Abstract
The coxsackie B virus and adenovirus receptor (CAR) and the major histocompatibility complex (MHC) class I α2 domain have been identified as high-affinity cell receptors for adenovirus type 5 (Ad5) fiber. In this study we show that CAR but not MHC class I allele HLA-A*0201 binds to Ad5 with high affinity when expressed on hamster cells. When both receptors are coexpressed on the cell surface of hamster cells, Ad5 fiber bind to a single high-affinity receptor, which is CAR.
The first step in human adenovirus (Ad) infection consists of virus-cell recognition and attachment, involving the viral fiber protein and host cell surface receptor(s) (3, 4, 15). The existence of two classes of fiber recognition sites with high and low affinities with respect to binding has been previously reported (3). The coxsackie B virus and Ad receptor (CAR) and the major histocompatibility complex (MHC) class I α2 domain have been identified as putative cellular receptors involved in Ad type 2 (Ad2) and Ad5 attachment (1, 6, 16). CAR is also a receptor for subgroup A, C, D, E, and F Ad fibers but not for subgroup B Ad fibers like those of serotype 3 (13, 13a). The mouse homologue of human CAR also functions as a receptor for coxsackie B viruses and Ads (2, 16).
The MHC class I α2 domain was characterized as a high-affinity receptor for Ad5 fiber knob after reverse antibody biopanning of a phage-displayed hexapeptide library identified a peptide motif that showed homology with the α2 domain of human MHC class I (6). This icosapeptide neutralized Ad5 in HeLa cells and interacted with Ad fibers in a subgroup-specific manner (6). In addition, Ad5 binding to B lymphoblastoid cells (Daudi cells) was found to be dependent on cell surface expression of HLA class I molecules (6). Daudi cells that lack surface expression of HLA class I molecules (Daudi HLA− cells) (12) showed a weak capacity for Ad5 binding, in contrast to β2-microglobulin-transfected Daudi cells (Daudi HLA+) (12), which bound to Ad5 with high affinity (6). However, Ad5 fibers were shown to bind to HeLa cells, which express both putative receptors (data not shown), and CAR-transfected hamster cells, which express only CAR, with the same affinity (1). This suggests that binding was to a common receptor.
In the present study we address the role of the α2 domain of human MHC class I as a high-affinity receptor for Ad5 fiber by permanently expressing in hamster cells human HLA-A*0201 molecules alone and in conjunction with CAR. The levels of cell surface expression of CAR and HLA-A*0201 were determined by indirect flow cytometric analysis (fluorescence-activated cell sorting [FACS]) using the anti-CAR monoclonal antibody (MAb) RmcB (1, 7) and the anti-MHC class I MAbs W6/32, BB7.2, and PA2.1, respectively. MAb W6/32 is specific for the HLA class I H chain associated with conformation-stabilizing β2-microglobulin (12) (Dako, Glostrup, Denmark), MAb BB7.2 recognizes the conformation-sensitive epitope on HLA-A2 (10), and MAb PA2.1 recognizes the conformation-sensitive epitope on HLA-A2 and HLA-A28 (9).
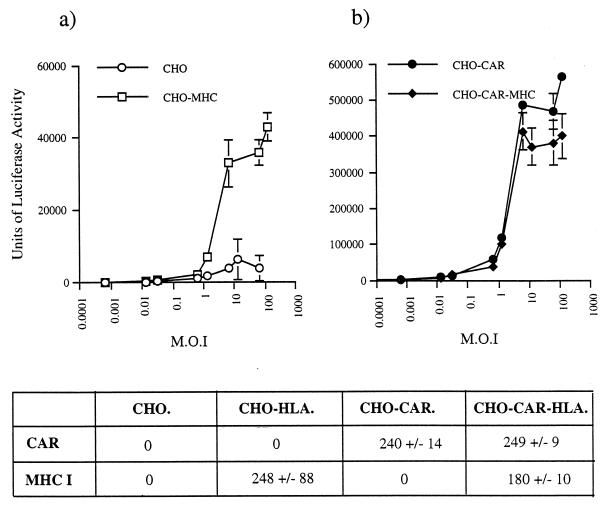
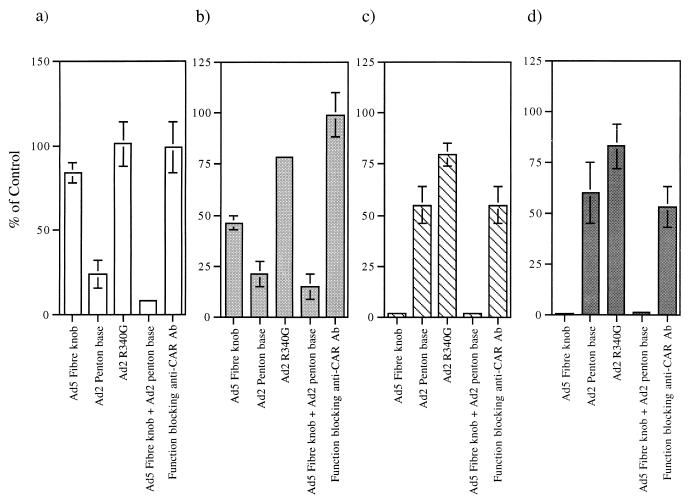
