Abstract
Purpose
Contact lens wear can induce corneal parainflammation involving CD11c+ cell responses (24 hours), γδ T cell responses (24 hours and 6 days), and IL-17-dependent Ly6G+ cell responses (6 days). Topical antibiotics blocked these CD11c+ responses. Because corneal CD11c+ responses to bacteria require transient receptor potential (TRP) ion-channels (TRPA1/TRPV1), we determined if these channels mediate lens-induced corneal parainflammation.
Methods
Wild-type mice were fitted with contact lenses for 24 hours or 6 days and compared to lens wearing TRPA1 (−/−) or TRPV1 (−/−) mice or resiniferatoxin (RTX)-treated mice. Contralateral eyes were not fitted with lenses. Corneas were examined for major histocompatibility complex (MHC) class II+, CD45+, γδ T, or TNF-α+ cell responses (24 hours) or Ly6G+ responses (6 days) by quantitative imaging. The quantitative PCR (qPCR) determined cytokine gene expression.
Results
Lens-induced increases in MHC class II+ cells after 24 hours were abrogated in TRPV1 (−/−) but not TRPA1 (−/−) mice. Increases in CD45+ cells were unaffected. Increases in γδ T cells after 24 hours of wear were abrogated in TRPA1 (−/−) and TRPV1 (−/−) mice, as were 6 day Ly6G+ cell responses. Contralateral corneas of TRPA1 (−/−) and TRPV1 (−/−) mice showed reduced MHC class II+ and γδ T cells at 24 hours. RTX inhibited lens-induced parainflammatory phenotypes (24 hours and 6 days), blocked lens-induced TNF-α and IL-18 gene expression, TNF-α+ cell infiltration (24 hours), and reduced baseline MHC class II+ cells.
Conclusions
TRPA1 and TRPV1 mediate contact lens-induced corneal parainflammation after 24 hours and 6 days of wear and can modulate baseline levels of resident corneal immune cells.
Keywords: TRPA1 and TRPV1, contact lens, parainflammation, cornea, major histocompatibility complex (MHC) class II+ cells, γδ T cells, Ly6G+ cells, IL-18, TNFα, resiniferatoxin (RTX)
Generally, contact lens wear is considered a safe form of vision correction. However, all modalities of contact lens wear from daily disposable to extended wear can be associated with adverse events, the most severe being microbial keratitis.1–4 To further understand the pathogenesis of contact lens-related microbial keratitis, we developed a murine model of lens wear.5 In this model, bacterial inoculation of contact lenses resulted in corneal infectious pathology after 1 to 13 days of wear, and prior to infection, all lens wearing corneas showed parainflammatory responses (i.e. without signs or symptoms of disease), namely CD11c+ (dendritic) cell infiltration after 24 hours, and Ly6G+ (neutrophil) cell infiltration after 5 to 6 days.5 Parainflammation is regarded as a low-grade inflammatory response to tissue stress residing between the basal homeostatic state and symptomatic inflammation.6 Corneal parainflammatory events have also been shown to occur during lens wear in humans,7–9 including changes in Langerhans cell density that bear resemblance to CD11c+ cell responses occurring in mice. The significance of corneal parainflammation during contact lens wear is yet to be determined. Although a primarily protective role has been postulated,10 the possibility that it could also enhance tissue susceptibility to infection and other adverse events cannot be excluded.5,11
Previously we have shown that CD11c+ cells are important for corneal defense against bacterial (Pseudomonas aeruginosa) adhesion.12 In that study, we also showed that corneal CD11c+ cells responded quickly to P. aeruginosa challenge (i.e. within 4 hours), but only in vivo, suggesting the potential for corneal sensory nerve involvement. In a more recent separate study, we have also shown that corneal defenses against bacterial adhesion require transient receptor potential (TRP) ion channels TRPA1 and TRPV1, with TRPA1 important for defense against P. aeruginosa.13 Importantly, TRP-mediated defenses against bacterial adhesion were also in vivo-dependent requiring nerve-associated TRP-channels, and correlated with TRP-dependent CD45+ and CD11c+ cell responses.13 Together, these findings suggest that corneal nerves expressing TRPA1 and TRPV1 allow rapid mobilization of CD11c+ and other immune cell defenses to defend against bacterial threats.
It has been well established that TRPA1 and TRPV1 ion channels play significant roles in the modulation of tissue inflammatory and immune responses and that these roles often rely upon extensive interactions between sensory neurons and immune cells.14–21 The cornea has been studied extensively in this regard with demonstrated involvement of TRPA1 and TRPV1 in corneal sensitivity, wound healing, and inflammation,22–26 and TRPV1 was also shown to be important for modulating neutrophil-mediated responses to P. aeruginosa corneal infection.27 Corneal sensory neurons have been shown to interact extensively with resident immune cells28 and are integral to maintenance of ocular immune privilege.29 Recently, a higher propensity of TRPV1-expressing corneal sensory nerves was observed after corneal injury, along with their interaction with intra-epithelial CD45+ and CD11c+ cells.30
Given the above findings, the present study tested the hypothesis that TPRA1 and/or TRPV1 are required for initiating corneal parainflammatory responses following contact lens wear for 24 hours and 6 days. Using a combination of TRPA1 and TRPV1 gene knockout mice, resiniferatoxin (RTX) to ablate TRPV1-expressing sensory nerves (and their subset that co-express TRPA1), and the contact lens wearing murine model, results show that both TRPA1 and TRPV1 ion channels are required for not only contact lens-induced corneal parainflammatory responses at 24 hours and 6 days, but also maintaining baseline levels of some resident corneal immune cells.
Materials and Methods
Murine Model of Contact Lens Wear
All procedures involving animals were carried out in accordance with the standards established by the Association for Research in Vision and Ophthalmology, under protocol AUP-2019-06-12322 approved by the Animal Care and Use Committee, University of California Berkeley. This protocol adheres to Public Health Service (PHS) policy on the humane care and use of laboratory animals, and the guide for the care and use of laboratory animals. This study is reported in accordance with the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments).
Six to 8 week old female and male C57BL/6J wild-type (WT) mice were used (purchased from Jackson Laboratories, then bred at the University of California Berkeley animal facility) along with age- and sex-matched TRPA1 (−/−) or TRPV1 (−/−) gene-knockout mice in the C57BL/6 background14,31 provided by Dr. Diana Bautista, University of California, Berkeley. Custom-made silicone-hydrogel mouse contact lenses were fitted onto the right eye of each mouse. Contralateral eyes were not fitted with contact lenses, and it was previously established in this model that those corneas were morphologically indistinguishable from naïve mice.5 Before lens fitting, contact lenses were removed from the packaging solution and soaked for 1 hour in phosphate buffered saline (PBS). Mice were anesthetized with 1.5% to 2% isoflurane delivered via a precision vaporizer (VetEquip Inc., Pleasanton, CA, USA). Following anesthesia, an Elizabethan collar (Kent Scientific) was placed on each mouse and contact lenses were fitted using a Handi-Vac suction pen (Edmund Optics, Barrington, NJ, USA). After lens insertion, mice were single-housed without enrichments to prevent lens removal. Pure-o'Cel paper bedding (The Andersons Inc., Maumee, OH, USA) was used to reduce dust levels. Contact lenses were worn for 24 hours or 6 days. After assigned times of lens wear, mice were euthanized with 5% isoflurane for 10 minutes followed by cervical dislocation and corneas subject to quantitative imaging ex vivo. Mice that had lost their contact lens or presented excessive weight loss, distress, or signs of keratitis were excluded from further experimentation.
Resiniferatoxin Ablation of TRPV1-Expressing Sensory Nerves
TRPV1-expressing sensory neurons were ablated by subcutaneous injection of RTX as described previously.13,32,33 An RTX stock solution was prepared (2 mg/mL) using 96% ethanol then used to prepare a 4 µg/mL solution by diluted in sterile PBS. Mice were anesthetized with isoflurane, and then RTX solution was injected subcutaneously; 20 µg/Kg body weight, daily for 3 days. Control mice were injected with vehicle only; with PBS containing the same level of 96% ethanol. Ocular surface health was assessed daily and contact lens fitting performed after the third day of RTX injection.
Fluorescein Staining
Eyes were stained with fluorescein, as described previously.13 Briefly, under isoflurane anesthesia (as above), a 5 µL drop of fluorescein solution (0.02%) was added to the ocular surface and corneal epithelial integrity was examined using a slit-lamp microscope with a blue filter. For contact lens wearing mice, fluorescein staining was performed after lens removal.
