Abstract
BACKGROUND:
Modulating myosin function is a novel therapeutic approach in patients with cardiomyopathy. Danicamtiv is a novel myosin activator with promising preclinical data that is currently in clinical trials. While it is known that danicamtiv increases force and cardiomyocyte contractility without affecting calcium levels, detailed mechanistic studies regarding its mode of action are lacking.
METHODS:
Permeabilized porcine cardiac tissue and myofibrils were used for X-ray diffraction and mechanical measurements. A mouse model of genetic dilated cardiomyopathy was used to evaluate the ability of danicamtiv to correct the contractile deficit.
RESULTS:
Danicamtiv increased force and calcium sensitivity via increasing the number of myosins in the ON state and slowing cross-bridge turnover. Our detailed analysis showed that inhibition of ADP release results in decreased cross-bridge turnover with cross bridges staying attached longer and prolonging myofibril relaxation. Danicamtiv corrected decreased calcium sensitivity in demembranated tissue, abnormal twitch magnitude and kinetics in intact cardiac tissue, and reduced ejection fraction in the whole organ.
CONCLUSIONS:
As demonstrated by the detailed studies of Danicamtiv, increasing myosin recruitment and altering cross-bridge cycling are 2 mechanisms to increase force and calcium sensitivity in cardiac muscle. Myosin activators such as Danicamtiv can treat the causative hypocontractile phenotype in genetic dilated cardiomyopathy.
Keywords: calcium, cardiomyopathies, myofibrils, myosin, sarcomeres
Novelty and Significance.
What Is Known?
Myosin-specific small molecules are an emerging therapeutic modality for cardiomyopathies.
Danicamtiv is a novel myosin modulator being considered for treatment of dilated cardiomyopathy.
Previous studied showed that Danicamtiv increased left ventricular systolic function in preclinical animal models and humans.
What New Information Does This Article Contribute?
Danicamtiv increases myosin recruitment and alters cross-bridge cycling by decreasing ADP release resulting in prolonged actomyosin interaction.
Danicamtiv recovers both tissue and organ level hypocontracility in a rodent genetic dilated cardiomyopathy model.
Novel myosin-specific small molecules have been developed for treatment of hypocontractility in heart failure. A better understating of the mechanisms of actions of these agents may help to elucidate subsets of patients that will most benefit from them. The work in this study demonstrates how a combination of structural and biomechanical studies informs on how Danicamtiv, a small molecule targeting myosin, increases cardiac contractility. Danicamtiv increases the structural recruitment of myosin and alters the biochemical cross-bridge cycling by decreasing ADP release, resulting in prolonged actomyosin interaction. Danicamtiv shares some of these mechanisms with Omecamtiv Mecarbil, which is the first myosin-specific molecule for increasing contractility. This detailed methodological framework can be used to connect how changes in myosin recruitment and cross-bridge cycling kinetics by small molecules affect cardiac myofibril activation and relaxation.
Meet the First Author, see p 375
Editorial, see p 444
The prevalence of heart failure (HF) continues to rise and despite new and improved treatments, there is still significant morbidity and cost.1 More than half of the patients with HF can be categorized by depressed systolic function or contractility.2 Traditional inotropic agents improve cardiomyocyte contractility via increased intracellular cAMP and calcium levels but do not improve patient survival.3,4 Directly targeting sarcomeric proteins can overcome adverse effects of traditional inotropes such as arrhythmias, increased myocardial oxygen demand, and activation of cell death pathways.3 Small molecules targeting myosin and modulating its activity have been developed over the last decade for the treatment of HF. The strategy for identifying these compounds has been to identify molecules that selectively bind to cardiac myosin and increase or inhibit the myosin ATPase activity. Omecamtiv mecarbil (OM) is a first-in-class myosin activator that was originally thought to work via increased phosphate release and priming myosin for binding to actin.5,6 More detailed biophysical measurements showed that OM inhibits the myosin working stroke and prolongs the detachment of a small population of nonforce generating myosin heads.7,8 The OM-bound myosin heads activate the thin filament and recruit more myosin heads with the net effect of having more myosin heads that are available for force generation. OM recently completed a phase III clinical trial in HF and was found to result in a modest decrease in HF events or death from cardiovascular causes.9,10 The successes and challenges of OM show the importance of a better understanding of the molecular mechanisms of new sarcomere modulators.
