Abstract
Aluminum, the third most abundant metal present in the earth’s crust, is present almost in all daily commodities we use, and exposure to it is unavoidable. The interference of aluminum with various biochemical reactions in the body leads to detrimental health effects, out of which aluminum-induced neurodegeneration is widely studied. However, the effect of aluminum in causing dyslipidemia cannot be neglected. Dyslipidemia is a global health problem, which commences to the cosmic of non-communicable diseases. The interference of aluminum with various iron-dependent enzymatic activities in the tri-carboxylic acid cycle and electron transport chain results in decreased production of mitochondrial adenosine tri-phosphate. This ultimately contributes to oxidative stress and iron-mediated lipid peroxidation. This mitochondrial dysfunction along with modulation of α-ketoglutarate and L-carnitine perturbs lipid metabolism, leading to the atypical accumulation of lipids and dyslipidemia. Respiratory chain disruption because of the accumulation of reduced nicotinamide adenine di-nucleotide as a consequence of oxidative stress and the stimulatory effect of aluminum exposure on glycolysis causes many health issues including fat accumulation, obesity, and other hepatic disorders. One major factor contributing to dyslipidemia and enhanced pro-inflammatory responses is estrogen. Aluminum, being a metalloestrogen, modulates estrogen receptors, and in this world of industrialization and urbanization, we could corner down to metals, particularly aluminum, in the development of dyslipidemia. As per PRISMA guidelines, we did a literature search in four medical databases to give a holistic view of the possible link between aluminum exposure and various biochemical events leading to dyslipidemia.
Keywords: Energy metabolism, estrogen, lipid metabolism, lipid peroxidation, metalloestrogen, oxidative stress
INTRODUCTION
Non-communicable diseases (NCDs) are a great challenge for the human population as they are incurable and need lifelong management. Dyslipidemia is commonly associated with many NCDs, and people with dyslipidemia are 18.1 times more prone to develop coronary heart disease compared to healthy people.[1] Dyslipidemia is a global health problem where the urban population is more vulnerable to it.[2,3] Some usual trends of dyslipidemic changes are high tri-glycerides (TGs), borderline high low-density lipoprotein cholesterol (LDL), and low high-density lipoprotein cholesterol (HDL).[2,3,4]
Aluminum (Al) exposure is a great threat to the community health, especially to the Al-exposed industrial workers.[5] Such workers showed elevated levels of serological indices of dyslipidemia compared to the unexposed ones.[6] The association between Al and dyslipidemia has been demonstrated experimentally. Acute and chronic exposure to aluminum chloride (AlCl3) produced dyslipidemic changes, showing a significant increase in total cholesterol (TC), TG, and LDL with decreased HDL and a decrease in TC/HDL and LDL/HDL ratios.[7,8,9] College-going male students reported an incidence of dyslipidemia linked with increased Al levels in their blood.[8] A specific dyslipidemic pattern has been linked to the hormonal changes associated with menopause. These are accountable for cardiovascular deaths and associated complications linked with disrupted estrogenic activity.[10]
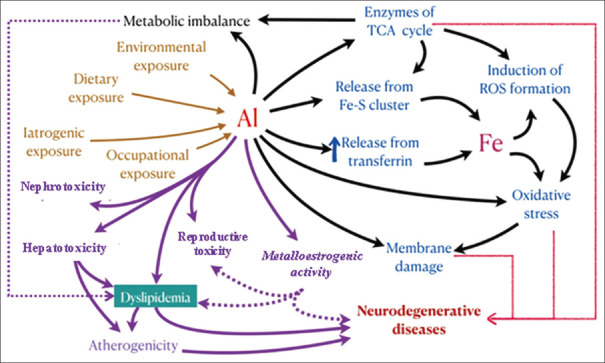
Al acts as an estrogen modulator disrupting the sexual and metabolic mechanisms, inducing an adipogenic environment in an individual.[11] Thus, in this review, we have addressed the possible metalloestrogenic role of Al in developing dyslipidemia. The potential interaction of Al with various enzymatic pathways related to energy metabolism in the generation of reactive oxygen species (ROS) and the misbalance of lipid metabolism is addressed. A composite view on the effect of Al in estrogen metabolism and disruption in lipid stores causing dyslipidemia is also highlighted [Figure 1].
Figure 1.
Possible link between aluminum and its effect on dyslipidemia. Aluminum exposure occurs through various routes. Aluminum toxicity causes nephrotoxicity, hepatotoxicity, neurodegenerative diseases, and reductive toxicity. Aluminum causes metabolic imbalance, disruption of the TCA cycle, perturbation of Fe homeostasis, and oxidative stress leading to dyslipidemia. Al: aluminium, Fe: iron
METHODS
According to the PRISMA guidelines for 2020, we did a literature search in four medical databases (PubMed, LAMA, Cochrane Central Register of Clinical Trials, Scopus, and the Google search engine).[12] We selected relevant studies from the past ten years till April 25, 2021. All the contributing authors independently searched the databases using the keywords “Aluminium” or “lipid metabolism” or “Aluminium and dyslipidemia” and “iron metabolism and lipid metabolism” and “estrogen or dyslipidemia” and “aluminium or metalloestrogen”. Some of the articles were also retrieved from the cross-references from previous published papers. The articles written in English were only selected. We selected the published peer-reviewed original articles, case series, and cross-sectional and observational studies. The inclusion criteria involved the articles were related to animal and human studies only. The exclusion criteria isolated case reports and case series with a sample size <5. Then, the eligible articles were analyzed and discussed further.
