Abstract
Background and objective
Cellular analysis of bronchoalveolar lavage (BAL) fluid may aid diagnosis in patients with undifferentiated interstitial lung disease (ILD). The utility of this test in the diagnostic process in conjunction with a multidisciplinary discussion (MDD) is not known. We aim to assess and compare interobserver agreement and diagnostic confidence before and after presenting BAL results in an ILD-MDD.
Methods
Patients undergoing investigations for ILD at Waikato Hospital were recruited. At the ILD-MDD two respiratory physicians and one respiratory radiologist participated in the discussion, and their diagnosis and diagnostic confidence were assessed at four sequential time points. Assessors were blinded to each others diagnosis and diagnostic confidence scores. The four sequential time points were (1) after clinical and radiology presentation; (2) after subsequent MDD; (3) after reviewing BAL results; (4) after final MDD with all results. Interobserver agreements were calculated using Fleiss κ statistic.
Results
36 patients were recruited, and 77.8% were male. In the first step, the interobserver agreement was substantial κ = 0.622 (95% CI 0.47–0.77), improving in step 2 following MDD to κ = 0.78 (95% CI 0.624–0.935), in step 3 κ = 0.776 (95% CI 0.614–0.937) and step 4 achieved almost perfect agreement of κ = 0.969 (95% CI 0.828-1.11). The diagnostic confidence for individual and group diagnosis increased with the presentation of BAL with and without multidisciplinary MDD.
Conclusion
We found that BAL cellular analysis improves interobserver agreement and confidence in diagnosis following MDD, thus aiding decision-making in cases with undifferentiated ILD.
Keywords: Multidisciplinary discussion, interstitial lung disease, decision making, bronchoalveolar lavage
Introduction
Interstitial lung disease (ILD) encompasses a diverse group of conditions that distort the lung interstitium with varying degrees of inflammation and fibrosis. Although they frequently display similar clinical, radiological and histological features, each form of ILD may have a distinctly different aetiology, natural history, response to treatment and burden upon the individual's life.1,2 An accurate diagnosis must be made so management can be tailored accordingly.3–5
Bronchoalveolar lavage (BAL) can play a role in making an accurate and confident diagnosis of certain forms of ILD. When used in conjunction with comprehensive clinical assessment, lung function testing, blood tests and imaging, BAL cellular analysis may provide additional diagnostic information that could obviate the need for high risk procedures such as surgical lung biopsy or cryobiopsy. Additionally, BAL may identify confounding conditions such as infection and malignancy.6–8
Current guidelines for idiopathic pulmonary fibrosis (IPF) and hypersensitivitiy pneumonitis (HP) recommend diagnoses be made following multidisciplinary discussion (MDD) with relevant investigations based on clinical evaluation, pulmonary function tests, and thoracic high resolution computed tomography (HRCT).9,10 Current guidelines for IPF recommend cellular analysis of BAL fluid for patients with newly detected ILD of apparently unknown cause who are clinically suspected of having IPF with an HRCT pattern of probable usual interstitial pneumonia (UIP), indeterminate for UIP, or an alternative diagnosis.11–19 However, this is a conditional recommendation based on very low-quality evidence. 9 Current guidelines for HP also recommend BAL with lymphocyte cellular analysis for newly identified ILD, whose differential diagnosis includes HP. These recommendations are made with very low confidence. 10
Both guidelines for IPF and HP also note the need to study the utility of BAL in the diagnostic process as a research priority going forward. No study has assessed the utility of BAL in conjunction with MDD in the diagnosis of undifferentiated ILD. Our study aimed to assess if BAL cellular analysis in conjunction with MDD improves inter-observer agreement and diagnostic confidence in the diagnosis of undifferentiated ILD.
Methods
Study design
This single-centre prospective observational study was conducted between 2019 and March 2021 at Waikato Hospital, a tertiary referral centre covering the middle third of North Island in New Zealand. The study was approved by the Waikato Hospital Clinical Audit and Support Unit. The study was deemed a low risk of breaching patient confidentiality, as bronchoscopy and BAL was part of standard practice at our institution and patient consent was being obtained. Hence, a formal Health and Disability Ethics Committee (HDEC) approval was not required.