Wheat Germ Agglutinin Labeling
Wheat germ agglutinin (WGA) was used to label the ocular surface glycocalyx and assess corneal surface morphology, as previously described.13 After euthanasia, mouse eyes were enucleated, rinsed in PBS, and then immersed in a 10 µg/mL solution of AlexFluor 647 conjugated to WGA (Invitrogen) for 5 minutes at room temperature. After washing 3 times with PBS, WGA-labeled eyes were fixed in 2% paraformaldehyde (PFA) overnight at 4°C before confocal imaging.
Immunolabeling of Whole Corneas
Major histocompatibility complex (MHC) class II+ cells, CD45+ cells, γδ T cells, TNF-α positive cells, or corneal nerves were labeled as follows. Enucleated eyes were fixed for 1 hour using 100% methanol, then washed in PBS for 10 minutes with rotation. Corneal dissections were performed under a dissecting microscope over ice, corneas were washed once in PBS for 10 minutes with rotation, then transferred to blocking solution (3% bovine serum albumin [BSA] with 0.3% Triton X-100 in PBS) for 1 hour at room temperature with rotation. To facilitate antibody penetration, corneas were then incubated in 20 mM EDTA in blocking solution for 1 hour at 37°C with rotation. The following primary antibodies were used, each diluted in blocking buffer; rat anti-mouse MHC class II+ (1:500; BD Pharmingen; #556999), rat anti-mouse CD45+ (1:500; BD Pharmingen; #550539), hamster anti-mouse TCR gamma/delta (1:500; BioRad; #MCA1366GA), rat anti-mouse TNF-α (1:500; Thermo Fisher; #14-7321-81) or rabbit anti-mouse β-tubulin III (1:500; Sigma-Aldrich; #T2200). Dissected corneas were incubated with primary antibody overnight at 4°C with rotation, then with appropriate secondary antibody diluted in blocking solution (1:800) for 2 hours at room temperature with rotation and covered with foil. The following secondary antibodies were used: goat anti-rat antibody (Life Technologies; #A21434), goat anti-hamster (Abcam; #175716), and goat anti-rabbit (Life Technologies; #A21434). DAPI (4,6-diamidino-2-phenylindole dihydrochloride; 12.5 µg/mL; Thermo Fisher; #D1306) was included in secondary antibody buffer solutions to label cell nuclei. Corneas were then transferred to fresh PBS and washed 3 times for 10 minutes with rotation at room temperature and flat-mounted with Prolong Diamond (Thermo Fisher; #P36970) to enhance visibility during confocal imaging.
Immunolabeling of Corneal Cryosections
Ly6G+ cells (neutrophils) were labeled and imaged using corneal cryosections, as previously described.5 Enucleated eyes were fixed in 2% PFA at 4°C overnight, followed by an overnight wash in PBS at 4°C to remove excess PFA. Fixed eyes were cryo-protected by immersion in sucrose (15% for 4 hours, and then 30% for an additional 4 hours) at room temperature, then embedded in optimal cutting temperature (OCT) compound (Tissue Tek), then flash frozen in liquid nitrogen, and stored at −80°C until sectioned. Embedded eyes were sectioned at 10 µm thickness using a Leica CM 1900 cryostat, placed on a glass slide and stored at −80°C until antibody labeling. To label Ly6G+ cells, corneal sections were washed with PBS for 5 minutes at room temperature, blocked with 3% BSA for 30 minutes at room temperature in a moist Petri dish, then incubated with primary antibody for labeling Ly6G+ cells: Rat NIMP-R14 antibody (10 µg/mL; Thermo Fisher; #1676494) at room temperature for 1 hour. Sections were washed 3 times with PBS, then incubated for 1 hour with secondary antibody in blocking solution: Alexa 647-conjugated goat anti-rat antibody (5 µg/mL; Life Technologies). Cornea sections were also counterstained with DAPI (12.5 µg/mL; Thermo Fisher) to visualize cell nuclei, and Cell Navigator (AAT Bioquest; #22661) diluted 1:400 for epithelial F-actin labeling. Sections were rinsed three times with PBS and mounted on a coverslip with Prolong Diamond (Thermo Fisher). Sections were allowed to dry for at least 30 minutes in the dark at room temperature before wide-field imaging.
Imaging
Confocal imaging was performed using a 20×/1.00 NA water-dipping objective and an upright Olympus FluoView FV1000 Confocal Microscope. Flat-mounted corneas were imaged using the following lasers: 555 nm red (MHC class II+ cells, CD-45+ cells, γδ T cells, or TNF-α+ cells), and 488 nm green (β-tubulin III, corneal nerves) and 405 nm blue (cell nuclei). For Z stacks at 0.80 µm or 1.0 µm steps, images were collected from 4 or more random fields per sample. In each instance, images included the central cornea located via β-tubulin III labeling, and four fields from the peripheral cornea. The 3-D and 4-D image reconstructions, cell quantification, cell morphology analysis, and video generation were performed using Image-J (MorpholibJ tools collection) and Imaris (Bitplane). Protocols were similar to those described previously5 and maximum intensity projections (i.e. reducing a 3-D image into 2-D by projecting the maximum intensity of each pixel in a specific channel to the z plane), was used where indicated to visualize and quantify MHC class II+ cells, CD-45+ cells, γδ T cells, and cell morphology.
Imaging corneal sections to observe Ly6G+ cells were performed using a Nikon Ti-E inverted wide-field fluorescence microscope equipped with Lumencor SpectraX illumination source, and CFI Plan APO VC 20×/0.75 NA objective. Image quantification was performed using Image-J (MorpholibJ tools collection) based on the maximum intensity projection of the Ly6G+ cells for at least 5 fields per sample, and at least 3 samples per condition and further confirmed by manual counts.
Real Time qPCR
Following enucleation, corneas were carefully dissected to remove all limbal tissue, flash frozen with liquid nitrogen and stored at −80°C until further analysis. Corneal dissection was performed using dithiothreitol (2% in PBS). Two corneas were pooled for RNA extraction for each condition. Corneas were homogenized in Trizol with a hand-held tissue homogenizer (Kinematica Polytron, Thermo Fisher) and RNA extracted using liquid-liquid extraction with the aqueous phase collected for RNA isolation and purification. The cDNA synthesis was performed using iScript (Bio-Rad) and RT-qPCR using Faststart Sybergreen (Roche) running on a Light Cycler 96 real-time PCR machine (Roche). Primers were designed to be separated by at least one intron to assure selective amplification of cDNA and tested for efficiency (greater than or equal to 1.90), and specificity under conditions used.
Statistical Analysis
Data analysis was performed using Prism 9.0 for Mac and Microsoft Excel 2010. Distribution of data was assessed using Shapiro-Wilk and Kolmogorov-Smirnov tests. Because most data were normally distributed, it was expressed as the mean with standard deviation (SD). Student's t-test was used for two group comparisons and a 1-way ANOVA with Tukey's multiple comparisons was used for three or more groups. The P values less than 0.05 were considered significant. All experiments were repeated twice unless otherwise stated.
Results
Corneas of TRPA1 (−/−) and TRPV1 (−/−) Mice Show Reduced Levels of Resident MHC Class II+ Cells: TRPV1 (−/−) Mice Lose MHC Class II+ Cell Responses to Lens Wear at 24 Hours
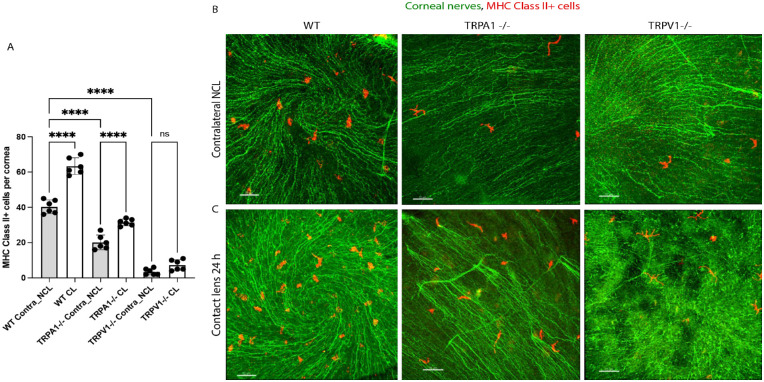
Previously we reported that contact lens wear increases Lyz2+ cell or CD11c+ cell responses in the murine cornea after 24 hours.5,34 We have also reported that TRPA1 and TRPV1 are required for corneal CD45+ and CD11c+ cell responses to P. aeruginosa inoculation.13 To determine if TRPA1 or TRPV1 were required for contact lens-induced corneal immune cell responses, lens wearing WT mice were compared to gene knockout mice in TRPA1 (−/−) or TRPV1 (−/−) for corneal MHC class II+ cell responses. Figure 1 shows that lens-induced MHC class II+ cell responses in the murine cornea after 24 hours of wear were present in both WT and TRPA1 (−/−) mice, but not TRPV1 (−/−) mice. Lens wearing eyes remained free of visible pathology, as previously observed.5,34 Surprisingly, baseline numbers of resident MHC class II+ cells in contralateral corneas (i.e. no lens wear), were significantly reduced in both TRPA1 (−/−) and TRPV1 (−/−) mice, the latter expressing five or fewer cells per cornea. Thus, both TRPA1 and TRPV1 are required for maintaining resident MHC class II+ cell numbers in the murine cornea, with TRPV1 required for their response to lens wear.
