Abstract
The objective of this study was to determine the effects of primary simian immunodeficiency virus (SIV) infection on the prevalence and phenotype of progenitor cells present in the gastrointestinal epithelia of SIV-infected rhesus macaques, a primate model for human immunodeficiency virus pathogenesis. The gastrointestinal epithelium was residence to progenitor cells expressing CD34 antigen, a subset of which also coexpressed Thy-1 and c-kit receptors, suggesting that the CD34+ population in the intestine comprised a subpopulation of primitive precursors. Following experimental SIVmac251 infection, an early increase in the proportions of CD34+ Thy-1+ and CD34+ c-kit+ progenitor cells was observed in the gastrointestinal epithelium. In contrast, the proportion of CD34+ cells in the thymus declined during primary SIV infection, which was characterized by a decrease in the frequency of CD34+ Thy-1+ progenitor cells. A severe depletion in the frequency of CD4-committed CD34+ progenitors was observed in the gastrointestinal epithelium 2 weeks after SIV infection which persisted even 4 weeks after infection. A coincident increase in the frequency of CD8- committed CD34+ progenitor cells was observed during primary SIV infection. These results indicate that in contrast to the primary lymphoid organs such as the thymus, the gastrointestinal epithelium may be an early extrathymic site for the increased prevalence of both primitive and committed CD34+ progenitor cells. The gastrointestinal epithelium may potentially play an important role in maintaining T-cell homeostasis in the intestinal mucosa during primary SIV infection.
Human immunodeficiency virus (HIV) infection has been demonstrated to cause a progressive loss in the frequency of hematopoietic progenitors in the bone marrow, thymus, and peripheral blood of HIV-seropositive individuals (2, 14, 15, 16, 23, 26, 29, 32, 48, 56, 57). Recent studies with the murine model have demonstrated that gastrointestinal epithelium can function as an extrathymic site for T-cell differentiation and maturation (41, 42). There is no information available, however, on the effects of HIV infection on T-cell maturation or differentiation and progenitor cell frequency in intestinal epithelium. The intestinal epithelium, like the thymic epithelium, has been shown to be capable of supporting T-cell differentiation and maturation (36) and also of carrying out negative and positive selection of T cells (42). Since gastrointestinal lymphoid tissue harbors more than 90% of the total lymphocytes in the body, a high level of T-cell turnover may occur at this site. The development and maturation of T cells from tissue progenitors that might have migrated into the gastrointestinal epithelium may be one of the mechanisms for maintaining T-cell homeostasis at these sites. Progenitor cells capable of generating mature T cells have been isolated from cryptopatches of the mouse intestinal epithelium (45). Murine intestinal epithelial cells were shown to secrete the c-kit ligand called stem cell factor (SCF), which supports T-cell differentiation (7, 9). Since HIV infection can cause severe CD4+ T-cell depletion, lymphopoiesis in the gastrointestinal epithelium may be involved in maintaining T-cell homeostasis. No information is available on the effects of HIV infection on lymphopoiesis in the intestinal epithelium. These studies have been limited by the inability to obtain sufficient amounts of intestinal tissue samples in early stages of HIV infection.
Simian immunodeficiency virus (SIV)-infected rhesus macaques provide a suitable animal model to determine the effect of HIV infection on the lymphopoietic potential of the gastrointestinal epithelium. Our previous studies have demonstrated that intestinal lymphoid tissue is an early target organ for SIV (20, 21, 34, 49). Severe depletion of CD4+ T cells coinciding with an increase in CD8+ T cells was detected in both intestinal intraepithelial lymphocytes and lamina propria during primary SIV infection (34, 46). The early depletion of intestinal CD4+ T cells was characterized by an attempt to maintain homeostasis by constantly replenishing the depleted T-cell pool, as was evidenced by the fact that there was an increase in CD8+ T cells following infection (34, 51). The intestinal epithelium may play an important role in this process. The potential of the immune system to generate T cells to maintain T-cell homeostasis during the course of HIV infection has been demonstrated (1, 33). One of the mechanisms may involve differentiation and maturation from T-cell precursors at thymic and extrathymic sites such as the gastrointestinal epithelium.
Based on the above-described findings, we hypothesized that the gastrointestinal epithelium may be an active site for the prevalence of progenitor cells and that HIV infection leads to an alteration in the phenotypic profile of these progenitor cells present in the gastrointestinal epithelium. We used SIV-infected rhesus macaques to enumerate and characterize the phenotype and frequency of CD34+ progenitor cells in the gastrointestinal epithelium and to determine whether SIVmac251 infection has an effect on the phenotype and frequency of these CD34+ progenitor cells.
