ABSTRACT
The 50% plaque reduction neutralization assay (PRNT50) has been previously used to assess the neutralization capacity of donor plasma against wild-type and variant of concern (VOC) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Emerging data suggest that plasma with an anti-SARS-CoV-2 level of ≥2 × 104 binding antibody units/mL (BAU/mL) protects against SARS-CoV-2 Omicron BA.1 infection. Specimens were collected using a cross-sectional random sampling approach. For PRNT50 studies, 63 previously analyzed specimens by PRNT50 versus SARS-CoV-2 wild-type, Alpha, Beta, Gamma, and Delta were analyzed by PRNT50 versus Omicron BA.1. The 63 specimens plus 4,390 specimens (randomly sampled regardless of serological evidence of infection) were also tested using the Abbott SARS-CoV-2 IgG II Quant assay (anti-spike [S]; Abbott, Chicago, IL, USA; Abbott Quant assay). In the vaccinated group, the percentages of specimens with any measurable PRNT50 versus wild-type or VOC were wild type (21/25 [84%]), Alpha (19/25 [76%]), Beta (18/25 [72%]), Gamma (13/25 [52%]), Delta (19/25 [76%]), and Omicron BA.1 (9/25 [36%]). In the unvaccinated group, the percentages of specimens with any measurable PRNT50 versus wild type or VOC were wild-type SARS-CoV-2 (16/39 [41%]), Alpha (16/39 [41%]), Beta (10/39 [26%]), Gamma (9/39 [23%]), Delta (16/39 [41%]), and Omicron BA.1 (0/39) (Fisher's exact tests, vaccinated versus unvaccinated for each variant, P < 0.05). None of the 4,453 specimens tested by the Abbott Quant assay had a binding capacity of ≥2 × 104 BAU/mL. Vaccinated donors were more likely than unvaccinated donors to neutralize Omicron when assessed by a PRNT50 assay.
IMPORTANCE SARS-CoV-2 Omicron emergence occurred in Canada during the period from November 2021 to January 2022. This study assessed the ability of donor plasma collected earlier (January to March 2021) to generate any neutralizing capacity against Omicron BA.1 SARS-CoV-2. Vaccinated individuals, regardless of infection status, were more likely to neutralize Omicron BA.1 than unvaccinated individuals. This study then used a semiquantitative binding antibody assay to screen a larger number of specimens (4,453) for individual specimens that might have high-titer neutralizing capacity against Omicron BA.1. None of the 4,453 specimens tested by the semiquantitative SARS-CoV-2 assay had a binding capacity suggestive of a high-titer neutralizing capacity against Omicron BA.1. These data do not imply that Canadians lacked immunity to Omicron BA.1 during the study period. Immunity to SARS-CoV-2 is complex, and there is still no wide consensus on correlation of protection to SARS-CoV-2.
KEYWORDS: COVID-19 convalescent plasma, SARS-CoV-2 antibody, Omicron, neutralizing antibody, plaque reduction neutralization, method comparisons
INTRODUCTION
The use of convalescent plasma to treat patients infected with emerging respiratory viruses, including severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and avian influenza, has been a topic of study for decades (1–3). Since the start of the SARS-CoV-2 pandemic, convalescent plasma was identified as a potential therapeutic candidate for clinical trials (4, 5). Those clinical trials identified mixed efficacy of convalescent plasma and the potential for early use of high-titer convalescent plasma in immunocompromised patients infected with SARS-CoV-2 (6–10). A revision of the U.S. Food and Drug Administration (FDA) emergency use authorization (EUA) for the use of COVID-19 convalescent plasma identified immunocompromised individuals as clinical trial candidates for high-titer COVID-19 convalescent plasma (11). Work on convalescent plasma has also led to further studies on protective immunity to SARS-CoV-2 (12–15) and has informed our incomplete understanding of the correlation of protection against SARS-CoV-2 (16, 17). Earlier convalescent plasma qualification approaches relied on low-throughput culture-based 50% plaque reduction neutralization (PRNT50) assays (18). Other, more rapid and easier-to-utilize approaches, including virus-like particle (VLP), competition assays, and enzyme-linked immunosorbent assays, were also used to identify high-titer plasma and study immune responses in individuals previously infected with SARS-CoV-2 (8, 12, 15, 19).
The Abbott SARS-CoV-2 IgG II Quant assay (Abbott anti-spike [S]; Abbott, Chicago, IL, USA; here referred to as the Abbott Quant assay) is a high-throughput assay that is simpler to operationalize than PRNT50. This assay generates semiquantitative results which can be converted into binding antibody units [BAU] per milliliter (20). A prior study noted that a cutoff of 7.1 × 103 BAU/mL might be used to screen for neutralizing high-titer plasma against wild-type, Alpha, Beta, Gamma, and Delta SARS-CoV-2 (12). High-throughput semiquantitative technologies enable researchers to screen large numbers of plasma donations for unique specimens that might contain high-titer anti-SARS-CoV-2 neutralizing plasma against wild-type and variant of concern (VOC) SARS-CoV-2 (21).
SARS-CoV-2 VOC Omicron has shown an ability to partially evade both infection and vaccine-generated pre-Omicron neutralizing antibody capacity (22–25). In individuals with a prior BA.1 or BA.2 infection, there is also a marked decrease in neutralizing capacity against BA.2.12.1, BA.4, and BA.5 (26). Compared to BA.5, Omicron BQ.1.1 and XBB.1 subvariants were more likely to escape neutralizing antibodies after both monovalent and bivalent mRNA vaccine boosting (27). There is growing evidence that screening plasma using high-throughput immunosorbent assays at a threshold of ≥2 × 104 BAU/mL may identify high-titer neutralizing plasma against Omicron BA.1 that could then be used in convalescent plasma clinical trials (28–30).
Assessments of neutralizing capacity of plasma or serum may be impacted by local and temporal factors. Prior to the emergence of Omicron, less than 10% of Canadians were estimated to have been naturally infected with SARS-CoV-2 (31, 32). Until January to March 2021, most infections in Canada were likely due to wild-type or Alpha SARS-CoV-2 (33). Vaccination campaigns were initiated in December 2020, with 96% of all Canadian blood donors showing evidence of measurable antibodies to anti-spike (S) by August 2021 (34). Canadian Blood Services was able to determine donor vaccination status most effectively for the time from January to March 2021 (12, 13).
This study used PRNT50 to determine the neutralizing capacity of vaccinated and unvaccinated donor plasma collected from January to March 2021against Omicron BA.1. This study also used the Abbott Quant assay to screen a larger number of donor plasma specimens collected from this time period for individual specimens potentially containing high-titer neutralizing capacity against Omicron BA.1.
RESULTS
All specimens were from donors with an anti-S or an anti-RBD serological signal.
Study specimens were subsamples of a larger repeated cross-sectional design with random cross-sectional sampling. Previously, 65 specimens were analyzed by PRNT50 (wild-type, Alpha, Beta, Gamma, and Delta SARS-CoV-2) (13) as well as the Abbott Quant assay (12). All specimens previously tested by PRNT50 had evidence of an anti-S or anti-receptor binding domain (RBD) signal (with or without anti-N) (12). Sixty-three specimens had sufficient sample volume to be tested by PRNT50 for Omicron SARS-CoV-2. For the 63 specimens, anti-N profiles, Abbott Quant assay results, donor vaccination histories, and PRNT50 (wild type, Alpha, Beta, Gamma, Delta, and Omicron SARS-CoV-2) are presented in Tables 1 to 4.
