Abstract
Background and Aims:
Locally advanced pancreatic cancer (LAPC) often causes obstruction. Verteporfin photodynamic therapy (PDT) can feasibly “debulk” tumor more safely than noncurative surgery and has multiple advantages over older PDT agents. We aimed to assess the feasibility of EUS-guided verteporfin PDT in ablating nonresectable LAPC.
Methods:
Adults with LAPC with adequate biliary drainage were prospectively enrolled. Exclusion criteria included significant metastatic disease burden, disease involving >50% duodenal or major artery circumference, and recent treatment with curative intent. CT was obtained between day −28 to 0. On day 0, verteporfin 0.4 mg/kg was infused 60 to 90 minutes before EUS, during which a diffuser was positioned in the tumor and delivered light at 50 J/cm for 333 seconds. CT was obtained on day 2, with adverse event monitoring occurring on days 1, 2, and 14. Primary outcome was presence of necrosis.
Results:
Of 8 patients (62.5% male, mean age 65±7.9 y) included in the study, 5 were staged at T3, 2 at T2, and 1 at T1. Most (4) had primary lesions in the pancreatic head. Mean pretrial tumor diameter was 33.3±13.4 mm. On day 2 CT, 5 lesions demonstrated a zone of necrosis measuring a mean diameter of 15.7±5.5 mm; 3 cases did not develop necrosis. No adverse events were noted during the procedure or postprocedure observation period (day 1–3), and no changes in patient reported outcomes were noted.
Conclusions:
In this pilot study, EUS-guided verteporfin PDT is feasible and shows promise as a minimally invasive ablative therapy for LAPC in select patients. Tumor necrosis is visible within 48 hours after treatment. Patient enrollment and data collection are ongoing.
Keywords: locally advanced pancreatic cancer, ablative therapy, photosensitizer, sodium porfimer
Introduction
Photodynamic therapy (PDT) is a localized ablative technique that involves administration of a photosensitizer to induce cell death via generation of free oxygen radicals after activation with light1. Interest in using PDT for solid gastrointestinal malignancies stems from its relatively selective nature for malignant cells, minimal effect on connective tissue, and maintenance of luminal gut integrity2. PDT has been approved by the U.S. Food and Drug Administration for the palliation of obstructing esophageal adenocarcinoma since 1995, with subsequent expansion to the treatment of Barrett’s esophagus with high-grade dysplasia as an alternative to esophagectomy in 20033-5.
Data supporting PDT use in the gastrointestinal tract are typically derived from studies with sodium porfimer, a first-generation photosensitizer that unfortunately is not a chemically pure compound. A recent phase 1 study demonstrated the safety of varying doses of sodium porfimer for PDT in 12 patients with locally advanced pancreatic cancer (LAPC)6, a disease in which patients often fail candidacy for curative surgical resection at the time of diagnosis but may benefit from a cytoreductive procedure7. Notably, the treatment was capable of producing measurable tumor necrosis on cross-sectional imaging obtained 18 days after PDT, and PDT in combination with subsequent Nab-paclitaxel and gemcitabine chemotherapy resulted in a median progression-free survival time of 2.6 months6.
Although sodium porfimer-mediated PDT has been shown to be effective, widespread application has been limited by multiple drawbacks, most notably a long half-life with consequent prolonged duration of cutaneous photosensitivity that requires patients to comply with avoidance of sunlight exposure and full skin coverage and eye protection for at least 30 days postprocedure2. Beyond acute toxicities, odynophagia, abdominal pain, and chest pain that may require narcotic use are common postprocedural complaints8.
In this study, we aimed to evaluate the safety and efficacy of verteporfin-mediated PDT administered under EUS guidance in patients with LAPC. Verteporfin is a United States Food and Drug Administration−approved second-generation photosensitizer that offers a significant patient safety advantage with a reduced half-life on the scale of hours, resulting in a short period of photosensitivity of approximately one day9. Specifically, the primary endpoint was the appearance and size of a post-PDT necrosis zone on CT imaging obtained 48 hours after verteporfin-mediated PDT.