RESULTS AND DISCUSSION
Aluminum exposure
Al exposure is considerably high among workers employed in the industry involving Al products.[13] The general population is also vulnerable to the wrath of Al exposure through common food items containing Al as additives.[14] Leaching of Al occurs from beverage cans and cookware, which significantly adds to the average dietary intake of Al.[15] The European Food Safety Authority (EFSA) recommends the tolerable weekly intake of 1 mg Al/kg BW in some individuals.[16] Besides EFSA, other institutions have recommended several assessments of Al as shown in Table 1 (adapted from ref[5]). Exposure through the intact skin and gastrointestinal tract is extremely mild in humans.[14] Internal exposure of Al is known to be a better predictor and is determined from the Al levels in urine or blood.[5] Breast milk also contains a small amount of this metal, which is ingested by human infants.[17] Animal experiments have shown that on injecting Al subcutaneously to pregnant and/or lactating rats, the fetuses and sucklings get exposed to the metal through the transplacental route and/or through maternal milk.[18] This huge Al load disrupts the general metabolism in an individual often interposing with iron (Fe) metabolism, which will further be discussed.
Table 1.
Current assessments and classification of Al (adapted from ref[5])
| Institution | Classification/Values | Rationale/Recommendation |
|---|---|---|
| World Health Organization (WHO) | 2 mg Al/kg BW | Provisional tolerable weekly intake (PTWI) based on a no-observed-adverse-effect level (NOAEL) |
| European Food Safety Authority (EFSA) | 1 mg Al/kg BW | Tolerable weekly intake (TWI) |
| German Research Foundation (GRF) | 50 µg Al/g creatinine | Biological tolerance value at the workplace (BAT value) based on neurotoxicity as the critical endpoint |
| German Federal Environmental Agency (GFEA) | <15 µg Al/L of urine,<5 µg Al/L of plasma | Provisional reference values for the general population |
Aluminum interfering with iron metabolism
Al intoxication disrupts the biological membranes, damages DNA, and enhances ROS formation, along with Fe homeostasis perturbation.[19,20,21,22] This disrupted Fe metabolism is associated with Al-induced dyslipidemia.[23] The two metals, Fe and Al, share common etiopathogenic pathways related to several pathological conditions,[24] where the similarity of Al and Fe in terms of their ionic radii makes them compete for their biological interactions. Experimentally, it has been shown that Al interferes with Fe absorption and transfer, leading to cytotoxicity and oxidative stress (OS). Animals exposed to Al showed a simultaneous increase in tissue Al and Fe contents in the liver, kidney, spleen, and heart and at some specific brain regions.[19] This increase in the content of tissue Fe is because of the Al-induced stabilization of iron regulatory element-binding protein 2, thus enhancing the synthesis of transferrin receptors and obstructing the synthesis of ferritin.[19] Often, Al toxicity leads to a picture similar to Fe deficiency anemia, accompanied by an increase in total Fe-binding capacity and decreased Fe absorption.[25]
Al influences the accumulation of tri-carboxylic acid (TCA) cycle intermediates as evident in Al-exposed human liver cells (Hep G2 cells), which showed depleted mitochondrial bioavailability of Fe and accumulation of succinate.[26] Two hypothesized pathways of Fe dyshomeostasis include [i] interventions to the regulatory proteins of Fe homeostasis[19] and [ii] displacement of Fe from transferrin and its release in the circulation. The body’s Fe regulatory system fails to understand these increased Fe serum levels and recognizes it as Fe overload.[27] Thus, a situation of Al exposure is misinterpreted as Fe excess, and the body responds by secreting hepcidin, which in turn ceases the Fe absorption from macrophages, hepatocytes, and enterocytes, leading to Fe deficiency in the long run.[28,29]
Spectrophotometric studies of Al-stressed cells report a sharp decline in aconitase, a mitochondrial Fe-S cluster-containing enzyme.[30] The interaction between Al and Fe generates labile Fe from these enzymes and other proteins containing the Fe-S cluster, leading to an elevated level of free intra-cellular Fe and ROS formation.[31] Hepatocytes treated with Al showed reduced activity and expression of fumarase and succinate dehydrogenase (SDH), the other two Fe-S cluster-containing enzymes.[32] This leads to succinate accumulation in the cell and drives it toward anaerobic respiration, eventually causing dyslipidemia.[33,34]
Al intoxication causes Fe-mediated lipid peroxidation by interfering with many Fe-dependent enzymes in the TCA cycle and electron transport chain (ETC), resulting in decreased production of mitochondrial adenosine tri-phosphate (ATP).[35] This ultimately creates OS and Fe-mediated lipid peroxidation, which damages cell membranes and other lipids. This event is considered to have an important implication in hepatic diseases, and these cells show abnormal fat depots resulting in obesity and dyslipidemia. The effect of Al on energy metabolism is further discussed.