TABLE 1.
Summary of Abbott Quant assay and PRNT50 results for vaccinated donors with anti-N signals (n = 5)a
| Specimen no. | Vaccination history | PRNT50 against variant: |
Abbott anti-S (BAU/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Wild type | Alpha | Beta | Gamma | Delta | Omicron | |||
| CIHR013654 | 1 dose (≥14 days) | 40 | <20 | <20 | <20 | <20 | <20 | 2 × 102 |
| CIHR015946 | Dose and timing NA | 20 | <20 | <20 | <20 | <20 | <20 | 7 × 101 |
| CIHR016894 | Dose and timing NA | 5,120 | 2,560 | 5,120 | 5,120 | 5,120 | 160 | 7 × 103 |
| CIHR017333 | Dose and timing NA | 1,280 | 640 | 160 | 160 | 160 | 40 | 3 × 103 |
| CIHR017730 | Dose and timing NA | 2,560 | 1,280 | 1,280 | 2,560 | 1,280 | 80 | 4 × 103 |
| Total donations with any neutralizing capacity (no. [%]) | 5 (100) | 3 (60) | 3 (60) | 3 (60) | 3 (60) | 3 (60) | 5 (100) | |
Median Abbott Quant assay values for this group were 3 × 103 BAU/mL (25th percentile to 75th percentile, 1 × 102 to 6 × 103 BAU/mL). NA, not available.
TABLE 2.
Summary of Abbott Quant assay and PRNT50 results for vaccinated donors without anti-N signals (n = 20)a
| Specimen no. | Vaccination history | PRNT50 against variant: |
Abbott anti-S (BAU/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Wild type | Alpha | Beta | Gamma | Delta | Omicron | |||
| CIHR013818 | Dose and timing NA | 40 | 80 | <20 | <20 | 80 | <20 | 2 × 102 |
| CIHR014329 | Dose and timing NA | 40 | 20 | 40 | <20 | 40 | <20 | 3 × 102 |
| CIHR015234 | 1 dose (≥14 days) | <20 | <20 | <20 | <20 | <20 | <20 | 1 × 102 |
| CIHR015533 | 1 dose (≥14 days) | 640 | 640 | 320 | 160 | 640 | 20 | 7 × 103 |
| CIHR015657 | Dose and timing NA | 40 | 20 | 20 | <20 | 20 | <20 | 8 × 102 |
| CIHR015884 | Fully vaccinated | 320 | 320 | 160 | 160 | 160 | <20 | 2 × 103 |
| CIHR015958 | Dose and timing NA | 40 | 40 | 20 | <20 | 20 | <20 | 5 × 102 |
| CIHR016698 | 1 dose (≥14 days) | 320 | 320 | 80 | 160 | 80 | <20 | 2 × 103 |
| CIHR016904 | Dose and timing NA | 20 | 20 | <20 | <20 | 20 | <20 | 2 × 101 |
| CIHR016905 | Fully vaccinated | 320 | 640 | 160 | 160 | 160 | 20 | 3 × 103 |
| CIHR016930 | 1 dose (≥14 days) | <20 | <20 | <20 | <20 | 20 | <20 | 0 |
| CIHR017087 | Dose and timing NA | 80 | 80 | 80 | 20 | 80 | <20 | 5 × 102 |
| CIHR017189 | 1 dose (≥14 days) | 160 | 80 | 80 | 80 | 80 | <20 | 1 × 103 |
| CIHR017229 | Dose and timing NA | <20 | <20 | 20 | <20 | <20 | <20 | 1 × 101 |
| CIHR017534 | Dose and timing NA | <20 | <20 | <20 | <20 | <20 | <20 | 5 × 101 |
| CIHR017540 | Dose and timing NA | 80 | 40 | 20 | <20 | <20 | 20 | 4 × 102 |
| CIHR017728 | Dose and timing NA | 1,280 | 640 | 320 | 160 | 640 | 80 | 4 × 103 |
| CIHR017824 | Dose and timing NA | 160 | 160 | 160 | 80 | 80 | <20 | 7 × 102 |
| CIHR017838 | Dose and timing NA | 640 | 640 | 640 | 320 | 640 | 40 | 3 × 103 |
| CIHR018126 | Dose and timing NA | 80 | 80 | 80 | 20 | 40 | 20 | 6 × 102 |
| Total donations with any neutralizing capacity (no. [%]) | 16 (80) | 16 (80) | 15 (75) | 10 (50) | 16 (80) | 6 (30) | 19 (95) | |
Median Abbott Quant assay values for this group were 5 × 102 BAU/mL (25th percentile to 75th percentile, 1 × 102 to 2× 103). NA, not available.
TABLE 3.
Summary of Abbott Quant assay and PRNT50 results for nonvaccinated donors with anti-N signals (n = 19)a
| Specimen no. | PRNT50 against variant: |
Abbott anti-S (BAU/mL) | |||||
|---|---|---|---|---|---|---|---|
| Wild type | Alpha | Beta | Gamma | Delta | Omicron | ||
| CIHR013757 | <20 | <20 | <20 | <20 | <20 | <20 | 2 × 100 |
| CIHR013936 | 80 | 40 | 40 | 40 | 40 | <20 | 7 × 101 |
| CIHR014110 | 80 | 20 | 40 | 40 | 40 | <20 | 2 × 102 |
| CIHR014113 | 80 | 20 | 20 | 40 | 40 | <20 | 2 × 102 |
| CIHR014235 | 160 | 40 | <20 | <20 | 320 | <20 | 3 × 102 |
| CIHR014309 | 80 | 40 | 20 | <20 | 80 | <20 | 3 × 102 |
| CIHR014840 | 40 | <20 | <20 | <20 | 20 | <20 | 1 × 102 |
| CIHR014884 | 160 | 160 | <20 | 80 | 80 | <20 | 2 × 102 |
| CIHR014993 | <20 | <20 | <20 | <20 | <20 | <20 | 1 × 101 |
| CIHR015094 | 320 | 320 | 640 | 80 | 640 | <20 | 5 × 101 |
| CIHR015434 | 40 | 40 | <20 | <20 | 40 | <20 | 6 × 101 |
| CIHR016024 | 20 | 20 | <20 | <20 | <20 | <20 | 7 × 101 |
| CIHR016624 | 20 | 20 | 40 | 20 | 20 | <20 | 2 × 101 |
| CIHR016979 | 40 | 40 | 20 | 80 | 80 | <20 | 1 × 102 |
| CIHR017127 | <20 | 20 | <20 | <20 | 40 | <20 | 2 × 101 |
| CIHR017305 | 80 | 40 | 20 | <20 | 80 | <20 | 1 × 102 |
| CIHR017724 | 160 | 80 | 80 | 80 | 160 | <20 | 3 × 102 |
| CIHR017894 | 40 | 20 | <20 | <20 | 20 | <20 | 5 × 101 |
| CIHR017990 | 80 | 40 | 20 | 20 | 40 | <20 | 8 × 101 |
| Total donations with any neutralizing capacity (no. [%]) | 16 (84) | 16 (84) | 10 (53) | 9 (47) | 16 (84) | 0 | 19 (100) |
Median Abbott Quant assay values for this group were 8 × 101 BAU/mL (25th percentile to 75th percentile, 5 × 101 to 2 × 102).