Patient selection
Patients older than 18 years with a radiologically or clinically suspected ILD at Waikato Hospital who were referred for BAL as part of the investigations deemed indicated by the treating clinician were considered eligible for inclusion. Recruitment occurred, and written consent was obtained prior to bronchoscopy and BAL fluid collection. BAL results were not disclosed until the time of discussion at step 3 of the MDD to ensure discussion participants remained blinded. Patients were excluded from the study if BAL results needed to be released prior to MDD due to clinical urgency. Respiratory physicians and speciality trainees at Waikato Hospital performed bronchoscopies with BAL collected and processed per guidelines. 7
Data collection
Before ILD MDD, all patients underwent clinical review (occupational history, organic-inorganic exposure, medications review), thoracic high resolution computed tomography (HRCT), lung function testing and blood evaluation for an underlying connective tissue disease or hypersensitivity reaction (antibodies, serum specific IgG). Referring clinicians also recorded a suspected diagnosis for the MDD at time of referral. The MDD consists of all respiratory physicians in the department, ILD clinical nurse specialists, a histopathologist, and a specialist radiologist with expertise in thoracic radiology. All discussion and diagnosis by each clinician or radiologist for the purpose of this study were performed within a single MDD session.
Assessment occurred at four sequential time points during the MDD as outlined below;
Step 1: Clinical data and HRCT, were reviewed without discussion.
Step 2: Clinical data, and HRCT were reviewed followed by a group discussion
Step 3: Clinical data, HRCT, and BAL results reviewed without discussion
Step 4: Clinical data, HRCT, BAL results reviewed followed by a group discussion.
Clinicians and radiologist were given a tabulated guide to interpreting BAL cellular analysis adapted from the recent American Thoracic Society clinical practice guideline. 7 At the end of all steps, participating clinicians (two respiratory physicians) and one chest radiologist recorded their diagnostic impressions and confidence on a dedicated pre-coded sheet. It contained diagnostic categories as per the Australasian ILD Registry and confidence categories as follows: 1 = Confident >90%, 2 = Provisional, High Confidence 70%–89%, 3 = Possible, low confidence 51%–69%, 4 = Unclassifiable ILD <50%.
The final diagnoses were categorised into predominantly fibrotic (Idiopathic pulmonary fibrosis, chronic Hypersensitivity pneumonia, Non-specific interstitial pneumonia, Cryptogenic organsising pneumonia, Combined pulmonary fibrosis and emphysema) predominantly inflammatory (acute Hypersensitivity pneumonia, drug-related, Idiopathic pulmonary fibrosis with autoimmune features) and unclassifiable for final analysis.
Statistical methods
Baseline statistics are presented as number and percentage for categorical variables and mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, as appropriate. A Fleiss κ statistic assessed intra- and interrater agreement in diagnosis. Fleiss κ scores were interpreted as almost perfect agreement (above 0.8), substantial agreement (scores between 0.6 and 0.8), moderate agreement (scores between 0.4 and 0.6), fair agreement (scores between 0.2 and 0.4), slight agreement (scores between 0.0 and 0.2), and poor agreement (scores below 0.0).20,21 A recorded confidence level of 1 was considered high (>90%), with decreasing order of confidence levels of 2 (70%–90%), 3 (50%–70%) and 4 (<50%). Chi-square statistics (χ2) were used to examine the confidence of each evaluator between four consecutive stages of the study and between categorical variables. All statistical analyses were performed in Excel, SPSS v26 or Statistica v13.1.
