Abstract
Animals exhibit behavioral changes during illness, including lethargy, anorexia, fever, adipsia, and anhedonia, which are believed to comprise an adaptive evolutionary strategy. Exploratory and social behaviors generally decrease during illness, but behavioral changes of dogs during illness have not been described. The objective of this study was to evaluate a novel canine behavior test during subclinical illness induced by dietary Fusarium mycotoxin. Twelve mature female beagle dogs received 3 treatment diets: a control diet (control), a diet formulated with grains contaminated with Fusarium mycotoxin (toxin), and the toxin diet together with a toxin binding agent (binder). All dogs received each diets for 14 d in a Latin square design with a 7-d washout period between diet trials. The test consisted of individually releasing dogs into the center aisle of the housing room for 4 min per day, during which interactions with familiar dogs in adjacent kennels were recorded by an observer outside the room who was blind to treatment groups. Total interactions, orientation, and attempted physical contact with other dogs were less frequent during the toxin and binder diet treatments. Conversely, frequencies of physical proximity and olfactory contact with familiar dogs in adjacent kennels were not associated with diet. In conclusion, induction of subclinical gastrointestinal illness influenced aspects of social interactions in beagle dogs. A clinical assessment sheet integrating these findings was developed to aid in early identification of subclinical illness in research dogs based on behavior.
Introduction
This study was initiated in response to a request from the authors’ institutional animal care and use committee to refine a clinical scoring protocol for use in canine challenge studies that are likely to cause malaise or sickness behavior. Specifically, we were invited to collaborate with another research team studying the ability of a nutritional intervention to mitigate mycotoxicosis associated with ingestion of Fusarium contaminated diets. Given the anticipated welfare impacts for the dogs on the primary study,20 a secondary study was performed concurrently to evaluate changes in canine exploratory motivation in response to mycotoxicosis. The secondary study (reported here) provided the opportunity to refine daily assessments, reevaluate criteria for humane endpoints, and examine the behavioral responses of these dogs in more detail.
Fusarium mycotoxins are found in temperate regions and can affect health and performance of domestic animals that ingest contaminated corn, wheat, and barley.19 A number of surveys of commercial cereal-based companion animal food have found varying levels of Fusarium mycotoxins.5,10,24 Deoxynivalenol (DON or vomitoxin) is known to induce emesis, feed refusal, gastrointestinal irritation,13 immunosuppression, and disruption of protein and DNA synthesis.23 Furthermore, the consumption of Fusarium mycotoxins can cause significant health effects, including reductions in canine blood pressure, heart rate, and serum concentrations of total protein, globulin, fibrinogen, alkaline phosphatase, and lipase.20 Mycotoxin research involving dogs to date has focused on clinical signs such as vomiting13 and anorexia,9 whereas relationships between DON and the social and exploratory motivations of singly housed research beagles has not been studied.
Sickness behaviors (for example, lethargy, anorexia, fever, adipsia, and anhedonia) arise when an animal is challenged by a pathogen that provokes a proinflammatory cytokine cascade. The change in behavior is the result of an adaptive strategy of a stimulated immune system to alter and facilitate the eradication of infection.12 Numerous animal species studied to date have demonstrated some of these sickness behavior elements, lending support to its existence as an evolutionarily beneficial strategy.17,21 The concept of sickness behavior as a motivational state suggests the expression of sickness behaviors such as anorexia are context dependent, influenced by both internal and external stimuli.1 Motivation is a central state that reorganizes perception and action through the integration of emotional and cognitive processes.6 The unconscious and systemic recognition of new relevant priorities allows animals to act in response to a serious stimulus, extinguish the threat, and revert to the expression of sickness behavior.1
Gastrointestinal conditions can be caused by Fusarium spp. and other dog food contaminants.4,27 Animals present with clinical signs that may include obvious signs of abdominal pain (that is, vocalization, restlessness, licking at or attempts to touch flank), emesis, nausea, large or small bowel diarrhea, anorexia, and lethargy.11 Despite this wealth of clinical signs during overt illness, little research has specifically focused on the effect of gastrointestinal conditions on canine behavior and its use for early detection of compromised welfare. Because dogs are a highly social species and often seek social interactions, there may be an opportunity to gain insight into canine health and welfare by monitoring changes in behavior in order to refine their care during experimental use.
