Abstract
A mouse member of the immunoglobulin superfamily, originally designated the murine poliovirus receptor homolog (Mph), was found to be a receptor for the porcine alphaherpesvirus pseudorabies virus (PRV). This mouse protein, designated here murine herpesvirus entry protein B (mHveB), is most similar to one of three related human alphaherpesvirus receptors, the one designated HveB and also known as poliovirus receptor-related protein 2. Hamster cells resistant to PRV entry became susceptible upon expression of a cDNA encoding mHveB. Anti-mHveB antibody and a soluble protein composed of the mHveB ectodomain inhibited mHveB-dependent PRV entry. Expression of mHveB mRNA was detected in a variety of mouse cell lines, but anti-mHveB antibody inhibited PRV infection in only a subset of these cell lines, indicating that mHveB is the principal mediator of PRV entry into some mouse cell types but not others. Coexpression of mHveB with PRV gD, but not herpes simplex virus type 1 (HSV-1) gD, inhibited entry activity, suggesting that PRV gD may interact directly with mHveB as a ligand that can cause interference. By analogy with HSV-1, envelope-associated PRV gD probably also interacts directly with mHveB during viral entry.
Members of the alphaherpesvirus subfamily, exemplified by herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), bovine herpesvirus 1 (BHV-1), and pseudorabies virus (PRV), have a very broad host range in cultured cells. They can also infect and cause disease in animal species other than the natural host. PRV has been used for experimental infections of mice and rats, in part to study aspects of PRV pathogenesis and in part to monitor viral spread in the nervous system as a means of tracing neuronal connections (3, 4, 7, 16, 25). For these studies, it is important to know the identities and distributions of rodent receptors for PRV entry into cells.
Human and animal representatives of the alphaherpesvirus subfamily exhibit common requirements for entry into cells (19, 29). The initial interaction of virus with cells is binding of the virion glycoprotein gC, and in some cases gB, to cell surface glycosaminoglycans, preferentially heparan sulfate. Although gC is dispensable for the infection of many cultured cells, gB, gD, gH, and gL are required for mediating the fusion between virion envelope and cell membrane that allows viral penetration. Cells expressing gD of HSV, BHV-1, or PRV can be resistant to infection by homologous virus and, in some cases, by heterologous alphaherpesviruses (6, 8, 14, 26). This phenomenon, termed gD-mediated interference, suggests that cell-associated gD may sequester or down-regulate a cellular factor required for viral entry.
Recently, four human cell surface proteins have been shown to mediate the entry into cells of one or more of the alphaherpesviruses including PRV (12, 21, 31). One of these proteins is a previously undescribed member of the tumor necrosis factor receptor family, designated HVEM originally (21) and later HveA (12, 31). The other three proteins are related members of the immunoglobulin (Ig) superfamily, a subfamily including the poliovirus receptor (CD155) (18), poliovirus receptor-related protein 1 (Prr1) (17), and poliovirus receptor-related protein 2 (Prr2) (11), which have also been designated CD155-HveD, HveC, and HveB, respectively (12, 31). Prr1-HveC and Prr2-HveB have no detectable activity as receptors for poliovirus entry (23). All three members of the subfamily are receptors for PRV entry, however, and subsets of the three also serve as receptors for HSV-1, HSV-2, and BHV-1 entry (12, 31).
A murine homolog of the poliovirus receptor subfamily is Mph (22). Two transmembrane glycoproteins differing only in the transmembrane and cytoplasmic domains, Mphα and Mphδ, are expressed from mRNAs generated by alternative splicing from the primary transcript (2, 10, 22). Although Mph was initially isolated on the basis of its homology to CD155-HveD, recent studies (1, 11) suggest that it is more closely related (69% identical and 84% similar) to human HveB than to CD155-HveD. In this paper, we use the term mHveB for Mphα. It has recently been reported that mHveB mediates homophilic cell aggregation (1). Male mice carrying a homozygous disruption of the mph gene produce morphologically aberrant spermatozoa and are infertile, indicating a role for this gene in spermatogenesis (5). Our results presented here demonstrate that mHveB can serve as a receptor for PRV entry.