We tested whether HLA-A*0201 is a receptor for Ad5 fiber by first exposing HLA-A*0201-transfected cells (CHO-HLA) to Ad5Luc3, a replication-competent Ad vector, at different multiplicities of infection (MOI) (Fig. 1a). Ad5Luc3, obtained from P. Boulanger, is a replication-competent virus which contains the luciferase gene under the control of the simian virus 40 early promoter inserted in the E3 region of the Ad5 genome. In a quantitative assay, using luciferase activity as the end point assay, we found an 8- to 10-fold increase in luciferase activity in HLA-A*0201-transfected cells compared to control cells at an MOI of 10 or more (Fig. 1a). In order to determine if the increased efficiency of Ad infection of CHO-HLA cells was due to a specific interaction between Ad5 fiber and cell surface receptors, we pretreated CHO-HLA and control cells with recombinant Ad5 fiber knob (100 μg/ml) and/or Ad2 penton base (100 μg/ml) and then exposed the cells to Ad5Luc3 at an MOI of 100 for 1 h at 4°C. Cells were then washed and incubated at 37°C to allow internalization and one cycle of virus replication (Fig. 2a and b). This assay measures fiber attachment to functional cell surface receptors and dissociates virus attachment from internalization, because at low temperatures only virus-cell attachment occurs whereas endocytosis requires physiological temperatures. We found that luciferase activity in control cells was predominantly inhibited by Ad2 penton base (75% inhibition) but not by Ad5 fiber (20% inhibition) (Fig. 2a). Pretreatment of control cells with Ad2 mutant penton base R340G, which is mutated in the Arg-Gly-Asp (RGD) integrin binding motif (8), failed to inhibit Ad5Luc3 infection of these cells (Fig. 2a). These results indicate that Ad infection of control cells is predominantly mediated by an interaction between the viral penton base protein and its cell surface receptors. In contrast, when CHO-HLA cells were preincubated with 100 μg/ml of Ad5 fiber, luciferase expression was reduced by 50% (Fig. 2b). These observations indicate that the increased susceptibility of HLA-A*0201-transfected hamster cells to Ad5 infection was due to an interaction between the Ad5 fiber protein and functional fiber receptors on CHO-HLA cells.
FIG. 1.
Ad-mediated gene delivery to control hamster cells (CHO cells) and HLA-A*0201-expressing CHO cells (CHO-HLA) (a) and CAR-expressing CHO cells (CHO-CAR) and CAR- and HLA-A*0201-expressing CHO cells (CHO-CAR-MHC). (b). Cells were seeded at 105 cells/well in triplicate in 24-well plates and 24 h later were incubated with various amounts of Ad5Luc3 (MOI 0.001 to 1,000) for 1 h at 4°C in serum-free Dulbecco’s modified Eagle’s medium. Cells were then washed, and after 18 h at 37°C, a cell extract was measured by being mixed with a 100-μl aliquot of beetle luciferase, being transferred to a luminometer tube, and measuring the light generated on a Turner TD-20e Luminometer for 60 s. Data are shown as means and standard errors of the means (error bars) (n = 3). Note that the y axes in the two panels differ. Below these panels, the CAR and MHC class I status of each cell line, as determined by flow cytometry, is described. CAR and MHC class I levels are represented as median fluorescence units ± standard deviations.
FIG. 2.