The purpose of this study was to evaluate a behavioral test for sickness behavior associated with Fusarium mycotoxin ingestion in dogs. We hypothesized that the ingestion of mycotoxins would be associated with reduced exploratory and social motivation of dogs.
Materials and Methods
Ethical approval for all experimental procedures was provided by the Animal Care Committee, University of Guelph under protocol 03R021. The facility is compliant with the Animals for Research Act of Ontario and holds a Good Animal Practice certificate issued by the Canadian Council on Animal Care.
Study design.
Our study was designed around the other research team’s experimental design and was expected to provide descriptive observations about changes in dog behavior. Variables of interest (sample size calculation, diet composition, toxin levels, diet palatability, food consumption, weight loss, and physiologic parameters) and additional study design details have been reported previously.20 Dogs were treated as the experimental units. All dogs received all treatments, were randomly assigned to one of 3 treatment diets in the first round of testing, and were systematically assigned to the remaining diet treatment according to a 3 × 3 Latin square design. Diets were fed for 14 d with a 7-d washout phase between diets. A pilot study found beagles fed DON contaminated food recovered from a 5% weight loss in 5 d.18 All dogs received the control diet during the washout phase.
Animals and husbandry.
Twelve female beagle dogs (Marshall BioResources, North Rose, NY) from the University of Guelph dog quarantine facility were enrolled in the study. All dogs had been previously enrolled in studies, but the number and topic were unknown. Under the supervision of a veterinarian, dogs underwent monthly parasitic screening and were deemed free of internal and external parasites. The dogs received yearly vaccinations for canine distemper, parvovirus, adenovirus 2, Bordetella, and rabies virus. Their mean ± SD age was 2.8 ± 1.6 y, and body weights were 10.1 ± 1.1 kg. Dogs were individually housed in kennels (1.21 m × 1.89 m) with raised plastic-coated wire mesh floors. Individual housing was required to measure consumption of the diets. Kennel walls were stainless steel with a solid lower section (0.81 m) and an upper section (0.90 m) that consisted of vertical bars (0.07 m apart). Resting boards (1.11 m × 0.51 m) were situated 0.21 m above the raised flooring at the rear of each kennel and permitted nose-to-nose contacts between dogs in adjacent kennels. The room was configured with 6 kennels on either side of a center aisle (1.41 m wide), with gutters (0.29 m wide, 0.05 m deep) directly in front of the pens (Figure 1). The room temperature ranged from 18 to 21 °C, with a relative humidity of 40% to 70% and a 12:12-h diurnal period (lights on, 0645).
Figure 1.
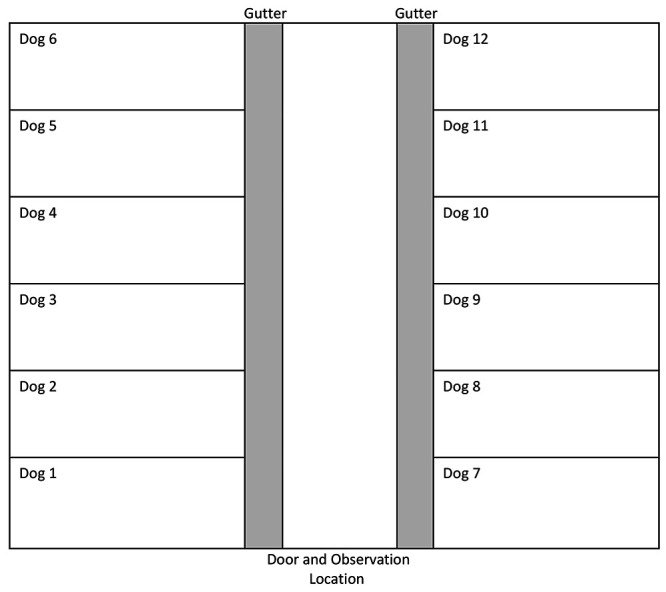
Configuration of housing and testing room for 4-min observation period of 12 female beagle dogs.