Murine HveB expression in CHO-K1 cells enhances PRV entry.
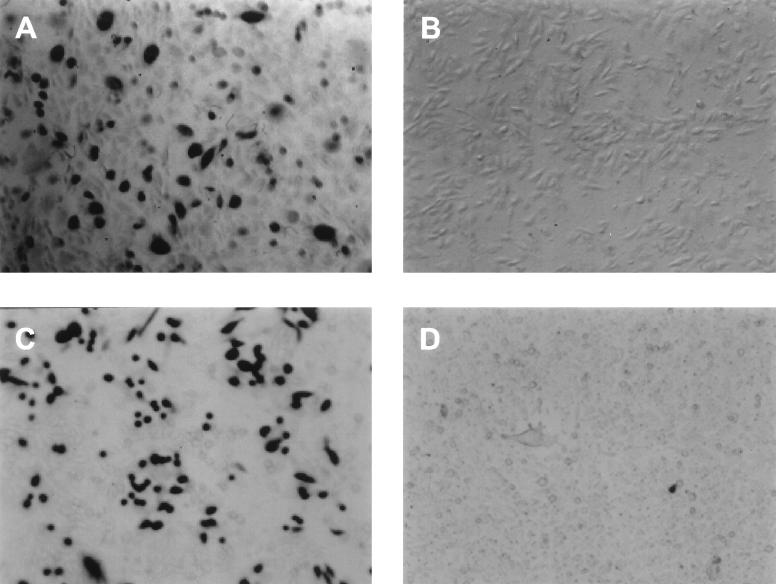
An mHveB-expressing plasmid, designated pDS6, was isolated from a day 19 fetal mouse (FVB strain) cDNA expression library, by a PCR-based technique called Rapid Screen (Origene Technologies, Inc.). Nucleotide sequencing was performed to confirm the presence of the mHveB insert, which proved to be identical in sequence to the cDNA described by Morrison and Racaniello (22). Because Chinese hamster ovary (CHO-K1) cells are highly resistant to the entry of several alphaherpesviruses despite the expression of cell surface glycosaminoglycans to which the viruses can bind (12, 21, 28, 31), these cells were transfected with the mHveB-expressing and control plasmids to test the alphaherpesvirus entry activity of mHveB. The transfected cells were inoculated with recombinants of HSV-1(KOS) (21), a mutant designated HSV-1(KOS)Rid1 (31), HSV-2(333) (27), BHV-1(Cooper) (20), and PRV(Kaplan) (3), each of which carried lacZ so that susceptibility of the cells to infection could be assessed by quantitation of β-galactosidase activity, by methods previously described (12, 21, 31). In multiple experiments (data not shown), mHveB consistently failed to enhance the entry of HSV-1(KOS), HSV-1(KOS)Rid1, HSV-2(333), or BHV-1. However, mHveB expression significantly enhanced the entry of PRV, as demonstrated by quantitation of β-galactosidase activity after exposure of mHveB-expressing and control CHO-K1 to serial dilutions of virus (data not shown) and as illustrated in Fig. 1. For this experiment, CHO-K1 cells were transiently transfected with the mHveB-expressing plasmid or a control plasmid and then either stained with anti-mHveB monoclonal antibody (MAb) 6B3 (1) or exposed to PRV, in order to determine whether the numbers of cells expressing mHveB and rendered susceptible to PRV entry were approximately the same. Whereas the cells transfected with control plasmid failed to react with the anti-mHveB antibody and resisted PRV entry (Fig. 1B and D), approximately 30% of the cells transfected with the mHveB plasmid expressed mHveB (Fig. 1A) and were infected by PRV as assessed by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining (Fig. 1C). The spectrum of alphaherpesvirus entry activity observed for mHveB is similar to that observed for human HveB except that human HveB could mediate entry of HSV-1 mutants, such as HSV-1(KOS)Rid1, that have amino acid substitutions at position 27 in gD, and also of some HSV-2 strains (31).