Figure 1.
Quantification (A) and representative immunofluorescence imaging (B, C) of MHC class II+ cells (red) in the corneas of wild-type or gene-knockout mice in TRPA1 (−/−) or TRPV1 (−/−) with or without contact lens wear for 24 hours. Corneal nerves were labeled with β-tubulin III (green). Scale bar = 70 µm. MHC class II+ cell responses to lens wear (white bars) were present in WT and TRPA1 (−/−) mice, but not TRPV1 (−/−) mice. Contralateral eyes of TRPA1 (−/−) and TRPV1 (−/−) mice (grey bars) showed a significant reduction in baseline numbers of MHC class II+ cells. **** P < 0.0001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test].
Figure 1 (and others below) also show β-tubulin III labeling of corneal nerves with the spiral pattern helping us to locate the center of each cornea for image acquisition (see Methods). Although not the focus of the present study, a preliminary analysis did not reveal any observable differences in nerve density between WT versus TRPA1 (−/−) or TRPV1 (−/−) corneas at baseline or after lens wear for 24 hours versus contralateral eyes (data not shown).
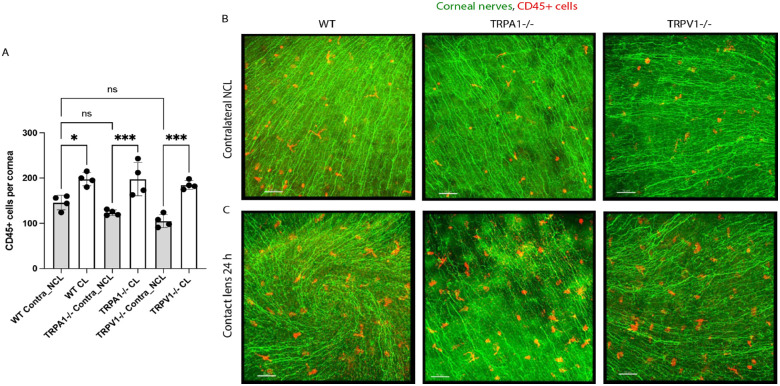
Corneal CD45+ Cell Responses to Lens Wear Do Not Depend on TRPA1 and TRPV1
Because we have previously shown that TRPA1 and TRPV1 were required for corneal CD45+ cell responses to P. aeruginosa inoculation,13 we next determined if corneal CD45+ cell responses to 24 hours of contact lens wear also depended on TRPA1 or TRPV1. Figure 2 shows that 24 hours of lens wear caused a significant increase in CD45+ cells in the corneas of WT, TRPA1 (−/−), and TRPV1 (−/−) mice compared to their respective no lens wear contralateral eyes. However, there was no significant difference among WT, TRPA1 (−/−), and TRPV1 (−/−) mice in baseline numbers of CD45+ cells in the cornea (i.e. under no lens wear conditions). Thus, the absence of TRPA1 or TRPV1 does not significantly affect CD45+ cell numbers in the murine cornea nor their response to 24 hours of contact lens wear.
Figure 2.
Quantification (A) and representative immunofluorescence imaging (B, C) of CD45+ cells (red) in the corneas of wild-type or gene-knockout mice in TRPA1 (−/−) or TRPV1 (−/−) with or without contact lens wear for 24 hours. Corneal nerves were labeled with β-tubulin III (green). Scale bar = 70 µm. CD45+ cell responses to lens wear (white bars) were present in all mice: WT, TRPA1 (−/−), and TRPV1 (−/−). Contralateral eyes of TRPA1 (−/−) and TRPV1 (−/−) mice (grey bars) did not show a significant reduction in baseline numbers of CD45+ cells * P < 0.05, *** P = 0.001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test].
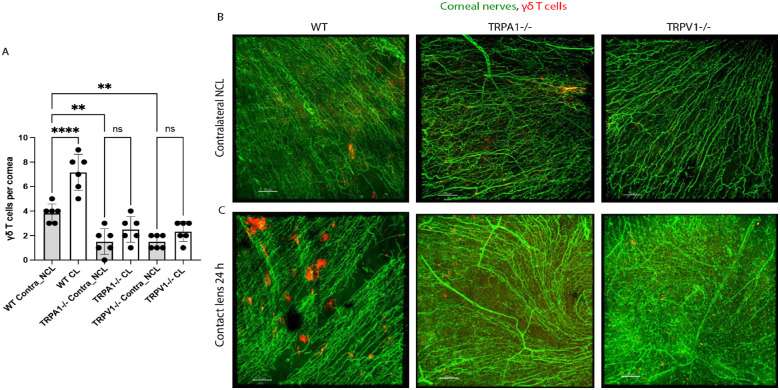
Corneas of TRPA1 (−/−) and TRPV1 (−/−) Mice Also Show Reduced Baseline Levels of γδ T Cells and Loss of Their Response to 24 Hours of Lens Wear
We have recently shown that contact lens-induced corneal parainflammation involving Ly6G+ cell (neutrophil) infiltration after 6 days of lens wear required γδ T cells and IL-17.35 Thus, we next explored if TRPA1 or TRPV1 were also required for γδ T cell infiltrative responses to lens wear after 24 hours. Figure 3 shows that contact lens-induced increases in corneal γδ T cells after 24 hours were abrogated in both TRPA1 (−/−) and TRPV1 (−/−) mice. Moreover, the baseline number of γδ T cells in contralateral corneas was also significantly reduced in TRPA1 and TRPV1 gene knockout mice (see Fig. 3). Thus, TRPA1 and TRPV1 are also involved in maintaining γδ T cell numbers in the murine cornea as well as their responses to 24 hours of lens wear.
Figure 3.
Quantification (A) and representative immunofluorescence imaging (B, C) of γδ T cells (red) in the corneas of wild-type or gene-knockout mice in TRPA1 (−/−) or TRPV1 (−/−) with or without contact lens wear for 24 hours. Corneal nerves were labeled with β-tubulin III (green). Scale bar = 70 µm. The γδ T cell responses to lens wear (white bars) were absent in both TRPA1 (−/−) and TRPV1 (−/−) mice. Contralateral eyes of TRPA1 (−/−) and TRPV1 (−/−) mice (grey bars) each also showed a significant reduction in baseline numbers of γδ T cells. ** P < 0.01, **** P < 0.0001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test].
Shape Analysis of MHC Class II+ and γδ T Cells After 24 Hours of Lens Wear Revealed the Presence of More Circular Cells
Having quantified numbers of MHC class II+, CD45+ and γδ T cells recruited into cornea after 24 hours of lens wear, cell shape analysis was also conducted under the same experimental conditions, as previously described.5,34 Figure 4 shows that 24 hours of lens wear in WT mice resulted in an increased circularity of both MHC class II+ (see Fig. 4A) and γδ T cells (see Fig. 4C) but not CD45+ cells (see Fig. 4B). Examples of these circularity changes are shown in Supplemental Figure S1. When circularity of MHC class II+, CD45+, and γδ T cells was assessed in TRPA1 (−/−) and TRPV1 (−/−) mice, a similar pattern of increased cell circularity was observed for MHC class II+ cells in lens wearing corneas after 24 hours, and no significant change was observed for CD45+ cells. However, increased circularity of corneal γδ T cells with 24 hours of lens wear was not observed in TRPA1 (−/−) and TRPV1 (−/−) mice.
Figure 4.
