultures were stimulated with estrogen in the absence or presence of cycloheximide for the indicated times. RNA was extracted and subjected to Northern blot analysis for c-myc and LMP1 expression. The loading of the samples was controlled by ethidium bromide (EtBr) staining.
LMP1 transcripts accumulated gradually within the first 6 h after estrogen stimulation in the absence and presence of cycloheximide, and comparison of both kinetics showed a weak delay only in the presence of cycloheximide. The transactivation of the LMP1 and other EBNA2-dependent promoters by EBNA2 requires multiple known and unknown DNA binding factors. Theoretically, the potentially low abundance of one or several of these factors in resting ER/EB cells could be limiting, and this could explain the delayed kinetics of LMP1 RNA induction. PU.1, Spi-B, and RBP-J, however, which have been shown to play a role in the regulation of expression of the LMP1 promoter, are abundantly expressed in primary B cells and in estrogen-deprived ER/EB cultures (13a) and are thus unlikely to be limiting. The limiting factors, potentially labile or not expressed, have yet to be identified.
There were, however, obvious differences when c-myc induction in the presence and absence of cycloheximide was compared. In the absence of cycloheximide, c-myc expression gradually increased over a period of 6 h, in a manner similar to the kinetics of LMP1 induction. In the presence of cycloheximide, c-myc RNA levels increased within the first 2 h postinduction. After this initial induction phase, c-myc was expressed at constant levels, which never equalled those reached in the presence of de novo protein synthesis. These results indicate that at least two independent mechanisms, characterized by their requirement for de novo protein synthesis, contribute to the induction of c-myc RNA expression by EBNA2. In order to define further these mechanisms of c-myc induction by EBNA2, we have tried to identify EBNA2-responsive cis elements within the upstream region of the c-myc promoter. So far, we have not been able to define such an element by transient transfection of c-myc promoter reporter constructs encompassing the 2.3 kb upstream of the P1 promoter (data not shown).
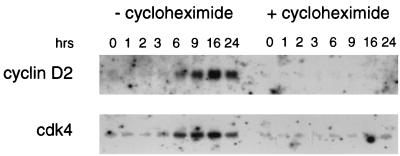
In ER/EB cells, LMP1 and c-myc expression is closely followed by cyclin D2 and cdk4 protein induction. Since EBNA2 in cooperation with EBNA-LP (leader protein) can induce cyclin D2 expression in resting B cells (17), we asked whether cyclin D2 is a direct target gene of EBNA2. Analysis of the RNA abundance of both genes after estrogen induction revealed, however, that although cyclin D2 and cdk4 RNAs are detectable after 3 to 6 h postinduction (Fig. 4), de novo protein synthesis is required for activation of cyclin D2 as well as cdk4 expression. EBNA-LP is unlikely to be a limiting factor, since it is expressed in estrogen-deprived ER/EB cultures and is not modulated upon estrogen addition (data not shown). We conclude that in contrast to LMP1 and c-myc, neither cyclin D2 nor cdk4 is a direct EBNA2 target.
FIG. 4.
Neither cyclin D2 nor cdk4 is a direct EBNA2 target gene. The Northern blots shown in Fig. 3 were stripped and reprobed for cyclin D2 and cdk4 expression.
Cyclin D2 and cdk4 are both elements of the basic cell cycle machinery and drive cell cycle progression in early G1. Since we and others have shown that cyclin D2 and cdk4 expression can be induced by various different B-cell activation protocols, we propose that induction of proliferation by EBNA2 is a secondary event potentially driven by the primary viral and cellular EBNA2 targets.
We show here for the first time that the proto-oncogene c-myc is a direct target gene of EBNA2. Our data clearly support the notion that activation of c-myc gene transcription is the primary cause for the observed elevated levels of c-myc RNA. However, our data do not formally exclude the possibility that secondary mechanisms such as stabilization of transcripts might cooperate with transcriptional induction. In addition, our data showing elevated levels of c-myc RNA in the absence of de novo protein synthesis do not exclude the possibility that EBNA2 acts in concert with other factors. Theoretically, EBNA2 could activate latent transcription factors which might cooperate with EBNA2 to activate transcription. Thus, the molecular mechanism by which EBNA2 activates transcription of the c-myc gene still has to be elucidated.
Ectopic expression of LMP1 in estrogen-deprived ER/EB cultures promotes cell survival but cannot substitute for functions provided by EBNA2 (26). In contrast, ectopic overexpression of c-myc, at very high levels, can drive proliferation in the absence of functional EBNA2 and LMP1 in estrogen-deprived ER/EB cells (16). This suggests that activation of c-myc by EBNA2 is an important step in the process of EBV-induced proliferation and immortalization.
Acknowledgments
This work was supported by Die Deutsche Forschungsgemeinschaft (SFB 217, SFB 455, and La948/2-2) and the Fonds der Chemischen Industrie. B.K. is a fellow of Das AIDS-Stipendienprogramm (Deutsches Krebsforschungszentrum).
