ABSTRACT
Schistosomiasis is a parasitic disease that afflicts approximately 250 million people worldwide. There is an urgent demand for new antiparasitic agents because praziquantel, the only drug available for the treatment of schistosomiasis, is not universally effective and may derail current progress toward the WHO goal of eliminating this disease as a public health problem by 2030. Nifuroxazide (NFZ), an oral nitrofuran antibiotic, has recently been explored to be repurposed for parasitic diseases. Here, in vitro, in vivo, and in silico studies were conducted to evaluate the activity of NFZ on Schistosoma mansoni. The in vitro study showed significant antiparasitic activity, with 50% effective concentration (EC50) and 90% effective concentration (EC90) values of 8.2 to 10.8 and 13.7 to 19.3 μM, respectively. NFZ also affected worm pairing and egg production and induced severe damage to the tegument of schistosomes. In vivo, a single oral dose of NFZ (400 mg/kg of body weight) to mice harboring either prepatent or patent S. mansoni infection significantly reduced the total worm burden (~40%). In patent infection, NFZ achieved a high reduction in the number of eggs (~80%), but the drug caused a low reduction in the egg burden of animals with prepatent infection. Finally, results from in silico target fishing methods predicted that serine/threonine kinases could be one of the potential targets for NFZ in S. mansoni. Overall, the present study revealed that NFZ possesses antischistosomal properties, mainly in terms of egg burden reduction in animals with patent S. mansoni infection.
IMPORTANCE The increasing recognition of the burden imposed by helminthiasis, associated with the limited therapeutic arsenal, has led to initiatives and strategies to research and develop new drugs for the treatment of schistosomiasis. One of these strategies is drug repurposing, which considers low-risk compounds with potentially reduced costs and shorter time for development. In this study, nifuroxazide (NFZ) was evaluated for its anti-Schistosoma mansoni potential through in vitro, in vivo, and in silico studies. In vitro, NFZ affected worm pairing and egg production and induced severe damage to the tegument of schistosomes. In vivo, a single oral dose of NFZ (400 mg/kg) to mice harboring either prepatent or patent S. mansoni infection significantly reduced the total worm burden and egg production. In silico investigations have identified serine/threonine kinases as a molecular target for NFZ. Collectively, these results implied that NFZ might be a potential therapeutic candidate for the treatment of schistosomiasis.
KEYWORDS: antibiotic, anthelmintic agent, drug discovery, drug repurposing, medicinal chemistry, parasitic diseases, schistosomiasis
INTRODUCTION
Schistosomiasis, a parasitic disease caused by a blood fluke of the genus Schistosoma, is a debilitating disease with a serious global burden (1). It is a chronic disease of poverty characterized by pain and disability that, collectively, exacerbates the already compromised situation of health care in tropical and subtropical areas. Estimates show that at least 230 million people are affected with schistosomiasis and approximately 10% of the world population is at risk of infection (2). Schistosoma mansoni, one of the three major human species, is responsible for public health problems in Africa, the Middle East, the Caribbean, and South America. The morbidity associated with schistosome infection is driven almost entirely by the parasite’s massive egg output, causing hepatosplenomegaly, liver fibrosis, and ascites; in severe cases, S. mansoni infection can be fatal (3).
Since no effective vaccine against schistosomiasis is available, the Word Health Organization (WHO) strategy for schistosomiasis morbidity and transmission focuses on large-scale treatment (preventive chemotherapy) with praziquantel (PZQ). For example, in 2019, an estimate of at least 236.6 million people required preventive treatment for schistosomiasis (2). Although schistosomiasis control has been generally successful across many countries, numerous persistent hot spots remain, and several studies reported the reduced efficacy of praziquantel (4–7). The WHO revised neglected tropical disease (NTD) roadmap targets the elimination of schistosomiasis as a public health problem in all areas of endemicity by 2030 (8). However, any possibility of selection of the parasites for praziquantel resistance or low sensitivity may hamper the 2030 target of global disease elimination. As a result, the WHO recognizes the importance of initiatives for the development of safe, affordable, and effective drugs for schistosomiasis (9–11).
Strategies that have been applied successfully to expedite the discovery process for drugs against parasitic diseases include drug repurposing (12, 13). Known as “new uses for old drugs,” drug repurposing is an approach for identifying new uses for approved or investigational drugs that are outside the scope of the original medical indication. Increasingly, several research groups around the world, including ours, are considering this strategy to alleviate the dilemma of drug shortages, including in the search for new antischistosomal agents (14–18).
Nitrofuran derivatives have been used for decades to treat infectious diseases. In the early 1960s, nitrofurans were used to treat patients with schistosomiasis in China, but toxicity and suboptimal activity led to the abandonment of these compounds (19). Although some nitrofuran drugs have been withdrawn from the market, most of them remain used today in human or veterinary medicine. Nifuroxazide (NFZ) is an oral nitrofuran antibiotic that has been used successfully for many decades for the treatment of infectious colitis and diarrhea. The drug has proved to be well tolerated and safe, and it is available in several countries worldwide. NFZ was also explored as a potential antiparasitic agent, particularly to be repurposed for diseases caused by protozoa (for review see reference 20 and 21). However, studies using NFZ against parasitic worms are scarce. As part of our continuous effort to identify drugs with anthelmintic properties, in this study, we evaluated the antiparasitic activity of NFZ against S. mansoni. Based on the phenotypic assay, NFZ was first tested against adult parasites ex vivo, and subsequently, the 50% effective concentration (EC50) and 90% effective concentration (EC90) values were determined. The motility and morphology of the schistosomes were also monitored using light and scanning electron microscopy. Furthermore, NFZ was administered orally in a murine model infected with either immature (prepatent infection) or adult (patent infection) S. mansoni to characterize the full spectrum of activity of this drug. Finally, target fishing simulations were applied to predict the potential targets and pathways for NFZ in schistosomes.
RESULTS
NFZ alters the viability of adult schistosomes in vitro.
The in vitro antiparasitic potential of NFZ was assessed initially at different concentrations on S. mansoni worm pairs (male and female). For comparison, the known antischistosomal drug PZQ was used as a positive control, whereas vehicle-treated parasites (0.5% DMSO, representing the highest concentration of solvent) were used as a negative control. In vitro EC50 and EC90 values (72 h of incubation) of NFZ and PZQ obtained against adult worms of S. mansoni are summarized in Table 1. EC50 values of NFZ were 8.28 and 13.79 μM at 72 h against male and female worms, respectively. The EC90 values of NFZ were above 10 and 15 μM for male and female schistosomes, respectively. In comparison, PZQ has an EC50 value below 1 μM against adult worm pairs. As expected, 0.5% DMSO had no toxic effect on adult S. mansoni.
TABLE 1.
In vitro activities of nifuroxazide and praziquantel against adult S. mansoni and cytotoxicitya
| Drug | Schistosome results |
Vero cell CC50 | SI | |||
|---|---|---|---|---|---|---|
| EC50 | EC90 | |||||
| Male | Female | Male | Female | |||
| NFZ | 8.28 (6.4–11.5) | 13.79 (11.4–16.7) | 10.84 (9.18–12.17) | 19.32 (17.43–23.57) | >200 | >14 |
| PZQ | 0.63 (0.56–0.72) | 0.81 (0.68–0.96) | 0.97 (0.89–1.19) | 1.21 (1.02–1.38) | >200 | >246 |
S. mansoni adult worms were exposed for 72 h to the tested compounds to calculate the EC50 and EC90 values. Data were calculated from three experiments, and each experiment was performed with three replicates. EC50, 50% effective concentration against adult worm pairs (male and female); CC50, 50% cytotoxic concentration against Vero cells; SI, selectivity index. Values are shown with 95% confidence intervals in parentheses.