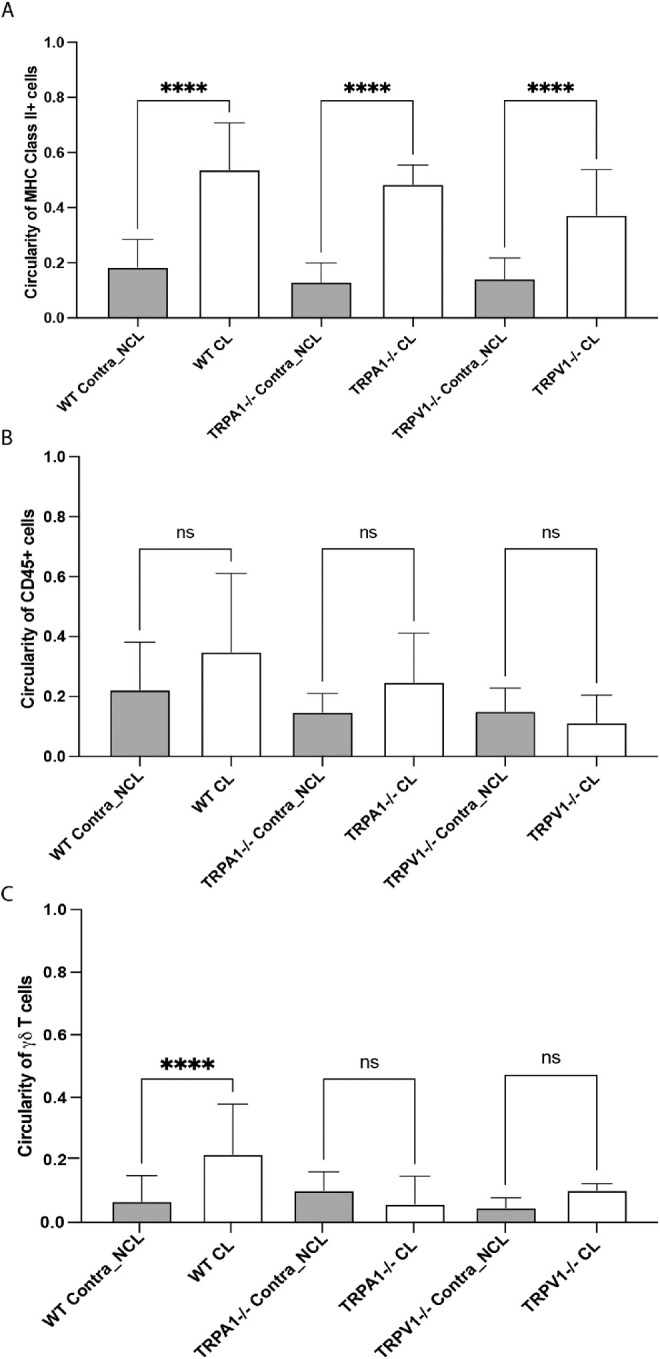
Quantification of cell circularity of (A) MHC class II+ cells, (B) CD45+ cells, and (C) γδ T cells in the corneas of WT, TRPA1 (−/−), or TRPV1 (−/−) mice after 24 hours of lens wear (CL) versus no lens wear contralateral eyes (NCL). Lens wear was associated with a significant increase in circularity (i.e. decreased dendritic cell shape) for MHC class II+ cells in all mice, but not for CD45+ cells. For γδ T cells, only lens wearing WT mice showed a significant increase in cell circularity. **** P < 0.0001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test].
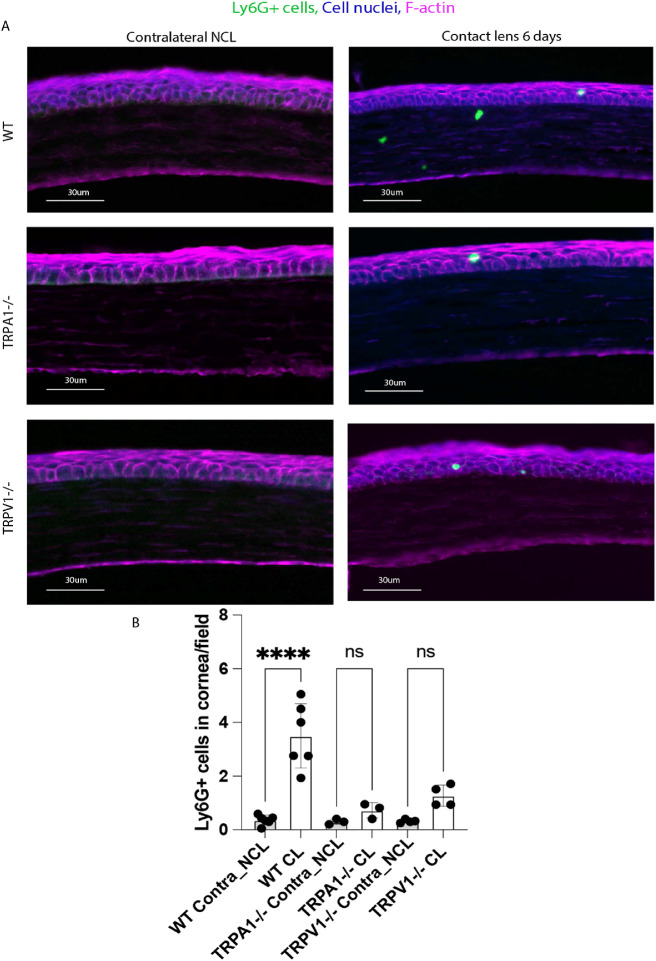
TRPA1 and TRPV1 Receptors Are Required for Ly6G+ Cell (Neutrophil) Responses in Lens Wearing Corneas After 6 Days
Because TRPA1 and TRPV1 are required for lens-induced γδ T cell responses (see Fig. 3), and our previous work has shown that γδ T cell responses are required for Ly6G+ cell infiltration after 6 days of lens wear,35 we explored Ly6G+ cell responses in WT, TRPA1 (−/−), and TRPV1 (−/−) mice. Figure 5 shows that 6 days of lens wear induced a Ly6G+ cell response in WT mice as expected, and that this response was abrogated in TRPA1 (−/−) and TRPV1 (−/−) mice. Few Ly6G+ cells were found in no lens wear contralateral corneas of any mice. Thus, consistent with their role in lens-induced γδ T cell responses after 24 hours of wear (see Fig. 3), TRPA1 and TRPV1 are also required for Ly6G+ (neutrophil) infiltration of contact lens wearing corneas.
Figure 5.
(A) Immunofluorescence imaging of corneal cryosections showing Ly6G+ cell (neutrophil) infiltration of a mouse cornea after 6 days of contact lens wear versus the no lens wear contralateral eye. Lens-induced Ly6G+ cell infiltration was reduced in the corneas of lens wearing TRPA1 (−/−) and TRPV1 (−/−) mice. Ly6G+ cells (green), cell nuclei (blue), and cell F-actin (red). Scale bar = 30 µm. (B) Quantification of Ly6G+ cells (per field of view) in WT versus TRPA1 (−/−) and TRPV1 (−/−) corneas after 6 days of contact lens wear versus contralateral eyes. The lens-induced Ly6G+ response was lost in both TRPA1 (−/−) and TRPV1 (−/−) mice. **** P < 0.0001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test].