Viral loads in intestinal tissue during primary SIV infection.
Our previous studies have demonstrated that lymphocytes located in the gastrointestinal epithelium were infected with SIV early during the course of SIV infection, which resulted in severe changes in their phenotypic and functional profiles (34). Higher numbers of SIV-positive cells were detected during the primary acute and terminal stages than during the asymptomatic stage of SIV infection. These infected cells were strongly positive for SIV nucleic acids, indicating that they may support active viral replication (34, 46). The viral burden in intestinal tissue samples was measured by the branched DNA signal amplification assay as previously described by Pachl et al. (40) and Harris et al. (19). High copy numbers of SIVmac251 RNA were detected in intestinal tissue samples during primary SIV infection between 1 and 2 weeks postinfection (p.i.). At 2 weeks p.i., the SIV RNA copy numbers in jejunum and colon tissues ranged from 423,000 to 596,000 and 410,000 to 674,000 per 25 mg of tissue, respectively. The viral burden steadily increased, and by 4 weeks p.i., copy numbers ranged from 1,717,000 to 21,997,000 and 1,095,000 to 2,230,000 per 25 mg of tissue in jejunum and colon tissues, respectively. The high viral loads, accompanied by severe CD4+ T-cell depletion during early SIV infection, may have a significant impact on the homeostatic mechanisms that operate in this extensive and severely affected lymphoid compartment.
Unlike in the thymus, the frequency of CD34+ progenitors did not decline in the gastrointestinal epithelium during primary SIV infection.
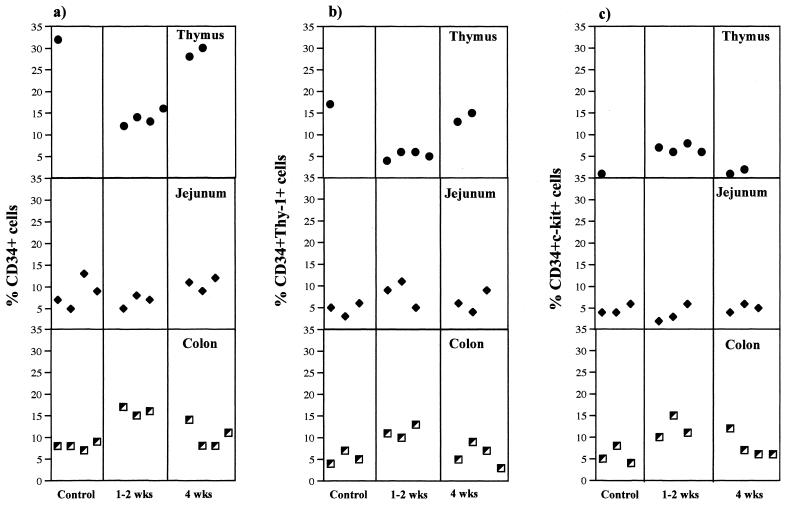
The frequency of CD34+ progenitors was determined by flow cytometry, using freshly isolated mononuclear cells from jejunum and colon epithelium and thymus tissue and peripheral blood from rhesus macaques (n = 4) 1 to 4 weeks after SIV infection. The cell isolation procedures and flow cytometric analysis were performed as previously described (34, 46). The frequencies of CD34+ progenitors in the intestinal epithelia of SIV-infected animals were compared with those of uninfected control animals. A minor proportion of mononuclear cells within the jejunum and colon epithelia of uninfected rhesus macaques expressed the CD34 antigen, whereas the thymus tissues was found to contain a higher proportion of CD34+ progenitors (Fig. 1a). The frequency of CD34+ progenitors in the colon epithelium increased at 1 to 2 weeks following SIV infection (Fig. 1a) but returned to preinfection levels at 4 weeks p.i. The percentages of CD34+ cells in the jejunal epithelium did not show any decline and remained similar to the preinfection values during primary SIV infection (Fig. 1a). The CD34 antigen has been shown to be expressed by a minor population of mononuclear cells in the bone marrow which exhibit multilineage progenitor cell activity in vitro (10, 11, 50). Previous studies have shown that the CD34+ bone marrow cells were enriched for primitive hematopoietic stem cells and may reconstitute long-term multilineage hematopoiesis in nonhuman primates (5, 6). In the murine model, progenitor cells were found to be present within the crypts found at the base of the villus epithelium (45). Further, gastrointestinal intraepithelial lymphocytes from humans (30) and mice (17) have been shown to express RAG-1 (recombination activation gene 1), suggesting that the intestinal epithelium may support extrathymic T-cell differentiation and maturation. It is difficult to determine at this point why the increase in CD34+ progenitors was more prominent only in the colon epithelium. It may be possible that the unique microenvironments of the jejunum and colon may play a role in this process and may contribute to the differences observed in the prevalence of CD34+ cells following SIV infection.