Danicamtiv or MYK-491 is a promising novel myosin activator that increases myofibril ATPase activity and calcium sensitivity.11 In vivo studies demonstrated improved atrial and ventricular function in a canine HF model as well as patients with HF with reduced ejection fraction.11,12 There is an ongoing clinical trial enrolling patients with genetic dilated cardiomyopathy (DCM) with sarcomeric variants for treatment with Danicamtiv. We and others have shown that a reduction in the force and kinetics of the cardiac twitch, the tension developed as a function of time, is predictive of the diagnosis and severity of DCM in rodent myocytes and humans.13–15 It is logical, therefore, that patients with DCM may benefit from treatment with myosin activators. A recent study in a human-engineered heart tissue platform showed that administration of Danicamtiv resulted in a larger increase in systolic contraction at a smaller lusitropic cost compared with OM.16 While this suggests possible differences in mechanisms, there is limited information available regarding Danicamtiv’s mechanisms of action other than that Danicamtiv increases the number of myosin heads available for force production without altering passive stiffness.17 Here, we present the first detailed mechanistic study of how Danicamtiv affects the cross-bridge cycle, cardiac muscle contractile kinetics, and myosin structure. We show that Danicamtiv increased calcium sensitivity and elevated force mostly at low calcium levels. Using X-ray diffraction, we demonstrate that treatment with Danicamtiv under resting conditions repositioned myosin heads closer to the thin filament. In myofibrils under load, Danicamtiv slowed the myosin cross-bridge cycle and prolonged relaxation kinetics by inhibiting the rate of ADP product release. Last, we demonstrate the ability of Danicamtiv to recover hypocontractility at the tissue and organ levels in a rodent DCM model.
METHODS
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal Use and Ethics
All experiments followed protocols approved by both the University of Washington and the Illinois Institute of Technology Institutional Animal Care and Use Committees according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Farm pig hearts were obtained immediately after the animal was euthanized and rinsed in cold oxygenated Tyrode’s buffer.
DCM Mouse Model
We used a previously published rodent genetic DCM model. The I61Q cTnC (cardiac troponin C) is an engineered cTnC variant that desensitizes the myofilament to calcium via altering cTnC calcium binding.18,19 Transgenic mice show progressive ventricular dilation and reduced contractility as they age.13 Experimental mice were between 3 and 5 months of age.
In Vivo Mouse Echocardiography
Transthoracic echocardiography was performed using Vevo 3100 high-frequency, high-resolution imaging system (VisualSonics) equipped with MS400 MicroScan Transducer. After initial m-mode measurement, a 27G butterfly needle was inserted into the tail vein and total 125 μL of N,N-dimethylacetamide: polyethylene glycol 400 (PEG-400): 30% 2-hydroxypropyl-β-cyclodextrin (5:25:70) containing 2 mg/kg of Danicamtiv or DMSO (vehicle) was delivered. This was followed by a 500 μL of normal saline and removal of the catheter. The mouse continued under sedation and a repeat measurement was performed 10 minutes after the injection.
Demembranated Tissue Mechanics
Tissue preparation and solutions are described in Supplemental Methods.
Isometric Force and Rate of Tension Redevelopment
Permeabilized trabeculae (mouse) or thin strips (pig) were dissected and mounted between a force transducer (Aurora Scientific, model 400A) and a motor (Aurora Scientific, model 315C) using aluminum T-clips (Aurora Scientific).14 Sarcomere length was set to ≈2.3 µm for the experiments. Experiments were conducted in physiological solution (pH 7.0) at 15 °C (mouse) or 21 °C (pig) containing a range of negative log of calcium concentration (pCa; −log10[Ca2+]) values from 9.0 to 4.0 with and without 1 μM Danicamtiv (MedChemExpress). Tissue was allowed to reach stead-state force (F) at each pCa. F-pCa curves were collected and analyzed with custom code using LabView software and fit to the Hill equation. The rate of tension redevelopment (ktr) and high-frequency stiffness was measured at each pCa (details in the Supplemental Material).