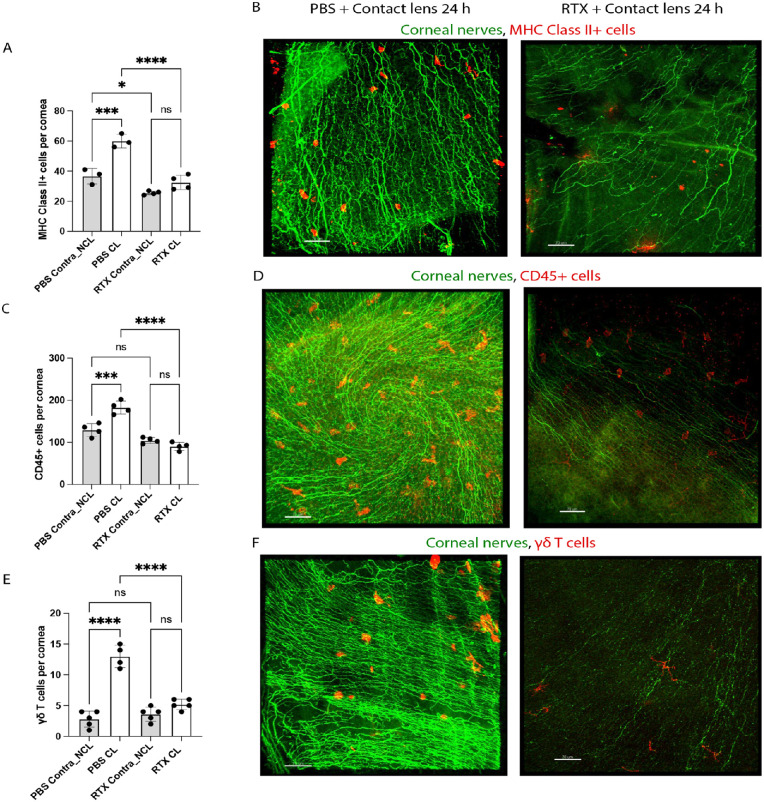
RTX Inhibits Lens-Induced MHC-Class II+, CD45+ and γδ T Cell Responses After 24 Hours of Wear and Ly6G+ Cell Responses After 6 Days of Wear
Given the above findings with TRPA1 and TRPV1 gene knockout mice, we also utilized a pharmacological approach to test the role of TRPA1 and TRPV1 in contact lens-induced parainflammatory responses in the cornea. Mice were treated with RTX, a potent TRPV1 agonist which ablates TRPV1-expressing nociceptors, along with a subset that express both TRPA1 and TRPV1.36,37 WT mice were subjected to subcutaneous application of RTX, 3 days prior to lens fitting while control group mice were injected with PBS. Consistent with our previous study,13 corneas of PBS or RTX-treated mice showed no epithelial injury or disruption as indicated by fluorescein staining and WGA-labeling with or without lens wear (data not shown). Figure 6 shows that RTX treatment attenuated each of the contact lens-induced cellular responses at 24 hours for MHC class II+ cells (see Figs. 6A, 6B), CD45+ cells (see Figs. 6C, 6D), and γδ T cells (see Figs. 6E, 6F). RTX mimicked the reduced baseline levels of resident MHC class II+ cells in no lens wear contralateral corneas seen in TRPA1 and TRPV1 gene-knockout mice (see Fig. 6A), but not that observed for γδ T cells (see Fig. 6E). Figure 7 shows that RTX treatment also abrogated the lens induced Ly6G+ cell response after 6 days of wear. Thus, RTX treatment mimicked some of the changes seen in TRPA1 (−/−) and TRPV1 (−/−) mice: loss of lens-induced MHC class II+ cell responses at 24 hours in TRPV1 (−/−) mice, loss of lens-induced γδ T cell responses at 24 hours in both TRPA1 (−/−) and TRPV1 (−/−) mice, and loss of lens-induced Ly6G+ cell responses after 6 days in both TRPA1 (−/−) and TRPV1 (−/−) mice.
Figure 6.
RTX treatment of mice to deplete TRPV1 nociceptors attenuates contact lens-induced corneal immune cell responses at 24 hours involving MHC class II+ cells (A, B), CD45+ cells (C, D) and γδ T cells (E, F). RTX also reduced baseline numbers of resident MHC class II+ cells but did not affect CD45+ or γδ T cells. (A, C, E) Quantification of corneal MHC class II+ cells (A), CD45+ cells (C) and γδ T cells (E) after 24 hours of lens wear versus no lens wear contralateral eyes each with PBS or RTX treatment. * P < 0.05, *** P = 0.001, **** P < 0.0001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test]. (B, D, F) Immunofluorescence imaging of lens wearing corneas (24 hours) after PBS treatment (left panels) versus RTX treatment (right panels). MHC class II+ cells (B), CD45+ cells (D), or γδ T cells (F). Respective immune cells (red). Corneal nerves were labeled with β-tubulin III (green). Scale bar = 70 µm.
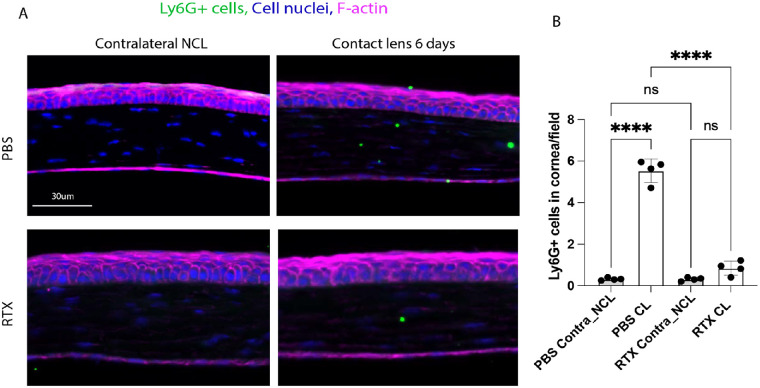
Figure 7.
(A) Immunofluorescence imaging of corneal cryosections showing Ly6G+ cell (neutrophil) infiltration of a mouse cornea after 6 days of contact lens wear versus the no lens wear contralateral eye with and without RTX treatment to deplete TRPV1 nociceptors. RTX treatment attenuated the lens-induced Ly6G+ cell infiltration. Ly6G+ cells (green), cell nuclei (blue), and cell F-actin (red). Scale bar = 30 µm. (B) Quantification of Ly6G+ cells (per field of view) in the corneas of WT mice treated with PBS (control) or RTX with and without 6 days of contact lens wear. RTX treatment prevented lens-induced Ly6G+ cell infiltration. **** P < 0.0001, ns = not significant [One-way ANOVA with Tukey's multiple comparisons test].
RTX Blocks Lens-Induced Expression of Corneal TNF-α and IL-18 Genes and Infiltration of TNF-α + Cells After 24 Hours of Wear
To begin to understand the mechanisms behind TRPA1/TRPV1-mediated recruitment of immune cells to the cornea after 24 hours of lens wear, the transcriptional profile of select proinflammatory cytokines was examined. Figure 8 shows changes gene expression in lens wearing corneas after 24 hours expressed relative to contralateral eyes (i.e. baseline normalized to zero) in PBS-treated versus RTX-treated mice. Contact lens wear was associated with a significant increase in gene expression for IL-18 (approximately 8.5-fold) and TNF-α (approximately 19.2-fold) versus respective contralateral corneas (see Fig. 8; black bars, red asterisks *, **). In each instance, this response was blocked in RTX-treated mice (see Fig. 8; compare black bars to white bars, black asterisks **, ****). IL-1β responses were also significantly reduced in RTX-treated corneas although increased expression of this gene in lens wearing (PBS-treated) controls did not reach statistical significance.
Figure 8.
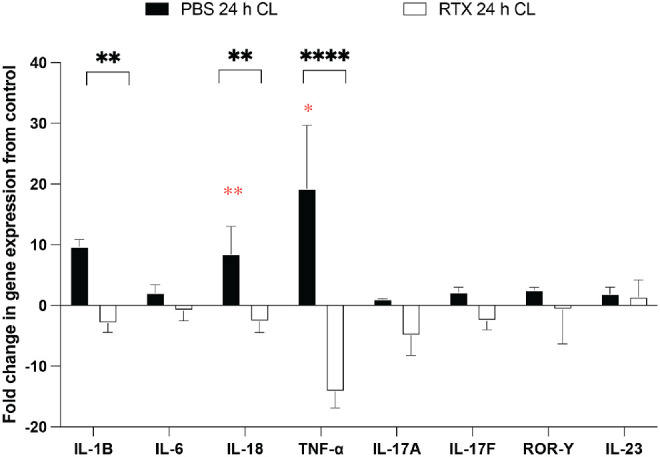
The qPCR analysis of cytokine gene expression in the mouse cornea after 24 hours of contact lens wear versus contralateral eyes in wild-type mice treated with RTX to deplete TRPV1-expressing nociceptors or with PBS as a control. Data are expressed as the fold-change in the lens wearing cornea relative to the contralateral eye (i.e. normalized to zero). Red asterisks * P < 0.05, ** P < 0.01 [Students t-test]. Lens wearing PBS-treated control mice (black bars) were also compared to lens wearing RTX-treated mice (white bars). ** P < 0.01, **** P < 0.0001 [Student's t-test].