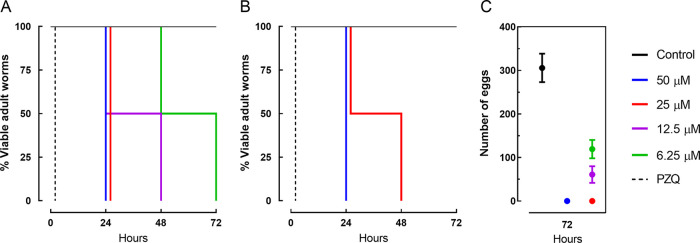
Assays regarding the survival times of adult worm pairs were also performed with NFZ to understand the kinetics and mode of action. As shown in Fig. 1, NFZ induced mortality in a time- and concentration-dependent manner. Similar to the results shown in Table 1, the antiparasitic assay revealed that male schistosomes are more susceptible to NFZ (Fig. 1A) than female worms (Fig. 1B). For example, when adult worm pairs were exposed to NFZ at a concentration of 25 μM for 48 or 72 h, 100% mortality was observed only for male parasites. Throughout the incubation period, parasites in the negative-control group (0.5% DMSO) remained viable. In contrast, PZQ caused the mortality of all worms immediately.
FIG 1.
Viability and egg output of adult S. mansoni worms during exposure to nifuroxazide (NFZ) and praziquantel (PZQ). Adult worm pairs were obtained from animals by perfusion at 42 days after infection. Each concentration was tested at least in triplicate, with the highest concentration of DMSO serving as the control. Male (A) and female (B) schistosomes were monitored for up to 72 h, and results are expressed as the percent mortality recorded by Kaplan-Meier survival curves. Eggs released by paired adult worms exposed to NFZ (C). Data are presented as the mean ± SD from three independent experiments (n = 3). Control, drug-free medium; PZQ, praziquantel at 2 μM.
NFZ affects S. mansoni egg output in vitro.
The capacity of NFZ to affect worm pairing and egg production was evaluated, and the number of eggs within 72 h is shown in Fig. 1C. During the assay, worm couples in the negative-control group moved actively and remained paired throughout the treatment. All adult worm pairs separated after incubation with NFZ at 25 and 50 μM, and they remained separated throughout the incubation period. Thus, a complete lack of oviposition was observed when adult schistosomes were exposed to NFZ at a concentration of ≥25 μM. At a concentration of 12.5 μM, the helminths remained coupled, but the number of eggs was significantly reduced compared with that of control worms (P < 0.001).
NFZ induces severe damage to the tegument of schistosomes.
Scanning electron microscopy examination revealed a normal tegument topography of control group schistosomes, with the typical preservation of tubercles and spines (Fig. 2A and B). In contrast, NFZ at lethal concentrations (50 and 12.5 μM) caused morphological alterations in the tegument of adult schistosomes, which were more pronounced in male (Fig. 2C, E, and G) than in female helminths (Fig. 2D, F, and H). Male parasites exhibited substantial tegumental damage throughout the whole body, with the tubercles and spicules losing their natural shape.
FIG 2.
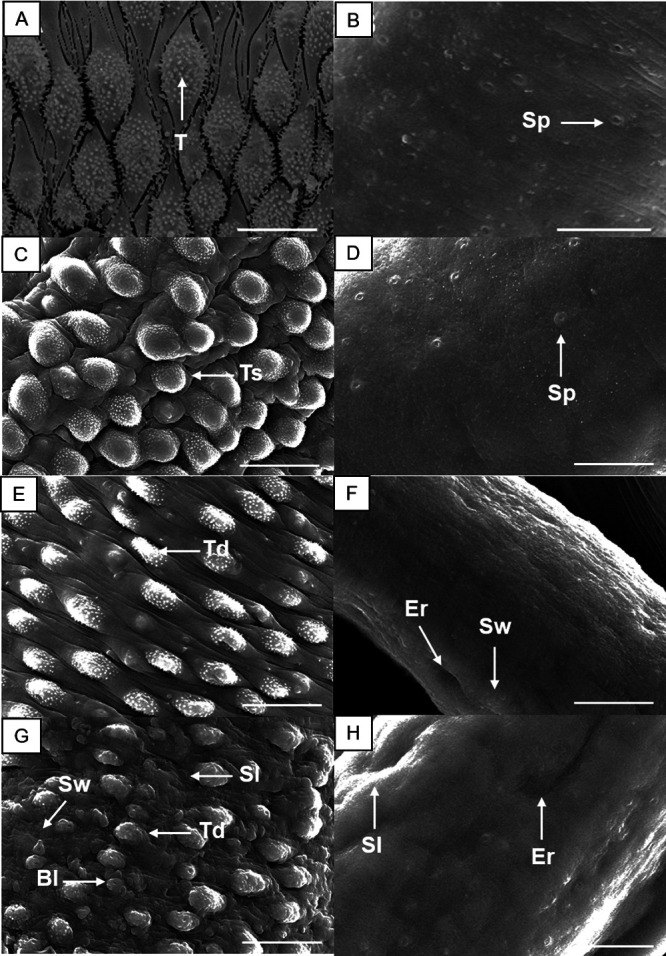
Scanning electron microscopy of adult S. mansoni following incubation with nifuroxazide (NFZ). Control male parasite showing intact tubercles (T) and spines on the surface (A), and control female worm (B) showing the sensory papillae (Sp). Schistosomes were exposed to NFZ at 12.5 μM (C and D), 25 μM (E and F), and 50 μM (G and H). Male (A, C, E, and G) and female (B, D, F, and H) schistosomes. The dorsal tegumental surface shows tubercle shortening (Ts), swelling (Sw), erosion (Er), sloughing (Sl), blisters (Bl), and tubercle disintegration (Td). Images were captured using a JEOL SM 6460LV scanning electron microscope after 72 h of incubation. Scale bars: 10 μm.
NFZ did not exhibit cytotoxicity on Vero cells.
A cytotoxicity assay was performed to evaluate the 50% cytotoxic concentration (CC50) and the selectivity index (SI; ratio between the CC50 values for the cells and the EC50 values for schistosomes). As shown in Table 1, NFZ did not exhibit toxicity at the maximum concentration tested (200 μM), with an SI of >10.
NFZ reduces worm burden and egg production in vivo.
Considering the in vitro results, we investigated the efficacy of NFZ and PZQ administered orally (400 mg/kg of body weight) to mice harboring either juvenile (prepatent infection) or adult (patent infection) S. mansoni. Results were compared with that of the control infected but untreated animal. Of note, all drugs were well tolerated, and all mice survived until the end of the experimental work.
Total worm burden reductions and male and female burden reductions following a single oral dose of NFZ and PZQ are presented in Fig. 3. NFZ significantly reduced worm burden (~40%; P < 0.01) in both prepatent and patent S. mansoni infections compared with control S. mansoni-infected mice (Fig. 3A and B). Administration of PZQ led to a low, but significant, reduction in the number of parasites in animals with prepatent infection (30.4%; P < 0.05) (Fig. 3A). In contrast, a high total worm burden reduction of 88.6% (P < 0.001) was observed when the mice were treated with PZQ (Fig. 3B).