Methods
General Study Design
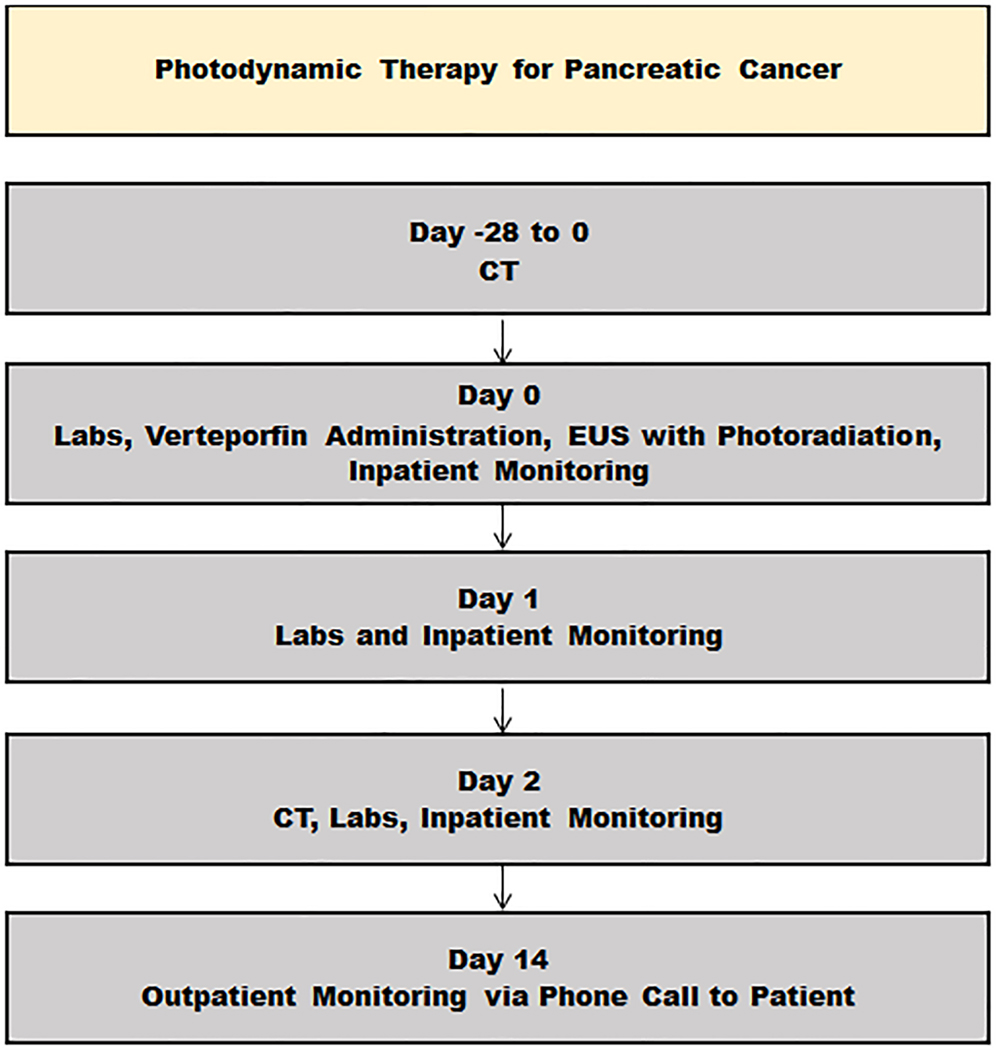
Figure 1 outlines the flow of study-related assessments in relation to the PDT procedure on day 0. The protocol was initially developed at University College London (SPP) and assessed in 15 inoperable patients with LAPC under CT guidance9. This protocol was then initiated at the Mayo Clinic as an EUS-guided verteporfin PDT study. In summary, upon enrollment, a high-resolution, contrast-enhanced pancreatic protocol CT scan was obtained between day −28 to 0. On day 0, patients were admitted to the Clinical Research Unit at Mayo Clinic (Rochester, MN), where a physical exam was performed, baseline quality of life was assessed with the validated EORTC QLQ-C30 questionnaire10, and baseline laboratory testing was obtained, including complete blood counts, comprehensive metabolic panel, fasting glucose, amylase, prothrombin time, and CA 19–9.
Figure 1.
Study data collection flow chart.
Admission to the Clinical Research Unit allowed for administration of the photosensitizer (described further below) and minimization of exposure to both natural and artificial light. Inpatient monitoring in the Clinical Research Unit continued for 48 hours thereafter, from day 0 to day 2, for the duration of the drug’s activity to permit gradual controlled light re-adaptation before discharge. Specifically, patients were gradually introduced to bright indoor lighting by the end of day 1 and were allowed exposure to sunlight by the end of day 2. On days 1 and 2, symptom assessment and adverse event monitoring were performed, and the following laboratory testing was obtained: complete blood counts, comprehensive metabolic panel, fasting glucose, and amylase. On day 2, a high-resolution, contrast-enhanced pancreatic protocol CT scan was obtained. On day 14, patients underwent symptoms assessment and adverse event monitoring via phone call.