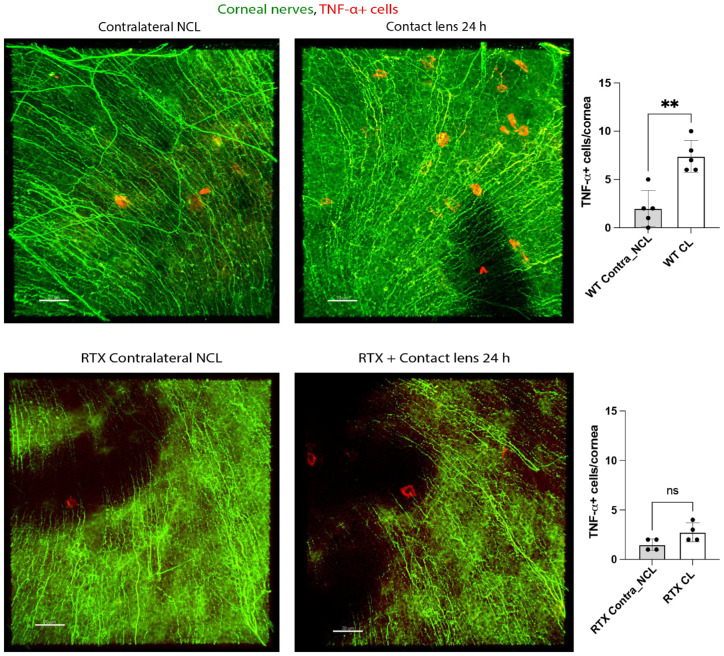
Given the significant increase in TNF-α gene expression induced by 24 hours of contact lens wear, we next examined corneal infiltration by TNF-α + cells. Figure 9 shows that lens wear induced a significant increase in TNF-α + cells after 24 hours consistent with the increase in gene expression. RTX treatment blocked lens-induced infiltration of TNF-α + cells also consistent with its effects on gene expression showing that this phenotype was also mediated by TRPA1 and/or TRPV1.
Figure 9.
RTX attenuates TNF-α+ cell infiltration of the murine cornea in response to 24 hours of contact lens wear. Immunofluorescence imaging (A) and quantification (B) shows infiltration of TNF-α+ cells in the contact lens wearing cornea of WT mice which was attenuated by RTX treatment (C, D). TNF-α+ cells (red). Corneal nerves were labeled with β-tubulin III (green). Scale bar = 70 µm. ** P < 0.01, ns = not significant [Student t-test].
Discussion
The results of this study demonstrate a role for TRPA1 and TRPV1 ion channels in contact lens-induced corneal parainflammation after 24 hours and 6 days of lens wear, along with a role in maintaining baseline levels of resident immune cells in the murine cornea. These conclusions were derived from experiments using a combination of single gene knockout mice, TRPA1 (−/−) or TRPV1 (−/−), and the use of RTX, the TRPV1 agonist, which ablates TPV1-expressing sensory nerves and along with the subset of those nerves that co-express TRPA1. Contact lens-induced MHC class II+ cell responses at 24 hours were abrogated in TRPV1 (−/−) mice, but not TRPA1 (−/−) mice, whereas lens-induced CD45+ responses remained in both gene knockout mice. Lens-induced γδ T cell responses at 24 hours were also abrogated in both TRPV1 (−/−) and TRPA1 (−/−) mice, as were Ly6G+ cell responses after 6 days of lens wear. Surprisingly, gene knockout of TRPA1 or TRPV1 decreased baseline numbers of MHC class II+ cells and γδ T cells in the murine cornea, but not those of CD45+ cells. RTX abrogated all contact lens-induced cellular responses at 24 hours: MHC class II+ cells, CD45+ cells and γδ T cells, and lens-induced 6 day Ly6G+ cell responses. RTX treatment also decreased baseline numbers of MHC class II+ cells in the murine cornea, but not those of γδ T cells, and RTX blocked 24 hours lens-induced increases in corneal expression of TNF-α and IL-18 and infiltration of TNF-α + cells. Whereas further studies are needed to understand the differences between TRPA1 or TRPV1 gene-knockout mice versus RTX-mediated phenotypes, together these data demonstrate novel roles for both TRPA1 and TRPV1 in mediating corneal parainflammation and immune cell homeostasis.
Recently we showed that the Ly6G+ cell parainflammatory response of the murine cornea to 6 days of contact lens wear requires IL-17A, and that γδ T cells are the likely source of this proinflammatory cytokine, because lens wear induced γδ T cell responses in the cornea after 1 and 6 days of wear, and antibody-mediated functional depletion of γδ T cells abrogated lens-induced IL-17A responses and Ly6G+ cell responses in the cornea after 6 days of wear.35 Results of the present study showing that lens-induced γδ T cell responses in the cornea (24 hours) and Ly6G+ responses (6 days) were also abrogated in either TRPA1 or TRPV1 gene knockout mice (see Figs. 3, 5, respectively) and by RTX treatment (see Figs. 6E, 6F, 7, respectively) suggest that both of these ion channels are required for contact lens-induced parainflammation after 6 days of wear via the following pathway: Lens wear (24 hours to 6 days) → TRPA1 and TRPV1 activation → γδ T cell recruitment → IL-17A secretion → Ly6G+ cell (neutrophil) infiltration. Given the extensive localization of TRPV1 and TRPA1 on subsets of sensory nerves in the corneal epithelium and stroma during health and after injury,25,30,38 these data suggest that corneal sensory nerves are required for initiation of contact lens-induced parainflammation, at least that involving γδ T cells and Ly6G+ cells. It remains to be determined why both of these ion channels are required since each have different physiological roles in nociception and respond to different endogenous and exogenous agonists.15,23,39 The answer to that question will likely become clearer as the trigger(s) for lens-induced TRPA1 and TRPV1-mediated parainflammation are identified.
Another significant finding from the present study is that TRPA1 and TRPV1 both influenced the baseline levels of resident MHC class II+ cells and γδ T cells in the murine cornea (see Figs. 1, 3, respectively). Without lens wear, numbers of resident MHC class II+ cells were significantly decreased in corneas of TRPA1 (−/−) mice (by approximately 50% versus WT), and further reduced (by approximately 90% versus WT) in TRPV1 (−/−) corneas, suggesting a distinct influence for each ion channel on these cells. For baseline numbers of corneal γδ T cells, both ion channels were required with an equal decline in resident cells observed in each gene knockout mouse. RTX produced a significant decrease in corneal MHC class II+ cells (see Fig. 6B) but did not affect γδ T cells, perhaps a reflection of the low numbers present. Baseline levels of corneal CD45+ cells declined slightly in both TRPA1 (−/−) and TRPV1 (−/−) mice versus WT but differences were not significant. This finding may reflect the broader distribution of the CD45+ cell population in the cornea with TRPA1 and TRPV1 only affecting subpopulations of CD45+ cells. It is also possible that a larger sample size might confirm a significant reduction in CD45+ cells in corneas of TRPA1 (−/−) or TRPV1 (−/−) mice at baseline. Unfortunately, limited lens supply precluded further experiments on this issue in the present study. As mentioned above, TRPV1 is expressed on numerous corneal sensory nerves and a subset of TRPV1-expressing nociceptors co-express TRPA1. Because corneal sensory nerves are fundamentally interconnected with resident immune cells in the cornea (e.g. dendritic cells and macrophages), and they exhibit cross-talk that influences each other's function in health and disease,28–30,40,41 it is perhaps not surprising that TRPA1 and TRPV1 were found to be required for maintenance of MHC class II+ and γδ T cell numbers in the murine cornea. It will be of interest in future studies to determine the signaling mechanisms involved in TRPV1 or TRPA1-mediated immune cell homeostasis in the cornea (e.g. does TRPV1 or TRPA1-mediated release of neuropeptides play a role in recruitment or maintenance of resident immune cell numbers?).
It was noted that some corneal immune cell responses to lens wear were unaffected by TRPA1 or TRPV1 gene knockout. For example, MHC class II+ cells still responded to 24 hours of lens wear in TRPA1 (−/−) mice despite the decline in baseline cell numbers (see Fig. 1). Likewise, CD45+ cell responses to 24 hours of lens wear remained intact in both TRP gene-knockout mice (see Fig. 2). However, RTX treatment abrogated all 24 hours of lens-induced responses, in MHC class II+ cells, CD45+ cells, and γδ T cells (see Fig. 6). Because RTX provides short-term ablation of TRPV1-expressing sensory nerves (and their subset that co-express TRPA1) in WT mice after 3 days of treatment, whereas gene knockout mice may have functional adaptations to compensate for the absence of TRPA1 or TRPV1, these data suggest that TRPA1 and TRPV1 could also affect MHC class II+ and CD45+ cell responses to 24 hours of lens wear, as well as γδ T cell responses. Indeed, MHC class II+ cell responses to lens wear were lost in TRPV1 (−/−) mice (see Fig. 1). However, it remains possible that RTX could exert some nonspecific effects beyond ablation of TRPV1-expressing sensory nerves. Thus, further investigation is warranted of the TRPA1- and TRPV1-mediated pathways involved in the response of each corneal immune cell type to 24 hours of lens wear.