Dogs had ad-libitum access to water using 2 water bowls that were refreshed daily. Each dog received 600 g of the assigned diet once daily and received a 20-min leash walk outside or inside the facility 5 d per week. On days 0, 5, 10, and 15 (0930 to 1230) dogs were assessed for body weight, nutrient digestibility, and physical and clinicopathologic variables.20 At the end of the study dogs returned to a general holding protocol for use in additional projects before being retired and adopted.
Behavioral test.
A standardized behavioral test was created for practical use in the research facility. Negative affect associated with sickness includes feelings of pain, malaise, lethargy, and thermal discomfort, with concomitant reduction in motivation for exploration, foraging, and social interaction.1 Consequently, we hypothesized that singly housed dogs would prioritize isolation and rest during periods of malaise, forfeiting opportunities to explore and interact with other dogs in the kennel area. The familiar home kennel environment was used to control for potential confounding effects of fear and anxiety associated with novel environments and unfamiliar dogs.
Behavioral tests were conducted once daily, 5 d per week during the 2 wk that the dogs were fed the treatment diets. Tests were scheduled after lights came on (0645) and before routine husbandry procedures were performed (0830). The test began when the technician entered the room, opened the kennel door for one focal dog, and exited the room to observe the dog through a glass window in the external door. The dog had a 4-min opportunity to leave its kennel, explore the center aisle of the room, and interact with 11 familiar dogs through kennel gates. The test duration of 4 min was selected for practical reasons because it allowed all dogs to be released, observed, and returned to their pens before daily husbandry began. Dogs were released in a random sequence determined by a random number generator. All dogs in the housing room were enrolled in the trial.
One technician conducted all observations and was blind to experimental treatments. Frequencies of exploratory and social behaviors were recorded for the focal dog. Ethogram elements corresponded to common and easily observable dog behaviors (Table 1). An interaction was categorized as any uninterrupted sequence of behavioral elements directed toward a particular stimulus dog. Multiple behavioral elements could be observed and recorded during a single interaction. An interaction was considered to have concluded when the focal dog changed orientation or moved out of the proximity of the stimulus dog. Focal dog behaviors not directed toward or in proximity to a stimulus dog were not recorded. A dog was categorized as a non-responder if 2 or more minutes of the 4-min test were spent in its home pen.
Table 1.
Ethogram for behavioral elements measured during each 4-min observation period of 11 female beagle dogs1
| Behavior | Description |
|---|---|
| Orientation | Focal dog’s head is directed toward a stimulus dog’s pen |
| Physical Proximity | Focal dog is standing with 2 paws in the gutter area immediately proximate (≤ 29 cm) to the gate of a stimulus dog’s kennel. |
| Olfactory Contact | Focal dog is standing with 2 paws in the gutter area immediately proximate (< 29 cm) to the gate of a stimulus dog’s kennel, and focal dog’s nose in ≤ 5 cm above the floor |
| Attempted Physical Contact | Focal dog’s nose touches or passes between the bars of a stimulus dog’s kennel gate |
| Arousal2 | Focal dog wags its tail laterally |
One interaction is any uninterrupted sequence of behaviors directed toward the same stimulus dog
Arousal was only measured in conjunction with other behavioral elements
Statistical procedure.
The frequencies of behavioral elements were calculated as the total number of observed acts per 4-min observation period. Due to the small number of non-responders, data collected during these periods were included. Initially the data was characterized with PROC UNIVARIATE in SAS (version 9.04; SAS Institute, Cary, NC). The normality of distribution for the response variables were visually scrutinized through the inspection of histograms. Residuals from all data were normalized by taking the natural logarithm of the means (log-link).