FIG. 1.
Entry of PRV mediated by mHveB. CHO-K1 cells in six-well tissue culture dishes were transfected with mHveB-expressing plasmid pDS6 (A and C) or control plasmid pcDNA3 (B and D). (A and B) At 48 h posttransfection, the cells were washed twice with phosphate-buffered saline and fixed (phosphate-buffered saline containing 2% formaldehyde and 0.2% gluteraldehyde). The fixed cells were immunostained by incubation with the anti-mHveB MAb 6B3 (1:100 dilution) for 1 h at room temperature followed by washing with phosphate-buffered saline and serial incubations with horseradish peroxidase-conjugated goat anti-rat IgG antibody (1:200 dilution for 1 h) and DAB (3,3′-diaminobenzidine tetrahydrochloride), which produces a brown insoluble end product. (B and D) At 48 h posttransfection, the cells were exposed to a β-galactosidase-expressing recombinant of PRV(Kaplan) (3) at 20 PFU/cell. After 6 h, the cells were washed three times with phosphate-buffered saline, fixed, permeabilized, and incubated with X-Gal, which yields an insoluble blue product upon hydrolysis by β-galactosidase.
Anti-mHveB antibody (6B3) and soluble mHveB block PRV entry.
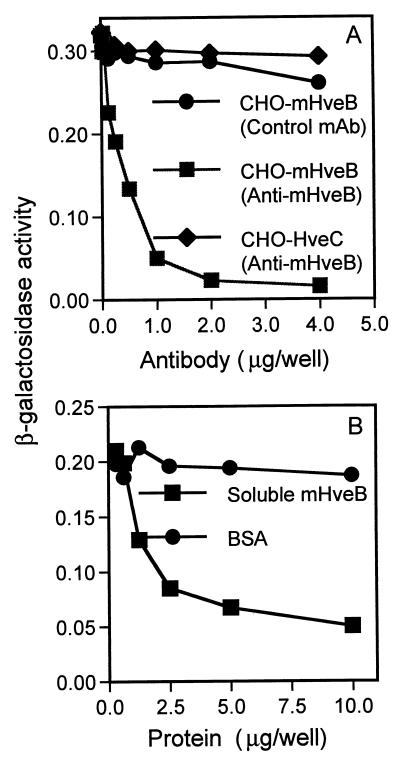
If the entry activity of mHveB depends on direct interaction with a PRV envelope glycoprotein, then antibodies specific for appropriate regions of mHveB could block viral entry and soluble mHveB could bind to virus, thereby preventing the virus from binding to cell surface membrane-bound mHveB. Two MAbs were tested for the ability to block mHveB-dependent entry of PRV. Antibody 6B3 binds to the N-terminal V-like domain of mHveB, and antibody 18C12 binds to the membrane-proximal C2-like domain (1). Figure 2A shows that incubation of mHveB-expressing CHO-K1 cells with antibody 6B3, but not the control antibody, almost completely inhibited PRV infection whereas antibody 6B3 had no effect on infection of human HveC-expressing cells. Antibody 18C12, tested at the same range of concentrations as shown for 6B3, had only partial inhibitory activity (data not shown).
FIG. 2.