TABLE 4.
Summary of Abbott Quant assay and PRNT50 results for nonvaccinated donors without anti-N signals (n = 19)a
| Specimen no. | PRNT50 against variant: |
Abbott anti-S (BAU/mL) | |||||
|---|---|---|---|---|---|---|---|
| Wuhan | Alpha | Beta | Gamma | Delta | Omicron | ||
| CIHR014238 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR014491 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR014632 | <20 | <20 | <20 | <20 | <20 | <20 | 1 × 101 |
| CIHR014664 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR014926 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR015079 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR015475 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR015843 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR015948 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR016403 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR016447 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR016548 | <20 | <20 | <20 | <20 | <20 | <20 | 2 × 101 |
| CIHR016557 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR016973 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR017530 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR017945 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| CIHR018000 | <20 | <20 | <20 | <20 | <20 | <20 | 1.4 × 101 |
| CIHR018166 | <20 | <20 | <20 | <20 | <20 | <20 | 1 × 100 |
| CIHR018178 | <20 | <20 | <20 | <20 | <20 | <20 | 0 |
| Total no. of donations with any neutralizing capacity | 0 | 0 | 0 | 0 | 0 | 4 (21) | |
Median Abbott Quant assay values for this group were 0 BAU/mL (25th percentile to 75th percentile, 0 to 1 × 101).
Neutralization of wild-type and VOC SARS-CoV-2 in vaccinated versus unvaccinated donors.
Since different cell lines were used to understand the neutralizing capacity of donor plasma against Omicron SARS-CoV-2, median PRNT50 results were not compared directly. Instead, the numbers of specimens producing any neutralizing antibodies (e.g., ≥20) were compared within vaccinated and unvaccinated groups.
Small numbers of specimens for individuals with a vaccine history and an anti-N signal (possible evidence of a past SARS-CoV-2 infection) led to the combination of data from donors vaccinated with an anti-N signal (Table 1) and donors vaccinated without an anti-N signal (Table 2). Data from unvaccinated donors with an anti-N signal (Table 3) and unvaccinated donors without an anti-N signal (Table 4) were also combined.
When neutralizing capacity was measured by PRNT50, plasma from vaccinated donors was more likely than plasma from unvaccinated donors to neutralize VOCs (including Omicron BA.1) and wild type. For wild-type neutralization, the proportions were vaccinated (21/25 [84%]) versus unvaccinated (16/39 [41%]) (P = 0.0008; odds ratio, 7.55; 95% confidence interval [CI], 2.31 to 22.67). For Alpha neutralization, the proportions were vaccinated (19/25 [76%]) versus unvaccinated (16/39 [41%]) (P = 0.01; odds ratio, 4.55; 95% CI, 1.42 to 14.80). For Beta neutralization, the proportions were vaccinated (18/25 [72%]) versus unvaccinated (10/39 [26%]) (P = 0.0003; odds ratio, 7.46; 95% CI, 2.25 to 23.02). For Gamma neutralization, the proportions were vaccinated (13/25 [52%]) versus unvaccinated (9/39 [23%]) (P = 0.03; odds ratio, 3.61; 95% CI, 1.29 to 10.03). For Delta neutralization, the proportions were vaccinated (19/25 [76%]) versus unvaccinated (16/39 [41%]) (P = 0.01; odds ratio, 4.55; 95% CI, 1.42 to 14.80). For Omicron (BA.1) neutralization, the proportions were vaccinated (9/25 [36%]) versus unvaccinated (0/39) (P ≤ 0.0001; odds ratio, ∞).
Assessment of residual specimens from January, February, and March 2021 using the Abbott Quant assay.
In addition to the 63 specimens tested by PRNT50 for Omicron and the Abbott Quant Assay, 4,390 randomly sampled specimens were tested by the Abbott Quant assay (n = 4,453). The monthly distribution of these 4,453 specimens collected in 2021 was 1,499 in January, 1,465 in February, and 1,489 in March. The BAU per milliliter values of these 4,453 specimens are presented in Fig. 1. None of the BAU per milliliter values reached a level of 2 × 104 BAU/mL.
FIG 1.
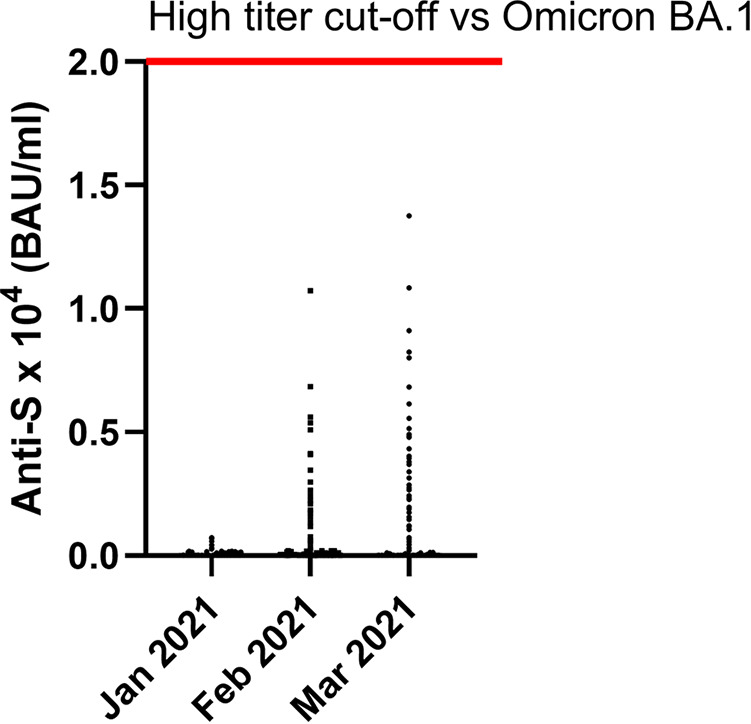
Anti-S BAU/mL levels for Canadian blood donors (April 2020 to March 2021). For this study, 4,453 retention specimens were available from January (n = 1,499), February (n = 1,465), and March (n = 1,489) for testing using the Abbott Quant assay. Anti-S BAU per milliliter values are on the y axis. Data are presented as scatterplots of BAU per milliliter values for each specimen monthly. The red line indicates a high-titer cutoff against SARS-CoV-2 Omicron BA.1 (≥2 × 104 BAU/mL). None of the 4,453 plasma specimens contained an anti-S BAU/mL value of ≥2 × 104 BAU/mL.
DISCUSSION
For the period from January to March 2021, plasma collected from vaccinated Canadian blood donors was more likely to have measurable neutralizing antibodies (measured by PRNT50 against wild type, Alpha, Beta, Gamma, Delta, and Omicron BA.1) than plasma from unvaccinated blood donors. In the unvaccinated group, none of the plasma specimens had measurable PRNT50 titers versus Omicron BA.1. As previously noted, specimens were collected when seroprevalence to SARS-CoV-2 was <10% and when most Canadians with a history of SARS-CoV-2 infection would have been infected with wild-type or Alpha SARS-CoV-2 (32, 33). Only a minority (8%) of vaccinated donors in this study claimed to be fully vaccinated (12, 32), and only 2% of Canadians had received two doses of a SARS-CoV-2 vaccine (35). Wastewater studies and clinical specimens suggest that Omicron emergence occurred much later in Canada, during the period from November 2021 to January 2022 (36–40).