Data were collected on patient demographics, baseline disease characteristics, and study related CT imaging. Tumor size was considered the largest diameter in any dimension. The necrosis zone was measured as the average of the length and width of any new hypodense lesions in the primary tumor that were not present on the pre-PDT CT scan.
This prospective study functioned as part of a National Cancer Institute funded protocol (P01 CA084203) for the evaluation of the role of PDT in pancreatic cancer and was approved by the Mayo Clinic Institutional Review Board (protocol number 16-001243) on December 6, 2016. This study is registered, and was first posted, on ClinicalTrials.gov under identifier NCT03033225 on January 26, 2017.
Patient Selection
The full inclusion and exclusion criteria are listed in Table 1. Adults with histologically proven locally advanced or advanced pancreatic cancer with adequate biliary drainage and no evidence of uncontrolled infection who were deemed by their oncologic provider as unsuitable for surgical resection and unable benefit from chemotherapy with curative intent were eligible and offered participation in the by a trained clinical research coordinator. Patients were expected to have an estimated life expectancy of at least 12 weeks from the time of enrollment and were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Exclusion criteria included metastases to areas other than the lung or liver, more than 3 lung metastases or lung metastases greater than 5 cm, disease involving greater than 50% of the circumference of the duodenum or a major artery, and treatment with curative intent within the past 2 weeks.
Table 1.
Study participant selection criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Histological evidence of locally advanced pancreatic cancer or small volume metastasis not amenable to systemic chemotherapy and surgical resection, if the patient is unfit or refuses surgical resection | Evidence of metastasis other than to lung or liver. If metastasis in the lung or liver, lesions must be <5 cm in diameter |
| Age >18 years | Age <18 years, pregnancy, breast feeding, or porphyria |
| Measurable tumor as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria | Locally advanced disease with more than 50% of the circumference of the duodenum involved or involvement of a major artery |
| ECOG performance stage 0–2 | ECOG performance status 3–4 |
| Estimated life expectancy of at least 12 weeks | Prior treatment with curative intent within the past 12 weeks before entry |
| Capable of giving informed consent | Any psychiatric condition that makes informed consent impossible |
| Adequate biliary drainage with total bilirubin <2.5 times the upper limit of normal | Documented hemorrhagic diathesis or coagulopathy, need for therapeutic anticoagulation, history of additional past or current malignancy that would interfere with treatment response evaluation |
| Women of childbearing age require a negative pregnancy test before study and must remain on contraception for the duration of the study | Evidence of uncontrolled systemic disease or laboratory finding that would in the investigator’s opinion undesirable for the patient to participate in the trial |
EUS-guided PDT Procedure
On day 0, verteporfin for injection (Visudyne, Bausch+Lomb, West Laval, Quebec, Canada) was administered intravenously at a dosing scheme of 0.4 mg/kg in the Clinical Research Unit 60 to 90 minutes before photoradiation. The treatment window was based on data from prior pharmacokinetic studies in animal models, as well as prior clinical PDT data2, 9. Patients were administered prophylactic oral ciprofloxacin 500 mg or an equivalent broad-spectrum antibiotic if an allergy was present, which was continued for 24 hours after the procedure for a total of 3 doses. Patients were then transferred to the endoscopy unit and sedated under monitored anesthesia care with propofol.