Data were analyzed using a generalized linear mixed model (GLMM), employing PROC GLIMMIX in SAS (version 9.04; SAS Institute). Within the framework of the GLMM, 2 models were used to examine the data. The means model was used to interpret data within and between days and employed a standard factorial by fitting Treatment, Day, and the Treatment × Day interaction as fixed effects. The linear regression in 3 treatments was used to interpret the data over time and included Treatment, Day, and Day × Day interaction as fixed effects. Both models included Treatment Period and Focal Dog as random effects. The GLMM accounted for potential autocorrelation between repeated measures because each dog had 12 observations per treatment by incorporating an autocorrelation structure. The autocorrelation structure that resulted in an Akaike Information Criterion (AIC) closest to zero was considered to be the best model. Initially all effects were included and factors that were not significant effects were systematically removed to produce the final linear model. Dog age and dog weight were not significant factors and were not included as effects in the final model.
To ensure that possible outliers were identified and model assumptions were met, residual plots of predicted against observed values were visually scrutinized. Outliers with residuals exceeding 3 standard deviations were excluded from the model, which resulted in removal of one observation from the visual contact model and 2 different observations from the olfactory contact model. The models did not converge for arousal; therefore, descriptive statistics are provided for arousal, and treatment effects were not assessed. The approximate power for the experiment to find an effect if one was present was 0.9, as determined by a calculation using a large sample approximation.
Results
A data deletion error caused loss of behavioral data for 1 dog across all treatments, such that data of 11 of the 12 dogs was available for analysis.
Dogs were easily observed, eagerly left their pens, and would briskly walk the length of the entire housing room before stopping in front of individual pens. While most dogs eagerly left their pens during all observation periods, 2 of 11 dogs were classified as non-responders on 8 separate occasions over the 30 tests performed. Non-responders represented 3.5% and 2% of the toxin and binder diet observations respectively. None of the dogs were non-responders during the control treatment.
During all 330 tests, the focal dogs performed 14.3 ± 0.3 (mean ± SE) total interactions with neighboring dogs in the room. Total interactions differed by treatment, occurring less frequently during toxin and binder diets (P < 0.01, Table 2). Similarly, orientation occurred 1.2 ± 0.1 times during all tests and occurred less frequently during toxin and binder diets (P = 0.01). Physical contact was attempted 5.5 ± 0.2 times during all tests and occurred less frequently during toxin and binder diets (P < 0.01).
Table 2.
Least squares mean frequency (± 95% confidence interval) of behavioral elements during a 4-min observation period for 11 female beagle dogs on treatment diets. LL = lower confidence limit. UL = upper confidence limit
| Behavioral Act | Control | Toxin1 | Binder2 | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower 95% CI | Mean | Upper 95% CI | Lower 95% CI | Mean | Upper 95% CI | Lower 95% CI | Mean | Upper 95% CI | ||
| Interactions3 | 12.7 | 15.6 | 19.1 | 10.3 | 12.7 | 15.6 | 10.9 | 13.3 | 16.4 | < 0.01 |
| Orientation4 | 0.7 | 1.2 | 2.1 | 0.3 | 0.6 | 1.2 | 0.6 | 1.1 | 1.9 | 0.01 |
| Physical Proximity5 | 7.8 | 10.3 | 13.8 | 6.3 | 8.5 | 11.3 | 6.5 | 8.7 | 11.6 | 0.07 |
| Olfactory Contact6 | 1.0 | 2.2 | 4.9 | 1.1 | 2.4 | 5.2 | 1.4 | 2.9 | 6.4 | 0.07 |
| Attempted Physical Contact7 | 5.2 | 6.7 | 8.6 | 3.6 | 4.7 | 6.1 | 3.3 | 1.4 | 5.7 | < 0.01 |
Diet was prepared with gains known to be naturally contaminated with Fusarium mycotoxins
Diet was prepared with gains known to be naturally contaminated with Fusarium mycotoxins, with the addition of 0.2% glucomannan mycotoxin absorbent
One interaction by the focal dog is defined as any uninterrupted sequence of behaviors with the same stimulus dog
Focal dog’s head is directed toward stimulus dog’s kennel gate
Focal dog is standing with 2 paws is gutter ≤ 29 cm proximate to the front of a stimulus dog’s kennel gate
Focal dog is standing with 2 paws is gutter ≤ 29 cm proximate to the front of a stimulus dog’s kennel gate with its dog’s nose ≤ 5 cm above the floor
Focal dog’s nose is touching or passed between door bars of a stimulus dog’s kennel gate
Physical proximity and olfactory contact did not differ based on diet and occurred 10.0 ± 0.3 and 3.1 ± 0.2 times over all tests, respectively. The diet being consumed by stimulus dogs did not significantly influence focal dog behavior in this study. Dogs wagged their tails an average of 4.1 ± 0.3 times in conjunction with other behavioral elements; tail wagging was not analyzed statistically. Aggressive behavior was not noted during these interactions.