FIG. 1.
Alterations in the prevalence of CD34+ progenitor cells in the gastrointestinal epithelium during primary SIV infection. Freshly isolated mononuclear cells from the jejunum and colon epithelium and thymus tissues of uninfected control and SIV-infected rhesus macaques at 1 to 2 weeks and 4 weeks p.i. were stained with CD34 and CDw90 (Thy-1) or CD117 (c-kit receptor) and analyzed by flow cytometry. Negative-control samples were stained with matched-isotype control antibodies. The frequencies of CD34+ (a), CD34+ Thy-1+ (b), and CD34+ c-kit+ (c) cells were determined as percentages of gated mononuclear cells.
In contrast to the gastrointestinal epithelium, an early depletion of CD34+ cells was observed in the thymus during primary SIV infection (Fig. 2). The frequency of CD34+ cells in the thymus was found to decline at 1 to 2 weeks p.i. compared to that of uninfected control animals (Fig. 1a). At 4 weeks p.i., the frequency of CD34+ cells returned to preinfection levels, indicating that CD34+ progenitors were repopulating the thymus later in SIV infection. Peripheral blood was found to contain only a minor proportion (less than 1%) of CD34+ progenitors in uninfected control animals. There was no significant change in the frequency of CD34+ progenitor cells in peripheral blood following SIV infection. Wykrzykowska et al. (55) have shown that the frequency of CD34+ progenitors in the thymus decreased early in SIV infection, followed by regeneration at 7 to 8 weeks p.i. Numerous studies have demonstrated that HIV-1 and SIV infections lead to a loss of thymocytes. This may be attributed to either direct virus infection or the inhibition of thymocyte maturation due to changes in the thymic microenvironment having an adverse effect on lymphopoiesis (3, 27, 37, 38, 44, 47). Our findings seemed to indicate that viral infection had contrasting effects on the thymus and the gastrointestinal epithelium during primary SIV infection. It is difficult to explain the opposite changes observed between the gastrointestinal epithelium and the thymus. Since there was no major change observed in CD34+ cells in the blood, cell trafficking may play only a minor role in this process. Though the essential role of the thymus in development of the immune system is beyond doubt, the role of the thymus in maintenance of the adult immune system is hard to define. It is possible that in adults the gastrointestinal epithelium may be another potential site for maintaining T-cell homeostasis during primary HIV and SIV infection.
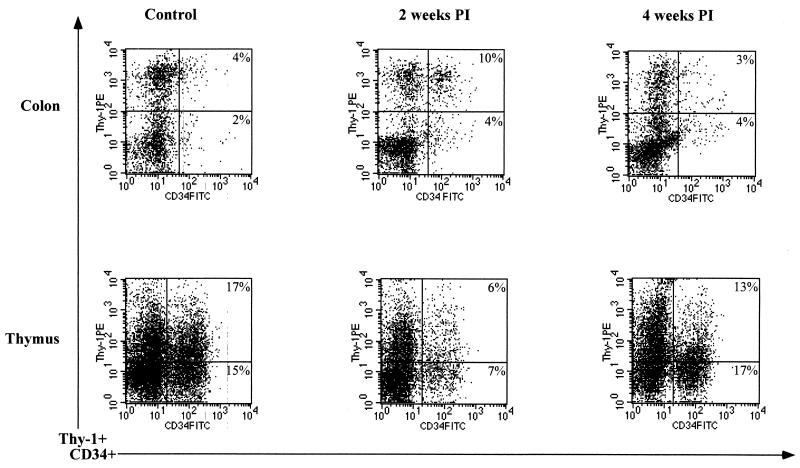
FIG. 2.
Flow cytometric analysis of mononuclear cells expressing CD34 and Thy-1 during primary SIV infection. Freshly isolated mononuclear cells from the colon epithelium and thymuses of uninfected and SIV-infected rhesus macaques at 2 and 4 weeks p.i. were stained for CD34 and CDw90 (Thy-1) antigens and analyzed by flow cytometry. Negative-control samples were stained with matched-isotype control antibodies. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
The prevalence of primitive CD34+ progenitors expressing Thy-1 and the c-kit receptor increased in the gastrointestinal epithelium during primary SIV infection.