Effect of Ad5 fiber knob, Ad2 penton base, Ad2 mutant penton base R340G, and anti-CAR functional blocking antibody (Ab) on Ad-mediated gene transfer to CHO (a), CHO-HLA (b), CHO-CAR (c), and CHO-CAR-HLA (d). Cells were seeded at 105 cells/well in triplicate in 24-well plates and were either left untreated (control cells) or were pretreated with Ad5 fiber knob (100 μg/ml), Ad2 penton base (100 μg/ml), Ad2 mutant penton base R340G (8) (100 μg/ml), and anti-CAR functional blocking antibody (1:200 dilution; gift of D. M. McDonald and G. E. Blair) for 10 min at 4°C prior to the addition of Ad5Luc3 for 1 h at 4°C. Luciferase activity was measured as described in the legend to Fig. 1. Results were expressed as percentages of the control cells (i.e., no antibody = 100%). Data are shown as means ± standard errors of the means (error bars) (n = 3). CHO-CAR, CHO-CAR-HLA, and CHO cells were infected with Ad5Luc3 at a constant MOI (10 PFU/cell). In the absence of competing proteins, luciferase gene expression in CHO cells infected with 10 PFU/cell of Ad5Luc3 was 1,333 relative light units (RLU), compared to 380,000 RLU obtained with CHO-CAR cells and 360,000 RLU obtained with CHO-CAR-HLA cells infected with Ad5Luc3 at the same MOI. When infected with Ad5Luc3 at 100 PFU/cell, luciferase activity was 36,980 RLU in CHO-HLA cells and 3,670 RLU in CHO cells.
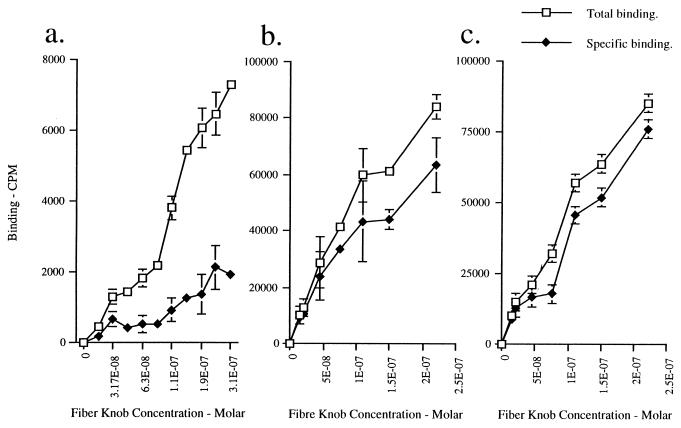
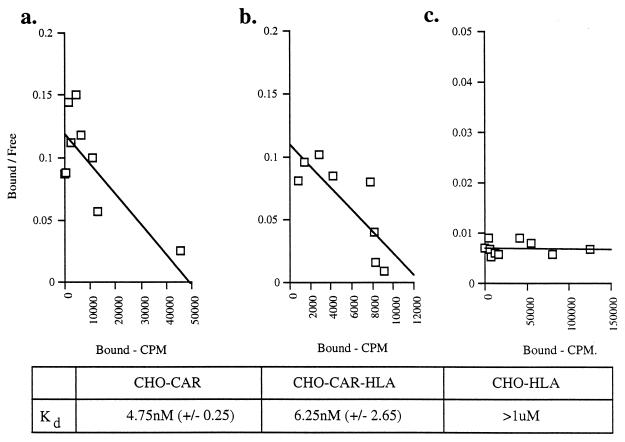
Ad5 fiber attachment to CHO-HLA cells was assessed further by direct binding experiments. Prechilled cells were incubated with increasing concentrations of 125I-labelled Ad5 fiber knob in the presence (nonspecific binding) and absence (total binding) of 100-fold excess of unlabelled fiber knob. Counts per minute associated with total and nonspecific 125I-labelled fiber knob binding to cells were determined. There was no specific binding to control CHO cells (data not shown), which is in agreement with previous findings (1). Although we observed specific binding of 125I-labelled Ad5 fiber knob to CHO-HLA cells, specific binding was at low levels (Fig. 3a). Scatchard analysis showed that 125I-labelled Ad5 fiber knob bound HLA-A*0201 with a binding constant that was higher than 10−6 M (Fig. 4c). This value is significantly higher than the binding constant reported for Ad2 and HeLa cells (11, 14) or KB cells (3) and for recombinant Ad5 fiber knob and HeLa cells (5). Our findings therefore strongly suggest that HLA-A*0201 expressed on hamster cells is not a high-affinity receptor for Ad5 fiber. The α2 domain of HLA-A*0201 shows absolute homology with the α2 domain of MHC class I consensus sequence and with the α2 domain of HLA-A and HLA-B molecules which are expressed on the surface of Daudi HLA+ cells (12). The fact that cell surface expression of HLA-A*0201 was confirmed by three different anti-MHC class I MAbs which recognize conformational sensitive epitopes in HLA class I indicates that HLA-A*0201 was correctly folded. Our findings therefore strongly suggest that the peptide domain around and including Try167 of the α2 domain of MHC class I molecules does not bind to Ad5 fiber with high affinity.