Quick Stretch Analysis
Permeabilized porcine myocardial strips (≈180×700 μm) were mounted between a piezoelectric motor (P841.40, Physik Instrumente, Auburn, MA) and a strain gauge (AE801, Kronex, Walnut Creek, CA) using aluminum T-clips. They were lowered into a 30 μL droplet of relaxing solution (pCa 8.0) maintained at 28 °C and set at 2.3 µm sarcomere length. Stress (force per cross-sectional area) was recorded following a step-length change of 0.5% muscle length to assess cross-bridge kinetics as a function of [MgATP] as previously described and repeated with varying [MgATP].20
Myofibril Mechanics
Myofibril activation and relaxation measurements were performed on a custom set-up as previously described.21,22 Briefly, myofibrils were mounted between 2 glass needles; one which acted as a force transducer and the other as an inflexible motor arm. A dual diode system measures force based on needle deflection, with force transducer needle stiffness measured at 7.98 mm/µN. A double-barreled glass pipette delivered relaxing (pCa=9.0) and activating (pCa=5.8 or 4.0) solutions to the mounted myofibril. Activation and relaxation data were collected at 21 °C and fitted as previously described.22
Intact Twitch Assay
Unbranched, intact trabeculae or papillary muscles were dissected from the right ventricular wall and mounted between a force transducer (Cambridge Technology, Inc, model 400A) and a rigid post. The tissue was submerged in a chamber continuously perfused with oxygenated modified Krebs buffer (1.8 mmol/L CaCl2) at 33 °C. Continuous twitch tension traces were recorded using LabView software at a sampling rate of 1 kHz and analyzed with custom code in MATLAB software (version 2021a, The MathWorks).14
In Vitro Motility Assay
Myosin and HMM (heavy meromyosin) were purified from pig left ventricular samples as previously described.22 In vitro motility assays were performed at 30 °C using unregulated Rhodamine Phalloidin labeled F-actin in the presence of 2 mmol/L ATP and either DMSO or 0.5 μM Danicamtiv.21,22 In a subset of experiments, the nucleotide composition was changed to 1 mmol/L ATP and 1 mmol/L ADP. Custom-built software analyzed images of the moving filaments as in our previous publications.21,22
X-Ray Diffraction
Wild-type Yucatan mini-pig hearts were provided by Exemplar Genetics LLC. Permeabilized tissue preparation and beamline specifications are described in Supplemental Methods. X-ray diffraction experiments were performed at the BioCAT beamline 18ID at the Advanced Photon Source, Argonne National Laboratory.23 Skinned muscle preparations were mounted in a custom rig allowing for simultaneous X-ray diffraction and force measurements as monitored by an ASI 610A data acquisition and control system (Aurora Scientific). The muscle was incubated in a customized chamber with the solution temperature between 28 °C and 30 °C. The sarcomere length of the muscles was set to 2.3 µm by monitoring the helium-neon laser (633 nm) diffraction pattern on a screen. X-ray fiber diffraction patterns were collected in pCa 8 solution in the absence or presence of 50 μM of Danicamtiv. Analysis of x-ray diffraction images was done as described previously24,25 and provided in the Supplemental Methods.24,25
Please see the Major Resources Table in the Supplemental Material.
Statistical Analysis
We used GraphPad Prism 9 for data presentation and statistical analysis. The data are presented as mean±SEM. We used Shapiro-Wilk test for normality. For data not normally distributed, we used Kruskal-Wallis with Dunn multiple comparison test or Wilcoxon matched-pairs signed rank test. For porcine demembranated tissue mechanics, we used a mixed-effect model with Šídák multiple comparisons test for pCa curves and paired 2-tailed t test for the remaining measures. For myofibrils, we used Welch unpaired 2-tailed t test (pCa, 4.0), or 2-way ANOVA followed by Tukey multiple comparisons test (pCa, 5.8±ADP). For X-ray diffraction, we used paired 2-tailed t tests. For in vitro motility and murine demembranated tissue mechanics, we used 2-way ANOVA with Šídák multiple comparisons. For quick-step analysis, we used 2-way ANOVA with Šídák multiple comparison for krel and 1-way ANOVA with Tukey multiple comparisons for the remaining measures. For sinusoidal length-perturbation assays, we used multiple unpaired t tests. For murine intact mechanics, we used mixed-effect analysis with Dunnett multiple comparisons.