The frequencies of CD34+ Thy-1+ progenitors were determined by flow cytometry, using freshly isolated mononuclear cells from jejunum and colon epithelium and with thymus tissue and peripheral blood from rhesus macaques (n = 4) at 1 to 4 weeks after SIV infection and compared to those from uninfected controls (Fig. 1b). A subpopulation of CD34+ cells in the gastrointestinal epithelium of uninfected control animals was found to express Thy-1, indicating that a subset of CD34+ progenitor cells in the intestinal epithelium consisted of primitive precursor cells. Studies have shown that the most primitive progenitor subsets of human bone marrow CD34+ cells were characterized by the expression of Thy-1 (4, 12, 18, 39). Further, these studies have demonstrated that Thy-1 antigen was expressed by a population of CD34high, CD45RAlow, and HLA-DRlow cells and that this population of cells was found to harbor high-proliferative-potential hematopoietic precursor cells and long-term-culture-initiating cells. Murray et al. (39) have demonstrated that the CD34+ Thy-1+ subset of peripheral blood stem cells is enriched for primitive stem cells. The frequencies of CD34+ Thy-1+ progenitors increased in both jejunum and colon epithelium at 1 and 2 weeks p.i. relative to those of uninfected controls (Fig. 1b) but returned to preinfection levels at 4 weeks p.i. In contrast to the case in the gastrointestinal epithelium, the frequency of CD34+ Thy-1+ progenitors in the thymus declined at 1 (n = 2) and 2 (n = 2) weeks p.i. and then increased to preinfection levels at 4 (n = 2) weeks p.i. (Fig. 2), suggesting that regeneration of CD34+ cells in the thymus was characterized by an increase in the frequency of CD34+ Thy-1+ progenitors. No significant differences in the levels of Thy-1 expression in intestinal epithelium and thymus tissues were observed between SIV-infected animals and control animals.
The primitive CD34+ progenitor cells have been shown to be enriched for the c-kit receptor. We determined the frequencies of CD34+ c-kit+ progenitors in freshly isolated mononuclear cells from jejunum and colon epithelium and in thymus and peripheral blood of rhesus macaques at 1 to 4 weeks after SIV infection and compared them with those of uninfected controls (Fig. 1c). The frequency of CD34+ c-kit+ cells was found to increase in the colon epithelium at 1 and 2 weeks p.i. but returned to preinfection levels at 4 weeks p.i. No decline was evident in the frequency of CD34+ c-kit+ cells within the jejunum epithelium relative to uninfected controls during primary SIV infection. Thymus was found to contain only a minor proportion of CD34+ c-kit+ progenitors (∼1 to ∼2%), which increased to ∼6 to ∼8% at 2 weeks p.i. and then returned to uninfected control levels at 4 weeks p.i. The c-kit receptor (CD117) is a tyrosine kinase-containing receptor that binds to SCF. Puddington et al. (43) have reported that the interactions between murine SCF and the c-kit receptor may play an important role in maintaining homeostasis in the gastrointestinal mucosa. Kanamori et al. (24) have identified tiny clusters of cells in the murine intestinal crypts. A large proportion of these cells expressed c-kit, the interleukin-7 receptor, Thy-1, and lymphocyte function-associated antigen 1 and were CD3−, T-cell receptor αβ and γδ negative, soluble immunoglobulin M negative, and B220−, indicating that the crypts may be the extrathymic site where the development of T and/or B progenitors takes place and that they may be a source of extrathymic T cells. Briddell et al. (8) have reported that in humans the c-kit receptor is expressed on both primitive and more differentiated hematopoietic progenitor cells.
Our results demonstrated that the frequency of CD34+ Thy-1+ and CD34+ c-kit+ primitive precursors in the gastrointestinal epithelium increased early in SIV infection. Klimpel et al. (25) demonstrated that the production of SCF was enhanced in the intestine following Salmonella infection and suggested that intestinal epithelial cells may be the major producer of SCF. It is likely that enhanced secretions of SCF may be observed following SIV infection. Serum SCF levels were found to be elevated in HIV-infected patients (31). As SCF has been shown to increase the colony-forming potential of hematopoietic progenitors obtained from HIV-infected individuals (35, 52), secreted SCF may play an important role in the differentiation of progenitor cells expressing the c-kit receptor and thereby contributing to the maintenance of homeostasis in the intestinal immune system. The role of cell trafficking in increasing the prevalence of CD34+ cells in the gastrointestinal epithelium cannot be completely ruled out. However, as there were no major changes in the frequency of CD34+ cells in peripheral blood during early infection, cell traffic may play only a minor role in this process. On the other hand viral infection may play a role in increasing the frequency of these subpopulations of precursor cells in the gastrointestinal epithelium. Hillyer et al. (22) have shown that the percentage of bone marrow CD34+ progenitors increased during primary SIV infection. Our data show that changes in the CD34+ progenitor cell percentages in intestine are similar to those observed in bone marrow during primary infection.