FIG. 3.
Binding of 125I-labelled Ad5 fiber knob to CHO-HLA (a), CHO-CAR (b), and CHO-CAR-HLA (c) cells. 125I labelling of purified Ad5 knob was performed with iodination beads (Pierce) according to the manufacturer’s standard protocol. Cells seeded at 105 cells well in triplicate in 24-well plates were washed in serum-free medium and chilled on ice for 2 h. Monolayers were incubated at 4°C for 1 h with increasing amounts of 125I-labelled Ad5 knob and a fixed excess of unlabelled Ad5 fiber knob (100-fold) in serum-free medium. The input ranged from 0 to 2.5 × 10−7 cpm. The specific activity of Ad5 fiber knob was approximately 4,030 cpm/ng (2.1 × 1013 cpm/mol). The cell monolayers were washed three times with phosphate-buffered saline and after being removed from tissue culture dishes, samples were placed into 5-ml aliquots of Ecoscint O (National Diagnostics) and read in a model 1900CA Tri-carb liquid scintillation analyzer (Packard) to determine β counts emitted per minute. Note that the y axis in panel a differs from the y axes in panels b and c.
FIG. 4.
Scatchard plot analysis of Ad5 fiber knob binding to CHO-CAR (a), CHO-CAR-HLA (b), and CHO-HLA (c). cells. Scatchard analysis was performed by using the Prism program as the standard method for determining the dissociation constant (Kd) (the concentration at equilibrium at which 50% of receptors are occupied). One representative experiment out of three is shown. Below these panels, the Kd values are shown as means, with standard errors of the means in parentheses (n = 3).
We then tested Daudi HLA− and Daudi HLA+ cells for CAR expression by FACS using anti-CAR MAb RmcB (1, 7). We found that CAR is expressed on the cell surface of both Daudi HLA− and Daudi HLA+ cells, at similar low levels (data not shown). It is therefore possible that the increased attachment and permissivity of Daudi HLA+ cells to Ad5 (6) may not be due to a direct interaction between MHC class I molecules and Ad5 fiber, but due to the increased accessibility of CAR as a consequence of expression of HLA class I molecules on the cell surface. We addressed this question by investigating Ad5 infection and Ad5 fiber knob attachment to hamster cells expressing CAR alone (CHO-CAR) (1) and hamster cells expressing both CAR and HLA-A*0201 (CHO-CAR-HLA) on their cell surface.
The susceptibility of CHO-CAR and CHO-CAR-HLA cells to Ad5 infection was assessed by exposing these cells to Ad5Luc3 at increasing MOI (Fig. 1b). Luciferase expression was 100-fold higher in CHO-CAR cells than in control cells at an MOI higher than 10 (Fig. 1b), which is in agreement with previous findings (1). There was no significant difference in luciferase activity between CHO-CAR and CHO-CAR-HLA cells (Fig. 1b), both of which were more susceptible to Ad5Luc3 infection than CHO-HLA cells (Fig. 1a). The luciferase signal was almost totally abolished (95% inhibition) by preincubation of CHO-CAR and CHO-CAR-HLA cells with the maximal inhibiting concentration of Ad5 fiber knob (100 μg/ml) (Fig. 2c and d), in contrast to control cells that showed no significant inhibition (Fig. 2a) and CHO-HLA cells in which luciferase activity was reduced by 50% (Fig. 2b). In order to assess if Ad5 infection was through a specific interaction with CAR, all transformed cells were preincubated with an anti-CAR rabbit polyclonal blocking antibody at a concentration of 1:200 (gift of D. M. McDonald and G. E. Blair). We found that in the presence of this antibody, luciferase activity was reduced by 50% in CHO-CAR (Fig. 2c) and CHO-CAR-HLA cells (Fig. 2d). In contrast, there was no reduction in luciferase expression in control hamster cells and CHO-HLA cells (Fig. 2a and b). We also found a similar, dose-dependent increase in specific binding of 125I-labelled Ad5 fiber knob to CHO-CAR (Fig. 3b) and CHO-CAR-HLA cells (Fig. 3c), which was 20- to 50-fold higher than the specific binding to CHO-HLA cells (Fig. 3a). Scatchard analyses showed that Ad5 fiber knob bound to CHO-CAR and CHO-CAR-HLA cells with affinity constants of 4.75 × 10−9 and 6.25 × 10−9 M, respectively (Fig. 4a and b). These values are similar to the binding constant reported for Ad5 fiber knob and HeLa cells (5). Our observations therefore demonstrate that when both CAR and the MHC-class I are coexpressed on the cell surface of hamster cells, Ad5 fiber binds to a single high-affinity receptor, which is CAR, and there is no functional cooperativity between the two receptors.