Anti-mHveB MAb and soluble mHveB blocked entry of PRV into mHveB-expressing CHO-K1 cells. The CHO cells were transiently transfected in six-well dishes with mHveB-expressing pDS6 (squares and circles) and human HveC-expressing pBG38 (12) (diamonds) with Lipofectamine (Gibco-BRL). At 24 h posttransfection, the cells were replated in 96-well tissue culture dishes. The following day, the cells were washed with phosphate-buffered saline and inoculated with PRV. (A) The cells were incubated for 30 min with serial dilutions of purified anti-mHveB MAb 6B3 (squares and diamonds) or control anti-myc MAb (circles), and then a constant dose of β-galactosidase-expressing PRV (106 PFU/well) was added. After 3 h of incubation, the virus-MAb mixtures were removed, unpenetrated virus was inactivated by brief treatment with 100 mM citrate buffer (pH 3.0), and incubation was continued for another 3 h. The cells were permeabilized, and ONPG (o-nitrophenyl-β-d-galactopyranoside) substrate was added for quantitation of β-galactosidase activity at 405 nm with a Dynatech enzyme-linked immunosorbent assay reader or a Spectromax 250 reader. (B) Purified soluble mHveB protein (squares) or BSA (circles) at amounts indicated was added to 5 × 105 PFU of β-galactosidase-expressing PRV and incubated for 30 min. Soluble protein-virus mixtures were added to mHveB-expressing CHO-K1 cells plated in 96-well dishes. Three hours postinfection, residual virus was neutralized by treatment of cells with 100 mM citrate, pH 3.0. Medium was replenished, and 4 h later, viral entry was assayed as described above.
The soluble mHveB was purified from the culture medium of 3T3 cells engineered to express the three extracellular Ig-like domains linked to six histidine residues (10). After chromatography on nickel-agarose, Q-Sepharose, methyl-Sepharose, and Sephacryl S-300, soluble mHveB was judged to be greater than 95% pure by silver staining (data not shown). The purified soluble mHveB and bovine serum albumin (BSA) as control were mixed at various concentrations with PRV, and then CHO-K1 cells expressing mHveB were inoculated with the protein-virus mixtures. Figure 2B shows that soluble mHveB, but not BSA, caused significant inhibition of PRV entry, as much as 80% inhibition at the highest dose tested. Similar blocking effects have been observed elsewhere with human HveA and HveC (12, 21), which were shown to bind directly to HSV gD (15, 24, 33, 34). Taken together, the results obtained with the anti-mHveB antibodies and soluble mHveB indicate that mHveB binds directly to PRV.
PRV entry via mHveB is sensitive to gD-mediated interference.
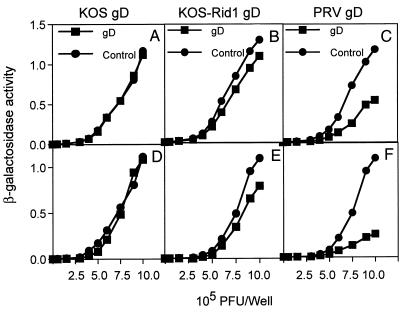
To determine whether PRV entry mediated by mHveB was subject to gD-mediated interference, CHO-K1 cells were transiently cotransfected with mixtures of mHveB expression plasmid and gD expression plasmids (or control plasmids) and then inoculated with various doses of β-galactosidase-expressing PRV. As shown in Fig. 3C and F, coexpression of PRV gD with mHveB strongly interfered with PRV infection, suggesting a direct interaction between the two proteins. Enhancement of interference was observed when the ratio of PRV gD to mHveB was increased from 1:1 (Fig. 3C) to 4:1 (Fig. 3F) during transfection. However, similar ratios of wild-type HSV-1 gD had no effect on PRV entry (Fig. 3A and D). This is consistent with the inability of HSV-1(KOS) to use mHveB for entry. It is interesting that the mutant gD expressed by HSV-1(KOS)Rid1 exhibited a small but reproducible level of interference (Fig. 3B and E), although the mutant appeared unable to use mHveB for entry. Human HveB is able to mediate entry of both PRV and HSV-1(KOS)Rid1 (31). Because the human and murine forms of HveB are highly homologous (84% similarity), it should be relatively straightforward to identify features of human HveB that permit entry of HSV-1(KOS)Rid1 and, presumably, more efficient interaction with Rid1 gD.