As previously described, Omicron BA.1 can escape neutralization from patients infected with non-Omicron strains. These trends are independent of specific geographic regions. In the United States, convalescent-phase serum collected from a small number of patients infected with Delta (n = 19) had lower levels of pseudovirus neutralization against BA.1 than convalescent-phase serum from BA.1-infected patients (n = 31) (41). In another U.S. study, postinfection serum panels (1 month postinfection [n = 64] and 6 months postinfection [n = 36]) collected prior to the emergence of BA.1 exhibited decreased neutralization against BA.1 than wild-type SARS-CoV-2 when measured with a 50% fluorescent focus reduction neutralization titer (FFRNT50) assay (42). Convalescent serum from Chinese patients hospitalized from January to April 2020 with no vaccination history (n = 24) or 1 dose of vaccine (n = 20) also exhibited reduced neutralization against BA.1 compared to wild type using a pseudovirus assay (43). A small number of specimens collected from Austrian patients with ancestral infection (March and April 2020 [n = 10]) had reduced neutralization of BA.1, using a focus-forming neutralization assay (44).
None of the specimens screened with the Abbott Quant assay had a value of ≥2 × 104 BAU/mL, which has been previously associated with high-titer plasma against Omicron BA.1 (28). This is not unexpected, as convalescent plasma collected during earlier waves of the pandemic may have reduced efficacy against Omicron subvariants as they arise (45). However, this finding does not imply that the donors tested lacked protection against SARS-CoV-2 disease and death. Immunity to SARS-CoV-2 is complex and involves neutralizing antibodies, binding antibodies, antibody-dependent cellular cytotoxicity (46), complex mechanisms of cell-mediated immunity (47), and elements of innate immunity (48). Due to this complexity, there is still no wide consensus on correlations of protection to SARS-CoV-2 (16, 17). Apart from a potential role as a cutoff for high-titer convalescent plasma by convalescent plasma trials (28, 29), there is also no international consensus on the protective utility of the binding antibody value of ≥2 × 104 BAU/mL (30, 49).
A full year of the pandemic would need to pass before the Canadian population developed high BAU per milliliter values. A larger Canadian seroprevalence study (10,000 to 40,000 specimens/month) first identified median BAU/mL levels of ≥2 × 104 BAU/mL in February of 2022 after the emergence of Omicron. However, the low frequency of anti-N and high frequency of anti-S in the population suggests that high BAU per milliliter values were being driven by COVID-19 vaccination programs rather than natural infection (50). This study does not discriminate between the impacts of boosters or new bivalent vaccines. However, it is important to note the benefit of SARS-CoV-2 vaccines in reducing disease burden and death in the Canadian population, even in an environment dominated by Omicron (51–53). The rollout of SARS-CoV-2 vaccines in Canada can be seen as a success story, with 85% of Canadians receiving at least one dose and 82% receiving a primary series by 11 September 2022. However, some Canadians expressed antivaccine sentiments, lacking understanding of vaccines and herd immunity (54), and vaccine-hesitant individuals often expressed a preference for natural immunity (55).
This study has several additional caveats. Different cell culture conditions were used for wild type, Alpha, Beta, Gamma, and Delta than for Omicron. To account for this, the study focused on identifying the presence or absence of any neutralizing antibody capacity against SARS-CoV-2 VOCs. This study included a small number of specimens for the time from January 2021 to March 2021 used for PRNT50 (13). Due to the time taken to develop Omicron BA.1 PRNT50 assays, this study did not assess donor plasma for neutralization against later sublineages of BA.1, BA.2, BA.3, BA.4, BA.5, or recombinants that have circulated in Canada (56). It is also important to acknowledge that donor-declared histories of vaccination may be confounded by recall bias and may be incomplete (57). The collection of vaccination histories, as approved in the study ethics proposal, was also limited to the specimens used for PRNT50 and not linked to data broadly tested with the Abbott Quant assay.
Although this work relies on specimens collected early in the pandemic, it does have applicability to understanding humoral immunity in individuals who are partially vaccine hesitant (receiving less than a full series of wild-type SARS-CoV-2 vaccine) or completely vaccine hesitant (relying on immunity from an earlier infection with wild-type or Alpha SARS-CoV-2). Those individuals may have impaired humoral protection against Omicron BA.1 SARS-CoV-2 infection. Therefore, even in populations with high rates of SARS-CoV-2 infection, vaccination (including boosting with monovalent or bivalent vaccines) is an important strategy in reducing the burden of severe disease and death (58, 59). This protection is broad and ensures the safety of adults and children in the population from outcomes including intensive care admission and death, even when Omicron is dominant (51).
MATERIALS AND METHODS
Ethical considerations.
Institutional ethics board clearance for this project was received from the University of Alberta and the following institutions: Canadian Blood Services and Sinai Health, Toronto (Mount Sinai Hospital).
CIHR Correlates of Immunity study participants and samples.
Canadian Blood Services collects retention EDTA plasma (Becton Dickson [BD], Mississauga, ON, Canada) specimens as previously described (12, 13, 32, 60). As previously described, this was a repeated cross-sectional design with random cross-sectional sampling of all available retention samples (n = 1,500/month) for a 12-month period from January, February, and March of 2021 (total n = 4,500) (20). Samples were then anonymized, aliquoted, transported to test sites, and then stored (−40 to −80°C) (12). A total of 4,453 retention specimens were available from January (n = 1,499), February (n = 1,465), and March (n = 1,489) for testing with the Abbott Quant assay.
Donor SARS-CoV-2 vaccination history and linking to specific specimens.
During the donation screening process, all donors were asked if they received a SARS-CoV-2 vaccine in the past 3 months. This was standard practice at Canadian Blood Services, did not collect information on the vaccine producer, and was not linked to provincial vaccination records. Donor vaccine information focused on donors with specimens linked to PRNT50 neutralization assays (12).
Specimens chosen for SARS-CoV-2 neutralization testing.
Specimens assessed for antibody neutralizing capacity of wild type and variant (Alpha, Beta, Gamma, Delta, and Omicron BA.1) were previously selected using a published tiered testing approach (12, 13).
Definitions of evidence of anti-N positivity.
Serological evidence of anti-N positivity was defined as the presence of an anti-N signal by at least one of the Abbott Architect anti-N SARS-CoV-2 IgG assay or the Sinai Health anti-N assay (see previous publication [12]).
PRNT50 assays: wild type and variants of concern.
Selected EDTA plasma specimens were used in PRNT50 experiments. Vero cell cultures were used for Wuhan wild type (hCoV-19/Canada/ON_ON-VIDO-01-2/2020, Global Initiative on Sharing All Influenza Data [GISAID, https://gisaid.org/] accession number EPI_ISL_425177) and variant of concern strains (Alpha [B.1.1.7], Beta [B.1.351], Gamma [P.1], and Delta [B.1.617.2]). Culture conditions for wild type, Alpha, Beta, Gamma, and Delta followed the experimental conditions previously described (12, 61). For Omicron PRNT50, experimental conditions varied only in that PRNT50 plates were incubated for 3 days prior to fixation with crystal violet-formaldehyde solution for at least 1 h. After rinsing with distilled water (dH2O), plates were air-dried, and plaques were counted on a lightbox (for the detailed PRNT50 procedure, please see Valcourt et al. [61] and Lin et al. [12]). The Omicron virus stock was a clinical isolate passaged in Vero E6 and TMPRSS2 cells, and next-generation sequencing (NGS) was used to confirm the Omicron BA.1 sequence.