CD34+ progenitors coexpressing CD4 undergo a dramatic depletion in the gastrointestinal epithelium during primary SIV infection.
A heterogeneous population of committed progenitor cells positive for CD4 antigen was detected in the gastrointestinal epithelium. We determined the effect of primary SIV infection on the frequency of CD34+ CD4+ cells. In uninfected control samples, the frequency of CD34 gated cells expressing CD4 antigen was found to be about 15 to 16% in the jejunum epithelium and 13 to 28% in the colon epithelium, whereas ∼28 to ∼39% of CD34 gated cells were found to coexpress CD8 antigen. Following SIV infection, the frequency of CD34+ CD4+ precursors was found to decline dramatically at 1 to 2 weeks p.i. in both jejunum and colon epithelium (∼2 to ∼5%) and remained low even at 4 weeks p.i. In contrast, the frequency of CD34+ progenitors coexpressing CD8 was found to increase at 1 to 2 weeks p.i. (∼59 to ∼79%) relative to values for uninfected controls and then to decrease to ∼48 to ∼50% at 4 weeks p.i. These changes in the phenotype and prevalence of this progenitor cell population following SIV infection suggest that the progenitor cells may play a major role in maintaining tissue homeostasis at intestinal lymphoid sites. Our previous studies have shown that the immunophenotypic profiles of IEL and LPL were significantly altered during primary SIV infection, which was not reflected in peripheral blood (34, 46). The frequency of the CD3+ CD4+ single-positive (∼0 to ∼5%) and CD3+ CD4+ CD8+ double-positive (∼2 to ∼5%) T-cell subsets declined in the jejunum epithelium as early as 2 weeks p.i. compared to the frequency of CD3+ CD4+ single-positive (∼8 to ∼20%) and CD3+ CD4+ CD8+ double-positive (∼5 to ∼13%) T-cell subsets in uninfected control animals. A similar decline was observed in the frequency of CD3+ CD4+ single-positive (∼0 to ∼1%) and CD3+ CD4+ CD8+ double-positive (∼2 to ∼5%) T-cell subsets in the colon epithelia compared to the frequency of CD3+ CD4+ single-positive (∼3 to ∼42%) and CD3+ CD4+ CD8+ double-positive (∼8 to ∼17%) T-cell subsets in the colon epithelium of uninfected animals. No major changes were observed in the frequency of CD3+ CD4+ single-positive (∼54 to ∼58%) and CD3+ CD4+ CD8+ double-positive (∼1 to ∼2%) T cells in the peripheral blood of SIV-infected animals at 4 weeks p.i. compared to uninfected control animals. An increase in the proportion of CD3+ CD8+ T cells coincided with CD4+ T-cell depletion, suggesting that homeostatic mechanisms may operate in the gastrointestinal epithelium to maintain T-cell numbers, as previously reported (34). The frequency of CD3+ CD8+ T cells increased from ∼39 to ∼75% in both the jejunum and the colon of uninfected control animals to >77% at 2 weeks p.i. It is possible that the selective increase in the frequencies of various primitive and committed subsets of progenitors coinciding with the dramatic changes observed in the gastrointestinal epithelium early in infection may be a mechanism for maintaining T-cell homeostasis in the gastrointestinal epithelium. Our results were found to support this hypothesis. Following SIV infection, the severe depletion may be the frequency of CD34+ cells coexpressing CD4 antigen was found to coincide with the severe depletion of CD4+ T cells. The levels of CD4+ T cells remained low throughout infection, suggesting that the depletion of progenitor CD34+ CD4+ cells may be a factor contributing to the failure of replenishment of CD4+ T cells in the intestinal epithelium. In mice, pluripotent hematopoietic stem cells and T-cell lineage precursors have been shown to express CD4 (53, 54). Low levels of CD4 antigen were shown to be expressed on subsets of human CD34+ cells (28), and a majority of the CD34+ CD4+ cells isolated from human peripheral blood was found to express the fusin receptor (13), indicating that these cells are susceptible to HIV infection. On the other hand, the increased frequency of CD34+ cells coexpressing CD8 antigen during primary SIV infection may be one of the mechanisms for the increased prevalence of CD8+ cells in the intestinal epithelium. The majority of the CD34+ progenitor cells in the thymus were found to coexpress both CD4 and CD8 antigens prior to and after SIV infection, indicative of their immature phenotype.