FIG. 3.
PRV entry is sensitive to gD-mediated interference. CHO cells cotransfected with mHveB-expressing plasmid (pDS6) and gD-expressing plasmids [pRE4 for wild-type HSV-1 gD (9), pMW13 for mutant HSV-1(KOS)Rid1 gD (32), pCMV-gD for PRV gD (13), and pcDNA3 as control] in a 1:1 ratio (A to C) or a 1:4 ratio (D to F) were infected with β-galactosidase-expressing PRV at the doses indicated. Six hours later, the cells were rinsed and solubilized by addition of 0.5% Nonidet P-40 in phosphate-buffered saline containing ONPG (o-nitrophenyl-β-d-galactopyranoside), and the enzymatic activity was monitored at 410 nm.
mHveB is expressed in many mouse cell lines.
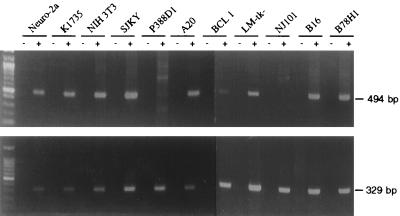
To assess expression of mHveB in various cell lines, total RNA was extracted from cells lysed with RNA STAT-60 (Tel-Test, Inc., Friendswood, Tex.), and the RNA was converted to first-strand cDNA with the 3′ rapid amplification of cDNA ends system (Gibco BRL), followed by PCR amplification with primers specific for mHveBα or mHveBδ and β2-microglobulin. As shown in Fig. 4, mHveBα expression was detected in most cell lines tested, including melanoma and neuronal lines. Only one cell line tested (NJ101 of B-cell origin) reproducibly gave negative results. Similar results were obtained with primers specific for mHveBδ cDNA except that mHveBδ cDNA was not readily detectable in BCL1 cells (data not shown). Overall, these results indicate that PRV entry into several cell types could be mediated by either mHveBα or mHveBδ.
FIG. 4.
Expression of mHveB mRNA in mouse cell lines. Total RNA was isolated from the cell lines indicated, and reverse transcription-PCR was performed as described in the text, generating a 494-bp fragment from mHveB cDNA and a 329-bp fragment from β2-microglobulin cDNA. The primers for mHveB were 5′-AGAGTGGAACACGAGAGCTT-3′ and 5′-GGATCCTCTGTCGCCATCAT-3′, and those for β2-microglobulin were 5′-GACCCTGGTCTTTCTGGTGC-3′ and 5′-AGTAGACGGTCTTGGGCTCG-3′ (30). (Upper panel) mHveB expression in mouse cell lines Neuro-2a (neuroblastoma), K1735 (melanoma), NIH 3T3 (fibroblast), SJKY (fibroblast), P388D1 (macrophage), A20 (B-cell lymphoma), BCL1 (B-cell lymphoma), LM-tk− (connective tissue), NJ101 (B-cell lymphoma), B16 (melanoma), and B78H1 (melanoma) plus or minus reverse transcriptase. SJKY, P388D1, A20, BCL1 and NJ101 were obtained from B. Kim; B16, B78H1, and K1735 were obtained from N. Fraser; and Neuro-2a, LM-tk−, and NIH 3T3 were obtained from the American Type Culture Collection. (Lower panel) β2-Microglobulin expression, plus or minus reverse transcriptase.
mHveB is the major mediator of PRV entry into mouse melanoma (B78H1) cells.