SARS-CoV-2 antibody testing using the Abbott Quant assay.
We tested 4,453 randomly selected retention specimens by using the Abbott Quant assay (Abbott Laboratories, Chicago, IL, USA) as per the manufacturer’s guidelines and as previously described (12). These specimens were not subjected to prior stratification based on anti-N, anti-RBD, or anti-S. Semiquantitative values (units per milliliter) generated by the Abbott Quant were converted to BAU per milliliter as described in a prior analysis (12, 20).
Data storage and statistical analysis.
A study identification number was assigned by the information technology team at Canadian Blood Services. All samples were labeled with a study identification number, and all data were stored with this number. Researchers did not have access to the donor-identifying data. Data were stored using a password-protected Microsoft Excel (Redmond, WA, USA) spreadsheet. Descriptive data (median, 25th percentile, and 75th percentile), Fisher's exact test (two-sided), odds ratios, and 95% CIs were calculated with GraphPad Prism (version 9.2.0; GraphPad Software, Inc., San Diego, CA, USA) was used to analyze data. PRNT50 values were assessed for the presence (yes or no) of any measurable neutralizing response against wild type and variant (Alpha, Beta, Gamma, Delta, BA.1 Omicron) SARS-CoV-2.
ACKNOWLEDGMENTS
Canadian Blood Services staff and leadership were instrumental in supporting this project. The operations of the University of Alberta biosafety level 3 (BSL3) laboratory were supported by M. Desaulniers.
The following authors have no conflicts of interest: Sheila F. O’Brien, Qi-Long Yi, Ashleigh Tuite, Karen Colwill, Bhavisha Rathod, Kento T. Abe, and Yi-Chan J. Lin. Stated conflicts of interest are as follows. David H. Evans consults for, and holds research contracts from, Tonix Pharmaceuticals, New York, related to the construction of COVID-19 vaccines. Support for the operations of the University of Alberta BSL3 facility was also received from the Li Ka Shing Institute of Virology, Canada Foundation for Innovation, and Alberta Innovates. Steven J. Drews has functioned as a content expert for respiratory viruses for Johnson & Johnson (Janssen) and has received funding in kind from Abbott. Guillermo Orjuela and Ninette F. Robbins are current employees and shareholders of Abbott Laboratories. Anne-Claude Gingras has received research funds from a research contract with Providence Therapeutics Holdings, Inc., for other projects.
S.J.D., D.H.E., and S.F.O. received funding through the Canadian Institutes of Health Research (CIHR; VR2-172723) and Alberta Innovates (G2020000360 Drews). Commercial Abbott Architect SARS-Cov-2 IgG assay kit costs were supported by Abbott Laboratories, Abbott Park, Illinois. Abbott analyzers used by Canadian Blood Services were supplied by the COVID-19 Immunity Task Force (CITF). Publication charges for this manuscript were funded by the CITF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Methodology, Y.-C.J.L., K.T.A., A.-C.G., B.R., K.C., A.T., D.H.E., and S.J.D.; Investigation, D.H.E., A.-C.G., K.C., A.T., S.F.O., and S.J.D.; Funding acquisition, S.F.O., D.H.E., G.O., N.F.R., A.T., A.-C.G., and S.J.D.; Supervision, D.H.E., A.-C.G., S.F.O., and S.J.D.; Manuscript drafting, S.J.D., Y.-C.J.L., S.F.O., A.T., A.-C.G., G.O., N.F.R., and D.H.E.; Data collation and analysis, A.-C.G., S.F.O., Q.-L.Y., D.H.E, Y.-C.J.L., and S.J.D.; and Administration, S.J.D.
Contributor Information
Steven J. Drews, Email: steven.drews@blood.ca.
Rosemary C. She, Keck School of Medicine of the University of Southern California
REFERENCES
- 1.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. 2005. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu XX, Gao HN, Wu HB, Peng XM, Ou HL, Li LJ. 2015. Successful treatment of avian-origin influenza A (H7N9) infection using convalescent plasma. Int J Infect Dis 41:3–5. doi: 10.1016/j.ijid.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Kong LK, Zhou BP. 2006. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J 12:3–5. [PubMed] [Google Scholar]
- 4.Roback JD, Guarner J. 2020. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA 323:1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 5.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. 2020. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bégin P, Callum J, Cook R, Jamula E, Liu Y, Finzi A, Arnold DM, CONCOR-1 Study Group . 2022. Reply to: concerns about estimating relative risk of death associated with convalescent plasma for COVID-19. Nat Med 28:53–58. doi: 10.1038/s41591-021-01639-5. [DOI] [PubMed] [Google Scholar]
- 7.Bégin P, Callum J, Jamula E, Cook R, Heddle NM, Tinmouth A, Zeller MP, Beaudoin-Bussières G, Amorim L, Bazin R, Loftsgard KC, Carl R, Chassé M, Cushing MM, Daneman N, Devine DV, Dumaresq J, Fergusson DA, Gabe C, Glesby MJ, Li N, Liu Y, McGeer A, Robitaille N, Sachais BS, Scales DC, Schwartz L, Shehata N, Turgeon AF, Wood H, Zarychanski R, Finzi A, Marceau D, Huang A, Carr H, Lin Y, Lall R, Graham C, Arsenault C, Sales V, Sidhu D, Semret M, Hamm C, Arhanchiague E, Solh Z, Srour N, Soliman K, Yee C, Laroche V, Nahirniak S, et al. 2021. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med 27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estcourt LJ, Turgeon AF, McQuilten ZK, McVerry BJ, Al-Beidh F, Annane D, Arabi YM, Arnold DM, Beane A, Bégin P, van Bentum-Puijk W, Berry LR, Bhimani Z, Birchall JE, Bonten MJM, Bradbury CA, Brunkhorst FM, Buxton M, Callum JL, Chassé M, Cheng AC, Cove ME, Daly J, Derde L, Detry MA, De Jong M, Evans A, Fergusson DA, Fish M, Fitzgerald M, Foley C, Goossens H, Gordon AC, Gosbell IB, Green C, Haniffa R, Harvala H, Higgins AM, Hills TE, Hoad VC, Horvat C, Huang DT, Hudson CL, Ichihara N, Laing E, Lamikanra AA, Lamontagne F, Lawler PR, Linstrum K, Litton E, et al. 2021. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA 326:1690–1702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmarets M, Hoffmann S, Vauchy C, Rijnders BJA, Toussirot E, Durrbach A, Körper S, Schrezenmeier E, van der Schoot CE, Harvala H, Brunotte G, Appl T, Seifried E, Tiberghien P, Bradshaw D, Roberts DJ, Estcourt LJ, Schrezenmeier H. 2023. Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19 (COVIC-19): protocol for a randomised, open-label trial. BMJ Open 13:e071277. doi: 10.1136/bmjopen-2022-071277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tayyar R, Wong LK, Dahlen A, Shu E, Pandey S, Liu AY. 2023. High-titer post-vaccine COVID-19 convalescent plasma for immunocompromised patients during the first omicron surge. Transpl Infect Dis 25:e14055. doi: 10.1111/tid.14055. [DOI] [PubMed] [Google Scholar]
- 11.O’Shaughnessy JA. 2021. Convalescent plasma EUA letter. https://www.fda.gov/media/141477/download. Retrieved 18 May 2023.