Parameters of the model as predictors of occurrence are presented in Table 3.
Table 3.
Parameters as predictors for occurrences of interactions, visual contact, and attempted physical contact during a 4-min observation period of 11 female beagle dogs
| Interactions1 | Orientation2 | Attempted Physical Contact3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Beta4 | Lower 95% CI | Upper 95% CI | P | Beta4 | Lower 95% CI | Upper 95% CI | P | Beta4 | Lower 95% CI | Upper 95% CI | P |
| Treatment Diet | < 0.01 | 0.03 | < 0.01 | |||||||||
| Control | Reference | — | — | — | Reference | — | — | — | Reference | — | — | — |
| Toxin 5 | 0.204 | 0.082 | 0.326 | < 0.01 | 0.547 | 0.141 | 0.952 | < 0.01 | 0.352 | 0.149 | 0.554 | < 0.01 |
| Binder 6 | 0.155 | 0.034 | 0.276 | 0.01 | 0.124 | −0.239 | 0.487 | 0.53 | 0.441 | 0.231 | 0.651 | < 0.01 |
| Day7 | 0.06 | < 0.01 | 0.02 | |||||||||
| Day × Day8 | 0.05 | < 0.01 | 0.06 | |||||||||
One focal dog interaction is any uninterrupted sequence of behaviors to a specific stimulus dog
Focal dog’s head is directed toward stimulus dog’s kennel gate
Focal dog’s nose is touching or passed between door bars of a stimulus dog’s kennel gate
Beta = fixed effect estimate.
Diet was prepared with gains known to be naturally contaminated with Fusarium mycotoxins
Diet was prepared with gains known to be naturally contaminated with Fusarium mycotoxins with added 0.2% glucomannan mycotoxin absorbent
Observation days over 14-d treatment period
Quadratic effect
A dog was less likely to engage in an interaction, orientation, or attempt to make physical contact with neighboring dogs when consuming the toxin diet. Dogs fed the binder diet were also less likely to show interactions and attempts at physical contact. The number of days on a treatment diet was a significant predictor of orientation and attempts to physically contact neighboring dogs. A concave quadratic effect was also significant for total interactions and orientation. Treatment diet and number of days on treatment diet were not significant predictors of physical proximity or olfactory contact.
Discussion
The objective of this study was to evaluate behavior test for identifying sickness motivation or malaise in dogs fed mycotoxins. At the onset of the study, we did not know how the dogs would react to the contaminated diets or whether they would develop clinical signs of illness. The dogs readily ate the control diet; after eating the contaminated diets for 14 d, their food intakes had decreased by 35%, they lost 5% of body weight, and they showed significant changes in several clinicopathological variables.20 These findings and the fact that 2 dogs did not leave their pens during several observation periods while consuming the DON contaminated diets suggest that these dogs were experiencing some degree of illness. Outside of the observation periods, one dog vomited twice while consuming the contaminated diets, but none of the dogs were removed from the study for medical and/or ethical concerns.