Aluminum and energy metabolism
Al tends to form complexes with ATP, blocking its availability as an energy source by inhibiting many enzymes that utilize ATP for their action.[36] Inhibition of hexokinase and isocitrate dehydrogenase by Al indicates its ability to alter the energy balance. Similarly, Al inhibits glycerol kinase, hindering the energy balance by preventing the lateral entry of glycerol into the glycolytic pathway.[37,38] Al was also found to inhibit an assay of hexokinase/G6PDH couple enzyme bioavailability in vitro.[20] The hexokinase in the rat brain showed a pH-dependent inhibition by Al.[39] It is obvious that Al causes energy imbalance by altering the performance of the most important ATP-utilizing enzyme of the body.[40] The most important mechanisms by which Al interferes with energy metabolism are listed below:
(a) Mitochondrial dysfunction and disruption of the Krebs Cycle
Al exposure perturbs the oxidative phosphorylation and production of ATP in Hep G2 cells.[26] Al has a stimulatory effect on SDH and α-ketoglutarate dehydrogenase (KGDH) and inhibits glutamate dehydrogenase. Glutamate dehydrogenase catalyzes α-ketoglutarate (AKG) synthesis from glutamate. The glutamate dehydrogenase inhibition results in decreased availability of AKG. Apart from this, Al also inhibits aconitase, a Fe-S cluster comprising protein that binds to citrate, catalyzing its isomerization to isocitrate, which is required to be converted to AKG, eventually leading to decreased availability of AKG.[41] Aconitase is also involved in the cellular regulation of Fe and energy production by mitochondria[42] by regulating and coding for proteins involved in the function of Fe availability.[43]
A substantial decrease in the action of SDH, KGDH, isocitrate dehydrogenase (IDH), fumarase, aconitase, and cytochrome oxidase in Al-exposed cells has been verified experimentally.[32] Findings from in vitro studies of Hep G2 cells cultured with Al show a diminished flux of the TCA cycle and oxidative phosphorylation, impeding production of ATP.[44]
(b) ROS and ɑ-Ketoglutarate
There are several mechanisms proposed to illustrate the pro-oxidative effects of Al, which include the interaction with (i) superoxide radicals, (ii) other pro-oxidants such as Fe, and (iii) the target substrate, such as membrane lipids.[21,45] These facts have ultimately resulted in the alleviation of (i) non-Fe- and Fe-mediated lipid peroxidation,[46,47] (ii) non-Fe-mediated hydroxyl radical formation,[48] and (iii) non-Fe-intervened oxidation of reduced nicotinamide adenine dinucleotide (NADH).[49,50] Al exposure is able to disrupt the Fe homeostasis inside the cell, resulting in ROS synthesis.[38]
ROS synthesis is linked to reduced KGDH activity, resulting in decreased NADH formation (pro-oxidant) and AKG aggregation (anti-oxidant) at the expense of energy production and mitochondrial dysfunction.[38] Al-exposed Hep G2 cells hoarded succinate, a derivative of AKG, which leads to ROS sequestration. AKG supplementation led to degradation and hydroxylation of the hypoxia-inducible factor (HIF).[33] The Al-mediated mitochondrial metabolism derangement and the subsequent commitment of AKG to ROS sequestration stabilize HIF-1α and enhance the ATP glycolytic efficiency.[51] In vivo, Al facilitates the activities of the superoxide radical, thus accelerating oxidative damage via Fenton reaction.[52] Mitochondria are the major sites of ROS formation, and the pathophysiology behind Al-induced neurodegeneration is determined by the oxidative damage caused to many mitochondrial proteins.[53]
Accumulation of TCA cycle intermediates is also an important hallmark of Al-induced OS. Metal toxicity-induced OS is known to hinder cellular ATP levels.[54] Cardiomyocyte exposure to hydrogen peroxide (H2O2) results in a reduction in the cellular ATP levels.[55] There is a metabolic shift toward glycolysis to meet the ATP demands following Al exposure.[56] Laboratory animals exposed to Al show changes in many OS indices, including an increased level of superoxide dismutase and catalase along with increased peroxidative biomarkers such as malondialdehyde and lipid hydroperoxides.[57,58] Anti-oxidants such as vitamin E and N-acetyl cysteine protect against the oxidative damage caused by Al in experimental animals.[59,60] Hepatocytes exposed to Al alter the TCA cycle flux by the inhibition of KGDH and NAD-dependent IDH.[44,61] This ultimately results in anaerobiosis, leading to adequate release of ROS.[62]
(c) Anaerobiosis
Under the normoxic conditions, prolyl hydroxylase (PHD) and HIF prolyl hydroxylase help in the degradation of HIF-1α. Decreased expression of PHD in Al-exposed cells caused HIF-1α stabilization.[62,63] ROS and succinate interfere with PHD activity, thereby stabilizing HIF-1α and relaying mitochondrial activity to the nucleus through the PHD-HIF signal.[34] Al-exposed Hep G2 cells show altered PHD levels by increasing the production of succinate and ROS as well as restricting the accessibility of AKG, a principal prosthetic group required for the activity of PHD.[50]
Furthermore, Al-exposed hepatocytes, when supplemented with AKG, trigger HIF-1α hydroxylation and degradation, suggesting mitochondrial dysfunction promoting anaerobiosis.[33] Acute exposure to Al inhibited various key enzymes for glycolysis and ATP production.[64] Therefore, to meet the ATP requirements of the Al-exposed cell, a metabolic shift toward glycolysis through anaerobic metabolism is required. This is further supported by a study by Kumar et al.,[65] where mitochondrial preparation from Al-exposed rats showed reduced activity of certain ETC complexes in the hippocampal cell. The various cytochrome levels were decreased following Al exposure, indicating altered mitochondrial metabolism. Al-exposed cells also exhibited disruption of β-oxidation of fatty acids (FAs), which promotes dyslipidemia and may result in several hepatic abnormalities.[50]
(d) Lipogenesis
Al-induced mitochondrial dysfunction contributes to the transition of metabolized carbohydrates toward the biosynthesis of lipids and the accumulation of TG. The presence of a high amount of very low-density lipoprotein cholesterol (VLDL) and lipids in Al-exposed cells indicates that Al enhances lipid biosynthesis and secretion.[26] Interruption of the TCA cycle results in the accumulation of citrate that is exported to the cytosol, and fatty acyl moieties are produced through the activity of lipogenic enzymes. Esterification of glycerol-containing fatty acyl groups results in the formation of TG, which is either transported extracellularly as VLDL or stored in the cytosol.[66]
Both Al exposure and ROS production critically diminish L-carnitine levels and cause lipid accumulation in hepatocytes and changes in L-carnitine levels.[67] Human astrocytes and hepatocytes exposed to Al and H2O2 showed decreased levels of L-carnitine, reduced β-oxidation, and increased accumulation of lipids.[62] During the Al and H2O2 challenge, reduction in the two key enzymes, butyrobetainealdehyde dehydrogenase and butyrobetainealdehyde dioxygenase, involved in L-carnitine biogenesis was observed. Conversely, on exposure to AKG, the Al- and H2O2-treated cells resulted in the increased production of L-carnitine with a significant reduction in ROS levels. Therefore, it seems that the channeling of available AKG to avert OS results in reduced synthesis of L-carnitine contributing to dyslipidemia.[62] Al-treated Hep G2 cells not only enhance lipid droplet generation but also are unable to oxidize palmitic acid.[68] In another instance, oxidation of palmitic acid is restored after a 24-hour incubation in 5 Mm AKG.[62] This L-carnitine recovery with AKG shows that the toxicity of Al or ROS diminishes the availability of AKG for it to be used in ROS detoxification. This failure to manage L-carnitine levels contributes to the accumulation of lipids in the heart, skeletal muscle, kidney, and liver.[69]
Thus, Al toxicity disrupts the enzymes in the TCA cycle disabling aerobic production of ATP, promoting anaerobic glycolysis, generation of ROS, and mitochondrial dysfunction. This mitochondrial dysfunction along with modulation of AKG and L-carnitine perturbs lipid metabolism, leading to the accumulation of lipids, dyslipidemia, and hepatic steatosis as observed in obesity and metabolic syndrome. Further, it is also considered that estrogen is a potent regulator of lipid metabolism and energy release. In the following sections, we will highlight how the metalloestrogenic property of Al might be the possible cause leading to dyslipidemia.