- 12.Lin Y-CJ, Evans DH, Robbins NF, Orjuela G, Hu Q, Samson R, Abe KT, Rathod B, Colwill K, Gingras A-C, Tuite A, Yi Q-L, O’Brien SF, Drews SJ. 2022. Utilization of the Abbott SARS-CoV-2 IgG II Quant assay to identify high-titer anti-SARS-CoV-2 neutralizing plasma against wild-type and variant SARS-CoV-2 viruses. Microbiol Spectr 10:e02811-22. doi: 10.1128/spectrum.02811-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drews SJ, Hu Q, Samson R, Abe KT, Rathod B, Colwill K, Gingras A-C, Yi Q-L, O’Brien SF. 2022. SARS-CoV-2 virus-like particle neutralizing capacity in blood donors depends on serological profile and donor-declared SARS-CoV-2 vaccination history. Microbiol Spectr 10:e02262-21. doi: 10.1128/spectrum.02262-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunau B, Golding L, Prusinkiewicz MA, Asamoah-Boaheng M, Armour R, Marquez AC, Jassem AN, Barakauskas V, O’Brien SF, Drews SJ, Haig S, Lavoie PM, Goldfarb DM. 2022. Comparative 6-month wild-type and delta-variant antibody levels and surrogate neutralization for adults vaccinated with BNT162b2 versus mRNA-1273. Microbiol Spectr 10:e02702-21. doi: 10.1128/spectrum.02702-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe K, Li Z, Samson R, Samavarchi-Tehrani P, Valcourt E, Wood H, Budylowski P, Dupuis A, II, Girardin R, Rathod B, Wang J, Barrios-Rodiles M, Colwill K, McGeer A, Mubareka S, Gommerman J, Durocher Y, Ostrowski M, McDonough K, Drebot M, Drews S, Rini JM, Gingras A. 2020. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 5:e142362. doi: 10.1172/jci.insight.142362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury DS, Schlub TE, Cromer D, Steain M, Fong Y, Gilbert PB, Subbarao K, Triccas JA, Kent SJ, Davenport MP. 2023. Correlates of protection, thresholds of protection, and immunobridging among persons with SARS-CoV-2 infection. Emerg Infect Dis 29:381–388. doi: 10.3201/eid2902.221422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benkeser D, Fong Y, Janes HE, Kelly EJ, Hirsch I, Sproule S, Stanley AM, Maaske J, Villafana T, Houchens CR, Martins K, Jayashankar L, Castellino F, Ayala V, Petropoulos CJ, Leith A, Haugaard D, Webb B, Lu Y, Yu C, Borate B, van der Laan LWP, Hejazi NS, Carpp LN, Randhawa AK, Andrasik MP, Kublin JG, Isaacs MB, Makhene M, Tong T, Robb ML, Corey L, Neuzil KM, Follmann D, Hoffman C, Falsey AR, Sobieszczyk M, Koup RA, Donis RO, Gilbert PB, on behalf of the AstraZeneca AZD1222 Clinical Study Group . 2023. Immune correlates analysis of a phase 3 trial of the AZD1222 (ChAdOx1 nCoV-19) vaccine. NPJ Vaccines 8:36. doi: 10.1038/s41541-023-00630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drews S, Devine D, McManus J, Mendoza E, Manguiat K, Wood H, Girardin R, Dupuis A, McDonough K, Drebot M. 2021. A trend of dropping anti-SARS-CoV-2 plaque reduction neutralization test titers over time in Canadian convalescent plasma donors. Transfusion 61:1440–1446. doi: 10.1111/trf.16364. [DOI] [PubMed] [Google Scholar]
- 19.Sheffield WP, Bhakta V, Howell A, Jenkins C, Serrano K, Johnson N, Lin YJ, Colwill K, Rathod B, Greenberg B, Gingras AC, Evans DH, Flaumenhaft E, Beckett A, Drews SJ, Devine DV. 2022. Retention of hemostatic and immunological properties of frozen plasma and COVID-19 convalescent apheresis fresh-frozen plasma produced and freeze-dried in Canada. Transfusion 62:418–428. doi: 10.1111/trf.16772. [DOI] [PubMed] [Google Scholar]
- 20.Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Marculescu R, Wolzt M, Wagner OF, Binder CJ, Haslacher H. 2021. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr 9:e00247-21. doi: 10.1128/Spectrum.00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe KT, Rathod B, Colwill K, Gingras A-C, Tuite A, Robbins NF, Orjuela G, Jenkins C, Conrod V, Yi Q-L, O’Brien SF, Drews SJ. 2022. A qualitative comparison of the Abbott SARS-CoV-2 IgG II Quant assay against commonly used Canadian SARS-CoV-2 enzyme immunoassays in blood donor retention specimens, April 2020 to March 2021. Microbiol Spectr 10:e01134-22. doi: 10.1128/spectrum.01134-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He P, Liu B, Gao X, Yan Q, Pei R, Sun J, Chen Q, Hou R, Li Z, Zhang Y, Zhao J, Sun H, Feng B, Wang Q, Yi H, Hu P, Li P, Zhang Y, Chen Z, Niu X, Zhong X, Jin L, Liu X, Qu K, Ciazynska KA, Carter AP, Briggs JAG, Chen J, Liu J, Chen X, He J, Chen L, Xiong X. 2022. SARS-CoV-2 Delta and Omicron variants evade population antibody response by mutations in a single spike epitope. Nat Microbiol 7:1635–1649. doi: 10.1038/s41564-022-01235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapointe HR, Mwimanzi F, Cheung PK, Sang Y, Yaseen F, Kalikawe R, Datwani S, Waterworth R, Umviligihozo G, Ennis S, Young L, Dong W, Kirkby D, Burns L, Leung V, Holmes DT, DeMarco ML, Simons J, Matic N, Montaner JSG, Brumme CJ, Prystajecky N, Niikura M, Lowe CF, Romney MG, Brockman MA, Brumme ZL. 2022. Serial infection with SARS-CoV-2 Omicron BA.1 and BA.2 following three-dose COVID-19 vaccination. Front Immunol 13:947021. doi: 10.3389/fimmu.2022.947021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basile K, Rockett RJ, McPhie K, Fennell M, Johnson-Mackinnon J, Agius JE, Fong W, Rahman H, Ko D, Donavan L, Hueston L, Lam C, Arnott A, Chen SC-A, Maddocks S, O’Sullivan MV, Dwyer DE, Sintchenko V, Kok J. 2022. Improved neutralisation of the SARS-CoV-2 Omicron variant following a booster dose of Pfizer-BioNTech (BNT162b2) COVID-19 vaccine. Viruses 14:2023–2028. doi: 10.3390/v14092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Focosi D, Franchini M, Joyner MJ, Casadevall A, Sullivan DJ. 2022. Analysis of anti-Omicron neutralizing antibody titers in different vaccinated and unvaccinated convalescent plasma sources. medRxiv. doi: 10.1101/2021.12.24.21268317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachmann NP, Miller J, Collier AY, Ventura JD, Yu J, Rowe M, Bondzie EA, Powers O, Surve N, Hall K, Barouch DH. 2022. Neutralization escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med 387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J, Hachmann NP, Collier AY, Lasrado N, Mazurek CR, Patio RC, Powers O, Surve N, Theiler J, Korber B, Barouch DH. 2023. Substantial neutralization escape by SARS-CoV-2 Omicron variants BQ.1.1 and XBB.1. N Engl J Med 388:662–664. doi: 10.1056/NEJMc2214314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimeglio C, Migueres M, Bouzid N, Chapuy-Regaud S, Gernigon C, Da-Silva I, Porcheron M, Martin-Blondel G, Herin F, Izopet J. 2022. Antibody titers and protection against Omicron (BA.1 and BA.2) SARS-CoV-2 infection. Vaccines (Basel) 10:1548. doi: 10.3390/vaccines10091548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toussirot E. 2022. Early high-titre convalescent plasma in clinically vulnerable individuals with mild COVID-19 (COVIC-19). NCT05271929. https://clinicaltrials.gov/ct2/show/NCT05271929. Retrieved 3 June 2023. [DOI] [PMC free article] [PubMed]
- 30.Harvala H, Nguyen D, Simmonds P, Lamikanra AA, Tsang HP, Otter A, Maes P, Webster M, Clarkson A, Kaloyirou F, Hopkins V, Laidlaw SM, Carroll M, Mora A, Griffiths A, MacLennan S, Estcourt L, Roberts DJ. 2022. Convalescent plasma donors show enhanced cross-reactive neutralizing antibody response to antigenic variants of SARS-CoV-2 following immunization. Transfusion 62:1347–1354. doi: 10.1111/trf.16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVID-19 Immunity Task Force. 2021. Recent blood donor data suggest that Canadians still remain vulnerable to SARS-CoV-2 infection. https://www.covid19immunitytaskforce.ca/recent-blood-donor-data-suggest-that-canadians-still-remain-vulnerable-to-sars-cov-2-infection/. Retrieved 19 October 2021.