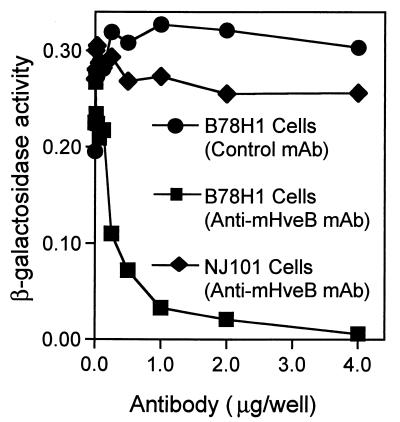
Most of the cell lines tested for Fig. 4 were found to be susceptible to PRV entry, including NJ101 cells, in which mHveB mRNA was not detected. Antibodies specific for mHveB could be expected to block PRV infection of mouse cells, provided that mHveB was the principal receptor available for viral entry. Two cell lines that could be infected by PRV, one of which expressed mHveB mRNA (B78H1) and the other of which apparently did not (NJ101), were preincubated with anti-mHveB MAb (6B3) or control antibody prior to inoculation with PRV. As shown in Fig. 5, PRV infection of B78H1 cells could be blocked almost completely by the anti-mHveB MAb in a dose-dependent manner. In contrast, the same antibody did not block infection of NJ101 cells, indicating expression of some other mediator(s) of PRV entry in these cells. The same experiment performed (data not shown) with other mouse cell lines produced no detectable inhibition (NIH 3T3) or only partial inhibition (LM-tk−), suggesting the expression of multiple mediators of PRV entry in these cells. This is not surprising given that multiple PRV coreceptors belonging to the poliovirus receptor subfamily have been identified in human cells.
FIG. 5.
Infection of mouse melanoma (B78H1) cells is dependent on mHveB. B78H1 cells were preincubated with anti-mHveB MAb (squares) or control anti-myc MAb (circles), and NJ101 cells were preincubated with anti-mHveB MAb (diamonds). After 30 min of incubation, the cells were challenged by the addition of a constant dose of β-galactosidase-expressing PRV. After 3 h of incubation, the virus-MAb mixtures were removed, unpenetrated virus was inactivated by brief treatment with 100 mM citrate buffer (pH 3.0), and incubation was continued for another 3 h. The cells were permeabilized, and ONPG (o-nitrophenyl-β-d-galactopyranoside) substrate was added for quantitation of β-galactosidase activity as described in the text.
Although humans and rodents are not normally infected by PRV, the existence of human and murine cell surface proteins that can mediate PRV entry provides an explanation for the broad host range of PRV in cultured cells. The fact that PRV can use both human and murine members of the poliovirus receptor subfamily as entry receptors strongly suggests that porcine and other animal homologs of these cell surface proteins are very likely to be functional entry proteins for PRV infection of cells of the natural hosts. The results suggest that PRV interacts with some highly conserved features of human and animal members of this receptor subfamily.
Although rodents including mice and rats are not regarded as the natural hosts, they can be infected by PRV and have been extensively used as models for studying PRV pathogenesis and also for tracing neuronal connections by the spread of viral infection (3, 4, 7, 16, 25). It is possible that infection of neurons could be mediated by mHveB or by another related member of the Ig superfamily not yet characterized. The discovery of mHveB as a cellular coreceptor for PRV entry, along with the results suggesting that PRV gD can interact with mHveB, is sure to open new avenues for understanding the mechanism of entry and spread of PRV in the cells of animals. At the same time, more experiments are needed to identify other mediators of PRV entry in mouse and their homologs in host animals to determine if the mechanism of entry is similar and interchangeable in mice and pigs, the natural hosts for PRV.
Acknowledgments
We thank L. Bello, G. Cohen, R. Eisenberg, N. Fraser, V. Gerdts, B. Kim, T. Mettenleiter, and A. Nomoto for providing some of the reagents used in this study; M. Warner for generation of pMW13; and N. Susmarski for excellent technical assistance.
This work was supported by NIH grant U01 AI31494 (P.G.S.). Support for trainees was provided by NIAID fellowship F32 AI09951 (D.S.) and a fellowship from the American Social Health Association (C.L.R.).