We successfully created and applied a 4-min test for all dogs enrolled in the study. A variety of behavior tests have been created for evaluating the suitability of dogs for rehoming,2,8,25 behavioral treatment,26 and genetic selection.22 Many of these tests occur in a novel arena and/or introduce stimuli into the dog’s home kennel or cage, with particular emphasis on outcomes associated with fear and anxiety responses. Conversely, our goal was to minimize fear and anxiety during the test itself, so as to titrate within-dog changes in sickness vs exploratory and social motivations. In a familiar environment, among familiar conspecifics, and in the absence of competition for resources, we hypothesized that dogs would be highly motivated to explore and interact with other dogs when given the opportunity. Indeed, all dogs participated actively during the control treatment. We hypothesized that malaise and lethargy would result in dogs fed the toxin diets, and that dogs would then be unwilling or unable to engage in these behaviors. However, only 2.75% of these tests resulted in non-response from only two dogs and associated with the dog that vomited when on the toxin and binder treatments. Therefore, the test was not sensitive enough to answer the binary question of whether or not a dog was experiencing subacute illness. We do not know whether malaise increases motivation for contact among closely bonded individuals, and we did not have information about prior affiliative and/or agonistic interactions between our dogs.
Attempted physical contacts and orientation were significantly lower on both mycotoxin-containing diets. Dogs were not forced into social situations in the current study, and social interactions were among familiar kennel mates. The focal dog was free to leave its kennel to initiate these interactions, which could be reciprocated by the stimulus dog through the kennel door bars. While our study did not detect a significant influence of stimulus dog diet on focal dog behavior, other research has demonstrated that female CD-1 mice use odor cues to avoid individuals infected with pathogens.14–16 Research that specifically determines whether dogs modify their social interactions due to the degree of sickness of a conspecific is encouraged.
Treatment duration was a significant predictor of decreases in orientation and attempted physical contact. These findings are consistent with a previous study in which other indicators of illness worsened after 14 d of consuming the contaminated diets.20 However, a significant quadratic effect of day for orientation and total interactions complicates the identification of a clear pattern. One possibility is that dogs were tested Monday through Friday and descriptively there appeared to be a rebound effect on the first Monday (Day 4) as dogs across all treatments demonstrated 6.8% more interactions on Mondays as compared with other days. However, a rebound effect was not evident on the second Monday (Day 11). Due to these different effects, strong conclusions concerning the day of the week are not possible for these behavioral elements. We encourage additional research to examine optimal test frequency, either as incorporated into daily husbandry or as an ad hoc diagnostic tool.
A limitation in the current study that could affect the motivation to explore was hunger. Diets containing mycotoxins have significantly reduce food intake and cause gastrointestinal distress in dogs.13,20 During the daily observational test, some focal dogs tried to retrieve control (non–mycotoxin-contaminated) diet from under the pens of neighboring stimulus dogs; this behavior observationally specific to some dogs during the toxin and binder diets. Conducting the test after, rather than before daily husbandry, may have alleviated some of this behavior but also could have affected focal dog exploratory and social motivations. A priori, we determined that olfactory social information would have been lost if the room had been cleaned before testing.
This current study focused on the effects of presumed subclinical illness on dog behavior. However, additional research is needed to explore any relationship between illness and specific personality traits or existing conspecific relationships. We used intact female beagle dogs, and although none of them displayed visible signs of estrous, an undetected estrous cycle could also have affected social and exploratory behaviors. Future research specifically addressing this possibility is encouraged. The current study focused on one breed of dog in a research environment, and generalizability of our findings to other breeds and environments (for example, companion dogs), should be conducted.
Behavior is perhaps the best indicator of animal welfare.7 Several validated quality of life surveys are available for dogs in clinical practice settings,3 however, a standardized protocol is currently not available for assessment of the welfare of dogs used in research. The current study was not intended to perform a comprehensive animal welfare test. However, extrapolating changes in exploratory and social behavior from this study could allow better assessment of subclinical illness in research dogs in noninfectious studies with test articles expected to produce illness or with unknown effects. Based on our findings, we have developed a modified clinical assessment sheet (Figure 2). Although our proposed assessment sheet should be validated by users, it does incorporate the most informative components of our results. Members of research teams can monitor research beagles for changes in willingness to interact, play, or look at neighboring dogs as an early indicator of disease. The use of these findings and the assessment sheet could represent a refinement in the care of research dogs by identifying reduced or declining welfare as expressed through changes in assertive social behavior.