- 32.Tuite AR, Fisman D, Abe KT, Rathod B, Pasculescu A, Colwill K, Gingras A-C, Yi Q-L, O’Brien SF, Drews SJ. 2022. Estimating SARS-CoV-2 seroprevalence in Canadian blood donors, April 2020 to March 2021: improving accuracy with multiple assays. Microbiol Spectr 10:e02563-21. doi: 10.1128/spectrum.02563-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Collaborating Centre for Infections Diseases. 2022. Updates on COVID-19 variants of concern. https://nccid.ca/covid-19-variants/#subMenuSection0. Retrieved 14 June 2022.
- 34.COVID-19 Immunity Task Force. 2021. Vaccine-induced seroprevalence hits highest level to date, yet early fourth wave hitting those most at-risk: Canadian Blood Services August report. https://www.covid19immunitytaskforce.ca/vaccine-induced-seroprevalence-hits-highest-level-to-date-yet-early-fourth-wave-hitting-those-most-at-risk-canadian-blood-services-august-report/. Retrieved 3 June 2023.
- 35.Government of Canada. 2021. COVID-19 vaccination: vaccination coverage. https://health-infobase.canada.ca/covid-19/vaccination-coverage/. Retrieved 19 October 2021.
- 36.Hubert CRJ, Acosta N, Waddell BJM, Hasing ME, Qiu Y, Fuzzen M, Harper NBJ, Bautista MA, Gao T, Papparis C, Van Doorn J, Du K, Xiang K, Chan L, Vivas L, Pradhan P, McCalder J, Low K, England WE, Kuzma D, Conly J, Ryan MC, Achari G, Hu J, Cabaj JL, Sikora C, Svenson L, Zelyas N, Servos M, Meddings J, Hrudey SE, Frankowski K, Parkins MD, Pang XL, Lee BE. 2022. Tracking emergence and spread of SARS-CoV-2 Omicron variant in large and small communities by wastewater monitoring in Alberta, Canada. Emerg Infect Dis 28:1770–1776. doi: 10.3201/eid2809.220476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawal OU, Zhang L, Parreira VR, Brown RS, Chettleburgh C, Dannah N, Delatolla R, Gilbride KA, Graber TE, Islam G, Knockleby J, Ma S, McDougall H, McKay RM, Mloszewska A, Oswald C, Servos M, Swinwood-Sky M, Ybazeta G, Habash M, Goodridge L. 2022. Metagenomics of wastewater influent from wastewater treatment facilities across Ontario in the era of emerging SARS-CoV-2 variants of concern. Microbiol Resour Announc 11:e00362-22. doi: 10.1128/mra.00362-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Aoust PM, Tian X, Towhid ST, Xiao A, Mercier E, Hegazy N, Jia JJ, Wan S, Kabir MP, Fang W, Fuzzen M, Hasing M, Yang MI, Sun J, Plaza-Diaz J, Zhang Z, Cowan A, Eid W, Stephenson S, Servos MR, Wade MJ, MacKenzie AE, Peng H, Edwards EA, Pang XL, Alm EJ, Graber TE, Delatolla R. 2022. Wastewater to clinical case (WC) ratio of COVID-19 identifies insufficient clinical testing, onset of new variants of concern and population immunity in urban communities. Sci Total Environ 853:158547. doi: 10.1016/j.scitotenv.2022.158547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell SL, Klaver BRA, Harrigan SP, Kamelian K, Tyson J, Hoang L, Taylor M, Sander B, Mishra S, Prystajecky N, Janjua NZ, Zlosnik JEA, Sbihi H. 2023. Clinical severity of Omicron subvariants BA.1, BA.2, and BA.5 in a population-based cohort study in British Columbia, Canada. J Med Virol 95:e28423. doi: 10.1002/jmv.28423. [DOI] [PubMed] [Google Scholar]
- 40.Harrigan SP, Wilton J, Chong M, Abdia Y, Velasquez Garcia H, Rose C, Taylor M, Mishra S, Sander B, Hoang L, Tyson J, Krajden M, Prystajecky N, Janjua NZ, Sbihi H. 2023. Clinical severity of severe acute respiratory syndrome coronavirus 2 Omicron variant relative to Delta in British Columbia, Canada: a retrospective analysis of whole-genome sequenced cases. Clin Infect Dis 76:e18–e25. doi: 10.1093/cid/ciac705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seaman MS, Siedner MJ, Boucau J, Lavine CL, Ghantous F, Liew MY, Mathews JI, Singh A, Marino C, Regan J, Uddin R, Choudhary MC, Flynn JP, Chen G, Stuckwisch AM, Lipiner T, Kittilson A, Melberg M, Gilbert RF, Reynolds Z, Iyer SL, Chamberlin GC, Vyas TD, Vyas JM, Goldberg MB, Luban J, Li JZ, Barczak AK, Lemieux JE. 2022. Vaccine breakthrough infection leads to distinct profiles of neutralizing antibody responses by SARS-CoV-2 variant. JCI Insight 7:e159944. doi: 10.1172/jci.insight.159944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou J, Xia H, Xie X, Kurhade C, Machado RRG, Weaver SC, Ren P, Shi PY. 2022. Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection. Nat Commun 13:852. doi: 10.1038/s41467-022-28544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Zhou B, Fan Q, Zhou X, Liao X, Lin J, Ma Z, Dong J, Wang H, Ge X, Ju B, Zhang Z. 2023. Omicron variants escape the persistent SARS-CoV-2-specific antibody response in 2-year COVID-19 convalescents regardless of vaccination. Emerg Microbes Infect 12:2151381. doi: 10.1080/22221751.2022.2151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rössler A, Netzl A, Knabl L, Schäfer H, Wilks SH, Bante D, Falkensammer B, Borena W, von Laer D, Smith DJ, Kimpel J. 2022. BA.2 and BA.5 omicron differ immunologically from both BA.1 omicron and pre-omicron variants. Nat Commun 13:7701. doi: 10.1038/s41467-022-35312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan DJ, Franchini M, Joyner MJ, Casadevall A, Focosi D. 2022. Analysis of anti-SARS-CoV-2 Omicron-neutralizing antibody titers in different vaccinated and unvaccinated convalescent plasma sources. Nat Commun 13:6478. doi: 10.1038/s41467-022-33864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson SI, Kgagudi P, Manamela NP, Kaldine H, Venter EM, Pillay T, Lambson BE, van der Mescht MA, Hermanus T, Balla SR, de