Aluminum as a metalloestrogen
Many chemicals and heavy metals exhibit estrogenic activity by changing the gene expression or altering the activity of estrogenic receptors.[70] Reports suggest that they initiate estrogen agonist responses both in vitro and in vivo, and these inorganic environmental estrogens and xenoestrogens are termed metalloestrogens. The metalloestrogens disrupt the sexual and metabolic mechanisms inducing an adipogenic environment. Besides, it is also hypothesized that Al hinders the normal physiological pathway of estrogen and results in severe dyslipidemia.[11,26] Before addressing these concepts, the relationship between estrogen and lipid metabolism as well as energy release is highlighted.
Estrogen and lipid metabolism
Estrogen and estrogen receptors are involved in glucose and lipid metabolism.[71] The absence of estrogen during the menopausal period leads to dyslipidemia and abdominal fat deposition.[72] A significant increase in TC, TG, LDL, and LDL/HDL ratio was seen in post-menopausal women.[73] 17 β estradiol (E2) is the principal circulating estrogen in the human body, and it acts through estrogen receptor α, β (ERα and ERβ) and also the G-protein-coupled estrogen receptor (GPER). Two pathways are induced by E2 binding to GPER; one increases cyclic adenosine mono-phosphate concentrations, and the other increases intra-cellular Ca2+ concentrations.[74] Atherosclerosis is promoted by the deletion of GPER and thus causes elevated levels of LDL in mice fed with an atherogenic diet.[74]
The human subcutaneous tissue and visceral adipose tissue (VAT) express both ERα and ERβ,[75] where ERα plays an essential role in the sexual dimorphism related to the distribution of body fat. Female and male mice missing ERα demonstrate central obesity and are susceptible to insulin resistance.[76] At the cellular level, estrogen appears to be involved in regulating messenger ribonucleic acid (mRNA) production for proteins involved in lipid metabolism.[77] E2 directly increases the synthesis of lipoprotein lipase, whereas the synthesis of hormone-sensitive lipase is lowered.
In the liver, E2 reduces apo B-100 synthesis while stimulating apolipoproteins AI, AII, and CIII synthesis, thereby regulating the synthesis of VLDL and HDL.[78] The HDL fraction containing apo AI and AII is essential for degradation of chylomicrons and VLDL apart from direct and indirect transport of cholesterol to hepatocytes. Estrogen mediates reductions in the delivery of FAs to hepatocytes and increases the export of VLDL.[79] Ovariectomy in rodents leads to TG accumulation in the liver.[79]
ERα in hepatocytes can reduce steatosis, and this function is lost with the deletion of ERα, resulting in enhanced expression of genes involved in lipid synthesis.[80] Treatment with E2 diminishes lipogenesis in the liver by maintaining phosphorylation of acetyl CoA carboxylase.[80] It also enhances FA oxidation in hepatocytes and increases the mRNA levels of carnitine palmitoyltransferase I, a protein to transport FA for β-oxidation in mitochondria.[81]
The defensive effects of estrogen in hepatocytes are presumably because of the adipose tissue signaling by estrogen to reduce the FA release as a response to insulin and to facilitate FA oxidation in the skeletal muscle, thereby reducing the FA supply to the hepatocytes. Estrogen enhances FA oxidation in muscles and blocks muscles as well as hepatic lipogenesis via the regulation of the peroxisome proliferator-activated receptor gamma receptor, activated by a peroxisome proliferator.[82] E2 has also been reported to accentuate the oxidative capacity of muscles by regulating uncoupling proteins (UCP-2, UCP-3) and acyl CoA oxidase, which enhances FA uptake without lipid accumulation.[83] An estrogen-responsive element has been identified in the inducer region of β-hydroxyl β-methylglutaryl-CoA (HMG CoA) reductase genes, which is an important rate-limiting enzyme in cholesterol synthesis. Animal studies have shown a lower level of HMG CoA reductase protein in females treated with E2. A previous report by Pedram et al.[84] suggests that expression of HMG-CoA reductase and cholesterol content in the liver is suppressed by the ERα agonist and is coupled with decreased expression of the sterol regulatory element binding transcription factor. Estrogen triggers the hepatic cholesterol secretion into bile and has been reported to undergo inhibition by parallel ERα antagonist treatment.[85] There seems to exist a down-regulation of the expression of certain enzymes involved in the biosynthesis of bile acid as seen in ovariectomized animals.[86] The pathway of the estrogen-governed elevation in the synthesis of bile acid gene expression requires hepatic ERα.[87]
Estrogen and energy metabolism
Estrogen regulates glucose/energy metabolism by controlling the expression and activity of various enzymes involved in the TCA cycle.[88] During sexual maturation, estrogens promote lipid accumulation and adjust the lipid composition. However, a post-menopausal rise in fat indicates that estrogens play a significant role in differentiating adipocytes. In the pre-menopausal period, women have a greater amount of total body fat than men, with most of it accumulating within subcutaneous (gluteofemoral), whereas males tend to have more fat in the visceral compartment as compared to pre-menopausal females.[89] The rise in cardiometabolic risk following menopause is aligned with body fat accumulation and has been reported to be reversed by hormone replacement therapy.[90] Usually, the functions of the adipose tissue include insulation of heat, mechanical cushioning, and tri-acylglycerol preservation, whereas visceral fat is considered a highly metabolic tissue. Visceral fat is highly vulnerable to lipolysis compared to subcutaneous tissues. VAT is accompanied by elevated production of tumor necrotic factor α (TNFα), plasminogen activator inhibitor 1, C-reactive protein, and interleukin-6.[91] Subcutaneous fat is more protective and produces anti-inflammatory substances such as insulin-sensitizing adipokine.