Lens wear for 24 hours was associated with a significant increase in IL-18 and TNF-α gene expression in the murine cornea and infiltration of TNF-α positive cells, all of which were blocked by RTX treatment indicating a dependence of these responses on TRPA1 and/or TRPV1. It is well known that TNF-α is a proinflammatory cytokine with a multitude of effects on cell and tissue functions that contribute to disease pathology associated with infection and autoimmune diseases.42 Macrophages, monocytes, neutrophils, T lymphocytes, dendritic cells, and epithelial cells are all recognized as sources of TNF-α.43–45 In our model, contact lens-induced changes in TNF-α gene expression in the cornea after 24 hours of wear may reflect the MHC class II+ cell responses observed (see Fig. 1), the latter likely including CD11c+ cell responses to 24 hours of lens wear shown in our previous work.5,34 Indeed, MHC class II+ cell responses were also abrogated by RTX. Lens wear-induced TNF-α-positive cells in the cornea (see Fig. 9) appeared to be isolated rather than part of the epithelium. However, further work will be needed to determine the nature of the TNF-α positive cells and other potential sources of lens wear-induced TNF-α. Interestingly, the TNF-α receptor TNFR1 can function to modulate neutrophil phenotype, with paracrine TNF-α exposure serving to “license” neutrophils to increase their production of cytokines (both pro-inflammatory and anti-inflammatory) and eicosanoids (e.g. leukotriene B4) in response to TLR activation.46 It will be of value in future work to determine if lens-induced increases in TNF-α in the cornea after 24 hours of wear have significance in the context of subsequent Ly6G+ cell (neutrophil) infiltration induced by lens wear after 6 days. Changes in IL-18 gene expression could indicate involvement of inflammasome activation in lens-induced corneal parainflammation,47,48 and will be part of a future study because they were also present after 6 days of lens wear,35 and may also have significance in the context of lens-induced Ly6G+ cell infiltration of the cornea.
In two previous studies using this lens wearing murine model, morphology of corneal immune cells with or without lens wear has been used to indicate potential changes in phenotype.5,34 The distribution of Lyz2+ cells in the cornea after 7 days of lens wear showed increased circularity versus no lens wear contralateral eyes,5 whereas no significant changes in corneal CD11c+ cell morphology were observed after 24 hours of lens wear.34 In the present study, increased circularity was observed for corneal MHC class II+ cells and γδ T cells after 24 hours of lens wear versus contralateral eyes, but only γδ T cell changes were lost in TRPA1 and TRPV1 gene-knockout mice. Although changes in γδ T cell circularity after 24 hours of lens wear (see Fig. 4C) correlated with changes in γδ T cell numbers (see Fig. 3), further work is needed to determine the significance of immune cell morphology changes to lens-induced para-inflammation.
In conclusion, this study demonstrates that contact lens-induced corneal parainflammatory responses after 24 hours and 6 days of wear require both TRPA1 and TRPV1 ion channels, and that these channels also modulate baseline numbers of some resident corneal immune cells (MHC class II+). Our data suggest that one role for TRPA1 and TRPV1 in lens-induced parainflammation is to mediate γδ T cell responses to 24 hours of lens wear which (along with IL-17) we have shown to be required for Ly6G+ (neutrophil) cell responses to 6 days of lens wear.35 It is likely that TRPA1 and TRPV1 expressed on corneal sensory nerves mediate the parainflammatory responses to lens wear, and effects on baseline immune cell numbers. Because we used a global TRPV1 gene-knockout mouse or RTX treatment, however, a role for TRPV1 in non-neuronal cells, such as epithelial cells or macrophages, cannot be excluded.30,49 Notwithstanding the tissue location of the TRPA1 and TRPV1 ion channels involved, the demonstrated close association between corneal sensory nerves and corneal immune cells28,30,41 would suggest functional roles for both in lens-induced parainflammation and maintenance of immune cell numbers shown in this study and is worthy of further investigation.
Interestingly, a recent study using a corneal abrasion model showed that TRPV1-expressing sensory nerves exert a suppressive role on corneal inflammation via CCR2− macrophages and IL-10 expression and preventing the recruitment of γδ T cells and neutrophils. Depletion of TRPV1-expressing sensory neurons using RTX, or a selective antagonist AMG-517, increased numbers of corneal CCR2+ macrophages, their production of TNF-α, decreased IL-10, and increased γδ T cell and neutrophil responses.50 Those findings clearly contrast with the present study and our previous findings35 using a contact lens wearing model which together show a parainflammatory role for TRPV1 (and TRPA1), along with their role shown here in the maintenance of baseline immune cell numbers. Together, these data suggest that the role of these TRP ion channels in maintenance of corneal homeostasis is context dependent and the mechanisms that define this versatility require further study.
Further studies are also needed to identify the specific trigger(s) for TRPA1/TRPV1-mediated corneal parainflammation after contact lens wear, and ligands that help maintain baseline numbers of some corneal immune cells (MHC class II+). First, our finding that CD11c+ responses to lens wear (24 hours) were blocked by topical antibiotic pretreatment34 suggested an involvement of bacteria at the ocular-surface (e.g. conjunctival commensals). In a different murine model, a conjunctival commensal (Corynebacterium mastitidis) was required for ocular γδ T cell responses, with IL-17 secretion driving neutrophil recruitment that elevated neutrophil numbers at steady-state and helped defend the cornea during bacterial and fungal infection.51 Thus, bacteria (or their ligands) could play a role in parainflammatory responses to lens wear and/or maintenance of baseline levels of corneal immune cells shown in the present study. Because TRPA1- and TRPV1-expressing sensory nerves are both capable of directly responding to bacterial ligands (e.g. LPS via TLR4),52–54 a direct role for bacterial-sensory nerve interactions in mediating these phenotypes is quite plausible. Second, our previous work has also shown that IL-1R and MyD88 are required for corneal Ly6G+ (neutrophil) responses to 6 days of lens wear.5 Because, in addition to TLR4, the IL-1R is also present on TRPV1-expressing neurons,55 it is possible that the role of the IL-1R in lens-induced parainflammation involves its co-expression with TRPA1/TRPV1 on corneal sensory nerves. Finally, in addition to elucidating the triggers and mechanisms of lens-induced parainflammation, it is important to determine the clinical consequence of this phenotype for lens wear in humans. Parainflammation that serves as a protective response that restores homeostasis in the cornea during lens wear could rapidly turn deleterious if conditions allow.
Supplementary Material
Acknowledgments
Supported by the National Eye Institute EY030350 (S.M.J.F.).