Figure 2.
Dog clinical assessment sheet including social behavior parameters as early indicator of disease.
In conclusion, mycotoxin-induced gastrointestinal illness in dogs caused significant reductions in orientation towards and attempted social contact between familiar dogs in a familiar kennel room. The use and continued refinement of our clinical assessment sheet is encouraged as a means to identify early subclinical illness and minimize negative welfare outcomes for research dogs. Although statistically significant, the changes in behavior were subtle and provide a basis for developing refining technologies and algorithms used to identify subclinical illness in group-housed dogs.
Acknowledgments
We wish to acknowledge the contributions of the 12 beagles enrolled in our study. We recognize Dr Trevor Smith and his lab team for their willingness for us to run our research project in conjunction with their study. We are also grateful to the hard work and insightful observations from Dr Janet Cutler for her assistance in data collection. We also recognize the Natural Science and Engineering Research Council of Canada, the Canadian Foundation for Innovation, and Alltech for funding support.
References
- 1.Aubert A. 1999. Sickness and behavior in animals: A motivational perspective. Neurosci Biobehav Rev 23:1029–1036. 10.1016/S0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 2.Barnard S, Flint H, Shreyer T, Croney C. 2021. Evaluation of an easy-to-use protocol for assessing behaviors of dogs retiring from commercial breeding kennels. PLoS One 16:e0255883. 10.1371/journal.pone.0255883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belshaw Z, Asher L, Harvey ND, Dean RS. 2015. Quality of life assessment in domestic dogs: An evidence-based rapid review. Vet J 206:203–212. 10.1016/j.tvjl.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff K, Rumbeiha WK. 2012. Pet food recalls and pet food contaminants in small animals. Vet Clin North Am Small Anim Pract 42:237–250. 10.1016/j.cvsm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Boermans HJ, Leung MCK. 2007. Mycotoxins and the pet food industry: Toxicological evidence and risk assessment. Int J Food Microbiol 119:95–102. 10.1016/j.ijfoodmicro.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 6.Bolles RC. 1975. Theory of motivation. 2nd ed. New York (NY): Harper & Row. [Google Scholar]
- 7.Broom DM, Johnson KG. 1993. Stress and animal welfare. London: Chapman & Hall. 10.1007/978-94-024-0980-2 [DOI] [Google Scholar]
- 8.Döring D, Nick O, Bauer A, Küchenhoff H, Erhard MH. 2017. How do rehomed laboratory beagles behave in everyday situations? Results from an observational test and a survey of new owners. PLoS One 12:e0181303. 10.1371/journal.pone.0181303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flannery BM, Clark ES, Pestka JJ. 2012. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol Sci 130:289–297. 10.1093/toxsci/kfs255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzotti T, Biagi G, Pagliuca G, Pinna C, Scardilli M, Grandi M, Zaghini G. 2015. Occurrence of mycotoxins in extruded commercial dog food. Anim Feed Sci Technol 202:81–89. 10.1016/j.anifeedsci.2015.02.004. [DOI] [Google Scholar]
- 11.Hall EJ, Day MJ. 2017. Diseases of the small intestine, p 1516–1564. In: Ettinger SJ, Feldman EC, Cote E, editors. Textbook of veterinary and internal medicine. 8th edition. Philadelphia (PA): Elsevier Health Sciences. [Google Scholar]