FIG 3.
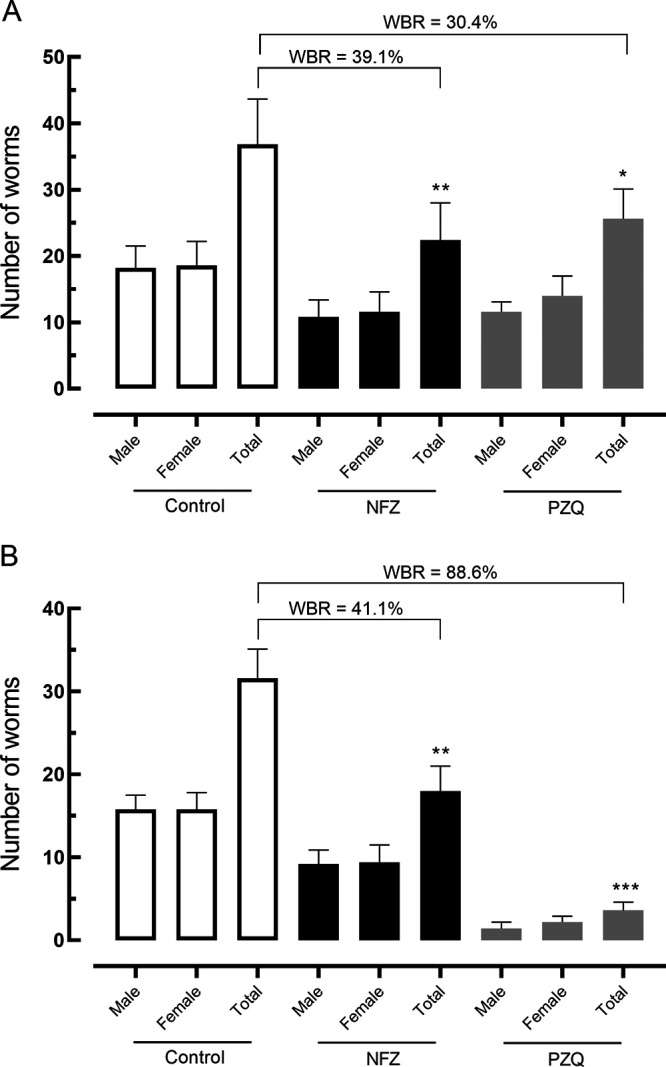
Effect of nifuroxazide (NFZ) and praziquantel (PZQ) on the parasite burden of mice with prepatent (A) and patent (B) Schistosoma mansoni infection. Vehicle (control), NFZ, and PZQ (400 mg/kg, single dose) were administered at 21 days (A) or 42 days (B) postinfection by oral gavage. On day 56 postinfection, all rodents were euthanized and parasite burdens were determined. Data are presented as the mean ± SD from five animals (n = 5 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001; P values were compared with infected untreated control. WBR, worm burden reduction.
The effect of NFZ on egg production was evaluated using the Kato-Katz method (eggs in the feces) and the oogram technique (eggs in the intestine). Results are summarized in Fig. 4.
FIG 4.
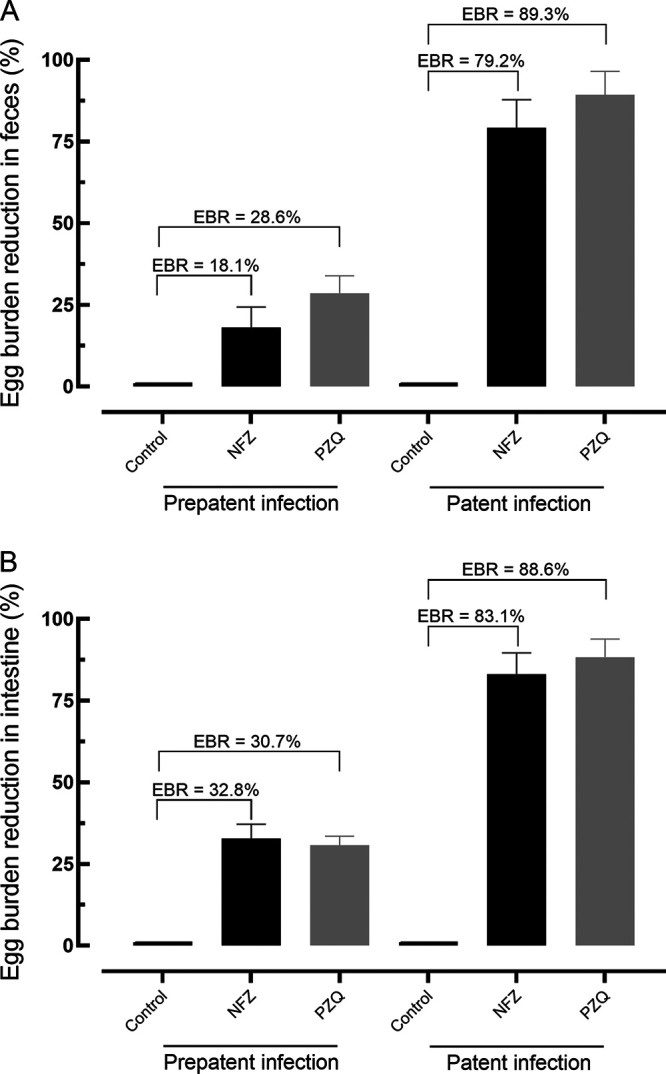
Effect of nifuroxazide (NFZ) and praziquantel (PZQ) on the egg burden in feces (A) and intestine (B) of mice with prepatent and patent Schistosoma mansoni infection. Vehicle (control), NFZ, and PZQ (400 mg/kg, single dose) were administered at 21 days (prepatent infection) or 42 days (patent) postinfection by oral gavage. On day 56 postinfection, all rodents were euthanized, and egg burden was determined by Kato Katz technique (egg in the feces) and oogram examination (immature eggs in the intestine). Data are presented as the mean ± SD from five animals (n = 5 per group). EBR, egg burden reduction.
For fecal examination, the oral dose of NFZ led to a high reduction in the number of eggs (~80%; P < 0.001) in the patent infection, whereas NFZ did not cause a significant reduction in the fecal egg burden of animals with prepatent infection. For comparison, PZQ achieved a fecal egg reduction of 89.3% and 28.6% in animals with patent and prepatent S. mansoni infection, respectively (Fig. 4A).
Regarding the egg burden in the intestine, a single dose of NFZ was able to reduce the number of immature eggs by 32.8% and 83.1% in mice with prepatent and patent infection, respectively. For comparison, PZQ resulted in a reduction of the egg burden by 30.7% and 88.6% during the prepatent and patent periods, respectively (Fig. 4B).
Target fishing studies have identified protein targets for NFZ.
Reverse docking studies were also performed to propose potential macromolecular targets for the action of NFZ. Its potential to produce reactive oxygen species employing nitroreductase catalysis is well known, but it is also known that more specific mechanisms of action, involving proteins as targets, can also take place (22, 23). To study potential receptors for NFZ, its structure was optimized, and its potential energy was calculated together with ChelpG charges by the HF/6-31G* computational method and subjected initially to pharmacophore screening using PharmMapper (Table 2).
TABLE 2.