Results
Baseline characteristics
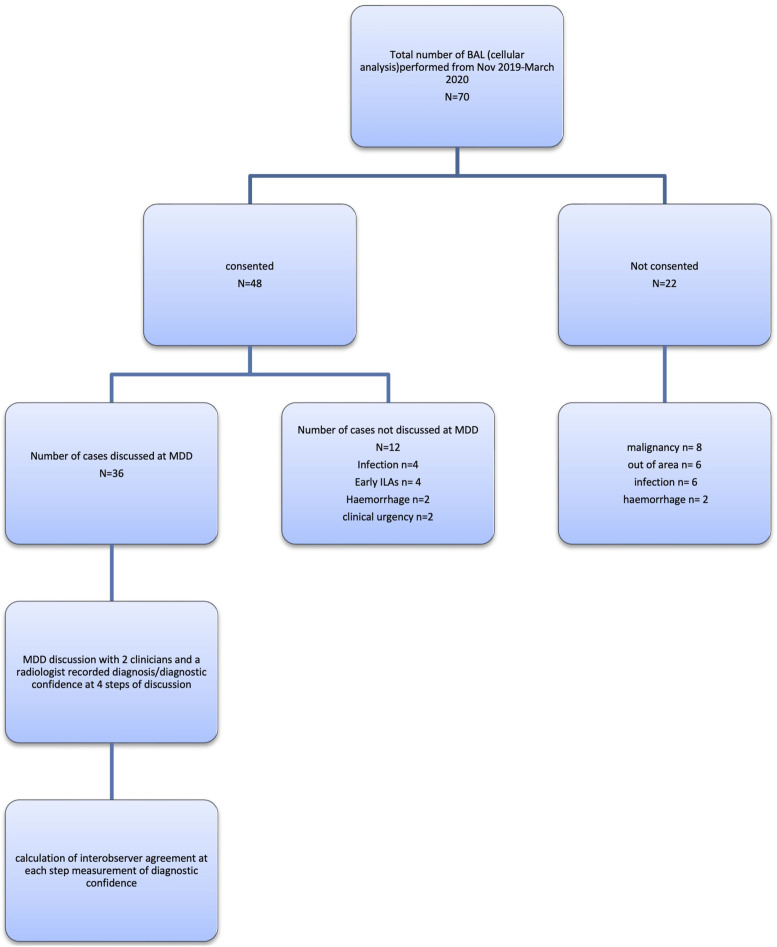
Between 1st November 2019 and 31st March 2021, of the 70 patients undergoing BAL at Waikato Hospital, 36 were recruited for our study (Figure 1). Baseline characteristics are presented in Table 1. The median age range was 37–83 years, and participants were predominantly male (77.8%). The most common pre-MDD suspected diagnosis was HP (15 cases, 36.5%), followed by IPF (13 cases, 31.7%). In the consensus agreement, 10/36 (27.8%) patients were diagnosed with a predominantly fibrotic disease, 19/36 (52.8%) with a predominantly inflammatory disease and 7/36 (19.4%) with an unclassifiable disease.
Figure 1.
ENROLLMENT (KEY: BAL: Bronchoalveolar lavage, MDD: Multidisciplinary discussion).
Table 1.
Baseline characteristics of study population. Continuous variables are presented as median (IQR) and categorical data presented as n (%).
| Demographics (N = 36) | |
| Females (%) | 8 (22.2) |
| Age (IQR) | 71 (63–76.5) |
| Ethnicity (%) | NZ European 31 (86); Maori 4 (11.2); Asian 1 (2.8) |
| Smoking | |
| Smoking status (%) | Smoker 2 (5.5); Ex-smoker 19 (52.8); Non-smoker 15 (41.7) |
| Pack-years (IQR) | 10.5 (0–22.5) |
| Co-morbidities | |
| Asthma (%) | 3 (8.3) |
| Chronic obstructive pulmonary disease (%) | 2 (5.6) |
| Obstructive sleep Apnoea (%) | 4 (11.1) |
| Hypertension (%) | 11 (30.5) |
| Ischemic heart disease (%) | 3 (8.3) |
| Gastro-oesophageal reflux disease (%) | 3 (8.3) |
| Lung function tests | |
| FVC (IQR) | 79.5 (68.5–9.5) |
| DLCO (IQR) (n=35) | 51/35, 37–70 |
| 6MWD (IQR) (n=10) | 420 (400–480) |
| BAL analysis | |
| % Epithelial cells (IQR) | 2.35 (0.5–5.5) |
| % Squamous cells (IQR) | 0.225 (0–0.75) |
| % Lymphocytes (IQR) | 7.5 (3–15) |
| % Neutrophiles (IQR) | 10.5 (5–22.5) |
| % Macrophages (IQR) | 61.5 (47–75.5) |
| % Eosinophils (IQR) | 1 (0–3) |
| Contamination (%) | 9 (25) |
| Suboptimal quality (%) | 9 (25) |
| Lobe (%) | Lingula 9 (25%), RML 9 (25%), RUL 7 (19.4%), LUL 10 (27.85), LLL 1 (2.8%) |
| Volume instilled (IQR) | 150 (120–180) |
| Volume returned (IQR) | 60 (50–70) |
Of the 36 BALs performed, 9 were considered suboptimal as per ATS guidelines 7 with >5% respiratory epithelial cells. 9 of 36 samples also had the presence of squamous cells suggesting contamination. Among BAL samples obtained, 18 of 36 fulfilled the criteria for adequate samples based on ATS 2012 guidelines. 7 BAL cellular analysis for IPF was predominantly neutrophilic. BAL cellular analysis was predominantly lymphocytic for other causes such as HP (acute), sarcoid, exposure secondary to vaping, silica, and connective tissue disease associated ILD.