RESULTS
Danicamtiv Increased Submaximal Force and Calcium Sensitivity While Prolonging Myofibril Relaxation
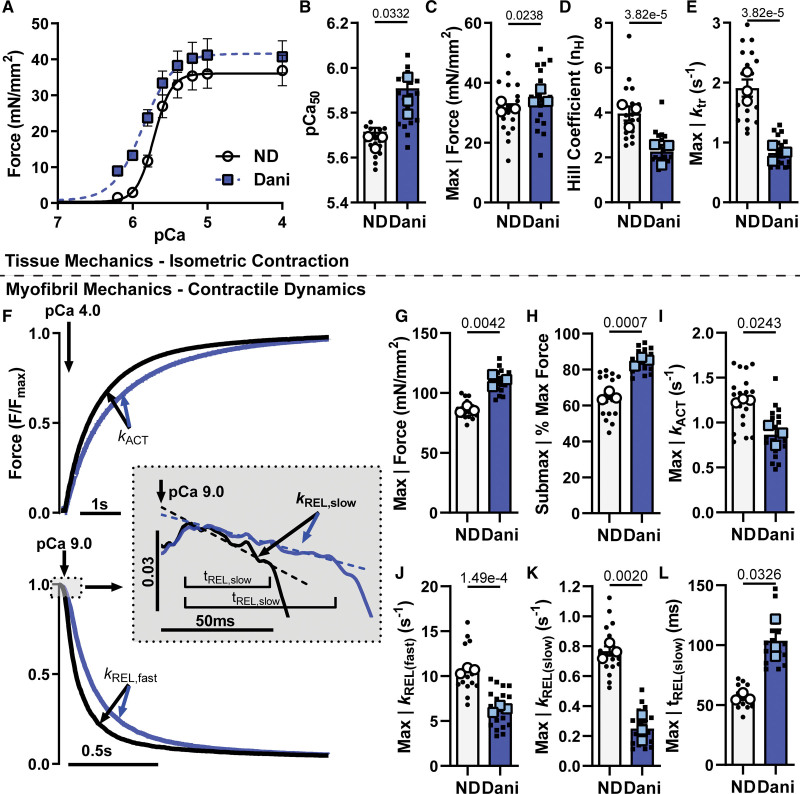
Permeabilized porcine ventricular muscle preparations showed a leftward shift in the force versus calcium (pCa) relationship in the presence of 1 µm Danicamtiv (Figure 1A; Table S1). This was quantified by an increase in pCa50 (Figure 1B; 5.72±0.02 versus 5.91±0.05) indicating an increase in the sensitivity of the myofilament to calcium. While there was a slight increase in maximally activated force (Figure 1C), there was a larger increase in force at submaximal calcium levels (Figure 1A). This leads to a reduction in the Hill coefficient (Figure 1D; 3.96±0.31 versus 2.27±0.29), suggesting an effect of Danicamtiv on myofilament cooperativity or recruitment. Force redevelopment rate constants(ktr) were significantly slower at each level of force (Figure S1B) suggesting that Danicamtiv reduced cross-bridge cycling kinetics. Incubation in Danicamtiv resulted in more than a 50% decrease in maximal ktr (Figure 1E; 1.91±0.14 versus 0.83±0.08). High-frequency small amplitude oscillation measurements of steady-state stiffness showed that Danicamtiv increased stiffness to the greatest degree at lower calcium levels (Figure S1C). The increased stiffness is proportional to the increase in force, suggesting that the increased stiffness is due to more myosin heads producing force rather than an increase in force per myosin head.
Figure 1.
Mechanics in permeabilized porcine cardiac tissue and myofibrils treated with 1 μM Danicamtiv (Dani). A, Force-pCa curves from isolated uniaxially aligned porcine ventricle tissue strips before and after incubation in 1 μM Dani. Summary data for (B) pCa50, (C) max force generation, (D) Hill coefficient, and (E) max kTR. F, Example myofibril mechanics traces at maximum calcium (pCa=4.0) showing activation (top) and relaxation (bottom). Summary myofibril data for (G) max force, (H) sub-max force, rate constants for (I) activation, (J) fast phase of relaxation, and (K) slow phase of relaxation, along with (L) duration of the slow phase of relaxation. Data are from 19 paired demembranated tissue preparations (small symbols) and 18 DMSO or 21 Dani-treated myofibrils (small symbols) from 3 biological replicates (large symbols). P values vs no drug (ND).
Isolation of subcellular myofibrils allows for measurements of force and kinetics of both activation and relaxation.21,22 Figure 1F shows representative normalized force traces of porcine myofibrils transitioning between maximally activated (high Ca2+) and relaxed (low Ca2+) conditions by rapid solution switching via a double-barreled pipette with and without Danicamtiv. Force is developed exponentially with a rate constant (kACT) that depends on thin filament activation, myosin recruitment, and myosin cross-bridge cycling. The rapid switch back to low calcium solution results in a biphasic relaxation relationship with an initial linear phase followed by a rapid exponential phase back to baseline. The rate constant during the linear slow phase of relaxation (kREL(slow)) reflects the rate of cross-bridge detachment, which is independent of the Ca2+ dissociation from troponin C. The duration of the slow phase (tREL(slow)) reflects the time it takes for the thin filament to deactivate and has been shown to be influenced by the properties of the thin filament proteins and the level of calcium.19,26 The subsequent fast exponential phase of relaxation (kREL(fast)) reflects inter-sarcomere dynamics and involves both active and passive elements that are influenced by multiple factors.