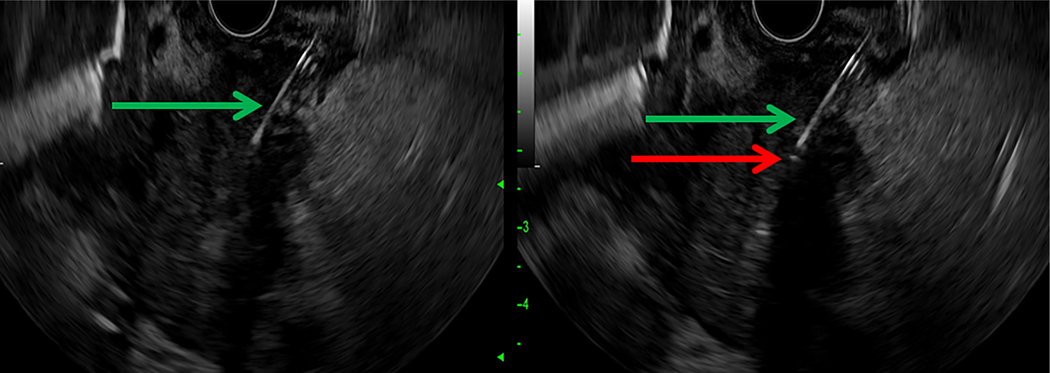
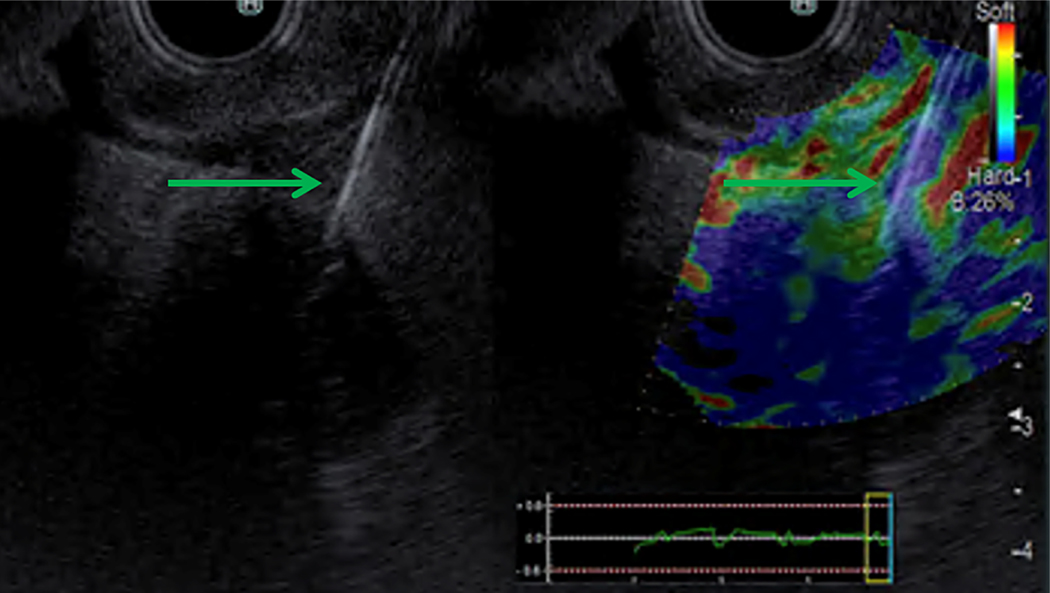
A linear ultrasound gastrovideoscope instrument (UCT180; Olympus, Center Valley, Pa, USA) and an advanced processing console (F75; Olympus, Center Valley, Pa, USA) were used to guide a 19-gauge fine-needle aspiration (FNA) needle (Echotip; Cook Medical, Bloomington, Ind, USA) into the point of the tumor mass where the desired point of photoradiation was to occur while providing a distance of at least 1 centimeter between blood vessels or the duodenal wall from this treatment zone. The needle was then advanced beyond this point; as the needle was subsequently withdrawn, a 0.4 mm core diameter optical fiber with a 1 cm long, echoic cylindrical diffusing tip (Pioneer Optics, Bloomfield, Conn, USA) was slowly advanced simultaneously to direct real-time placement of the diffusing tip directly into the desired point. The fiber was calibrated before insertion into the FNA needle for precise advancement with respect to distance. Placement of the photoradiation fiber is further illustrated in Figure 2. Elastography (Hitachi Arietta 850 System; Olympus, Center Valley, Pa, USA) was used when available to ensure needle placement within the tumor, as demonstrated in Figure 3.
Figure 2.
EUS-guided placement of a diffusing fiber for delivery of PDT. A, The 19-gauge FNA needle (green arrow) is visualized within the pancreatic head mass under endosonography. B, The diffusing tip of the optical fiber is seen after introduction through the needle under endosonography as a small hyperechoic point (red arrow).
Figure 3.
Elastography confirmation of EUS-guided needle insertion into tumor for delivery of PDT. A, The 19-gauge FNA needle (green arrow) is visualized within the pancreatic head mass under endosonography. B, The pancreatic head mass is visualized under elastography, with the mass delineated by increased stiffness (blue coloration) as compared with the surrounding tissue.
A diode laser (Model PSU-FC; Changchun New Industries Optoelectronics Technology Co. Ltd., Changchun, Jilin, China) generating 690-nm light was calibrated independently by our collaborator (BP) before clinical usage and was found to be stable and reproducible with respect to output and wavelength. The power output was set to 150 mW before each procedure using an integrating sphere that measured output from the fiber (Model PM 200; Thorlabs, Inc, Newton, NJ, USA). Once the diffusing fiber was in place at the desired point within the tumor, the laser was activated. To complete a light dose of 50 J, the tumor was illuminated for a total of 333 seconds. After photoradiation, the fiber was withdrawn and the FNA needle retracted. The fiber was checked for intactness and the power output confirmed with the integrating sphere after removal.