There were two deaths and one serious adverse event in the cohort. One death was likely due to BAL, and the other was unrelated to BAL. The serious adverse event was hospitalisation with type 1 acute respiratory failure requiring high flow oxygen therapy post-BAL. On baseline pulmonary function tests, both cases experiencing BAL-related adverse outcomes had poor gas transfer (DLCO <25% predicted).
Agreement
The interobserver agreement between clinicians and radiologists in steps 1 and 2 was 0.622 (95% CI 0.47–0.77) and 0.78 (95% CI 0.624–0.935), respectively. This initially dropped to 0.776 (95% CI 0.614–0.937) in step 3, followed by a rise to 0.969 (95% CI 0.828–1.11) in step 4 (Table 2). The results of an intra-observer agreement are reported in Table 3. Clinicians had a lower intra-observer agreement between steps 1 and 4 compared to radiologists (k = 0.490 and 0.526 respectively for Clinician A and Clinician B vs k = 0.636 for the Radiologist).
Table 2.
2X2 table to demonstrate the effect of discussion and bronchoalveolar lavage (BAL) on interobserver agreement during ILD-MDD.
| Without BAL | With BAL | |
|---|---|---|
| Without MDM discussion | 0.622 | 0.776 |
| STEP 1 | STEP 3 | |
| With MDM discussion | 0.780 | 0.969 |
| STEP 2 | STEP 4 |
Table 3.
Intra-observer agreement between each step.
| Steps | Clinician A | Clinician B | Radiologist |
|---|---|---|---|
| Step 1 to 2 | 0.717 | 0.877 | 0.880 |
| Step 2 to 3 | 0.799 | 0.828 | 0.815 |
| Step 3 to 4 | 0.853 | 0.799 | 0.789 |
| Step 1 to 4 | 0.490 | 0.526 | 0.636 |
Confidence
The proportion of patients with high confidence (>90%) increases significantly between each step for clinicians and radiologists (Table 4). The overall proportion of patients with >90% confidence is displayed in Figure 2.
Table 4.
Confidence in the diagnosis of evaluators during all 4 steps of the study.
| Clinician | Confidence, % | Step 1 | Step 2 | Step 3 | Step 4 | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| A | >90 | 3 | 8.3 | 7 | 19.4 | 15 | 41.7 | 20 | 55.6 | 0.014 |
| 70–90 | 14 | 38.9 | 14 | 38.9 | 15 | 41.7 | 6 | 16.7 | ||
| 50–70 | 8 | 22.2 | 9 | 25.0 | 2 | 5.6 | 5 | 13.9 | ||
| <50 | 11 | 30.6 | 6 | 16.7 | 4 | 11.1 | 5 | 13.9 | ||
| B | >90 | 5 | 13.9 | 10 | 27.8 | 13 | 36.1 | 18 | 50.0 | 0.004 |
| 70–90 | 21 | 58.3 | 15 | 41.7 | 15 | 41.7 | 10 | 27.8 | ||
| 50–70 | 7 | 19.4 | 9 | 25.0 | 7 | 19.4 | 4 | 11.1 | ||
| <50 | 3 | 8.3 | 2 | 5.6 | 1 | 2.8 | 4 | 11.1 | ||
| R | >90 | 13 | 36.1 | 15 | 41.7 | 20 | 55.6 | 22 | 61.1 | 0.002 |
| 70–90 | 13 | 36.1 | 12 | 33.3 | 6 | 16.7 | 5 | 13.9 | ||
| 50–70 | 8 | 22.2 | 7 | 19.4 | 9 | 25.0 | 5 | 13.9 | ||
| <50 | 2 | 5.6 | 2 | 5.6 | 1 | 2.8 | 4 | 11.1 | ||
Figure 2.