As in the demembranated tissue preparations, Danicamtiv increased myofibril force at maximal (pCa=4) and submaximal calcium (pCa=5.8). The ratio of submaximal to maximal activation was also significantly increased with Danicamtiv (Figure 1G and 1H). Treatment with Danicamtiv resulted in a slight decrease in kACT (Figure 1I). Relaxation kinetics analysis (Figure 1J) revealed that Danicamtiv decreased the fast rate of myofibril relaxation (kREL(fast); 10.64±0.20 versus 6.34±0.22 s−1), which is consistent with the slowed cellular and organ level kinetics in preclinical models.12 Our analysis also revealed that the initial linear slope (kREL(slow); 0.77±0.03 versus 0.25±0.05 s−1) of myofibril relaxation was decreased while the thin filament deactivation duration (tREL(slow); 56.5±1.9 versus 103.7±9.4 ms) increased (Figure 1K and 1L). These results showing prolonged relaxation kinetics suggest that Danicamtiv impacts cross-bridge turnover kinetics by inhibiting product release, either inorganic phosphate (Pi) or ADP. Previously reported mechanisms of the myosin activator OM showed that slower cross-bridge turnover can result in increased force generation and prolonged myosin head activation, requiring more time for the thin filament to transition from an active to an inactive state.7,27 The rate of cross-bridge turnover has been previously identified as a critical regulator for transitioning the thin filament from an active to an inactive state.28,29
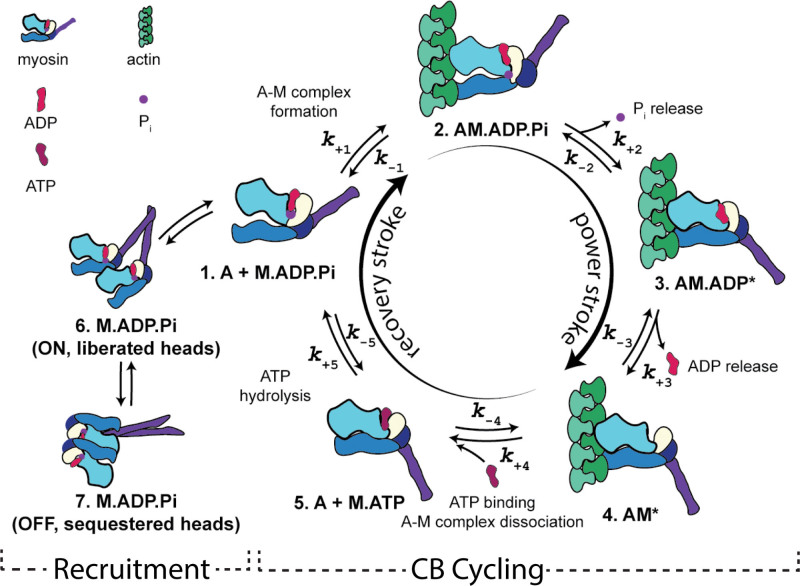
While it is known that Danicamtiv increases myofibril ATPase rate,11 a detailed understanding of the exact mechanisms is lacking. Together, our tissue mechanics and myofibril measurements suggest that Danicamtiv impacts cardiac function through increased myosin head recruitment and altered cross-bridge cycling kinetics (Figure 2). We will investigate this further through the following reductionist assays.
Figure 2.
Scheme illustrating a 5-step cross-bridge model of contraction. Figure art by Matthew Childers, University of Washington, 2023, licensed under a Creative Commons Attribution-Non-Commercial 4.0 International License.
Danicamtiv Altered Resting Myosin Thick Filament Structure and Both Passive and Active Elastic and Viscous Moduli
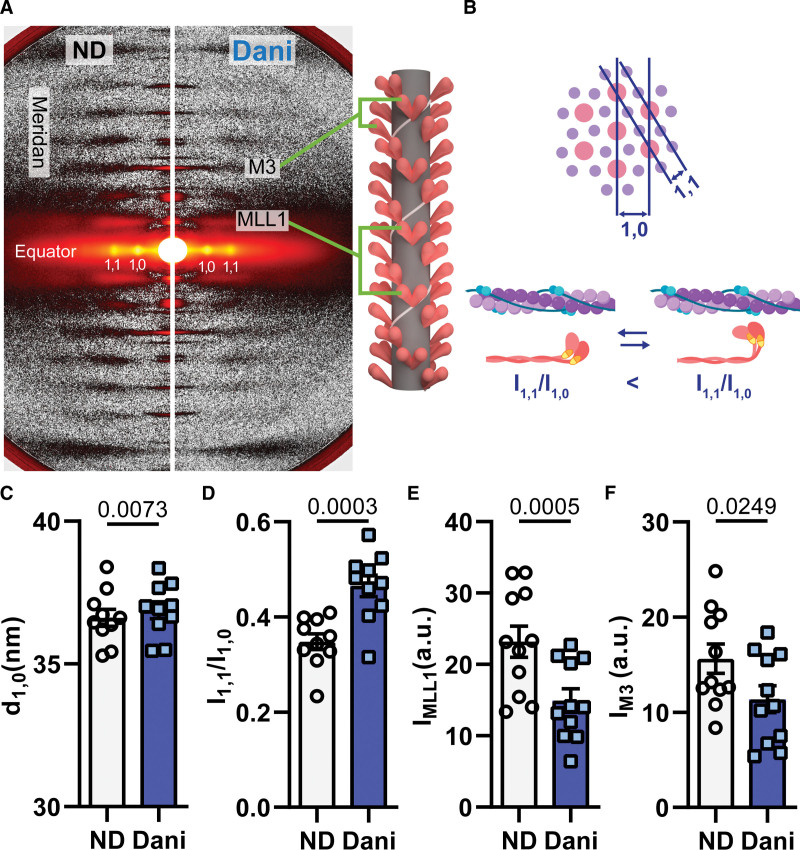
Previous studies have shown that myosin modulators can affect myosin structure.25,30 We used small-angle-x-ray diffraction patterns from permeabilized porcine myocardium to study the structural changes induced by Danicamtiv under relaxed conditions (pCa8, Figure 3A). The lattice spacing (d1,0; Figure 3B, top), directly proportional to interfilament spacing,31 increased after Danicamtiv treatment (Figure 3C). The increase of d1,0 could be a result of increased electrostatic repulsion between the myofilaments when myosin heads move away from the thick filament backbone toward actin filaments. The equatorial intensity ratio (I1,1/I1,0) reflects the proximity of myosin heads to the thin filament (Figure 3B, bottom).25,30,31 As seen in Figure 3D, Danicamtiv significantly increased I1,1/I1,0 under relaxed conditions suggesting that myosin heads moved away from the thick filament backbone and closer to the thin filament.
Figure 3.