Statistical Analyses
This study was designed as a feasibility study to assess the ability of EUS-guided verteporfin-mediated PDT to produce tumor necrosis and a safety study to assess potential adverse events. Therefore, the statistics are primarily descriptive, with the primary endpoint as the diameter of the necrosis zone, if visible, on the day 2 CT image. The necrosis zone was determined based on the appearance of hypoperfusion within the primary tumor seen on the day 2 CT image that was not previously seen on the day −28 to 0 CT image. The largest diameter across the necrosis zone was recorded. The secondary endpoint was overall tumor size; tumor sizes from the day −28 to 0 and day 2 CT images were compared with the Student t test.
Results
Between March 15, 2017 and July 20, 2019, 623 potential patients were examined for eligibility. Of these, 54 were confirmed eligible and approached for consideration. Eight patients proceeded to inclusion. Reasons for nonparticipation included nonresponse, unsuitable timing of study for personal reasons, unsuitable timing of study due to decision to initiate a chemotherapeutic agent, unsuitable location of study/unwillingness to travel to study site, and entry into a different research study.
Patient demographics and malignancy characteristics are summarized in Table 2. Of the 8 patients (62.5% male, mean age 65 ± 7.9 y) included, 5 (62.5%) were staged at T3, 2 (25%) at T2, and 1 (12.5%) at T1. Primary lesions were located in the pancreatic head (4, 50%), uncinate (2, 25%), and body/tail (1, 12.5%), whereas 1 (12.5%) patient had a recurrent lesion at the pancreaticojejunostomy site. The mean pretrial tumor diameter was 33.3 ± 13.4 mm.
Table 2.
Overall cohort characteristics.
| Overall cohort characteristics | |
|---|---|
| Sex | n (%) |
| Male | 5 (62.5) |
| Female | 3 (37.5) |
| Age at EUS-guided PDT | y |
| Mean | 65.0 ± 7.9 |
| Median | 64 |
| T Stage at initial diagnosis | n (%) |
| T1 | 1 (12.5) |
| T2 | 2 (25.0) |
| T3 | 5 (62.5) |
| Pre-trial treatment regimen | n (%) |
| FOLFIRINOX | 4 (50.0) |
| FOLFIRI | 1 (12.5) |
| Gemcitabine +/− Abraxane | 2 (25.0) |
| 5-FU with radiation | 1 (12.5) |
| Tumor location | n (%) |
| Head | 4 (50.0) |
| Uncinate | 2 (25.0) |
| Body/tail | 1 (12.5) |
| Other: pancreaticojejunostomy site | 1 (12.5) |
| Metastatic disease | n (%) |
| Liver | 3 (37.5) |
| Liver and Lung | 1 (12.5) |
| Vascular involvement | n (%) |
| Arterial | 6 (75.0) |
| Superior mesenteric artery | 4 (50.0) |
| Celiac artery | 2 (25.0) |
| Common hepatic artery | 4 (50.0) |
| Splenic and/or renal artery | 3 (37.5) |
| None | 2 (25.0) |
| Venous | 5 (62.5) |
| Superior mesenteric vein | 4 (50.0) |
| Portal Vein | 3 (37.5) |
| Splenic and/or renal vein | 3 (37.5) |
| None | 3 (37.5) |
| Portal hypertension | n (%) |
| Present | 4 (50.0) |
| Not present | 4 (50.0) |
| Pre-trial tumor diameter | mm |
| Mean | 33.3 ± 13.4 |
| Median | 38.5 |
Metastatic disease was found in 4 (50.0%) patients. These patients all had liver involvement, whereas one patient additionally had lung involvement. Arterial involvement was present in 6 (75%) patients: 4 with superior mesenteric artery involvement, 4 with common hepatic artery involvement, 3 with splenic and/or renal artery involvement, and 2 with celiac artery involvement. Venous involvement was present in 5 (62.5%) patients: 4 with superior mesenteric vein involvement, 3 with portal vein involvement, and 3 with splenic and/or renal vein involvement. Evidence of sinistral portal hypertension was present in 4 (50.0%).
On day 2 CT, mean tumor diameter was 33.9 ± 12.9 mm. Thus, no significant changes in mean tumor diameter between pre-PDT CT and day 2 CT images were identified. However, 5 lesions (62.5%) demonstrated a zone of necrosis pertaining to the PDT site measuring a mean diameter of 15.7 ± 5.5 mm; 3 (37.5%) cases did not develop necrosis. A prior study showed that the degree of necrosis on a day 5 CT scan after PDT does not appreciably change as compared with follow-up at day 289. However, given the nature of the underlying disease, the majority of patients (87.5%) had a repeat CT of the abdomen and pelvis for reasons unrelated to the study; 1 patient did not undergo any further known CT scans. These CT scans were obtained over a median duration of 54 days (interquartile range 26–74.5) after the procedure. Three (37.5%) patients experienced a decrease in the overall size of the primary pancreatic tumor and 4 (50.0%) had stable findings with respect to the primary pancreatic tumor.