List of protein targets predicted for nifuroxazide using the reverse pharmacophore approach with PharmMapper and similarity by Basic Local Alignment Search Tool
| PharmMapper results |
BLAST results |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Target | No. of features | Fit score | Z score | Corresponding target in S. mansoni | Max scorea | Query cover (%)b | E valuec | Identity (%)d | |
| PMID | Name | ||||||||
| 2VTI | Cell division protein kinase 2 | 7 | 4.674 | 5.40965 | Serine/threonine kinase | 374 | 98 | 4e-128 | 60.54 |
| 1U4O | l-lactate dehydrogenase | 5 | 3.897 | 4.31237 | Putative l-lactate dehydrogenase | 132 | 84 | 8e-35 | 28.25 |
| 1WBS | Mitogen-activated protein kinase 14 | 7 | 3.969 | 3.79749 | Serine/threonine kinase | 305 | 95 | 1e-100 | 45.43 |
| 1J4H | Peptidyl-prolyl cis-trans isomerase FKBP1A | 9 | 4.173 | 3.50129 | Putative immunophillin FK506 binding protein FKBP12 | 152 | 99 | 3e-48 | 66.98 |
| 1G45 | Carbonic anhydrase 2 | 5 | 3.72 | 3.4792 | Putative carbonic anhydrase II | 196 | 98 | 3e-61 | 40.23 |
| 1CXV | Collagenase 3 | 7 | 3.906 | 3.05545 | Matrix metallopeptidase-7 (M10 family) | 132 | 95 | 2e-36 | 45.78 |
Max score, the highest alignment score calculated from the sum of the rewards for matched nucleotides or amino acids and penalties for mismatches and gaps.
Query cover, the percentage of the query sequence (our specimen) that overlaps the reference sequence.
E value, the number of expected hits of similar quality (score) that could be found just by chance.
Identity, the number of matching bases over the number of alignment columns (64).
Unfortunately, Schistosoma molecular targets are uncommon among the well-described targets or pharmacophores screened by the PharmMapper database. For this reason, none of the retrieved targets corresponded directly to any Schistosomatidae protein but rather corresponded to humans and other more complex organisms. A search for local similarity between protein sequences was then performed to find homologous proteins among the known proteome of Schistosoma species, employing the basic local alignment search tool (BLAST). Table 2 lists the targets for which any proteome correspondence was found. Among protein targets predicted, serine/threonine kinases (STK) appeared twice among the results, when presenting the highest values of max scores, indicating that they were the best alignments obtained. Moreover, expected values (E values) were also the lowest among the results, which translates to their lower probability of arbitrary alignments. Matrix metallopeptidase-7 from S. mansoni and three putative targets (l-lactate dehydrogenase [LDH], immunophilin FK506 binding protein FKBP12, and carbonic anhydrase II) were also predicted to interact with NFZ.
DISCUSSION
The increasing recognition of the burden imposed by helminthiasis, associated with the limited therapeutic arsenal, has led to initiatives and strategies to research and develop new drugs for the treatment of schistosomiasis (9, 10). One of these strategies is drug repurposing, which considers low-risk compounds with potentially reduced costs and shorter time for development (24). NFZ, a well-known and often used antidiarrheal medicine, has been explored for its antiparasitic capabilities against parasites, such as Leishmania and Trypanosoma species (20). To our knowledge, in this study, we demonstrate for the first time that the antibacterial drug NFZ exhibits anthelmintic properties against the flatworm S. mansoni in vitro and in an animal model of schistosomiasis, for both prepatent and patent schistosome infections.
In vitro experiments revealed concentration-dependent, detrimental effects of NFZ on S. mansoni worm pairs, with male parasites being more susceptible to NFZ than females. Although this difference in drug sensitivity concerning the sexes of schistosomes was unknown, several antischistosomal agents are known to act more on male than on female worms (25–28). In addition, it is known that PZQ is more effective in male than in female worms (29), corroborating the results obtained in this study. NFZ also had detrimental effects on egg production in vitro, which is significant because PZQ, when administered at sublethal concentrations, is considerably less effective at reducing the egg count.
The tegumental outer surface of S. mansoni is of crucial importance for parasite survival, thus being a prime target for antischistosomal drug discovery studies (14, 18). Indeed, several studies have shown morphological alterations in schistosomes tegument following exposure to antiparasitic compounds, including PZQ (e.g., reference 28). Phenotypic studies using scanning electron microscopy revealed that NFZ induced substantial tegumental alterations in schistosomes, supporting the hypothesis that the parasite surface is one of the main targets of antischistosomal agents. These results regarding morphological changes could be due to the high lipophilicity of NFZ; consequently, this drug can cross the tegument of schistosomes and reach its molecular target(s).
To obtain further evidence for the importance of NFZ as an antischistosomal compound, NFZ was evaluated in both early and patent S. mansoni infection models in mice using a single oral dose of 400 mg/kg, following the protocol recommended for experimental schistosomiasis in a murine model (19). The treatment with NFZ revealed significant worm burden reductions in rodents with either prepatent or patent infection. It should be noted that PZQ treatment exerts varied cure rates (70 to 90%) (30), but it is concerning that some infections in humans and various other species of animals appear to be refractory to treatment (31, 32). Importantly, it is known that PZQ has low efficacy against juvenile parasites (prepatent infection) (33). Comparatively, oral treatment with NFZ is more effective in early infection than PZQ. These data suggested the advantage of using NFZ instead of PZQ in immature S. mansoni stages.
The efficacy of NFZ therapy in S. mansoni egg production was also evaluated. Interestingly, similar to PZQ, an oral dose of NFZ led to a high reduction in the number of eggs in patent infection (~80%). Since NFZ did not cause a high reduction in the number of worms in patent S. mansoni infections, these results show that the drug interferes directly with egg production, similar to what was observed in our in vitro experiments. Consistent with the results obtained, a significant egg burden reduction, even when worm reduction was low, has been reported with other antischistosomal compounds (34). Collectively, considering that egg production is important for both disease transmission and pathogenesis (1), the negative impact on parasite egg laying is of interest when seeking drugs with anthelmintic properties (9, 11).
The maximum plasma concentration (Cmax) for NFZ is achieved typically within 2 to 3 h after oral administration. The specific Cmax can vary depending on factors such as the formulation, dose, and individual patient characteristics, but information regarding the Cmax of NFZ is scarce. In mice treated intraperitoneally with NFZ at a dose of 15 mg/kg three times per week for 2 weeks, the mean levels of NFZ in the plasma corresponded to 8.5 μM (35), a concentration close to the EC50 described in this study. In rats, about 17% of orally administered NFZ reaches systemic circulation (36). The effectiveness of oral NFZ in S. mansoni-infected animals supports this concept, suggesting that NFZ may be effective in a clinically relevant dose range. In fact, in clinical trials using adult patients, NFZ has been used at a dose of 800 mg per day (37, 38). However, further studies are necessary to evaluate the pharmacokinetic properties and antischistosomal role of orally administered NFZ.
Literature on the mechanism of action of NFZ in bacteria is limited. NFZ exerts bactericidal or bacteriostatic effects, depending on concentration (39). The drug disrupts pathogenic metabolism by interfering with the nucleic acids of pathogens (21). In cancer cells, NFZ has been reported as a potent inhibitor of the signal transducer and activator of transcription 3 (STAT3) (40). To analyze the mechanism of action of NFZ on schistosomes, we performed target fishing studies. STK was one of the main targets of the NFZ, and its activity has been associated with the surface molecules of schistosomes (41). It is known that STKs are enzymes widely found in Apicomplexa (42) but are still under study in helminths (43). They seem to be related to sexual development and maturation and their modulation could affect oogenesis, spermatogenesis, and oviposition which, in the case of S. mansoni, can halt the pathological progress.