Fifty micromolar Danicamtiv (Dani) partially activated the thick filament in resting cardiac muscle (pCa=8.0). A, Sample X-ray diffraction patterns of demembranated porcine cardiac tissue exposed to X-ray beams while bathed in low calcium solution (pCa=8.0) containing DMSO (left) or Dani (right). B, Schematics defining how actin and myosin relationships relate to equatorial measurements. C, Summary data for lattice spacing (d1,0), (D) intensity ratio of the primary equatorial reflections (I1,1/I1,0), intensity of (E) the first-order myosin-based layer line (IMLL1), and (F) the third-order myosin-based meridional reflection (IM3). Data are from 10 paired tissue preparations. The illustrations were created with BioRender.com. a.u. indicates arbitrary units.
The meridional reflections arise from axially repeating structures in the myofilaments (Figure 3A).31 The distance of these reflections to the beam center are inversely related to the spacing of the axial periodicities along the myofilaments. When cardiac muscle was exposed to Danicamtiv under relaxing conditions, the intensity of the first-order myosin-based layer line (IMLL1; Figure 3E) and the third-order myosin-based meridional reflection (IM3; Figure 3F), both of which correlate with the ordering of myosin heads on the thick filament backbone, decreased by 27% and 35%, respectively. The decrease of IM3 and IMLL1 reflects a reduction in the number of ordered myosin heads. The intensity of the sixth-order myosin-based meridional reflection (M6) arises primarily from structures within the thick filament backbone.31 The spacing of the M6 reflection (SM6) increased by 0.2% with Danicamtiv (Figure S2; Table S2). Both the loss of the helical ordering of the myosin heads and the increase in thick filament backbone periodicity are suggestive of a transition in myosin structure and its position on the thick filament from the OFF to ON state.32 It is well recognized that resting thick filaments can be characterized by different structural and biochemical states that can be affected by both disease and small molecules.25,33,34 Here, the increase in the number of ON state thick filaments in the presence of Danicamtiv contributes to increased force generation via more myosin motors available for contraction.30
Our x-ray diffraction findings were supported by performing sinusoidal length-perturbation analysis to porcine cardiac tissue with and without Danicamtiv under resting and activating conditions. The resultant elastic and viscous moduli responses provide insight into the number of bound cross bridges and characteristics such as kinetics of force-generating cross-bridges, respectively. As seen in Figure S3, under relaxed conditions (pCa 8.0, 5 mmol/L [MgATP]), elastic moduli were not significantly changed by Danicamtiv. There was a significant increase in the viscous moduli at a subset of oscillatory frequencies for the Danicamtiv-treated strips. Under maximally activated conditions (pCa, 4.8–5 mmol/L [MgATP]), there was an increase in viscoelastic myocardial stiffness for the Danicamtiv-treated strips versus the untreated. Both elastic and viscous moduli values were greater in the presence of Danicamtiv across a wide range of frequencies, suggesting greater cross-bridge binding in the presence of Danicamtiv. The observed leftward shift toward lower frequencies for the elastic and viscous moduli responses also suggests Danicamtiv slowed cross-bridge cycling, consistent with the slowed ktr (Figure 1E).
