Abstract
Aims
A standardized sedation protocol for pulsed-field ablation (PFA) of atrial fibrillation (AF) through irreversible cellular electroporation has not been well established. We report our experience of a protocol for deep sedation with ketamine in spontaneous respiration during the PFA of AF.
Methods and results
All consecutive patients undergoing PFA for AF at our center were included. Our sedation protocol involves the intravenous administration of fentanyl (1.5 mcg/kg) and midazolam (2 mg) at low doses before local anesthesia with lidocaine. A ketamine adjunct (1 mg/kg) was injected about 5 minutes before the first PFA delivery. We enrolled 66 patients (age = 59 ± 9 years, 78.8% males, body mass index = 28.8 ± 5 kg/m2, fluoroscopy time = 21[15–30] min, skin-to-skin time = 75[60–100] min and PFA LA dwell time = 25[22–28] min). By the end of the procedure, PVI had been achieved in all patients by means of PFA alone. The mean time under sedation was 56.4 ± 6 min, with 50 (76%) patients being sedated for less than 1 hour. A satisfactory Ramsey Sedation Scale level before ketamine infusion was achieved in all patients except one (78.8% of the patients with rank 3; 19.7% with rank 2). In all procedures, the satisfaction level was found to be acceptable by both the patient and the primary operator (Score = 0 in 98.5% of cases). All patients reported none or mild pain. No major procedure or anesthesia-related complications were reported.
Conclusion
Our standardized sedation protocol with the administration of drugs with rapid onset and pharmacological offset at low doses was safe and effective, with an optimal degree of patient and operator satisfaction.
Clinical trial registration
Advanced TecHnologies For SuccEssful AblatioN of AF in Clinical Practice (ATHENA). URL: http://clinicaltrials.gov/Identifier: NCT05617456.
Keywords: Atrial fibrillation, Pulsed-field ablation, Cellular electroporation, Sedation strategy, Anaesthesia, Ketamine
Graphical Abstract
Graphical Abstract.
What’s new?
In the treatment of atrial fibrillation (AF) by means of pulsed-field ablation (PFA), a standardized sedation protocol has not been defined yet.
A structured protocol for deep sedation with ketamine in spontaneous respiration in PFA of AF is presented, showing efficiency and optimization of mapping and ablation conditions.
Sedation drugs with rapid onset and pharmacological offset at low doses demonstrated safety and effectiveness, with an optimal degree of patient and operator satisfaction.
A new ablation technique, called pulsed-field ablation (PFA), is now arousing increasing interest in the field of cardiac tissue ablation. Recent experiences demonstrated that PFA was efficacious for pulmonary vein isolation (PVI) and expressed a safety profile consistent with preferential tissue ablation1 and high tissue selectivity.2 To date, however, when PFA is performed, no standardized sedation protocol has been well established.3–8 Here, we present our experience of a protocol of deep sedation with ketamine in spontaneous respiration during the PFA of atrial fibrillation (AF), with particular regard to the efficiency and optimization of mapping and ablation conditions. Sixty-six consecutive patients undergoing AF ablation with the FARAPULSE™ PFA system (Boston Scientific) at our centre from July 2022 to January 2023 were included. A standard PFA protocol was applied.9 By the end of the procedure, PVI had been achieved in all patients by means of FARAPULSE™ PFA alone (Table 1).
Table 1.