- 12.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137. 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 13.Hughes DM, Gahl MJ, Graham CH, Grieb SL. 1999. Overt signs of toxicity to dogs and cats of dietary deoxynivalenol. J Anim Sci 77:693–700. 10.2527/1999.773693x. [DOI] [PubMed] [Google Scholar]
- 14.Kavaliers M, Choleris E. 2011. Sociality, pathogen avoidance, and the neuropeptides oxytocin and arginine vasopressin. Psychol Sci 22:1367–1374. 10.1177/0956797611420576. [DOI] [PubMed] [Google Scholar]
- 15.Kavaliers M, Choleris E, Ågmo A, Pfaff DW. 2004. Olfactory-mediated parasite recognition and avoidance: Linking genes to behavior. Horm Behav 46:272–283. 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Kavaliers M, Choleris E, Pfaff DW. 2005. Genes, odors and the recognition of parasitized individuals by rodents. Trends Parasitol 21:423–429. 10.1016/j.pt.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Kelley KW, Bluthé R-M, Dantzer R, Zhou J-H, Shen W-H, Johnson RW, Broussard SR. 2003. Cytokine-induced sickness behavior. Brain Behav Immun 17:112–118. 10.1016/S0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 18.Leung MCK. 2007. Physiological and proteomics effects of Fusarium mycotoxins in mature Beagles, MSc. Guelph (ON): University of Guelph. [Google Scholar]
- 19.Leung MCK, Díaz-Llano G, Smith TK. 2006. Mycotoxins in pet food: A review on worldwide prevalence and preventative strategies. J Agric Food Chem 54:9623–9635. 10.1021/jf062363+. [DOI] [PubMed] [Google Scholar]
- 20.Leung MCK, Smith TK, Karrow NA, Boermans HJ. 2007. Effects of foodborne Fusarium mycotoxins with and without a polymeric glucomannan mycotoxin adsorbent on food intake and nutrient digestibility, body weight, and physical and clinicopathologic variables of mature dogs. Am J Vet Res 68:1122–1129. 10.2460/ajvr.68.10.1122. [DOI] [PubMed] [Google Scholar]
- 21.Lopes PC, French SS, Woodhams DC, Binning SA. 2021. Sickness behaviors across vertebrate taxa: Proximate and ultimate mechanisms. J Exp Biol 224:jeb225847. 10.1242/jeb.225847. [DOI] [PubMed] [Google Scholar]
- 22.Overall KL, Hamilton SP, Chang ML. 2006. Understanding the genetic basis of canine anxiety: Phenotyping dogs for behavioral, neurochemical, and genetic assessment. J Vet Behav 1:124–141. 10.1016/j.jveb.2006.09.004. [DOI] [Google Scholar]
- 23.Pestka JJ, Bondy GS. 1990. Alteration of immune function following dietary mycotoxin exposure. Can J Physiol Pharmacol 68:1009–1016. 10.1139/y90-154. [DOI] [PubMed] [Google Scholar]
- 24.Shao M, Li L, Gu Z, Yao M, Xu D, Fan W, Yan L, Song S. 2018. Mycotoxins in commercial dry pet food in China. Food Addit Contam Part B Surveill 11:237–245. 10.1080/19393210.2018.1475425. [DOI] [PubMed] [Google Scholar]
- 25.Stella J, Shreyer T, Ha J, Croney C. 2019. Improving canine welfare in commercial breeding (CB) operations: Evaluating rehoming candidates. Appl Anim Behav Sci 220:104861. 10.1016/j.applanim.2019.104861. [DOI] [Google Scholar]
- 26.Stellato AC, Flint HE, Widowski TM, Serpell JA, Niel L. 2017. Assessment of fear-related behaviors displayed by companion dogs (Canis familiaris) in response to social and non-social stimuli. Appl Anim Behav Sci 188:84–90. 10.1016/j.applanim.2016.12.007. [DOI] [Google Scholar]
- 27.Witaszak N, Stępień Ł, Bocianowski J, Waśkiewicz A. 2019. Fusarium species and mycotoxins contaminating veterinary diets for dogs and cats. Microorganisms 7:26. 10.3390/microorganisms7010026. [DOI] [PMC free article] [PubMed] [Google Scholar]