According to reports, glucose intolerance, hyperinsulinemia, and hyperglycemia are linked with a lack of activity in ERα receptors or aromatase genes.[89] It is also established that obese post-menopausal females have a greater serum estrogen concentration relative to slim post-menopausal women, and somewhere, the explanation for this could be the pro-inflammatory syndrome associated with obesity because aromatase is triggered by cytokines such as TNFα.[92] E2 modulates both the concentration of lipid substances in plasma and atherogenicity by controlling the metabolism of lipids in adipocytes and hepatocytes.
Aluminum and dyslipidemia
Metalloestrogens such as Al may specifically activate estrogen receptors, contributing to detrimental health effects.[70] Tsialtasa reported that Al-chlorohydrate (ACH) exposure to MCF-7 breast cancer cells had no specific effect on the transcription of estrogen receptors but caused an increase in ERα protein levels, whereas administration of E2 resulted in a reduction in ERα protein levels.[93] ACH caused a reduction in ERβ protein levels and an increase in its mitochondrial localization.[93] Because ERα is known to control ERβ expression negatively, an increase in ERα may lead to a decline in ERβ levels.[94] On the other hand, increased localization of mitochondrial ERβ may induce mitochondrial metabolism and down-regulate gluconeogenesis as indicated by a reduction in the phosphoenolpyruvate carboxylase protein level, eventually leading to ROS generation.[95] Also supported by Dabre and his colleagues, long-term exposure to Al salts caused a two-fold upsurge in reporter gene expression driven by estrogen response elements in the MCF-7 cells.[96]
From the above pieces of evidence, it can be concluded that Al not only affects estrogenic receptors differentially and increases ERα proteins but also causes mitochondrial localization of ERβ, which affects mitochondrial metabolism and eventually results in the stimulation of the respiratory chain and ROS generation. Modulation of the estrogen receptors disrupts the lipid metabolism, bringing about changes in adipose tissue depots and increasing the pro-inflammatory responses. All these taken together are responsible for the dyslipidemic changes, observed in long-term Al exposure in the human population at large.
CONCLUSION
Thus, Al as an active pro-oxidant interrupts aerobic metabolism by dispersing Fe bioavailability within the mitochondria, eventually inducing OS. This ultimately disrupts the flux of the TCA cycle along with oxidative phosphorylation, obstructing the ATP production. The respiratory chain dishevels because of the accumulation of NADH as a consequence of OS. These mechanisms along with the stimulatory effect of Al exposure on glycolysis are conducive to many health issues including fat accumulation, obesity, and other hepatic disorders as depicted in Figure 1. The metalloestrogenic property of Al might be a possible cause to these effects, which lead to dyslipidemic changes in the populations exposed to the menace of Al. Although the exposure to Al is unavoidable, on knowing the enormous amount of interference that Al does with the normal lipid metabolism causing life-long health problems, we should limit the use of Al.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ariyanti R, Besral B. Dyslipidemia associated with hypertension increases the risks for coronary heart disease: A case-control study in Harapan Kita Hospital, National Cardiovascular Center, Jakarta. J Lipids. 2019;2019:2517013. doi: 10.1155/2019/2517013. doi:10.1155/2019/2517013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan NC, Koh KH, Goh CC, Koh YLE, Goh SCP. Asian patients with dyslipidemia in an urban population: Effect of ethnicity on their LDL-cholesterol treatment goals. J Clin Lipidol. 2016;10:410–9. doi: 10.1016/j.jacl.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18:689–700. doi: 10.1038/s41569-021-00541-4. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Rao RS, Misra A, Sharma SK. Recent trends in epidemiology of dyslipidemias in India. Indian Heart J. 2017;69:382–92. doi: 10.1016/j.ihj.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H. The health effects of aluminum exposure. Dtsch Aerzteblatt Online. 2017;114:653–9. doi: 10.3238/arztebl.2017.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.If G, Sf H, La R, Aa A. Dyslipidemia and disruption of L-Carnitine in aluminum exposed workers. Egypt J Occup Med. 2013;37:33–46. [Google Scholar]
- 7.Nampoothiri M, John J, Kumar N, Mudgal J, Nampurath GK, Chamallamudi MR. Modulatory role of simvastatin against aluminium chloride-induced behavioural and biochemical changes in rats. Behav Neurol. 2015;2015:210169. doi: 10.1155/2015/210169. doi:10.1155/2015/210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung E, Hyun W, Ro Y, Lee H, Song K. A study on blood lipid profiles, aluminum and mercury levels in college students. Nutr Res Pract. 2016;10:442–7. doi: 10.4162/nrp.2016.10.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghorbel I, Elwej A, Chaabane M, Jamoussi K, Zeghal N. Protective effect of selenium against aluminium chloride induced cardiotoxicity in rats. Pharm Biomed Res. 2017;3:19–25. [Google Scholar]
- 10.Toth P, Phan BA. Dyslipidemia in women: Etiology and management. Int J Womens Health. 2014;6:185–94. doi: 10.2147/IJWH.S38133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darbre PD. Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006;26:191–7. doi: 10.1002/jat.1135. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. doi:10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesdock JC, Arnold IMF. Occupational and environmental health in the aluminum industry: Key points for health practitioners. J Occup Environ Med. 2014;56((Suppl 5S)):S5–11. doi: 10.1097/JOM.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokel RA, McNamara PJ. Aluminium toxicokinetics: An updated mini review. Pharmacol Toxicol. 2001;88:159–67. doi: 10.1034/j.1600-0773.2001.d01-98.x. [DOI] [PubMed] [Google Scholar]