Table 3 summarizes the demographics and malignancy characteristics of responders, as defined by the demonstration of a zone of necrosis, versus nonresponders. Although analysis was limited by the small number of study participants, responders to PDT generally had lesions located in the pancreatic head with smaller lesions with respect to the diameter (Table 3). The majority of PDT responders had lower incidences of sinistral portal hypertension and arterial vascular involvement (Table 3). Of the 4 patients with metastatic disease, the 3 patients with only liver involvement were PDT responders, whereas the 1 patient with both liver and lung involvement was a nonresponder (Table 3).
Table 3.
Characteristics of PDT responders, as defined by induction of necrosis, versus PDT nonresponders.
| PDT responders versus PDT nonresponders | ||
|---|---|---|
| PDT responders (n=5) | PDT nonresponders (n=3) | |
| Demographics | ||
| Male (%) | 60.0 | 66.7 |
| Mean age at PDT (y) | 67.2±9.2 | 61.3±4.0 |
| Baseline disease characteristics | ||
| T Stage (%) | ||
| T1 | 0.0 | 33.3 |
| T2 | 40.0 | 0.0 |
| T3 | 60.0 | 66.7 |
| Tumor Location (%) | ||
| Head | 60.0 | 33.3 |
| Uncinate | 20.0 | 33.3 |
| Body/Tail | 0.0 | 33.3 |
| Other: pancreaticojejunostomy site | 20.0 | 0.0 |
| Metastatic disease (%) | ||
| Liver | 60.0 | 0.0 |
| Liver and Lung | 0.0 | 33.3 |
| Vascular involvement: arterial (%) | ||
| Superior mesenteric artery | 40.0 | 66.7 |
| Celiac Artery | 20.0 | 33.3 |
| Common hepatic artery | 40.0 | 66.7 |
| Splenic and/or renal artery | 20.0 | 66.7 |
| None | 40.0 | 0.0 |
| Vascular involvement: venous (%) | ||
| Superior mesenteric vein | 60.0 | 33.3 |
| Portal Vein | 40.0 | 33.3 |
| Splenic and/or renal vein | 20.0 | 66.7 |
| None | 40.0 | 33.3 |
| Portal hypertension (%) | ||
| Present | 40.0 | 66.7 |
| Tumor diameter (mm) | ||
| Mean | 32.6±13.0 | 34.3±17.0 |
| Median | 36.0 | 41.0 |
There were no intraprocedural adverse events, including with introduction and placement of the diffusing fiber. No adverse events were noted on photosensitivity assessments conducted through the postprocedure inpatient observation period (day 0 to 2). On symptom assessments through the postprocedure inpatient observation period (day 0 to 2), only 1 patient (12.5%) noted moderate levels of abdominal pain on day 2; 5 (52.5%) noted minimal pain and 2 (25%) noted no pain. Through day 14, 4 (50.0%) patients did not report any new symptoms, whereas 1 patient (12.5%) reported mild levels of abdominal pain and diarrhea, 1 (12.5%) patient required an emergency department visit on day 7 for progressive abdominal pain and nausea, which were treated with conservative measures, and 1 (12.5%) patient required an emergency department visit on day 8 for hematochezia, which was not felt by the evaluating clinician to be related to the procedure; 1 (12.5%) patient was not able to be reached for a follow-up visit or call and documentation from the patient’s local provider regarding procedure follow-up was not available. All 7 patients with medical contact did not report any concerns regarding photosensitivity. Last, no differences in ECOG scores obtained at day 2 as compared with baseline were observed.
As of November 2020, 7 patients (87.5%) died of pancreatic adenocarcinoma, with a median time to death from the procedure date of 209 days (interquartile range 132.5–288.5). One patient (12.5%) was alive with a survival duration from the procedure date of 407 days.