Clinical characteristics of the study population
| Parameter | n = 66 |
|---|---|
| Age, years | 59 ± 9 |
| Male gender, n (%) | 52 (78.8) |
| First-line therapy, n (%) | 17 (25.8) |
| Indication for ablation | |
| Paroxysmal AF, n (%) | 47 (71.2) |
| Early persistent AF, n (%) | 12 (18.2) |
| Long-standing persistent AF, n (%) | 7 (10.6) |
| History of atrial flutter/atrial tachycardia, n (%) | 3 (4.5) |
| Cardiomyopathy, n (%) | 6 (9.1) |
| Hypertension, n (%) | 43 (65.2) |
| Coronary artery disease, n (%) | 4 (6.1) |
| History of heart failure, n (%) | 1 (1.5) |
| Chronic obstructive pulmonary diseases, n (%) | 1 (1.5) |
| CKD, n (%) | 0 (0.0) |
| Sleep apnoea, n (%) | 3 (4.5) |
| Obesity | |
| BMI, kg/m2 | 28.8 ± 5 |
| Normal weight (stage 2, 18.5–24.9), n (%) | 14 (21.2) |
| Pre-obesity (stage 3, 25–29.9), n (%) | 26 (39.4) |
| Obesity (stage 4, ≥30.0), n (%) | 26 (39.4) |
| ASA Physical Status Classification ≥ 3 | 35 (53.0) |
| Beta-blockers, n (%) | 34 (51.5) |
| Antiarrhythmics, n (%) | 28 (42.4) |
AF, atrial fibrillation; ASA, American Society of Anesthesiologists; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
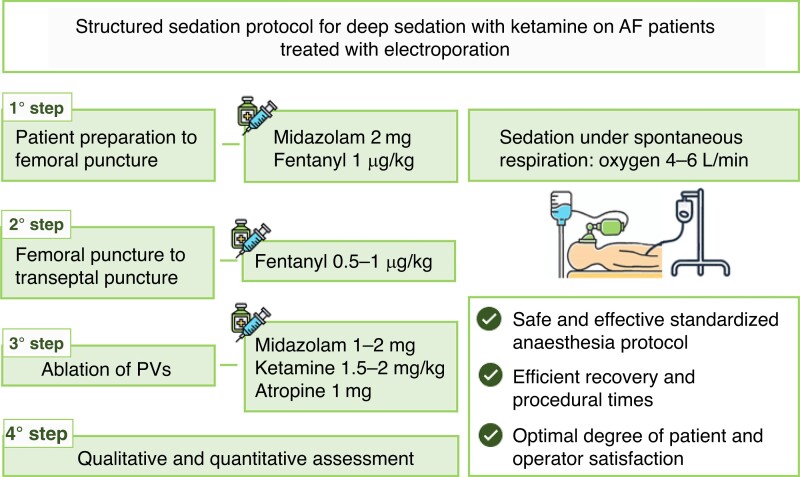
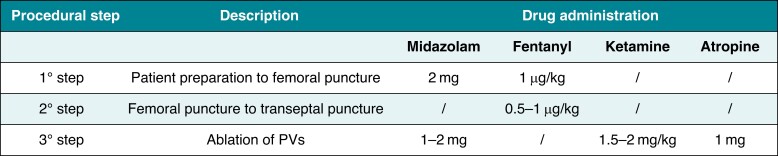
The anaesthesia procedure was managed by an anaesthetist and an operating room nurse. A four-step anaesthesia approach was implemented (Figure 1). In brief, our sedation protocol consists of the intravenous administration of midazolam (2 mg) and fentanyl (1.5 µg/kg) at low doses before local anaesthesia with lidocaine (200 mg). Patients underwent sedation under spontaneous respiration by administering oxygen (4–6 L/min) through a face mask or a nasal cannula during the whole procedure to ensure a safe spontaneous breathing sedation. To improve tolerance, the total dose of fentanyl was administered in small 5-min boluses, distributed between the first and second steps. Soon after the trans-septal puncture, heparin (1 mg/kg) and atropine (1 mg, to mitigate anticipated bradycardia) were injected, followed by a second bolus of midazolam (2 mg). The ketamine adjunct (1 mg/kg) was then injected 5 min before the first PFA delivery and was titrated to effect on the basis of patient’s condition, response, and changes in vital signs (e.g. SPO2, systolic and diastolic blood pressure, sign of pain, coughing, and heart rate). Once the effective anaesthetic level was reached, it was maintained by injecting 10 mg boluses of ketamine (Table 2).
Figure 1.
Sedation protocol.
Table 2.