Prolonged Myofibril Relaxation Induced by Danicamtiv Was due to the Altered ADP Release Rate
Using a combination of reductionistic approaches, we investigated whether Danicamtiv affects the product release rates of Pi and ADP during cross-bridge cycling. Either of these mechanisms could explain the decreased cross-bridge turnover time suggested by the decreased myofibril relaxation kinetics.
It is well accepted that increasing Pi results in decreasing force.35,36 As seen in Figure S4, a linear dependence of relative force on Log [Pi] was found in demembranated porcine cardiac tissue. The difference between the slopes was not significant between the Danicamtiv and no drug group. Hence, the relation between force and [Pi] was unchanged with Danicamtiv. These results are different than what is reported for OM, where 1-μM OM counteracts the inhibitory effects of Pi under similar conditions.37
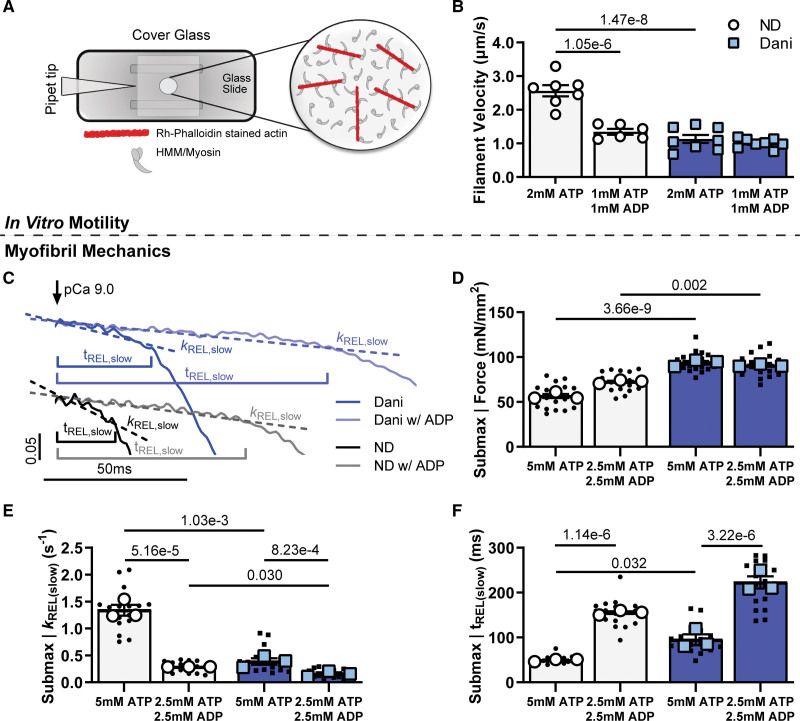
To test the hypothesis that ADP release is affected by Danicamtiv, we quantified myosin cross-bridge activity in the presence of elevated ADP conditions. Initial experiments utilized the in vitro motility assay, which directly measures cross-bridge activity (Figure 4A). Previous studies have shown that when ADP levels are elevated, filament velocity is decreased because ADP release is inhibited, promoting the strongly bound actin-myosin state.38,39 As seen in Figure 4B, Danicamtiv also resulted in a substantial (≈55%) decrease in filament velocity (2.65±0.17 versus 1.13±0.12 µm/s). There was no further decrease in velocity with nucleotide mixtures containing 50% ATP and 50% ADP in the presence of Danicamtiv (1.01±0.05 µm/s), suggesting no additive effect. These results support the hypothesis that Danicamtiv slows cross-bridge cycling rate through slowed ADP release.
Figure 4.
Effect of Danicamtiv (Dani) and ADP on cross-bridge cycling. A, The in vitro motility assay was used to look at cross-bridge cycling in isolated myosin and actin. B, Filament sliding velocity with 0.5 μM Dani-treated ATP-bound myosin (left columns), and with (50:50 ADP:ATP)-bound myosin (right columns). C, Example tracings of the slow phase of myofibril relaxation with and without 1 μM Dani and with ATP or 50:50 ADP:ATP mixture at pCa=5.8. Example tracings are offset for visual clarity. Summary data of the effect of Dani and ADP on (D) submaximal force, and the (E) rate constant and (F) duration for the slow phase of relaxation. Data are from 7 to 8 slides per condition for in vitro motility and 18 DMSO or 21 Dani-treated myofibrils (small symbols) from 3 biological replicates (large symbols).