In conclusion, our study has drawn attention to the role of extrathymic sites such as the gastrointestinal epithelium during primary SIV infection. We observed contrasting changes in the frequency of progenitor cells during primary SIV infection which may have significant implications in understanding how these lymphopoietic sites may contribute to maintenance of tissue homeostasis during primary HIV and SIV infection. Taken together our results clearly demonstrate that in contrast to the thymus, the gastrointestinal epithelium is an early site for the increased prevalence of CD34+ Thy-1+ and CD34+ c-kit+ progenitor cells during primary SIV infection and may play an important role in the maintenance of T-cell homeostasis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-DK43183, R01-AI43274, and RR-00169) and the Universitywide AIDS Research Program, University of California.
We are thankful to Linda Hirst, Ross Tarara, and Don Canfield at the California Regional Primate Research Center for valuable assistance in this project.
REFERENCES
- 1.Adleman L M, Wofsy D. T-cell homeostasis: implications in HIV infection. J Acquired Immune Defic Syndr. 1993;6:144–152. [PubMed] [Google Scholar]
- 2.Bagnara G P, Zauli G, Giovannini M, Re M C, Furlini G, La Placa M. Early loss of circulating hemopoietic progenitors in HIV-1-infected subjects. Exp Hematol (New York) 1990;18:426–430. [PubMed] [Google Scholar]
- 3.Baskin G B, Murphey-Corb M, Martin L N, Davison-Fairburn B, Hu F S, Kuebler D. Thymus in simian immunodeficiency virus-infected rhesus monkeys. Lab Investig. 1991;65:400–407. [PubMed] [Google Scholar]
- 4.Baum C M, Weissman I L, Tsukamoto A S, Buckle A M, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson R J, Andrews R G, Bensinger W I, Kalamasz D, Knitter G, Buckner C D, Bernstein I D. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Investig. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenson R J, Bensinger W I, Hill R S, Andrews R G, Garcia-Lopez J, Kalamasz D F, Still B J, Spitzer G, Buckner C D, Bernstein I D, et al. Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood. 1991;77:1717–1722. [PubMed] [Google Scholar]
- 7.Brandt J, Briddell R A, Srour E F, Leemhuis T B, Hoffman R. Role of c-kit ligand in the expansion of human hematopoietic progenitor cells. Blood. 1992;79:634–641. [PubMed] [Google Scholar]
- 8.Briddell R A, Broudy V C, Bruno E, Brandt J E, Srour E F, Hoffman R. Further phenotypic characterization and isolation of human hematopoietic progenitor cells using a monoclonal antibody to the c-kit receptor. Blood. 1992;79:3159–3167. [PubMed] [Google Scholar]
- 9.Briddell R A, Bruno E, Cooper R J, Brandt J E, Hoffman R. Effect of c-kit ligand on in vitro human megakaryocytopoiesis. Blood. 1991;78:2854–2859. [PubMed] [Google Scholar]
- 10.Civin C, Fackler T T M, Bernstein I, Buhring H, Campos L, Greaves M F, Kamoun M, Katz D, Landsorp P, Look T, Seed B, Sutherland D R, Tindle R, Vchanska-Ziegler B. Leukocyte typing IV. Oxford, United Kingdom: Oxford University Press; 1989. Summary of CD34 cluster workshop section; pp. 818–825. [Google Scholar]
- 11.Civin C I, Strauss L C, Brovall C, Fackler M J, Schwartz J F, Shaper J H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 12.Craig W, Kay R, Cutler R L, Lansdorp P M. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deichmann M, Kronenwett R, Haas R. Expression of the human immunodeficiency virus type-1 coreceptors CXCR-4 (fusin, LESTR) and CKR-5 in CD34+ hematopoietic progenitor cells. Blood. 1997;89:3522–3528. [PubMed] [Google Scholar]
- 14.De Luca A, Teofili L, Antinori A, Iovino M S, Mencarini P, Visconti E, Tamburrini E, Leone G, Ortona L. Haemopoietic CD34+ progenitor cells are not infected by HIV-1 in vivo but show impaired clonogenesis. Br J Haematol. 1993;85:20–24. doi: 10.1111/j.1365-2141.1993.tb08640.x. [DOI] [PubMed] [Google Scholar]
- 15.Donahue R E, Johnson M M, Zon L I, Clark S C, Groopman J E. Suppression of in vitro haematopoiesis following human immunodeficiency virus infection. Nature. 1987;326:200–203. doi: 10.1038/326200a0. [DOI] [PubMed] [Google Scholar]
- 16.Ganser A, Ottmann O G, von Briesen H, Volkers B, Rubsamen-Waigmann H, Hoelzer D. Changes in the haematopoietic progenitor cell compartment in the acquired immunodeficiency syndrome. Res Virol. 1990;141:185–193. doi: 10.1016/0923-2516(90)90020-j. [DOI] [PubMed] [Google Scholar]