Line plot of percentage of diagnoses each evaluator gave at each study step with >90% confidence.
Discussion
In this study of patients with undifferentiated ILD being discussed at an MDD, we demonstrated that BAL cellular analysis improves interobserver agreement and increases diagnostic confidence of the final diagnosis.
We measured interobserver agreement among three participants (two respiratory physicians and one radiologist) and recorded their diagnostic confidence pre and post BAL cellular analysis MDD. Overall, we have demonstrated that the interobserver agreement improves from pre-to post-BAL from 0.78 to 0.969. Although it also highlights the importance of discussion in diagnosing ILD, which is in concordance with previous findings from Flaherty et al. in 2004. 19 The diagnostic confidence increased significantly with each step with a higher proportion of diagnostic confidence >90% among both clinicians and radiologists at step 4 compared to previous steps. This suggests the utility of BAL in affirming suspected diagnoses among clinicians and consolidating treatment decisions. Unfortunately, despite a thorough multidisciplinary evaluation, up to 15% of ILD patients had unclassifiable ILD and could not be given a specific diagnosis.
Of note, the adverse event rate in this study is relatively high. We believe this can be attributed to two factors. The first is the illness severity and prognosis in this cohort of patients with suspected ILD which remains undifferentiated based on routine non-invasive assessments including history, physical exam, cross-sectional imaging and serological testing. Secondly, it highlights the risk of BAL in patients with severe impairment in gas transfer.
To our knowledge, this is the first study to evaluate the utility of BAL cellular analysis in conjunction with an MDD in diagnosing undifferentiated ILD. Previous studies have assessed the diagnostic utility of BAL cellular analysis in specific conditions in isolation.11–18 Our study results are consistent with these previous findings, showing current guidance on BAL interpretation leads to an improved diagnostic yield by MDD. The methods of our study are also consistent with previous studies evaluating diagnostic adjuncts in undifferentiated ILD such as the MDD process, 19 and transbronchial Cryobiopsy. 22 Our results show a similar utility of BAL in the diagnostic process as previous studies.
Our main limitation of the study is the relatively small sample size when compared to the study by Flaherty et. al. in 2004 19 and the COLD-ICE trial, 22 but are reasonable for a single centre. With one ILD specialist, our centre had limited expertise in interpreting BAL cellular proportions, and there is a risk of a learning curve. However we argue this level of expertise is representative of a majority of centres in the region, possibly increasing the applicability of our findings. In addition, there was a selection bias of patients, as patients were missed from other district health boards who participate in the regional MDD, and private practices. Bronchoscopists with different levels of expertise performing the procedure might impact BAL fluid recovery and cell count, however the rate of adequate sampling was within acceptable limits. The external validity of our findings are also limited by the single centre nature of our study. The use of BAL in the investigation of patients with suspected ILD may differ compared to other centres with different resources.
The proportion of patients in our study with a diagnosis of unclassifiable ILD is slightly higher than previously reported in the literature. 23 This could be because not all patients referred for discussion at MDD at our centre were also referred for BAL. This presents a selection bias of cases where the diagnosis is less clear increasing the chances of a diagnosis of unclassifiable ILD.
Whether incorporation of BAL into the evaluation of patients with undifferentiated ILD leads to improved patient centred outcomes remains unkown and cannot be inferred from our study. Future research should focus on if the improvement in diagnostic consensus and confidence leads to improved outcomes. Future studies could also evaluate if BAL use averts the need for further high risk procedures such as transbronchial cryobiopsy or surgical lung biopsy.
In conclusion, our prospective study shows that BAL cellular analysis used in conjunction with an MDD, improves interobserver agreement and increases diagnostic confidence in the diagnosis of undifferentiated ILD.