Discussion
This pilot study is the first case series to assess EUS-guided verteporfin-mediated PDT for pancreatic cancer in humans. The procedure was able to induce a tumor necrosis zone visible on CT imaging within the 48 hours after the procedure in the majority of patients. Although analysis is limited by the small number of study participants, responders to PDT generally had smaller lesions located in the pancreatic head. The majority of responders to PDT also had lower incidences of sinistral portal hypertension and arterial vascular involvement. In the setting of 37.5% of our cohort failing to respond, it is hypothesized that a combination of individual patient variations in verteporfin pharmacokinetics and perfusion, which is impacted by tumor size and acquired malignancy-related vascular abnormalities, accounts for the differences in effect9. Patient enrollment and data acquisition are ongoing, with the goal of identifying patient and tumor characteristics that may make a tumor more amenable to the induction of necrosis.
EUS-guided PDT has previously been successfully performed with sodium porfimer as the photosensitizer, but there are limitations to its use. PDT must be delayed approximately 20 to 50 hours after injection and the long half-life of sodium porfimer results in photosensitivity effect up to approximately 30 days6. In a recent phase I study, 4 grade 1 or grade 2 adverse events were attributed to sodium porfimer; of these events, 2 were specifically related to photosensitivity and 1 to skin hyperpigmentation6. The authors hypothesize that extensive patient counseling and follow-up on the risks of photosensitivity likely accounted for the low rate of related adverse events, but this may be difficult to replicate in a setting outside of a clinical study. Sodium porfimer also requires immediate use after reconstitution due to instability in its chemical composition derived from its difficult-to-reproduce mixture11, 12.
Verteporfin overcomes these challenges. It is rapidly eliminated in the bile with a half-life of approximately 5 to 6 hours that translates to a period of cutaneous photosensitivity of 24 to 48 hours13. Verteporfin is additionally characterized by a single compound form with constant composition that promotes chemical stability12. Peak tissue concentration occurs 1 to 2 hours of administration, and therefore patients are able to undergo PDT within a more reasonable timeframe as compared with first-generation photosensitizers9.
Verteporfin appears to have inherent tumor killing properties that enhance its candidacy as the photosensitizer of choice for such applications in the gastrointestinal tract, in combination with its absorption profile along the far-red wavelength that allows for increased tissue penetration12. Our group has shown in a series of in vitro experiments that verteporfin-mediated PDT is more effective, even at lower concentrations, than sodium porfimer-mediated PDT at inducing cell death, even among K-ras negative cell lines14. Verteporfin also inhibits cancer signaling pathways that confer drug resistance, giving verteporfin-mediated PDT the added advantage of synergism with chemotherapeutic agents, which has been demonstrated in vitro and in in vivo xenograft mouse models with gemcitabine and irinotecan15–18.
A recent United Kingdom study by our group found that verteporfin-mediated PDT administered under CT guidance was feasible9. This study was also able to successfully and consistently induce tumor necrosis. However, there is a distinct advantage to delivering PDT via EUS as opposed to a percutaneous CT-guided approach. EUS is a dynamic procedure that allows for real-time visualization and positioning of the needle to ensure appropriate targeting of the lesion while avoiding critical structures. In particular, sinistral portal hypertension, which results from malignant infiltration and obstruction of vascular structures, can result in extensive varices that can be easily visualized and avoided by EUS with conventional Doppler. Another potential advantage to EUS is decreased risk of clinically impactful seeding. EUS-guided FNA does not appear to be associated with an increased rate of peritoneal recurrence19, whereas there is evidence to suggest that patients who undergo CT guided FNA for the diagnosis of pancreatic cancer subsequently develop a higher frequency of peritoneal carcinomatosis as compared with patients who underwent EUS-guided FNA20. Although the selection criteria for this study included unresectable disease, the potential for seeding should remain a consideration, as the accelerated involvement of, for example, the peritoneum has implications for survival time and quality of life21.
Notably, there is a question of the value of a limited therapy to a primary tumor in the face of metastatic disease. However, along with the potential of reducing the local effect and consequences of tumor obstruction via direct tumor cell killing and direct tumor vasculature destruction, there is the potential for abscopal effect on distal metastasis22. The PDT-induced immune response is highly complex, with triggering of both local and systemic inflammation and activation of both the innate and adaptive immune systems22. These immune-mediated effects are particularly relevant given the potential of immune checkpoint inhibitors to be used in conjunction with PDT.
There are limitations to this study, most significantly, a small number of participants treated at a single institution, which limits our interpretation of the data particularly with respect to the comparison between responders and nonresponders. The study is also prone to selection bias, as patients required the ability to travel to our specific study center to undergo the study related treatment.