An interesting target that has been pointed out is the already characterized matrix metallopeptidase-7 from S. mansoni. This protein is from the M10 family and, as such, is probably involved in the degradation of the extracellular matrix when it is required, such as in tissue remodeling and embryonic development. It can break down proteoglycans, fibronectin, elastin, and casein to achieve this matrix decomposition. In S. mansoni, this enzyme is involved in collagen degradation, which allows the eggs to circulate within the host organism. McCrudden and Iredale (44) showed that an imbalance between the action of degradative metalloproteinases and tissue inhibitors of metalloproteinases leads to liver fibrosis. Moreover, Singh and coworkers (45) proposed that the same imbalance could take place in S. mansoni egg-induced fibrosis, which suggests that the worm metalloproteinase develops a role in egg delivery throughout the host body. Modulation of this enzyme could thus lead to an interesting way of helping the resorption of the eggs by the host tissues.
Three of the correspondents found (LDH, immunophillin FK506 binding protein FKBP12, and carbonic anhydrase II) are putative proteins. In other words, these proteins are probable proteins still to be isolated and characterized within the worm proteome, and their existence is attested only by similarity to genome sequences. NFZ acts by interfering with the activity of enzymes, such as NADH dehydrogenase and certain enzymes involved in electron transport chains within bacterial and protozoal cells (21, 46). By disrupting these key metabolic processes, NFZ disrupts the energy production and survival of the microorganisms (20). Since the parasitic stages of Schistosoma depend on anaerobic energy metabolism, LDH appears to be an interesting target for the development of novel anthelmintic agents. Recent studies have acknowledged the potential of LDH as a target for antiparasitic drug development (47). Carbonic anhydrase II is another putative enzyme that our group has already identified as a potential target for the N-acylhydrazone scaffold (43). This enzyme is supposed to be involved in the osmotic balance and acid-base homeostasis of the worms, and it is located mainly on the tegument surface.
It is important to note that the precise mode of action of NFZ may vary depending on the specific microorganism being targeted (48). Furthermore, research on the mechanism of action of NFZ is ongoing, and further studies may provide additional insights into its exact molecular interactions and pathways (49). All the targets discussed here must be validated experimentally, but the target fishing studies can, at least, point out some directions toward understanding the mechanism of action.
Many studies have revealed that NFZ is well tolerated and safe (38, 50). Advantageously, even at high dosages, NFZ does not affect the integrity of intestinal microbiota (50). In this study, we provide important information regarding the antiparasitic activities of NFZ against S. mansoni. We demonstrated that NFZ affected parasite viability and egg production, and it induced severe tegumental damage in schistosomes. In an animal model of schistosomiasis, we further demonstrate that NFZ is orally effective in both prepatent and patent infections. Moreover, target fishing investigations have identified molecular targets for NFZ, a finding that needs to be validated in experimental target deconvolution studies. Therefore, these results implied that NFZ might be a potential therapeutic candidate for the treatment of schistosomiasis.
MATERIALS AND METHODS
Drugs and reagents.
RPMI 1640 medium, Dulbecco’s modified Eagle medium (DMEM), heat-inactivated fetal calf serum, and penicillin G-streptomycin solutions (10,000 U/mL penicillin G sodium salt and 10 mg/mL streptomycin sulfate) were obtained from Vitrocell (Campinas, SP, Brazil). HEPES buffer, dimethyl sulfoxide (DMSO), and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Praziquantel was kindly provided by Ecovet Indústria Veterinária Ltda (São Paulo, SP, Brazil). NFZ was synthesized as describe in the published protocols (22, 51). In all in vitro experiments, compounds were solubilized in DMSO.
Animals, parasites, and cells.
The life cycle of S. mansoni (BH strain) is maintained by routine passage through Biomphalaria glabrata snails and Swiss mice at Guarulhos University (UNG, Guarulhos, SP, Brazil). Both rodents and snails were kept at 25°C and 50% humidity with an artificial 12-h/12-h day/night cycle and provided with water and food ad libitum. The 4-week-old mice were infected subcutaneously with S. mansoni cercariae, which were collected from S. mansoni-infected snails (39, 52).
Vero cells (monkey kidney epithelial cells) were obtained from the American Type Culture Collection (ATCC CCL-81; Manassas, VA). Cells were cultured in DMEM containing 2 mM l-glutamine, antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin), and 10% heat-inactivated fetal bovine serum and were kept at 37°C in a humidified atmosphere containing 5% CO2. Cells were maintained in 25-cm2 culture flasks (Corning, Tewksbury, MA) and harvested using 0.25% trypsin in 0.2 g/L EDTA solution (28).
In vitro antiparasitic assay.
Adult S. mansoni were collected from mice by dissection at 42 days postinfection and were maintained in RPMI 1640 culture medium supplemented with 5% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C and 5% CO2. Compounds were diluted to 50 μM in supplemented RPMI medium in 24-well plates (Corning, New York, NY), to which one worm of each sex was added per well (53, 54). Each concentration was tested at least in triplicate, and the experiments were repeated three times. Parasites incubated with drug-free DMSO (0.5%) served as a control. The egg output and viability of adult schistosomes were assessed via microscopic readout at 1, 24, 48, and 72 h (33) using a Motic AE2000 inverted microscope (Vancouver, Canada) equipped with a Motic ultrahigh definition (UHD) camera and with a 48-inch 4K-UHD monitor system (LG Electronics, Taubaté, SP, Brazil) (55). The death of adult schistosomes was defined as no movement observed for at least 1 to 2 min of examination, whereas parasites with any body movement were considered viable (28). The percentage of viable parasites was calculated considering schistosomes exposed to compounds versus control worms.
Scanning electron microscopy investigation.
Scanning electron microscopy studies were performed as described previously (26, 56). Briefly, schistosomes (treated and control groups) were fixed in 2.5% glutaraldehyde, and mounted specimens were coated with gold sputter (Denton Vacuum LLC, Moorestown, NJ) and photographed using a JEOL JSM-6460LV scanning electron microscope (Tokyo, Japan).
In vitro cytotoxicity assay.
The MTT assay was used to evaluate the cytotoxic activity as described previously (57). Briefly, cells were plated in 96-well plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) at a density of 2 × 103 cells/well in the presence of NFZ at different concentrations (starting at 200 μM and following a 3-fold dilution series) for 72 h at 37°C and 5% CO2. After the addition of MTT solution, the cells were maintained at 37°C for 4 h. Absorbance was measured at 595 nm using a spectrophotometer (Epoch, BioTek Instruments, Winooski, VT), and the percentage of viable cells was determined concerning the control wells. At least two independent experiments in triplicate were carried out for each test compound. The selectivity indices (SIs) were calculated by dividing the 50% cytotoxic concentration (CC50) obtained on cells with 50% effective concentration (EC50) values determined on schistosomes (58).
In vivo studies in S. mansoni-infected mice.