Ad5Luc3 infection of Daudi HLA− and Daudi HLA+ cells, which express CAR at similar low levels, was inhibited (50% inhibition) by the anti-CAR function blocking polyclonal antibody (gift of D. M. McDonald and G. E. Blair; data not shown). This suggests that Ad5Luc3 infects Daudi cells, at least in part, through an interaction with CAR. Why Ad5 binds to and infects Daudi HLA+ cells with increased efficiency (6) is therefore unclear. FACS analysis showed that Daudi HLA+ cells express more HLA class I but fewer CAR molecules on their cell surface than CHO-CAR-HLA cells (data not shown). This suggests that HLA class I-dependent Ad attachment and permissivity may only be observed when there is little or no surface expression of CAR. Alternatively, in human cells such as Daudi HLA+ cells, MHC class I molecules may interact with other cell surface cofactors, either to bind with high affinity to Ad5 fiber directly or to increase CAR accessibility.
Acknowledgments
We thank J. M. Bergelson and R. W. Finberg for CHO-CAR cells; S.-S. Hong and P. Boulanger for Ad5Luc3, recombinant Ad2 penton base, and recombinant mutant Ad2 penton base R340G; and D. M. McDonald and G. E. Blair for the anti-CAR functional blocking polyclonal antibody.
This work was funded by grants from the Wellcome Trust and the Special Trustees for Guy’s and St. Thomas’ Hospitals to George Santis.
REFERENCES
- 1.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Defer C, Belin M-T, Caillet-Boudin M-L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennache B, Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977;166:237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry L J, Xia D, Wilke M E, Deisenhofer J, Gerard R D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class I alpha 2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu K-H L, Lonberg-Holm K, Alstein B, Crowell R L. A monoclonal antibody specific for the cellular receptor for the group B coxsackie viruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karayan L, Hong S S, Gay B, Tournier J, Dupuy d’Angeac A, Boulanger P. Structural and functional determinants in adenovirus type 2 penton base recombinant protein. J Virol. 1997;71:8678–8689. doi: 10.1128/jvi.71.11.8678-8689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parham P, Bodmer W F. Monoclonal antibody to a human histocompatibility alloantigen HLA-A2. Nature. 1978;276:397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- 10.Parham P, Brodsky F M. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–678. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- 11.Persson R, Svensson U, Everitt E. Virus-receptor interaction in the adenovirus system: characterization of the positive cooperative binding of virions in HeLa cells. J Virol. 1985;54:92–97. doi: 10.1128/jvi.54.1.92-97.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quillet A, Presse F, Marchiol-Fournigault C, Haren-Bellan A, Benbunan M, Ploegh H, Fradelizi D. Increased resistance to non-MHC-restricted cytotoxicity related to HLA A, B expression: direct demonstration using β2-microglobulin-transfected Daudi cells. J Immunol. 1988;141:17–20. [PubMed] [Google Scholar]
- 13.Roelvink P W, Lizonova A, Lee J G M, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackie-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Santis, G., V. Legrand, S. S. Hong, E. Davison, I. Kirby, J. L. Impez, R. W. Finberg, J. M. Bergelson, M. Mehtali, P. Boulanger. Molecular determinants of adenovirus type 5 fiber bindings to its cellular receptor CAR. J. Gen. Virol., in press. [DOI] [PubMed]
- 14.Seth P, Rosenfeld M, Higginbotham J, Crystal R G. Mechanism of enhancement of DNA expression consequent to cointernalization of a replication-deficient adenovirus and unmodified plasmid DNA. J Virol. 1994;68:933–940. doi: 10.1128/jvi.68.2.933-940.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svensson U, Persson R, Everitt E. Virus-receptor interaction in the adenovirus system. I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981;38:70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomko R, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]