REFERENCES
- 1.Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- 2.Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- 3.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A, Mettenleiter T C. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 4.Babic N, Mettenleiter T C, Ugolini G, Flamand A, Coulon P. Propagation of pseudorabies virus in the nervous system of the mouse after intranasal inoculation. Virology. 1994;204:616–625. doi: 10.1006/viro.1994.1576. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard, M. J., Y. Dong, and V. R. Racaniello. 1998. Unpublished results.
- 6.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurol. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chase C C L, Lohff C, Letchworth G J. Resistance and susceptibility of bovine cells expressing herpesviral glycoprotein D homologs to herpesviral infections. Virology. 1993;194:365–369. doi: 10.1006/viro.1993.1269. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G H, Wilcox W C, Sodora D L, Long D, Levin J Z, Eisenberg R J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988;62:1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, Y., and V. Racaniello. 1998. Unpublished results.
- 11.Eberlé F, Dubreuil P, Mattei M G, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- 12.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 13.Gerdts V, Jones A, Makoschey B, Visser N, Mettenleiter T C. Protection of pigs against Aujeszky’s disease by DNA vaccination. J Gen Virol. 1997;78:2139–2146. doi: 10.1099/0022-1317-78-9-2139. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R M, Spear P G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor related protein 1 (PRR1/HveC) or herpes virus entry mediator (HVEM/HveA), two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loewy A D, Bridgman P C, Mettenleiter T C. Beta-galactosidase expressing recombinant pseudorabies virus for light and electron microscopic study of transneuronally labeled CNS neurons. Brain Res. 1991;555:346–352. doi: 10.1016/0006-8993(91)90364-2. [DOI] [PubMed] [Google Scholar]
- 17.Lopez M, Eberlé F, Mattei M G, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- 18.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 19.Mettenleiter T C. Molecular properties of alphaherpesviruses used in transneuronal pathway tracing. In: Kaplitt M G, editor. Viral vectors. New York, N.Y: Academic Press, Inc.; 1995. pp. 367–393. [Google Scholar]
- 20.Miller J M, Whetstone C A, Bello L J, Lawrence W C, Whitbeck J C. Abortion in heifers inoculated with a thymidine kinase-negative recombinant of bovine herpesvirus 1. Am J Vet Res. 1995;56:870–874. [PubMed] [Google Scholar]
- 21.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 22.Morrison M E, Racaniello V R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen, K., and V. Racaniello. 1998. Unpublished results.
- 24.Nicola A V, Ponce de Leon M, Xu R, Hou W, Whitbeck J C, Krummenacher C, Montgomery R I, Spear P G, Eisenberg R J, Cohen G H. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol. 1998;72:3595–3601. doi: 10.1128/jvi.72.5.3595-3601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrovskis E A, Meyer A L, Post L E. Reduced yield of infectious pseudorabies virus and herpes simplex virus from cell lines producing viral glycoprotein gp50. J Virol. 1988;62:2196–2199. doi: 10.1128/jvi.62.6.2196-2199.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe, C. L., and P. G. Spear. 1999. Unpublished results.
- 28.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 30.Takahama Y, Sugaya K, Tsuda S, Hasegawa T, Hashimoto Y. Regulation of early T cell development by the engagement of TCR-β complex expressed on fetal thymocytes from TCR-β-transgenic mice. J Immunol. 1995;154:5862–5869. [PubMed] [Google Scholar]
- 31.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 32.Warner, M. S., and P. G. Spear. 1999. Unpublished results.
- 33.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis S H, Rux A J, Peng C, Whitbeck J C, Nicola A V, Lou H, Hou W, Salvador L, Eisenberg R J, Cohen G H. Binding kinetics of herpes simplex virus (HSV) glycoprotein D to the herpes virus entry mediator (HVEM/HveA) using surface plasmon resonance. J Virol. 1998;72:5937–5947. doi: 10.1128/jvi.72.7.5937-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]