- 15.Ja-Liang L, Yu-Jen Y, Sun-Shen Y, Mei-Ling L. Aluminum utensils contribute to aluminum accumulation in patients with renal disease. Am J Kidney Dis. 1997;30:653–8. doi: 10.1016/s0272-6386(97)90489-3. [DOI] [PubMed] [Google Scholar]
- 16.European Food Safety Authority (EFSA) Safety of aluminium from dietary intake - scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC) EFSA J. 2008;6 doi: 10.2903/j.efsa.2008.754. doi:10.2903/j.efsa. 2008.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak P. Aluminum: Impacts and disease. Environ Res. 2002;89:101–15. doi: 10.1006/enrs.2002.4352. [DOI] [PubMed] [Google Scholar]
- 18.Yumoto S, Nagai H, Matsuzaki H, Matsumura H, Tada W, Nagatsuma E, et al. Aluminium incorporation into the brain of rat fetuses and sucklings. Brain Res Bull. 2001;55:229–34. doi: 10.1016/s0361-9230(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 19.Ward RJ, Zhang Y, Crichton RR. Aluminium toxicity and iron homeostasis. J Inorg Biochem. 2001;87:9–14. doi: 10.1016/s0162-0134(01)00308-7. [DOI] [PubMed] [Google Scholar]
- 20.Exley C, Birchall JD, Price NC. Aluminum inhibition of hexokinase activity in vitro: A study in biological availability. J Inorg Biochem. 1994;54:297–304. doi: 10.1016/0162-0134(94)80035-9. [DOI] [PubMed] [Google Scholar]
- 21.Exley C. The pro-oxidant activity of aluminum. Free Radic Biol Med. 2004;36:380–7. doi: 10.1016/j.freeradbiomed.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V, Bal A, Gill KD. Aluminium-induced oxidative DNA damage recognition and cell-cycle disruption in different regions of rat brain. Toxicology. 2009;264:137–44. doi: 10.1016/j.tox.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, He B, Xiao Y, Chen Y. Iron metabolism and its association with dyslipidemia risk in children and adolescents: A cross-sectional study. Lipids Health Dis. 2019;18:50. doi: 10.1186/s12944-019-0985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J-L, Fan Y-G, Yang Z-S, Wang Z-Y, Guo C. Iron and Alzheimer's disease: From pathogenesis to therapeutic implications. Front Neurosci. 2018;12 doi: 10.3389/fnins.2018.00632. doi:10.3389/fnins. 2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannata JB, Gómez Alonso C, Fernández Menéndez MJ, Fernández Soto I, McGregor S, Menéndez-Fraga P, et al. Iron uptake in aluminium overload: In vivo and in vitro studies. Nephrol Dial Transplant. 1991;6:637–42. doi: 10.1093/ndt/6.9.637. [DOI] [PubMed] [Google Scholar]
- 26.Mailloux R, Lemire J, Appanna V. Aluminum-induced mitochondrial dysfunction leads to lipid accumulation in human hepatocytes: A link to obesity. Cell Physiol Biochem. 2007;20:627–38. doi: 10.1159/000107546. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DF. The regulation of iron absorption and homeostasis. Clin Biochem Rev. 2016;37:51–62. [PMC free article] [PubMed] [Google Scholar]
- 28.Bignucolo A, Lemire J, Auger C, Castonguay Z, Appanna V, Appanna VD. The molecular connection between aluminum toxicity, anemia, inflammation and obesity: Therapeutic cues. Anemia. 2012 doi:10.5772/30273. [Google Scholar]
- 29.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–33. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mailloux RJ, Appanna VD. Aluminum toxicity triggers the nuclear translocation of HIF-1alpha and promotes anaerobiosis in hepatocytes. Toxicol Vitro Int J Publ Assoc BIBRA. 2007;21:16–24. doi: 10.1016/j.tiv.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Nayak P, Sharma SB, Chowdary NVS. Augmentation of aluminum-induced oxidative stress in rat cerebrum by presence of pro-oxidant (graded doses of ethanol) exposure. Neurochem Res. 2010;35:1681–90. doi: 10.1007/s11064-010-0230-3. [DOI] [PubMed] [Google Scholar]
- 32.Mailloux RJ, Hamel R, Appanna VD. Aluminum toxicity elicits a dysfunctional TCA cycle and succinate accumulation in hepatocytes. J Biochem Mol Toxicol. 2006;20:198–208. doi: 10.1002/jbt.20137. [DOI] [PubMed] [Google Scholar]
- 33.MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–9. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Quinlan GJ, Halliwell B, Moorhouse CP, Gutteridge JM. Action of lead (II) and aluminium (III) ions on iron-stimulated lipid peroxidation in liposomes, erythrocytes and rat liver microsomal fractions. Biochim Biophys Acta. 1988;962:196–200. doi: 10.1016/0005-2760(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 36.Ganrot PO. Metabolism and possible health effects of aluminum. Environ Health Perspect. 1986;65:363–441. doi: 10.1289/ehp.8665363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zatta P, Lain E, Cagnolini C. Effects of aluminum on activity of Krebs cycle enzymes and glutamate dehydrogenase in rat brain homogenate. Eur J Biochem. 2000;267:3049–55. doi: 10.1046/j.1432-1033.2000.01328.x. [DOI] [PubMed] [Google Scholar]
- 38.Mailloux RJ, Lemire J, Appanna VD. Hepatic response to aluminum toxicity: Dyslipidemia and liver diseases. Exp Cell Res. 2011;317:2231–8. doi: 10.1016/j.yexcr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Frances CW, Sidney PC. Proton-dependent inhibition of yeast and brain hexokinases by aluminum in ATP preparations. Proc Natl Acad Sci USA. 1979;76:5080–4. doi: 10.1073/pnas.76.10.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health Part B. 2007;10((Suppl 1)):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zatta P, Lain E, Cagnolini C. Effects of aluminum on activity of krebs cycle enzymes and glutamate dehydrogenase in rat brain homogenate. Eur J Biochem. 2000;267:3049–55. doi: 10.1046/j.1432-1033.2000.01328.x. [DOI] [PubMed] [Google Scholar]