- 17.Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas R, Mohle R, Pforsich M, Fruehauf S, Witt B, Goldschmidt H, Hunstein W. Blood-derived autografts collected during granulocyte colony-stimulating factor-enhanced recovery are enriched with early Thy-1+ hematopoietic progenitor cells. Blood. 1995;85:1936–1943. [PubMed] [Google Scholar]
- 19.Harris M, Patenaude P, Cooperberg P, Filipenko D, Thorne A, Raboud J, Rae S, Dailey P, Chernoff D, Todd J, Conway B, Montaner J S. Correlation of virus load in plasma and lymph node tissue in human immunodeficiency virus infection. INCAS Study Group. Italy, The Netherlands, Canada, Australia, and (United) States. J Infect Dis. 1997;176:1388–1392. doi: 10.1086/517328. [DOI] [PubMed] [Google Scholar]
- 20.Heise C, Miller C J, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 21.Heise C, Vogel P, Miller C J, Lackner A, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 22.Hillyer C D, Lackey D A D, Villinger F, Winton E F, McClure H M, Ansari A A. CD34+ and CFU-GM progenitors are significantly decreased in SIVsmm9 infected rhesus macaques with minimal evidence of direct viral infection by polymerase chain reaction. Am J Hematol. 1993;43:274–278. doi: 10.1002/ajh.2830430409. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins M, Hanley M B, Moreno M B, Wieder E, McCune J M. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood. 1998;91:2672–2678. [PubMed] [Google Scholar]
- 24.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klimpel G R, Langley K E, Wypych J, Abrams J S, Chopra A K, Niesel D W. A role for stem cell factor (SCF): c-kit interaction(s) in the intestinal tract response to Salmonella typhimurium infection. J Exp Med. 1996;184:271–276. doi: 10.1084/jem.184.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koka P S, Fraser J K, Bryson Y, Bristol G C, Aldrovandi G M, Daar E S, Zack J A. Human immunodeficiency virus inhibits multilineage hematopoiesis in vivo. J Virol. 1998;72:5121–5127. doi: 10.1128/jvi.72.6.5121-5127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kourtis A P, Ibegbu C, Nahmias A J, Lee F K, Clark W S, Sawyer M K, Nesheim S. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. . (Erratum, 336:595, 1997.) [DOI] [PubMed] [Google Scholar]
- 28.Louache F, Debili N, Marandin A, Coulombel L, Vainchenker W. Expression of CD4 by human hematopoietic progenitors. Blood. 1994;84:3344–3355. [PubMed] [Google Scholar]
- 29.Lunardi-Iskandar Y, Nugeyre M T, Georgoulias V, Barre-Sinoussi F, Jasmin C, Chermann J C. Replication of the human immunodeficiency virus 1 and impaired differentiation of T cells after in vitro infection of bone marrow immature T cells. J Clin Investig. 1989;83:610–615. doi: 10.1172/JCI113924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundqvist C, Baranov V, Hammarstrom S, Athlin L, Hammarstrom M L. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–1487. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- 31.Manegold C, Jablonowski H, Armbrecht C, Strohmeyer G, Pietsch T. Serum levels of stem cell factor are increased in asymptomatic human immunodeficiency virus-infected patients and are associated with prolonged survival. Blood. 1995;86:243–249. [PubMed] [Google Scholar]
- 32.Marandin A, Katz A, Oksenhendler E, Tulliez M, Picard F, Vainchenker W, Louache F. Loss of primitive hematopoietic progenitors in patients with human immunodeficiency virus infection. Blood. 1996;88:4568–4578. [PubMed] [Google Scholar]
- 33.Margolick J B, Donnenberg A D, Munoz A, Park L P, Bauer K D, Giorgi J V, Ferbas J, Saah A J. Changes in T and non-T lymphocyte subsets following seroconversion to HIV-1: stable CD3+ and declining CD3− populations suggest regulatory responses linked to loss of CD4 lymphocytes. The Multicenter AIDS Cohort Study. J Acquired Immune Defic Syndr. 1993;6:153–161. [PubMed] [Google Scholar]
- 34.Mattapallil J J, Smit-McBride Z, McChesney M, Dandekar S. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1β expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J Virol. 1998;72:6421–6429. doi: 10.1128/jvi.72.8.6421-6429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles S A, Lee K, Hutlin L, Zsebo K M, Mitsuyasu R T. Potential use of human stem cell factor as adjunctive therapy for human immunodeficiency virus-related cytopenias. Blood. 1991;78:3200–3208. [PubMed] [Google Scholar]