In vivo studies were performed according to drug discovery programs for schistosomiasis (59). Animal studies are reported in compliance with the National Centre for the Replacement and Refinement & Reduction of Animals in Research (NC3Rs) Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. For experimental protocols, 30 Swiss mice, at 3 weeks old, were infected subcutaneously with 80 S. mansoni cercariae each. Animals were then divided randomly into six experimental groups (five mice per group), and drugs (NFZ and PZQ) or a vehicle (2% ethanol in water) was administered for 21 days (immature parasite, prepatent infection) or 42 days (adult parasite, patent infection) postinfection by oral gavage using a single oral dose of 400 mg/kg (28, 60). On day 56 postinfection, animals in all groups were euthanized using CO2; worms were picked, sexed, and counted; and the worm burden reduction was calculated (61). Therapeutic efficacy was also based on the technique of qualitative and quantitative oograms in the intestine, as well as the Kato-Katz method for quantitative fecal examination, as reported previously (62).
All parameter (worm counts, quantitative and qualitative oogram, and quantitative fecal examination) measurements were performed by different people (by at least two different investigators). To eliminate bias in interpretation, the manipulators of the experiments were not the same researchers as the data analysts (16).
Target fishing studies.
The NFZ structure was constructed using GaussView 5.0 and optimized by employing the ab initio method HF/6-31G* with energy calculated considering ChelpG charges (Gaussian 09W). A mol2 file was constructed within an open babel converter, considering ChelpG as the atomic charges, and was input into the PharmMapper Web-based software (63) for pharmacophore screening. Simulation conditions were adjusted to “generate conformers,” employing the charges and initial conformation from the input file, screening the “All Targets” pharmacophore database, and “retrieving the best 300 results.” The top 25 results were analyzed by their Z score and FitScore values, and only those with the highest values (<2.5) were considered for the Basic Local Alignment Search Tool (BLAST) study (45). Each target was then aligned with known and putative proteins from the Schistosomatidae family database, through their FASTA files (protein primary sequences), employing the BLAST Web-based tool (64).
Statistical analysis.
Statistical analyses were performed using Graph Pad Prism software 8.0 (San Diego, CA). EC50, EC90, and CC50 values were calculated using sigmoid dose-response curves (32). For the experimental analysis of animal studies, the nonparametric Kruskal Wallis test was applied to compare the control group with the treated group (34). The level of statistical significance was set to a P value of <0.05. The data and statistical analysis comply with the recommendations on experimental design and analysis in the pharmacology field (14).
Ethical approval.
Animal studies are reported in compliance with the ARRIVE guidelines. The protocol for experimental design was reviewed and approved by the Committee for the Ethical Use of Animals in Experimentation of Guarulhos University (Guarulhos, SP, Brazil; protocol identifier [ID] 47/20) in conformity with the Brazilian law for Guidelines for Care and Use of Laboratory Animals.
Data availability.
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
We thank all members of the Research Center on Neglected Diseases.
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2016/22488-3 and 2020/01441-4 to J.d.M. and 2019/26686-2 to D.G.G.R.). V.R. and R.A.C. thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for fellowships. A.C.M. was supported by a fellowship from FAPESP (grant 2019/25905-2). M.F.M.-d.-S. received a scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (PIBIC/CNPq). J.d.M. also received an established investigator fellowship from the CNPq. The funding institutions did not have any role in the study design, data collection, data analysis, interpretation, or writing of the report in this study.
We are also grateful to Mariana B. A. Silva for technical assistance.
We declare no competing interests.
Contributor Information
Josué de Moraes, Email: moraesnpdn@gmail.com.
Kevin SW Tan, National University of Singapore.
REFERENCES
- 1.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. 2018. Schistosomiasis. Nat Rev Dis Primers 4:13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2022. Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet 383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster BL, Diaw OT, Seye MM, Faye DS, Stothard JR, Sousa-Figueiredo JC, Rollinson D. 2013. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns. Acta Trop 128:292–302. doi: 10.1016/j.actatropica.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand RE, Mwinzi PNM, Montgomery SP, Chan YL, Andiego K, Omedo M, Muchiri G, Ogutu MO, Rawago F, Odiere MR, Karanja DMS, Secor WE. 2017. A persistent hotspot of Schistosoma mansoni infection in a five-year randomized trial of praziquantel preventative chemotherapy strategies. J Infect Dis 216:1425–1433. doi: 10.1093/infdis/jix496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deol AK, Fleming FM, Calvo-Urbano B, Walker M, Bucumi V, Gnandou I, Tukahebwa EM, Jemu S, Mwingira UJ, Alkohlani A, Traoré M, Ruberanziza E, Touré S, Basáñez MG, French MD, Webster JP. 2019. Schistosomiasis—assessing progress toward the 2020 and 2025 global goals. N Engl J Med 381:2519–2528. doi: 10.1056/NEJMoa1812165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaré RK, N'Tamon RN, Bellai LG, Koffi JA, Mathieu TI, Ouattara M, Hürlimann E, Coulibaly JT, Diabaté S, N'Goran EK, Utzinger J. 2020. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d'Ivoire. Parasit Vectors 13:337. doi: 10.1186/s13071-020-04188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2021. A road map for neglected tropical diseases 2021–2030. https://www.who.int/publications/i/item/9789240010352.
- 9.Ferreira LLG, de Moraes J, Andricopulo AD. 2022. Approaches to advance drug discovery for neglected tropical diseases. Drug Discov Today 27:2278–2287. doi: 10.1016/j.drudis.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Mengarda AC, Iles B, F Longo JP, de Moraes J. 2022. Recent trends in praziquantel nanoformulations for helminthiasis treatment. Expert Opin Drug Deliv 19:383–393. doi: 10.1080/17425247.2022.2051477. [DOI] [PubMed] [Google Scholar]
- 11.Mengarda AC, Iles B, Longo JPF, de Moraes J. 2023. Recent approaches in nanocarrier-based therapies for neglected tropical diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol 15:e1852. doi: 10.1002/wnan.1852. [DOI] [PubMed] [Google Scholar]