- 42.Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–32. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alén C, Sonenshein AL. Bacillus subtilis aconitase is an RNA-binding protein. Proc Natl Acad Sci U S A. 1999;96:10412–7. doi: 10.1073/pnas.96.18.10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mailloux RJ, Bériault R, Lemire J, Singh R, Chénier DR, Hamel RD, et al. The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLos One. 2007;2:e690. doi: 10.1371/journal.pone.0000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Exley C. A biogeochemical cycle for aluminium? J Inorg Biochem. 2003;97:1–7. doi: 10.1016/s0162-0134(03)00274-5. [DOI] [PubMed] [Google Scholar]
- 46.Verstraeten SV, Oteiza PI. Effects of Al3+and related metals on membrane phase state and hydration: Correlation with lipid oxidation. Arch Biochem Biophys. 2000;375:340–6. doi: 10.1006/abbi.1999.1671. [DOI] [PubMed] [Google Scholar]
- 47.Gutteridge JMC, Quinlan GJ, Clark I, Halliwell B. Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim Biophys Acta BBA-Lipids Lipid Metab. 1985;835:441–7. doi: 10.1016/0005-2760(85)90113-4. [DOI] [PubMed] [Google Scholar]
- 48.Méndez-Alvarez E, Soto-Otero R, Hermida-Ameijeiras A, López-Real AM, Labandeira-García JL. Effects of aluminum and zinc on the oxidative stress caused by 6-hydroxydopamine autoxidation: Relevance for the pathogenesis of Parkinson's disease. Biochim Biophys Acta. 2002;1586:155–68. doi: 10.1016/s0925-4439(01)00077-1. [DOI] [PubMed] [Google Scholar]
- 49.Meglio L, Oteiza PI. Aluminum enhances melanin-induced lipid peroxidation. Neurochem Res. 1999;24:1001–8. doi: 10.1023/a:1021000709082. [DOI] [PubMed] [Google Scholar]
- 50.Kong S, Liochev S, Fridovich I. Aluminum (III) facilitates the oxidation of NADH by the superoxide anion. Free Radic Biol Med. 1992;13:79–81. doi: 10.1016/0891-5849(92)90168-g. [DOI] [PubMed] [Google Scholar]
- 51.Morten KJ, Badder L, Knowles HJ. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J Pathol. 2013;229:755–64. doi: 10.1002/path.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zatta P, Kiss T, Suwalsky M, Berthon G. Aluminium (III) as a promoter of cellular oxidation. Coord Chem Rev. 2002;228:271–84. [Google Scholar]
- 53.Kumar V, Bal A, Gill KD. Susceptibility of mitochondrial superoxide dismutase to aluminium induced oxidative damage. Toxicology. 2009;255:117–23. doi: 10.1016/j.tox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Yuan C-Y, Lee Y-J, Hsu G-SW. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J Biomed Sci. 2012;19:51. doi: 10.1186/1423-0127-19-51. doi:10.1186/1423-0127-19-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janero DR, Hreniuk D, Sharif HM. Hydrogen peroxide-induced oxidative stress to the mammalian heart-muscle cell (cardiomyocyte): Nonperoxidative purine and pyrimidine nucleotide depletion. J Cell Physiol. 1993;155:494–504. doi: 10.1002/jcp.1041550308. [DOI] [PubMed] [Google Scholar]
- 56.Exley C, Derek Birchall J. The cellular toxicity of aluminium. J Theor Biol. 1992;159:83–98. doi: 10.1016/s0022-5193(05)80769-6. [DOI] [PubMed] [Google Scholar]
- 57.Atienzar F, Desor D, Burnel D, Keller JM, Lehr P, Vasseur P. Effect of aluminum on superoxide dismutase activity in the adult rat brain. Biol Trace Elem Res. 1998;65:19–30. doi: 10.1007/BF02784111. [DOI] [PubMed] [Google Scholar]
- 58.Swain C, Chainy GB. Effects of aluminum sulphate and citric acid ingestion on lipid peroxidation and on activities of superoxide dismutase and catalase in cerebral hemisphere and liver of developing young chicks. Mol Cell Biochem. 1998;187:163–72. doi: 10.1023/a:1006831409769. [DOI] [PubMed] [Google Scholar]
- 59.Abubakar MG, Taylor A, Ferns GAA. Aluminium administration is associated with enhanced hepatic oxidant stress that may be offset by dietary vitamin E in the rat. Int J Exp Pathol. 2003;84:49–54. doi: 10.1046/j.1365-2613.2003.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009;1275:87–95. doi: 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mailloux RJ, Puiseux-Dao S, Appanna VD. Alpha-ketoglutarate abrogates the nuclear localization of HIF-1alpha in aluminum-exposed hepatocytes. Biochimie. 2009;91:408–15. doi: 10.1016/j.biochi.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Lemire J, Mailloux R, Darwich R, Auger C, Appanna VD. The disruption of L-carnitine metabolism by aluminum toxicity and oxidative stress promotes dyslipidemia in human astrocytic and hepatic cells. Toxicol Lett. 2011;203:219–26. doi: 10.1016/j.toxlet.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Pollard P, Yang M, Su H, Soga T, Kranc K. Prolyl hydroxylase domain enzymes: Important regulators of cancer metabolism. Hypoxia. 2014;2:127–42. doi: 10.2147/HP.S47968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dua R, Kumar V, Sunkariya A, Gill KD. Altered glucose homeostasis in response to aluminium phosphide induced cellular oxygen deficit in rat. Indian J Exp Biol. 2010;48:722–30. [PubMed] [Google Scholar]
- 65.Kumar V, Bal A, Gill KD. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res. 2008;1232:94–103. doi: 10.1016/j.brainres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 66.Cox RA, García-Palmieri MR. Cholesterol, triglycerides, and associated lipoproteins. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; 1990. [[Last accessed on 2020 Jun 19]]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK351/ [Google Scholar]