Beer Z, de Villiers TR, Bodenstein A, van den Berg G, Du Pisanie M, Burgers WA, Ntusi NAB, Abdullah F, Ueckermann V, Rossouw TM, Boswell MT, Moore PL. 2023. Antibody-dependent cellular cytotoxicity against SARS-CoV-2 Omicron sub-lineages is reduced in convalescent sera regardless of infecting variant. Cell Rep Med 4:100910. doi: 10.1016/j.xcrm.2022.100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruhl L, Kühne JF, Beushausen K, Keil J, Christoph S, Sauer J, Falk CS. 2023. Third SARS-CoV-2 vaccination and breakthrough infections enhance humoral and cellular immunity against variants of concern. Front Immunol 14:1120010. doi: 10.3389/fimmu.2023.1120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob IB, Gemmiti A, Xiong W, Reynolds E, Nicholas B, Thangamani S, Jia H, Wang G. 2023. Human surfactant protein A alleviates SARS-CoV-2 infectivity in human lung epithelial cells. bioRxiv. doi: 10.1101/2023.04.03.535215:1-43. [DOI] [PMC free article] [PubMed]
- 49.Huygens S, Preijers T, Swaneveld FH, Budde IK, GeurtsvanKessel CH, Koch BCP, Rijnders BJA. 2023. Dosing of convalescent plasma and hyperimmune anti-SARS-CoV-2 immunoglobulins: a phase I/II dose finding study. medRxiv. doi: 10.1101/2023.03.07.23286893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien SF, Caffrey N, Yi Q-L, Pambrun C, Drews SJ. 2022. SARS-CoV-2 seroprevalence among Canadian blood donors: the advance of Omicron. Viruses 14:2336–2315. doi: 10.3390/v14112336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell R, Cayen J, Thampi N, Frenette C, Bartoszko J, Choi KB, Comeau JL, Conly J, Ellis C, Ellison J, Embil J, Evans G, Johnston L, Johnstone J, Katz KC, Kibsey P, Lee B, Lefebvre M-A, Longtin Y, McGeer A, Mertz D, Minion J, Rudnick W, Silva A, Smith SW, Srigley JA, Suh KN, Tomlinson J, Wong A, Pelude L. 2023. Trends in severe outcomes among adult and pediatric patients hospitalized with COVID-19 in the Canadian Nosocomial Infection Surveillance Program, March 2020 to May 2022. JAMA Netw Open 6:e239050. doi: 10.1001/jamanetworkopen.2023.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grewal R, Nguyen L, Buchan SA, Wilson SE, Nasreen S, Austin PC, Brown KA, Fell DB, Gubbay JB, Schwartz KL, Tadrous M, Wilson K, Kwong JC. 2023. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat Commun 14:1273. doi: 10.1038/s41467-023-36566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, Kwong JC. 2022. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the Omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ 378:e071502. doi: 10.1136/bmj-2022-071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffith J, Marani H, Monkman H. 2021. COVID-19 vaccine hesitancy in Canada: content analysis of tweets using the theoretical domains framework. J Med Internet Res 23:e26874. doi: 10.2196/26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. 2013. Vaccine hesitancy: an overview. Hum Vaccin Immunother 9:1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Government of Canada. 2022. COVID-19 epidemiology update. https://health-infobase.canada.ca/covid-19/. Retrieved 3 June 2023.
- 57.Murphy D, Hotopf M, Wessely S. 2008. Multiple vaccinations, health, and recall bias within UK armed forces deployed to Iraq: cohort study. BMJ 337:a220. doi: 10.1136/bmj.a220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeCuir J, Surie D, Zhu Y, Gaglani M, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Phan M, Prekker ME, Gong MN, Mohamed A, Johnson NJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Bender WS, Duggal A, Wilson JG, Qadir N, Chang SY, Mallow C, Kwon JH, Exline MC, Lauring AS, Shapiro NI, Columbus C, Gottlieb R, Vaughn IA, Ramesh M, Lamerato LE, Safdar B, Halasa N, Chappell JD, Grijalva CG, Baughman A, Womack KN, Rhoads JP, Hart KW, Swan SA, Lewis N, et al. 2023. Effectiveness of monovalent mRNA COVID-19 vaccination in preventing COVID-19-associated invasive mechanical ventilation and death among immunocompetent adults during the Omicron variant period—IVY Network, 19 U.S. states, February 1, 2022-January 31, 2023. MMWR Morb Mortal Wkly Rep 72:463–468. doi: 10.15585/mmwr.mm7217a3. [DOI] [PubMed] [Google Scholar]
- 59.Arbel R, Peretz A, Sergienko R, Friger M, Beckenstein T, Duskin-Bitan H, Yaron S, Hammerman A, Bilenko N, Netzer D. 2023. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect Dis doi: 10.1016/S1473-3099(23)00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drews SJ, Abe KT, Hu Q, Samson R, Gingras AC, Colwill K, Rathod B, Wang J, Fazel-Zarandi M, Yi QL, Robinson A, Wood H, Tuite A, Fisman D, Evans DH, Lin YJ, O'Brien SF. 2022. Resistance of SARS-CoV-2 Beta and Gamma variants to plasma collected from Canadian blood donors during the spring of 2020. Transfusion 62:37–43. doi: 10.1111/trf.16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valcourt EJ, Manguiat K, Robinson A, Lin Y-C, Abe KT, Mubareka S, Shigayeva A, Zhong Z, Girardin RC, DuPuis A, Payne A, McDonough K, Wang Z, Gasser R, Laumaea A, Benlarbi M, Richard J, Prévost J, Anand SP, Dimitrova K, Phillipson C, Evans DH, McGeer A, Gingras A-C, Liang C, Petric M, Sekirov I, Morshed M, Finzi A, Drebot M, Wood H. 2021. Evaluating humoral immunity against SARS-CoV-2: validation of a plaque-reduction neutralization test and a multilaboratory comparison of conventional and surrogate neutralization assays. Microbiol Spectr 9:e00886-21. doi: 10.1128/Spectrum.00886-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