In conclusion, this pilot study has demonstrated that EUS-guided, verteporfin-mediated PDT is safe and capable of inducing tumor necrosis that is visible within 48 hours after treatment. The procedure shows promise as a minimally invasive ablative therapy to enhance tumor response in select patients with pancreatic cancer refractory to chemotherapy. It is possible that, based on this pilot data, this procedure be targeted for consideration in patients with specific lesion characteristics such as smaller size, pancreatic head location, absence of arterial involvement, and absence of sinistral portal hypertension. Additional data will help establish the optimal patient related factors, disease related conditions, and concurrent systemic immunotherapies under which verteporfin-mediated PDT can be used to affect systemic disease.
Acknowledgements
We would like to thank the patients and their families, without whom this trial would not have been possible. This work was supported by the National Institutes of Health grant P01 CA084203. SPP was partly supported by the University College London Hospitals/University College London Comprehensive Biomedical Centre, which receives a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Centre funding scheme. None of these organizations were involved in the statistical analysis, interpretation of the results, or writing of this manuscript.
Financial Support:
NIH grant P01 CA084203.
Potential Competing Interests:
Dr. Wang receives research funding from Fuji Medical.
List of Abbreviations
- PDT
photodynamic therapy
- LAPC
locally advanced pancreatic cancer
- EUS
endoscopic ultrasound
- CT
computed tomography
- ECOG
Eastern Cooperative Oncology Group
- FNA
fine needle aspiration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang AY, Yachimski PS. Endoscopic Management of Pancreatobiliary Neoplasms. Gastroenterology 2018;154:1947–1963. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf TE, Matthes K, Brugge WR. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: a pilot study in a porcine model (with video). Gastrointest Endosc 2008;67:957–61. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds T. Photodynamic therapy expands its horizons. J Natl Cancer Inst 1997;89:112–4. [DOI] [PubMed] [Google Scholar]
- 4.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology 2007;132:1226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488–98. [DOI] [PubMed] [Google Scholar]
- 6.DeWitt JM, Sandrasegaran K, O’Neil B, et al. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest Endosc 2019;89:390–398. [DOI] [PubMed] [Google Scholar]
- 7.Bahra M, Pratschke J, Klein F, et al. Cytoreductive Surgery for Pancreatic Cancer Improves Overall Outcome of Gemcitabine-Based Chemotherapy. Pancreas 2015;44:930–6. [DOI] [PubMed] [Google Scholar]
- 8.Higa JT, Hwang JH. History of Ablative Therapies for Barrett’s and Superficial Adenocarcinoma. In: Pleskow DK, Erim T, eds. Barrett’s Esophagus: Emerging Evidence for Improved Clinical Practice: Academic Press, 2016:133–149. [Google Scholar]
- 9.Huggett MT, Jermyn M, Gillams A, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer 2014;110:1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EORTC. EORTC QLQ-C30 (version 3). Questionnaires: Quality of Life of Cancer Patients. Volume 2020, 1995. [Google Scholar]
- 11.Administration UFaD. Photofrin Injection - Highlights of Prescribing Information. Volume 2020, 2011. [Google Scholar]
- 12.Bamfield P, Hutchings MG. Phenomena Involving Absorption of Light and Energy Transfer. Chromic Phenomena: Technological Applications of Colour Chemistry. 2 ed: Royal Society of Chemistry Publishing, 2010:366–470. [Google Scholar]
- 13.Houle JM, Strong A. Clinical pharmacokinetics of verteporfin. J Clin Pharmacol 2002;42:547–57. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Roy B, Anderson M, et al. Verteporfin- and sodium porfimer-mediated photodynamic therapy enhances pancreatic cancer cell death without activating stromal cells in the microenvironment. J Biomed Opt 2019;24:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy B, Anderson M, Genere JR, et al. Mo1390 – Synergistic Effect of Verteporfin-Pdt with Gemcitabine on Pancreatic Cancer Cell Death In-Vitro. Gastroenterology 2019;156:S–761. [Google Scholar]
- 16.Celli JP, Solban N, Liang A, et al. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg Med 2011;43:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Q, Jia L, Liu Y-H, et al. Synergetic anticancer effect of combined gemcitabine and photodynamic therapy on pancreatic cancer in vivo. World journal of gastroenterology 2009;15:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang HC, Mallidi S, Liu J, et al. Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res 2016;76:1066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, Woo YS, Lee KH, et al. Preoperative EUS-guided FNA: effects on peritoneal recurrence and survival in patients with pancreatic cancer. Gastrointest Endosc 2018;88:926–934. [DOI] [PubMed] [Google Scholar]
- 20.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690–5. [DOI] [PubMed] [Google Scholar]
- 21.Morizane C, Okusaka T, Morita S, et al. Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas 2011;40:415–21. [DOI] [PubMed] [Google Scholar]
- 22.Hwang HS, Shin H, Han J, et al. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J Pharm Investig 2018;48:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]