- 12.Janes J, Young ME, Chen E, Rogers NH, Burgstaller-Muehlbacher S, Hughes LD, Love MS, Hull MV, Kuhen KL, Woods AK, Joseph SB, Petrassi HM, McNamara CW, Tremblay MS, Su AI, Schultz PG, Chatterjee AK. 2018. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc Natl Acad Sci USA 115:10750–10755. doi: 10.1073/pnas.1810137115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Moraes J, Geary TG. 2020. FDA-Approved antiparasitic drugs in the 21st century: a success for helminthiasis? Trends Parasitol 36:573–575. doi: 10.1016/j.pt.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Lago EM, Silva MP, Queiroz TG, Mazloum SF, Rodrigues VC, Carnaúba PU, Pinto PL, Rocha JA, Ferreira LLG, Andricopulo AD, de Moraes J. 2019. Phenotypic screening of nonsteroidal anti-inflammatory drugs identified mefenamic acid as a drug for the treatment of schistosomiasis. EBioMedicine 43:370–379. doi: 10.1016/j.ebiom.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra RA, Silva MP, Silva TC, Salvadori MC, Teixeira FS, de Oliveira RN, Rocha JA, Pinto PLS, de Moraes J. 2019. In vitro and in vivo studies of spironolactone as an antischistosomal drug capable of clinical repurposing. Antimicrob Agents Chemother 63:e01722-18. doi: 10.1128/AAC.01722-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xavier RP, Mengarda AC, Silva MP, Roquini DB, Salvadori MC, Teixeira FS, Pinto PL, Morais TR, Ferreira LLG, Andricopulo AD, de Moraes J. 2020. H1-antihistamines as antischistosomal drugs: in vitro and in vivo studies. Parasit Vectors 13:278. doi: 10.1186/s13071-020-04140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Brito MG, Mengarda AC, Oliveira GL, Cirino ME, Silva TC, de Oliveira RN, Allegretti SM, de Moraes J. 2020. Therapeutic effect of diminazene aceturate on parasitic blood fluke Schistosoma mansoni infection. Antimicrob Agents Chemother 64:e01372-20. doi: 10.1128/AAC.01372-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porto R, Mengarda AC, Cajas RA, Salvadori MC, Teixeira FS, Arcanjo DDR, Siyadatpanah A, Pereira ML, Wilairatana P, Moraes J. 2021. Antiparasitic properties of cardiovascular agents against human intravascular parasite Schistosoma mansoni. Pharmaceuticals 14:686. doi: 10.3390/ph14070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lago EM, Xavier RP, Teixeira TR, Silva LM, da Silva Filho AA, de Moraes J. 2018. Antischistosomal agents: state of art and perspectives. Future Med Chem 10:89–120. doi: 10.4155/fmc-2017-0112. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser M, Mäser P, Tadoori LP, Ioset JR, Brun R. 2015. Antiprotozoal activity profiling of approved drugs: a starting point toward drug repositioning. PLoS One 10:e0135556. doi: 10.1371/journal.pone.0135556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailly C. 2019. Toward a repositioning of the antibacterial drug nifuroxazide for cancer treatment. Drug Discov Today 24:1930–1936. doi: 10.1016/j.drudis.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Rando DG, Avery MA, Tekwani BL, Khan SI, Ferreira EI. 2008. Antileishmanial activity screening of 5-nitro-2-heterocyclic benzylidene hydrazides. Bioorg Med Chem 16:6724–6731. doi: 10.1016/j.bmc.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 23.da Costa KM, Marques da Fonseca L, Dos Reis JS, Santos M, Previato JO, Mendonça-Previato L, Freire-de-Lima L. 2021. Trypanosoma cruzi trans-sialidase as a potential vaccine target against Chagas disease. Front Cell Infect Microbiol 11:768450. doi: 10.3389/fcimb.2021.768450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. 2019. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 25.Silva MP, Oliveira GL, de Carvalho RB, de Sousa DP, Freitas RM, Pinto PL, de Moraes J. 2014. Antischistosomal activity of the terpene nerolidol. Molecules 19:3793–3803. doi: 10.3390/molecules19033793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva MP, Silva TM, Mengarda AC, Salvadori MC, Teixeira FS, Alencar SM, Luz Filho GC, Bueno-Silva B, de Moraes J. 2021. Brazilian red propolis exhibits antiparasitic properties in vitro and reduces worm burden and egg production in an mouse model harboring either early or chronic Schistosoma mansoni infection. J Ethnopharmacol 264:113387. doi: 10.1016/j.jep.2020.113387. [DOI] [PubMed] [Google Scholar]
- 27.de Carvalho LSA, Alves I, Jr., Junqueira LR, Silva LM, Riani LR, de Faria Pinto P, da Silva Filho AA. 2019. ATP-diphosphohydrolases in parasites: localization, functions and recent developments in drug discovery. Curr Protein Pept Sci 20:873–884. doi: 10.2174/1389203720666190704152827. [DOI] [PubMed] [Google Scholar]
- 28.Brito JR, Wilairatana P, Roquini DB, Parra BC, Gonçalves MM, Souza DCS, Ferreira EA, Salvadori MC, Teixeira FS, Lago JHG, de Moraes J. 2022. Neolignans isolated from Saururus cernuus L. (Saururaceae) exhibit efficacy against Schistosoma mansoni. Sci Rep 12:19320. doi: 10.1038/s41598-022-23110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cioli D, Pica-Mattoccia L. 2003. Praziquantel. Parasitol Res 90:S3–S9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- 30.Coulibaly JT, Panic G, Silué KD, Kovač J, Hattendorf J, Keiser J. 2017. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial. Lancet Glob Health 5:e688–e698. doi: 10.1016/S2214-109X(17)30187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL. 1996. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg 55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 32.Jesudoss Chelladurai J, Kifleyohannes T, Scott J, Brewer MT. 2018. Praziquantel resistance in the zoonotic cestode Dipylidium caninum. Am J Trop Med Hyg 99:1201–1205. doi: 10.4269/ajtmh.18-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergquist R, Elmorshedy H. 2018. Artemether and praziquantel: origin, mode of action, impact, and suggested application for effective control of human schistosomiasis. Trop Med Infect Dis 3:125. doi: 10.3390/tropicalmed3040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xavier ES, de Souza RL, Rodrigues VC, Melo CO, Roquini DB, Lemes BL, Wilairatana P, Oliveira EE, de Moraes J. 2022. Therapeutic efficacy of carvacrol-loaded nanoemulsion in a mouse model of schistosomiasis. Front Pharmacol 13:917363. doi: 10.3389/fphar.2022.917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceruti T, D’Alessandris QG, Frapolli R, Gopalakrishnan J, Buccarelli M, Meroni M, Lauretti L, Ricci-Vitiani L, Pallini R, Zucchetti M. 2022. Development and validation of a HPLC-MS/MS method to measure nifuroxazide and its application in healthy and glioblastoma-bearing mice. Pharmaceutics 14:2071. doi: 10.3390/pharmaceutics14102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labaune JP, Moreau JP, Byrne R. 1986. Comparative physiological disposition of two nitrofuran anti-microbial agents. Biopharm Drug Dispos 7:431–441. doi: 10.1002/bdd.2510070504. [DOI] [PubMed] [Google Scholar]
- 37.Bourée P, Chaput JC, Krainik F, Michel H, Trépo C. 1989. Double-blind controlled study of the efficacy of nifuroxazide versus placebo in the treatment of acute diarrhea in adults. Gastroenterol Clin Biol 13:469–472. [PubMed] [Google Scholar]
- 38.Begovic B, Ahmedtagic S, Calkic L, Vehabović M, Kovacevic SB, Catic T, Mehic M. 2016. Open clinical trial on using nifuroxazide compared to probiotics in treating acute diarrhoeas in adults. Mater Sociomed 28:454–458. doi: 10.5455/msm.2016.28.454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarpignato C, Rampal P. 1995. Prevention and treatment of traveler’s diarrhea: a clinical pharmacological approach. Chemotherapy 41:48–81. doi: 10.1159/000239397. [DOI] [PubMed] [Google Scholar]