- 36.Mosley R L, Klein J R. Peripheral engraftment of fetal intestine into athymic mice sponsors T cell development: direct evidence for thymopoietic function of murine small intestine. J Exp Med. 1992;176:1365–1373. doi: 10.1084/jem.176.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller J G, Krenn V, Czub S, Stahl-Hennig C, Coulibaly C, Hunsmann G, Muller-Hermelink H K. The thymic epithelial reticulum and interdigitating cells in SIV-induced thymus atrophy and its comparison with other forms of thymus involution. Res Virol. 1993;144:93–98. doi: 10.1016/s0923-2516(06)80017-8. [DOI] [PubMed] [Google Scholar]
- 38.Muller J G, Krenn V, Schindler C, Czub S, Stahl-Hennig C, Coulibaly C, Hunsmann G, Kneitz C, Kerkau T, Rethwilm A, et al. Alterations of thymus cortical epithelium and interdigitating dendritic cells but no increase of thymocyte cell death in the early course of simian immunodeficiency virus infection. Am J Pathol. 1993;143:699–713. [PMC free article] [PubMed] [Google Scholar]
- 39.Murray L, Chen B, Galy A, Chen S, Tushinski R, Uchida N, Negrin R, Tricot G, Jagannath S, Vesole D, et al. Enrichment of human hematopoietic stem cell activity in the CD34+Thy-1+Lin− subpopulation from mobilized peripheral blood. Blood. 1995;85:368–378. [PubMed] [Google Scholar]
- 40.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S J, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 41.Poussier P, Julius M. T-cell development and selection in the intestinal epithelium. Semin Immunol. 1995;7:321–334. doi: 10.1016/1044-5323(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 42.Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol. 1994;12:521–553. doi: 10.1146/annurev.iy.12.040194.002513. [DOI] [PubMed] [Google Scholar]
- 43.Puddington L, Olson S, Lefrancois L. Interactions between stem cell factor and c-Kit are required for intestinal immune system homeostasis. Immunity. 1994;1:733–739. doi: 10.1016/s1074-7613(94)80015-4. [DOI] [PubMed] [Google Scholar]
- 44.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 46.Smit-McBride Z, Mattapallil J J, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stella C C, Ganser A, Hoelzer D. Defective in vitro growth of the hemopoietic progenitor cells in the acquired immunodeficiency syndrome. J Clin Investig. 1987;80:286–293. doi: 10.1172/JCI113071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone J D, Heise C C, Canfield D R, Elices M J, Dandekar S. Differences in viral distribution and cell adhesion molecule expression in the intestinal tract of rhesus macaques infected with pathogenic and nonpathogenic SIV. J Med Primatol. 1995;24:132–140. doi: 10.1111/j.1600-0684.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 50.Sutherland D R, Keating A. The CD34 antigen: structure, biology, and potential clinical applications. J Hematother. 1992;1:115–129. doi: 10.1089/scd.1.1992.1.115. [DOI] [PubMed] [Google Scholar]
- 51.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson P R, Desrosiers C R, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg R S, Chusid E D, Galperin Y, Thomson J C, Cheung T, Sacks H S. Effect of antiviral drugs and hematopoietic growth factors on in vitro erythropoiesis. Mt Sinai J Med. 1998;65:5–13. [PubMed] [Google Scholar]
- 53.Wineman J P, Gilmore G L, Gritzmacher C, Torbett B E, Muller-Sieburg C E. CD4 is expressed on murine pluripotent hematopoietic stem cells. Blood. 1992;80:1717–1724. [PubMed] [Google Scholar]
- 54.Wu L, Scollay R, Egerton M, Pearse M, Spangrude G J, Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 1991;349:71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
- 55.Wykrzykowska J J, Rosenzweig M, Veazey R S, Simon M A, Halvorsen K, Desrosiers R C, Johnson R P, Lackner A A. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J Exp Med. 1998;187:1767–1778. doi: 10.1084/jem.187.11.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zauli G, Re M C, Davis B, Sen L, Visani G, Gugliotta L, Furlini G, La Placa M. Impaired in vitro growth of purified (CD34+) hematopoietic progenitors in human immunodeficiency virus-1 seropositive thrombocytopenic individuals. Blood. 1992;79:2680–2687. [PubMed] [Google Scholar]
- 57.Zauli G, Re M C, Visani G, Furlini G, Mazza P, Vignoli M, La Placa M. Evidence for a human immunodeficiency virus type 1-mediated suppression of uninfected hematopoietic (CD34+) cells in AIDS patients. J Infect Dis. 1992;166:710–716. doi: 10.1093/infdis/166.4.710. [DOI] [PubMed] [Google Scholar]