- 67.Infante JP, Huszagh VA. Secondary carnitine deficiency and impaired docosahexaenoic (22:6n-3) acid synthesis: A common denominator in the pathophysiology of diseases of oxidative phosphorylation and beta-oxidation. FEBS Lett. 2000;468:1–5. doi: 10.1016/s0014-5793(00)01083-8. [DOI] [PubMed] [Google Scholar]
- 68.Xu H-Y, Yu L, Chen J-H, Yang L-N, Lin C, Shi X-Q, et al. Sesamol alleviates obesity-related hepatic steatosis via activating hepatic PKA pathway. Nutrients. 2020;12:329. doi: 10.3390/nu12020329. doi:10.3390/nu12020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. 2016;1863:2422–35. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace D. Nanotoxicology and metalloestrogens: Possible involvement in breast cancer. Toxics. 2015;3:390–413. doi: 10.3390/toxics3040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coyoy A, Guerra-Araiza C, Camacho-Arroyo I. Metabolism regulation by estrogens and their receptors in the central nervous system before and after menopause. Horm Metab Res. 2016;48:489–96. doi: 10.1055/s-0042-110320. [DOI] [PubMed] [Google Scholar]
- 72.Lizcano F, Guzmán G. Estrogen deficiency and the origin of obesity during menopause. BioMed Res Int. 2014;2014:757461. doi: 10.1155/2014/757461. doi:10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reddy Kilim S, Chandala SR. A comparative study of lipid profile and oestradiol in pre- and post-menopausal women. J Clin Diagn Res. 2013;7:1596–8. doi: 10.7860/JCDR/2013/6162.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi H, Dharshan Senthil Kumar SP, Liu X. G protein-coupled estrogen receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:193–250. doi: 10.1016/B978-0-12-386933-3.00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–42. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: An emerging regulator of insulin action and mitochondrial function. J Diabetes Res. 2015;2015:916585. doi: 10.1155/2015/916585. doi:10.1155/2015/916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szafran H, Smielak-Korombel W. [The role of estrogens in hormonal regulation of lipid metabolism in women. Przegl Lek. 1998;55:266–70. [PubMed] [Google Scholar]
- 78.Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2000. [[Last accessed on 2020 Jun 25]]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK305896/ [Google Scholar]
- 79.Palmisano BT, Zhu L, Stafford JM. Estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227–56. doi: 10.1007/978-3-319-70178-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu L, Shi J, Luu TN, Neuman JC, Trefts E, Yu S, et al. Hepatocyte estrogen receptor alpha mediates estrogen action to promote reverse cholesterol transport during Western-type diet feeding. Mol Metab. 2018;8:106–16. doi: 10.1016/j.molmet.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mauvais-Jarvis F. Biomed Central, London: Springer; 2017. Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; p. 630. [Google Scholar]
- 82.Huss JM, Torra IP, Staels B, Giguère V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–91. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ježek P, Holendová B, Garlid KD, Jabůrek M. Mitochondrial uncoupling proteins: Subtle regulators of cellular redox signaling. Antioxid Redox Signal. 2018;29:667–714. doi: 10.1089/ars.2017.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedram A, Razandi M, O'Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal. 2013;6:ra36. doi: 10.1126/scisignal.2004013. [DOI] [PubMed] [Google Scholar]
- 85.Wang HH, Afdhal NH, Wang DQ-H. Estrogen receptor alpha, but not beta, plays a major role in 17beta-estradiol-induced murine cholesterol gallstones. Gastroenterology. 2004;127:239–49. doi: 10.1053/j.gastro.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 86.Phelps T, Snyder E, Rodriguez E, Child H, Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol Sex Differ. 2019;10:52. doi: 10.1186/s13293-019-0265-3. doi:10.1186/s13293-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Della Torre S, Mitro N, Fontana R, Gomaraschi M, Favari E, Recordati C, et al. An essential role for liver ER? in coupling hepatic metabolism to the reproductive cycle. Cell Rep. 2016;15:360–71. doi: 10.1016/j.celrep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J-Q, Brown TR, Russo J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim Biophys Acta. 2009;1793:1128–43. doi: 10.1016/j.bbamcr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues-the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. doi:10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014;2014:615917. doi: 10.1155/2014/615917. doi:10.1155/2014/615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr Metab. 2004;1:12. doi: 10.1186/1743-7075-1-12. doi:10.1186/1743-7075-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res Phila Pa. 2011;4:329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Tsialtas I, Gorgogietas VA, Michalopoulou M, Komninou A, Liakou E, Georgantopoulos A, et al. Neurotoxic effects of aluminum are associated with its interference with estrogen receptors signaling. Neurotoxicology. 2020;77:114–26. doi: 10.1016/j.neuro.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Zhao C, Matthews J, Tujague M, Wan J, Ström A, Toresson G, et al. Estrogen receptor ?2 negatively regulates the transactivation of estrogen receptor ? in human breast cancer cells. Cancer Res. 2007;67:3955–62. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 95.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Darbre PD. The potential for estrogen disrupting chemicals to contribute to migration, invasion and metastasis of human breast cancer cells. J Cancer Metastasis Treat. 2019;5 doi:10.20517/2394-4722.2019.22. [Google Scholar]