Acknowledgements
Megon Schubel, Anne Darlington, Michelle Galbraith, Ayan Sabih, Christine Tuffery.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Dilip Jayasimhan https://orcid.org/0000-0002-8322-9227
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Walsh SLF. Multidisciplinary evaluation of interstitial lung diseases: current insights. Eur Respir Rev 2017; 26(144): 170002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaner RJ, Brown KK, Martinez FJ. Progress in interstitial lung disease. Am J Respir Crit Care Med 2017. May 1; 195(9): 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001; 164(9): 1722–1727. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KR, Toews GB, Travis WD, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002; 19(2): 275–283. [DOI] [PubMed] [Google Scholar]
- 5.Mikolasch TA, Garthwaite HS, Porter JC. Update in diagnosis and management of interstitial lung disease. Clin Med 2017. Apr; 17(2): 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: Is it clinically useful? Eur Respir J 2011; 38(4): 761–769. [DOI] [PubMed] [Google Scholar]
- 7.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012; 185(9): 1004–1014. [DOI] [PubMed] [Google Scholar]
- 8.Bollmann BA, Seeliger B, Drick N, et al. Cellular analysis in bronchoalveolar lavage: Inherent limitations of current standard procedure. Eur Respir J 2017; 49(6): 1601844. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis An Official ATS/ERS/JRS/ALAT Clinical practice guideline. Am J Respir Crit Care Med. 2018. Sep 1;198(5):e44–e68. [DOI] [PubMed] [Google Scholar]
- 10.Diagnosis of hypersensitivity pneumonitis in adults: An Official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2021. Jan 1;203(1):150–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee W, Chung WS, Hong KS, et al. Clinical usefulness of bronchoalveolar lavage cellular analysis and lymphocyte subsets in diffuse interstitial lung diseases. Ann Lab Med. 2015/02/12 ed. 2015. Mar;35(2):220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildge J, Frank J, Klar B. The role of bronchoalveolar lavage in the diagnosis of idiopathic pulmonary fibrosis: An investigation of the relevance of the protein content. Pneumologie. 2016. Jul 1;70(7):435–441. [DOI] [PubMed] [Google Scholar]
- 13.Nagai S, Kitaichi M, Itoh H, et al. Idiopathic nonspecific interstitial pneumonia/fibrosis: Comparison with idiopathic pulmonary fibrosis and BOOP. Eur Respir J 1998; 12(5): 1010–1019. [DOI] [PubMed] [Google Scholar]
- 14.Efared B, Ebang-Atsame G, Rabiou S, et al. The diagnostic value of the bronchoalveolar lavage in interstitial lung diseases. J Negat Results Biomed. 2017. Mar 1;16(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welker L, Jörres RA, Costabel U, et al. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. Eur Respir J 2004; 24(6): 1000–1006. [DOI] [PubMed] [Google Scholar]
- 16.Ryu YJ, Chung MP, Han J, et al. Bronchoalveolar lavage in fibrotic idiopathic interstitial pneumonias. Respir Med 2007; 101(3): 655–660. [DOI] [PubMed] [Google Scholar]
- 17.Veeraraghavan S, Latsi PI, Wells AU, et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J 2003; 22(2): 239–244. [DOI] [PubMed] [Google Scholar]
- 18.Tzilas V, Tzouvelekis A, Bouros E, et al. Diagnostic value of BAL lymphocytosis in patients with indeterminate for usual interstitial pneumonia imaging pattern. Eur Respir J 2019; 54(5): 1901144. [DOI] [PubMed] [Google Scholar]
- 19.Flaherty KR, King TE, Raghu G, et al. Idiopathic interstitial pneumonia: What is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med 2004; 170(8): 904–910. [DOI] [PubMed] [Google Scholar]
- 20.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 2016. Jul 2; 33(3): 613–619, https://dx.doi.org.nottingham.idm.oclc.org/101177/001316447303300309 [Google Scholar]
- 21.Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977. Mar [cited 2023 Jan 24];33(1): 159. Available from: https://pubmed.ncbi.nlm.nih.gov/843571/ [PubMed] [Google Scholar]
- 22.Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med. 2020. Feb 1;8(2):171–181. [DOI] [PubMed] [Google Scholar]
- 23.Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013. Sep;42(3):750–757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.