Procedural and sedation parameters
| Parameter | n = 66 |
|---|---|
| Procedure type | |
| De novo, n (%) | 51 (77.3) |
| Repeated ablation, n (%) | 15 (12.7) |
| Lab occupancy time, min | 115 [100–140] |
| Skin-to-skin time, min | 75 [60–100] |
| Fluoroscopy time, min | 21 [15–30] |
| Mapping system used, n (%) | 5 (7.6) |
| PFA applications to reach PVI, n | 32 [32–33.5] |
| Time to achieve PVI, min | 25 [22–28] |
| First-pass isolation rate per vein, % | 100 |
| Complications during the procedure, n (%) | 0 |
| Midazolam, mg | 4 ± 0.6 |
| Fentanyl, µg | 116.4 ± 15 |
| Ketamine, mg | 116.1 ± 11 |
| Diastolic PA, mmHg | 76.8 ± 9 |
| Systolic PA, mmHg | 131.7 ± 16 |
| HR, b.p.m. | 74.0 ± 12 |
| Time under sedation, min | 56.4 ± 6 |
| Time taken to sedate the patients adequately and insert the ablation catheter (steps 1 and 2), min | 22.9 ± 3 min |
| Duration of ketamine infusion (step 3), min | 21.8 ± 5 min |
| Time to recover from drug infusion under acceptable sedation (i.e. time from the end of ablation to the achievement of a Ramsey score of 2–3), min | 11.7 ± 3 min |
HR, heart rate; PA, pulmonary artery pressure; PFA, pulsed field ablation; PVI, pulmonary vein isolation.
In all cases except one (n = 65, 98.5%), the targeted dosage of sedative drugs was successfully administered. The mean time under sedation was 56.4 ± 6 min, with 50 (76%) patients being sedated for less than 1 h. The mean time taken to sedate the patients adequately and insert the ablation catheter (steps 1 and 2) was 22.9 ± 3 min. A satisfactory level of sedation before ketamine infusion was achieved in all patients except one. After ketamine infusion, all patients (100%) exhibited a sluggish response. During the post-operative phase, recovery (Steward recovery score = 6)10 was obtained on admission to the post-anaesthesiological care unit (PACU) in 26 (39.4%) patients, within 5 min in 27 (40.9%). In all procedures, the satisfaction level was found to be acceptable by both the patient and the primary operator (score = 0 in 98.5% of cases). All patients reported none or mild pain. No major procedure or anaesthesia-related complications were reported. In one case (1.5%), we decided to change the sedation strategy during anaesthesia, owing to an adverse reaction (vomiting) after fentanyl administration. Continuous infusion of sedative medication (propofol) and inhalational anaesthetic (sevoflurane) was successfully applied.
This single-centre, prospective evaluation of deep sedation with intravenous ketamine in spontaneous respiration is the first to evaluate adult patients undergoing PFA of AF. The main findings of our study are as follows: (i) a standardized anaesthetic protocol with the administration of drugs with rapid onset and pharmacological offset at low doses proved effective and safe during AF ablation; (ii) up-titration of intravenous ketamine through extended dilution time (i.e. not in one-shot boluses) yielded safe and effective anaesthesia, with very efficient recovery times and procedural times in line with those of thermal energy-source ablation systems; and (iii) applying this protocol during AF ablation with the novel FARAPULSE™ PFA system resulted in an optimal degree of patient and operator satisfaction. There are few reports of the use of ketamine in electrophysiology, owing to its limited use in general and, to date, its absence during PFA procedures. We chose to use ketamine during our FARAPULSE™ PFA procedures as its pharmacological characteristics make it a potentially excellent choice, in that its adequate dosage and timing of administration reduce its adverse effects. In our experience, we did not observe severe hypoxaemia and no intubation was required in any patient. In only one case did we decide to adopt an alternative sedation strategy, owing to an adverse reaction that was not related to ketamine adjunction. After its intravenous administration, anaesthesia occurs within 60 s, with analgesic/dissociative coverage of 10–15 min. Our series included a number of potentially complex patients in terms of sedation management with multiple associated critical factors (asthma, chronic obstructive pulmonary diseases, obesity, and obstructive sleep apnea syndrome (OSAS)).11,12 However, the protocol with ketamine as the main inducer required only observation on the part of the anaesthetist, without supporting intervention in the PACU. The FARAPULSE™ PFA procedure was short and was safe and effective. In this context, our structured protocol may optimize laboratory utilization and procedural time without compromising safety. In addition, in most of the procedures, the level of satisfaction was found to be good by the primary operator, confirming the applicability of this approach in routine clinical practice, even in complex cases. All of these aspects—shorter procedure times, patient satisfaction, and shorter hospitalizations—are important considerations for hospitals and have significant financial implications.13
Our observations were limited to the ablation of AF by means of the novel FARAPULSE™ PFA system. We cannot therefore transfer our findings to other cardiac interventions or other PFA systems, and we are not powered to estimate the occurrence of serious adverse events as these are rare events during sedation for AF ablation. However, this observational prospective study may provide evidence that deep sedation with ketamine adjunction is safe and feasible.