- 40.Nelson EA, Walker SR, Kepich A, Gashin LB, Hideshima T, Ikeda H, Chauhan D, Anderson KC, Frank DA. 2008. Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3. Blood 112:5095–5102. doi: 10.1182/blood-2007-12-129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rando DGG, da Costa MOL, Pavani TFA, Oliveira T, Dos Santos PF, Amorim CR, Pinto PLS, de Brito MG, Silva MPN, Roquini DB, de Moraes J. 2019. Vanillin-related N-Acylhydrazones: synthesis, antischistosomal properties and target fishing studies. Curr Top Med Chem 19:1241–1251. doi: 10.2174/1568026619666190620163237. [DOI] [PubMed] [Google Scholar]
- 42.Hui R, El Bakkouri M, Sibley LD. 2015. Designing selective inhibitors for calcium-dependent protein kinases in apicomplexans. Trends Pharmacol Sci 36:452–460. doi: 10.1016/j.tips.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira Moreira B, Weber MHW, Haeberlein S, Mokosch AS, Spengler B, Grevelding CG, Falcone FH. 2022. Drug repurposing and de novo drug discovery of protein kinase inhibitors as new drugs against schistosomiasis. Molecules 27:1414. doi: 10.3390/molecules27041414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCrudden R, Iredale JP. 2000. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol Histopathol 15:1159–1168. doi: 10.14670/HH-15.1159. [DOI] [PubMed] [Google Scholar]
- 45.Singh A, Singh A, Chaudhri SS. 2004. Visceral schistosomiasis of domestic animals in India: humoral immune status of infected cattle, sheep and goats against major polypeptide antigens of Schistosoma indicum and S. spindale. Parasite Immunol 26:167–175. doi: 10.1111/j.0141-9838.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 46.Blumenstiel K, Schöneck R, Yardley V, Croft SL, Krauth-Siegel RL. 1999. Nitrofuran drugs as common subversive substrates of Trypanosoma cruzi lipoamide dehydrogenase and trypanothione reductase. Biochem Pharmacol 58:1791–1799. doi: 10.1016/s0006-2952(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 47.Kayamba F, Faya M, Pooe OJ, Kushwaha B, Kushwaha ND, Obakachi VA, Nyamori VO, Karpoormath R. 2021. Lactate dehydrogenase and malate dehydrogenase: potential antiparasitic targets for drug development studies. Bioorg Med Chem 50:116458. doi: 10.1016/j.bmc.2021.116458. [DOI] [PubMed] [Google Scholar]
- 48.Althagafy HS, El-Aziz MKA, Ibrahim IM, Abd-Alhameed EK, Hassanein EHM. 2023. Pharmacological updates of nifuroxazide: promising preclinical effects and the underlying molecular mechanisms. Eur J Pharmacol 951:175776. doi: 10.1016/j.ejphar.2023.175776. [DOI] [PubMed] [Google Scholar]
- 49.Gan C, Zhang Q, Liu H, Wang G, Wang L, Li Y, Tan Z, Yin W, Yao Y, Xie Y, Ouyang L, Yu L, Ye T. 2022. Nifuroxazide ameliorates pulmonary fibrosis by blocking myofibroblast genesis: a drug repurposing study. Respir Res 23:32. doi: 10.1186/s12931-022-01946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X, Li Y, Jie H, Liu C, Xiong Y, Zuo Z, Zeng A, Li Y, Yu L, Shen G, Wang D, Xie Y, Ye T, Wei Y. 2015. Nifuroxazide induces apoptosis and impairs pulmonary metastasis in breast cancer model. Cell Death Dis 6:e1701. doi: 10.1038/cddis.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rando DG, Sato DN, Siqueira L, Malvezzi A, Leite CQ, do Amaral AT, Ferreira EI, Tavares LC. 2002. Potential tuberculostatic agents. Topliss application on benzoic acid [(5-nitro-thiophen-2-yl)-methylene]-hydrazide series. Bioorg Med Chem 10:557–560. doi: 10.1016/s0968-0896(01)00313-3. [DOI] [PubMed] [Google Scholar]
- 52.de Moraes J, Keiser J, Ingram K, Nascimento C, Yamaguchi LF, Bittencourt CR, Bemquerer MP, Leite JR, Kato MJ, Nakano E. 2013. In vitro synergistic interaction between amide piplartine and antimicrobial peptide dermaseptin against Schistosoma mansoni schistosomula and adult worms. Curr Med Chem 20:301–309. doi: 10.2174/092986713804806694. [DOI] [PubMed] [Google Scholar]
- 53.de Moraes J, Dario BS, Couto RA, Pinto PL, da Costa Ferreira AM. 2015. Antischistosomal activity of oxindolimine-metal complexes. Antimicrob Agents Chemother 59:6648–6652. doi: 10.1128/AAC.01371-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Almeida LM, de Carvalho LS, Gazolla MC, Silva Pinto PL, da Silva MP, de Moraes J, Da Silva Filho AA. 2016. Flavonoids and sesquiterpene lactones from Artemisia absinthium and Tanacetum parthenium against Schistosoma mansoni worms. Evid Based Complement Alternat Med 2016:9521349. doi: 10.1155/2016/9521349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roquini DB, Silva GL, Ferreira LLG, Andricopulo AD, Wilairatana P, De Moraes J. 2022. Susceptibility of Angiostrongylus cantonensis larvae to anthelmintic drugs. Front Pharmacol 13:901459. doi: 10.3389/fphar.2022.901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guimarães MA, de Oliveira RN, de Almeida RL, Mafud AC, Sarkis ALV, Ganassin R, da Silva MP, Roquini DB, Veras LM, Sawada TCH, Ropke CD, Muehlmann LA, Joanitti GA, Kuckelhaus SAS, Allegretti SM, Mascarenhas YP, de Moraes J, Leite JRSA. 2018. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. PLoS One 13:e0196667. doi: 10.1371/journal.pone.0196667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sessa DP, Mengarda AC, Simplicio PE, Antar GM, Lago JHG, de Moraes J. 2020. 15β-Senecioyl-oxy-ent-kaur-16-en-19-oic acid, a diterpene isolated from Baccharis lateralis, as promising oral compound for the treatment of schistosomiasis. J Nat Prod 83:3744–3750. doi: 10.1021/acs.jnatprod.0c01050. [DOI] [PubMed] [Google Scholar]
- 58.Amorim CR, Pavani TFA, Lopes AFS, Duque MD, Mengarda ACA, Silva MP, de Moraes J, Rando DGG. 2020. Schiff bases of 4-Phenyl-2-Aminothiazoles as hits to new antischistosomals: synthesis, in vitro, in vivo and in silico studies. Eur J Pharm Sci 150:105371. doi: 10.1016/j.ejps.2020.105371. [DOI] [PubMed] [Google Scholar]
- 59.Probst A, Biendl S, Keiser J. 2022. Improving translational power in antischistosomal drug discovery. Adv Parasitol 117:47–73. doi: 10.1016/bs.apar.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Mengarda AC, Mendonça PS, Morais CS, Cogo RM, Mazloum SF, Salvadori MC, Teixeira FS, Morais TR, Antar GM, Lago JHG, Moraes J. 2020. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop 205:105350. doi: 10.1016/j.actatropica.2020.105350. [DOI] [PubMed] [Google Scholar]
- 61.Roquini DB, Cogo RM, Mengarda AC, Mazloum SF, Morais CS, Xavier RP, Salvadori MC, Teixeira FS, Ferreira LE, Pinto PL, Morais TR, de Moraes J. 2019. Promethazine exhibits antiparasitic properties in vitro and reduces worm burden, egg production, hepato-, and splenomegaly in a schistosomiasis animal model. Antimicrob Agents Chemother 63:e01208-19. doi: 10.1128/AAC.01208-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mengarda AC, Silva MP, Cirino ME, Morais TR, Conserva GAA, Lago JHG, de Moraes J. 2021. Licarin A, a neolignan isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), exhibited moderate preclinical efficacy against Schistosoma mansoni infection. Phytother Res 35:5154–5162. doi: 10.1002/ptr.7184. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Zhou JL, Liu P, Sun S, Li P. 2010. Chemical markers’ fishing and knockout for holistic activity and interaction evaluation of the components in herbal medicines. J Chromatogr A 1217:5239–5245. doi: 10.1016/j.chroma.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 64.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.