REFERENCES
- 1.Beato M, Truss M, Chavez S. Control of transcription by steroid hormones. Ann NY Acad Sci. 1996;784:93–123. doi: 10.1111/j.1749-6632.1996.tb16231.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J I, Wang F, Mannick J, Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci USA. 1989;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell P J. Epstein-Barr virus immortalizing genes. Trends Microbiol. 1995;3:105–109. doi: 10.1016/s0966-842x(00)88891-5. [DOI] [PubMed] [Google Scholar]
- 4.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 5.Henkel T, Ling P D, Hayward S D, Peterson M G. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science. 1994;265:92–95. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- 6.Jochner N, Eick D, Zimber-Strobl U, Pawlita M, Bornkamm G W, Kempkes B. Epstein-Barr virus nuclear antigen 2 is a transcriptional suppressor of the immunoglobulin mu gene: implications for the expression of the translocated c-myc gene in Burkitt’s lymphoma cells. EMBO J. 1996;15:375–382. [PMC free article] [PubMed] [Google Scholar]
- 7.Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman S R. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by Jκ and PU.1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempkes B, Pawlita M, Zimber-Strobl U, Eissner G, Laux G, Bornkamm G W. Epstein-Barr virus nuclear antigen 2-estrogen receptor fusion proteins transactivate viral and cellular genes and interact with RBP-J kappa in a conditional fashion. Virology. 1995;214:675–679. doi: 10.1006/viro.1995.0084. [DOI] [PubMed] [Google Scholar]
- 9.Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart J W, Kremmer E, Delecluse H J, Rottenberger C, Bornkamm G W, Hammerschmidt W. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 1995;14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempkes B, Zimber-Strobl U, Eissner G, Pawlita M, Falk M, Hammerschmidt W, Bornkamm G W. Epstein-Barr virus nuclear antigen 2 (EBNA2)-oestrogen receptor fusion proteins complement the EBNA2-deficient Epstein-Barr virus strain P3HR1 in transformation of primary B cells but suppress growth of human B cell lymphoma lines. J Gen Virol. 1996;77:227–237. doi: 10.1099/0022-1317-77-2-227. [DOI] [PubMed] [Google Scholar]
- 11.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Monath T P, Melnick J L, Roizmann B, Straus S E, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 12.Laux G, Adam B, Strobl L J, Moreau-Gachelin F. The Spi-1/PU.1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-J kappa interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. EMBO J. 1994;13:5624–5632. doi: 10.1002/j.1460-2075.1994.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laux G, Dugrillon F, Eckert C, Adam B, Zimber-Strobl U, Bornkamm G W. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane protein and terminal protein 2 genes. J Virol. 1994;68:6947–6958. doi: 10.1128/jvi.68.11.6947-6958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Laux, G. Unpublished data.
- 14.Ling P D, Hsieh J J, Ruf I K, Rawlins D R, Hayward S D. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J Virol. 1994;68:5375–5383. doi: 10.1128/jvi.68.9.5375-5383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picard D. Regulation of protein function through expression of chimaeric proteins. Curr Opin Biotechnol. 1994;5:511–515. doi: 10.1016/0958-1669(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 16.Polack A, Hortnagel K, Pajic A, Christoph B, Baier B, Falk M, Mautner J, Geltinger C, Bornkamm G W, Kempkes B. c-myc activation renders proliferation of Epstein-Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc Natl Acad Sci USA. 1996;93:10411–10416. doi: 10.1073/pnas.93.19.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjöblom A, Jansson A, Yang W, Lain S, Nilsson T, Rymo L. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. J Gen Virol. 1995;76:2679–2692. doi: 10.1099/0022-1317-76-11-2679. [DOI] [PubMed] [Google Scholar]
- 19.Sjöblom A, Yang W, Palmqvist L, Jansson A, Rymo L. An ATF/CRE element mediates both EBNA2-dependent and EBNA2-independent activation of the Epstein-Barr virus LMP1 gene promoter. J Virol. 1998;72:1365–1376. doi: 10.1128/jvi.72.2.1365-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong X, Drapkin R, Reinberg D, Kieff E. The 62- and 80-kDa subunits of transcription factor IIH mediate the interaction with Epstein-Barr virus nuclear protein 2. Proc Natl Acad Sci USA. 1995;92:3259–3263. doi: 10.1073/pnas.92.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong X, Drapkin R, Yalamanchili R, Mosialos G, Kieff E. The Epstein-Barr virus nuclear protein 2 acidic domain forms a complex with a novel cellular coactivator that can interact with TFIIE. Mol Cell Biol. 1995;15:4735–4744. doi: 10.1128/mcb.15.9.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong X, Wang F, Thut C J, Kieff E. The Epstein-Barr virus nuclear protein 2 acidic domain can interact with TFIIB, TAF40, and RPA70 but not with TATA-binding protein. J Virol. 1995;69:585–588. doi: 10.1128/jvi.69.1.585-588.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D Y, Kalpana G V, Goff S P, Schubach W H. Epstein-Barr virus nuclear protein 2 (EBNA2) binds to a component of the human SNF-SWI complex, hSNF5/Ini1. J Virol. 1996;70:6020–6028. doi: 10.1128/jvi.70.9.6020-6028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimber-Strobl U, Kremmer E, Grasser F, Marschall G, Laux G, Bornkamm G W. The Epstein-Barr virus nuclear antigen 2 interacts with an EBNA2 responsive cis-element of the terminal protein 1 gene promoter. EMBO J. 1993;12:167–175. doi: 10.1002/j.1460-2075.1993.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimber-Strobl U, Strobl L J, Meitinger C, Hinrichs R, Sakai T, Furukawa T, Honjo T, Bornkamm G W. Epstein-Barr virus nuclear antigen 2 exerts its transactivating function through interaction with recombination signal binding protein RBP-J kappa, the homologue of Drosophila Suppressor of Hairless. EMBO J. 1994;13:4973–4982. doi: 10.1002/j.1460-2075.1994.tb06824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimber-Strobl U, Kempkes B, Marschall G, Zeidler R, Van Kooten C, Banchereau J, Bornkamm G W, Hammerschmidt W. Epstein-Barr virus latent membrane protein (LMP1) is not sufficient to maintain proliferation of B cells but both it and activated CD40 can prolong their survival. EMBO J. 1996;15:7070–7078. [PMC free article] [PubMed] [Google Scholar]