In conclusion, our standardized sedation protocol with the administration of drugs with rapid onset and pharmacological offset at low doses in the context of cellular electroporation for AF ablation proved safe and effective, with an optimal degree of patient and operator satisfaction.
Contributor Information
Saverio Iacopino, Electrophysiology Unit, GVM Care&Research, Maria Cecilia Hospital, Cotignola, 48033 RA, Italy.
Jacopo Colella, Electrophysiology Unit, GVM Care&Research, Maria Cecilia Hospital, Cotignola, 48033 RA, Italy.
Daniele Dini, Electrophysiology Unit, GVM Care&Research, Maria Cecilia Hospital, Cotignola, 48033 RA, Italy.
Lorenzo Mantovani, Electrophysiology Unit, GVM Care&Research, Maria Cecilia Hospital, Cotignola, 48033 RA, Italy.
Paolo Francesco Sorrenti, Electrophysiology Unit, GVM Care&Research, Maria Cecilia Hospital, Cotignola, 48033 RA, Italy.
Maurizio Malacrida, Medical Education & Scientific Affairs, Boston Scientific, 20134, Milan, Italy.
Pasquale Filannino, Electrophysiology Unit, GVM Care&Research, Maria Cecilia Hospital, Cotignola, 48033 RA, Italy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner Aet al. . Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moshkovits Y, Grynberg D, Heller E, Maizels L, Europace ME. Differential effect of high-frequency electroporation on myocardium vs. non-myocardial tissues. Europace 2023;25:748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia R, Waldmann V, Vanduynhoven P, Nesti M, Jansen de Oliveira Figueiredo M, Narayanan Ket al. . Worldwide sedation strategies for atrial fibrillation ablation: current status and evolution over the last decade. Europace 2021;23:2039–45. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 5. Parameswaran R, Al-Kaisey A, Kalman J. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol 2021;18:210–25. [DOI] [PubMed] [Google Scholar]
- 6. Di Biase L, Conti S, Mohanty P, Bai R, Sanchez J, Walton Det al. . General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm 2011;8:368–72. [DOI] [PubMed] [Google Scholar]
- 7. Chikata A, Kato T, Yaegashi T, Sakagami S, Kato C, Saeki Tet al. . General anesthesia improves contact force and reduces gap formation in pulmonary vein isolation: a comparison with conscious sedation. Heart Vessels 2017;32:997–1005. [DOI] [PubMed] [Google Scholar]
- 8. Yokokawa M, Chugh A, Dubovoy A, Engoren M, Jongnarangsin K, Latchamsetty Ret al. . A comparison of clinical outcomes and cost of radiofrequency catheter ablation for atrial fibrillation with monitored anesthesia care versus general anesthesia. J Cardiovasc Electrophysiol 2022;33:1714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kueffer T, Baldinger SH, Servatius H, Madaffari A, Seiler J, Mühl Aet al. . Validation of a multipolar pulsed-field ablation catheter for endpoint assessment in pulmonary vein isolation procedures. Europace 2022;24:1248–55. [DOI] [PubMed] [Google Scholar]
- 10. Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J 1975;22:111–3. [DOI] [PubMed] [Google Scholar]
- 11. Stowe DF, Bosnjak ZJ, Kampine JP. Comparison of etomidate, ketamine, midazolam, propofol, and thiopental on function and metabolism of isolated 7 hearts. Anesth Analg 1992;74:547–58. [DOI] [PubMed] [Google Scholar]
- 12. Morris C, Perris A, Klein J. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia 2009;64:532–9. [DOI] [PubMed] [Google Scholar]
- 13. Osorio J, Rajendra A, Varley A, Henry R, Cunningham J, Spear Wet al. . General anesthesia during atrial fibrillation ablation: standardized protocol and experience. Pacing Clin Electrophysiol 2020;43:602–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.