Disclosure: A. Datta, None; J.H. Lee, None; O. Flandrin, None; H. Horneman, None; J. Lee, None; M.M.E. Metruccio, None; D. Bautista, None; D.J. Evans, None; S.M.J. Fleiszig, None
References
- 1. Stapleton F, Naduvilath T, Keay L, et al.. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS One. 2017; 12(8): e0181343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willcox MDP. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007; 84(4): 273–278. [DOI] [PubMed] [Google Scholar]
- 3. Lim CHL, Carnt NA, Farook M, et al.. Risk factors for contact lens-related microbial keratitis in Singapore. Eye. 2016; 30(3): 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yildiz EH, Airiani S, Hammersmith KM, et al.. Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea. 2012; 31(10): 1097–1102. [DOI] [PubMed] [Google Scholar]
- 5. Metruccio MME, Wan SJ, Horneman H, et al.. A novel murine model for contact lens wear reveals clandestine IL-1R dependent corneal parainflammation and susceptibility to microbial keratitis upon inoculation with Pseudomonas aeruginosa. Ocul Surf. 2019; 17(1): 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008; 454(7203): 428–435. [DOI] [PubMed] [Google Scholar]
- 7. Alzahrani Y, Pritchard N, Efron N.. Changes in corneal Langerhans cell density during the first few hours of contact lens wear. Contact Lens Anterior Eye. 2016; 39(4): 307–310. [DOI] [PubMed] [Google Scholar]
- 8. Golebiowski B, Chao C, Bui KA, Lam WYW, Richdale K, Stapleton F.. Effect of age and contact lens wear on corneal epithelial dendritic cell distribution, density, and morphology. Cont Lens Anterior Eye. 2020; 43(1): 84–90. [DOI] [PubMed] [Google Scholar]
- 9. Chao C, Stapleton F, Willcox MDP, Golebiowski B, Richdale K.. Preinflammatory signs in established reusable and disposable contact lens wearers. Optom Vis Sci. 2017; 94(11): 1003–1008. [DOI] [PubMed] [Google Scholar]
- 10. Efron N. Contact lens wear is intrinsically inflammatory. Clin Exp Optom. 2017; 100(1): 3–19. [DOI] [PubMed] [Google Scholar]
- 11. Fleiszig SMJ, Kroken AR, Nieto V, et al.. Contact lens-related corneal infection: intrinsic resistance and its compromise. Prog Retin Eye Res. 2020; 76: 100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metruccio MME, Tam C, Evans DJ, Xie AL, Stern ME, Fleiszig SMJ.. Contributions of MyD88-dependent receptors and CD11c-positive cells to corneal epithelial barrier function against Pseudomonas aeruginosa. Sci Rep. 2017; 7(1): 13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan S, Datta A, Flandrin O, et al.. Nerve-associated transient receptor potential ion channels can contribute to intrinsic resistance to bacterial adhesion in vivo. FASEB. 2021; 35(10): e21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bautista DM, Jordt S-E, Nikai T, et al.. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006; 124(6): 1269–1282. [DOI] [PubMed] [Google Scholar]
- 15. Bautista DM, Pellegrino M, Tsunozaki M.. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol. 2012; 75(1): 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López-Requena A, Boonen B, Van Gerven L, Hellings PW, Alpizar YA, Talavera K.. Roles of neuronal TRP channels in neuroimmune interactions. In: Neurobiology of TRP Channels, Frontiers in Neuroscience . 1st ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2017: Chapter 15, doi: 10.4324/9781315152837-15. [DOI] [PubMed] [Google Scholar]
- 17. Khalil M, Alliger K, Weidinger C, et al.. Functional role of transient receptor potential channels in immune cells and epithelia. Front Immunol. 2018; 9: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talavera K, Startek JB, Alvarez-Collazo J, et al.. Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev. 2020; 100(2): 725–803. [DOI] [PubMed] [Google Scholar]
- 19. Riol-Blanco L, Ordovas-Montanes J, Perro M, et al.. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014; 510: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshida A, Furube E, Mannari T, et al.. TRPV1 is crucial for proinflammatory STAT3 signaling and thermoregulation-associated pathways in the brain during inflammation. Sci Rep. 2016; 6: 26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baral P, Umans BD, Li L, et al.. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat Med. 2018; 24: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang F, Yang H, Wang Z, et al.. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007; 213(3): 730–739. [DOI] [PubMed] [Google Scholar]
- 23. Okada Y, Reinach PS, Shirai K, et al.. Transient receptor potential channels and corneal stromal inflammation. Cornea. 2015; 34(suppl 11): S136–S141, doi: 10.1097/ICO.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 24. Okada Y, Reinach PS, Shirai K, et al.. TRPV1 involvement in inflammatory tissue fibrosis in mice. Am J Pathol. 2011; 178(6): 2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. González-González O, Bech F, Gallar J, Merayo-Lloves J, Belmonte C.. Functional properties of sensory nerve terminals of the mouse cornea. Investig Opthalmology Vis Sci. 2017; 58(1): 404. [DOI] [PubMed] [Google Scholar]
- 26. Eguchi H, Hiura A, Nakagawa H, Kusaka S, Shimomura Y.. Corneal nerve fiber structure, its role in corneal function, and its changes in corneal diseases. Biomed Res Int. 2017; 2017: 3242649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin T, Quellier D, Lamb J, et al.. Pseudomonas aeruginosa-induced nociceptor activation increases susceptibility to infection. PLoS Pathog. 2021; 17(5): e1009557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Gao N, Lee P, Yu FS.. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci Rep. 2016; 6: 36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niederkorn JY. Corneal nerves, CD11c+ dendritic cells and their impact on ocular immune privilege. Front Immunol. 2021; 12: 701935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiao H, Ivanusic JJ, McMenamin PG, Chinnery HR.. Distribution of corneal TRPV1 and its association with immune cells during homeostasis and injury. Investig Opthalmology Vis Sci. 2021; 62(9): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caterina MJ, Leffler A, Malmberg AB, et al.. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000; 288(5464): 306–313. [DOI] [PubMed] [Google Scholar]
- 32. Jeffry JA, Yu S-Q, Sikand P, Parihar A, Evans MS.. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009; 4(9): 7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raisinghani M, Pabbidi RM, Premkumar LS.. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J Physiol. 2005; 567: 771–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Datta A, Lee J, Truong T, Evans DJ, Fleiszig SMJ.. Topical antibiotics reduce CD11c+ cell numbers in the healthy murine cornea and modulate their response to contact lens wear. Sci Rep. 2022; 12(1): 10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Datta A, Truong T, Lee JH, et al. . Contact lens-induced corneal parainflammation involving Ly6G+ cell infiltration requires IL-17A and γδ T cells. Ocul Surf. 2023; 28: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caudle RM, Karai L, Mena N, et al.. Resiniferatoxin-induced loss of plasma membrane in vanilloid receptor expressing cells. Neurotoxicology. 2003; 24(6): 895–908. [DOI] [PubMed] [Google Scholar]
- 37. Pecze L, Pelsoczi P, Kecskés M, et al.. Resiniferatoxin mediated ablation of TRPV1+ neurons removes TRPA1 as well. Can J Neurol Sci. 2009; 36(2): 234–241. [DOI] [PubMed] [Google Scholar]
- 38. Schecterson LC, Pazevic AA, Yang R, Matulef K, Gordon SE.. TRPV1, TRPA1, and TRPM8 are expressed in axon terminals in the cornea: TRPV1 axons contain CGRP and secretogranin II; TRPA1 axons contain secretogranin 3. Mol Vis. 2020; 26: 576–587. [PMC free article] [PubMed] [Google Scholar]
- 39. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013; 29: 355–384. [DOI] [PubMed] [Google Scholar]
- 40. Seyed-Razavi Y, Chinnery HR, McMenamin PG.. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Investig Ophthalmol Vis Sci. 2014; 55: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 41. Wu M, Hill LJ, Downie LE, Chinnery HR.. Neuroimmune crosstalk in the cornea: the role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog Retin Eye Res. 2022; 91: 101105. [DOI] [PubMed] [Google Scholar]
- 42. Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008; 214(2): 149–160. [DOI] [PubMed] [Google Scholar]
- 43. McRitchie DI, Isowa N, Edelson JD, et al.. Production of tumour necrosis factor alpha by primary cultured rat alveolar epithelial cells. Cytokine. 2000; 12(6): 644–654. [DOI] [PubMed] [Google Scholar]
- 44. Fei M, Bhatia S, Oriss TB, et al.. TNF-α from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci USA. 2011; 108(13): 5360–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grivennikov SI, Tumanov A V, Liepinsh DJ, et al.. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005; 22(1): 93–104. [DOI] [PubMed] [Google Scholar]
- 46. Deguine J, Wei J, Barbalat R, Gronert K, Barton GM.. Local TNFR1 Signaling licenses murine neutrophils for increased TLR-dependent cytokine and eicosanoid production. J Immunol. 2017; 198: 2865–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE.. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013; 31: 73–106. [DOI] [PubMed] [Google Scholar]
- 48. Dinarello CA, Novick D, Kim S, Kaplanski G.. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013; 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sumioka T, Okada Y, Reinach PS, et al.. Impairment of corneal epithelial wound healing in a TRPV1-deficient mouse. Investig Opthalmology Vis Sci. 2014; 55(5): 3295. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Huang S, Yu R, et al.. TRPV1+ sensory nerves modulate corneal inflammation after epithelial abrasion via RAMP1 and SSTR5 signaling. Mucosal Immunol. 2022; 15(5): 867–881. [DOI] [PubMed] [Google Scholar]
- 51. St Leger AJ, Desai J V, Drummond RA, et al.. An ocular commensal protects against corneal infection by driving an Interleukin 17 response from mucosal γδ T cells HHS Public Access. Immunity. 2017; 47(1): 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Diogenes A, Ferraz CCR, Akopian AN, Henry MA, Hargreaves KM.. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 53. Chiu IM, Heesters BA, Ghasemlou N, et al.. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013; 501(7465): 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meseguer V, Alpizar YA, Luis E, et al.. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014; 5: 3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mailhot B, Christin M, Tessandier N, et al.. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med. 2020; 217: e20191430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.