Next, we performed measurements in myofibrils at submaximal calcium (pCa=5.8). Under isometric conditions, ADP release is the rate-limiting step in the cross-bridge cycle and cardiac muscle relaxation kinetics. Furthermore, by increasing ADP levels, there is an increase in maximal force and calcium sensitivity.36,38,39 To answer the specific question of whether Danicamtiv directly affects ADP release from myosin, we made our myofibril measurements with Danicamtiv in the presence of increased ADP levels. As expected, ADP (Figure 4C through 4F; Figure S6) increased force (56.6±3.2 versus 72.6±2.4 mN/mm2) and slowed myofibril relaxation rates (kREL(slow): 1.340±0.101 versus 0.286±0.001 s-1; kREL(fast): 12.81±1.05 versus 4.34±0.32), while prolonging the duration of the slow phase of relaxation (tREL(Slow): 49.1±1.7 versus 155.2±3.0 ms). Treatment with Danicamtiv at a submaximal calcium level (pCa=5.8) resulted in a similar increase in force and slowing of activation and relaxation kinetics that was seen at a maximal calcium level (Figure 4C through 4F; Table S1). Combined treatment with Danicamtiv and elevated ADP did not further increase force and minimally decreased cross-bridge detachment kREL(slow) compared with Danicamtiv and no ADP (Figure 4D and 4E). This suggests that ADP release is the key step in the cross-bridge cycle that is affected by Danicamtiv. Interestingly, the addition of elevated ADP to Danicamtiv-treated myofibrils further prolonged thin filament deactivation duration (tREL(Slow): 94.9±12.2 versus 222.5±13.6 ms), likely due to more strongly bound cross bridges.
Nucleotide Handling Rates Were Modified in the Presence of Danicamtiv
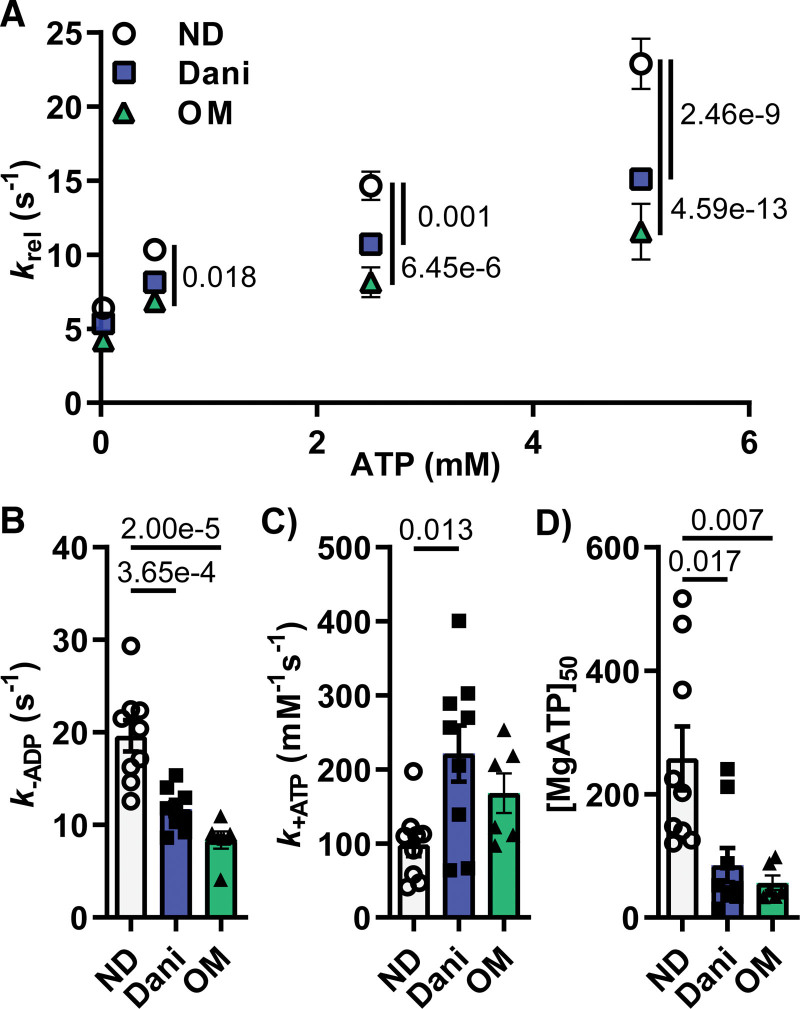
To confirm our myofibril results, we measured changes in nucleotide binding and release rates in the presence of Danicamtiv. We calculated ATP binding and ADP release rates in porcine cardiac tissue using a step-length protocol in the presence of increasing [MgATP] (Figure S7). Cross-bridge detachment rates (krel) increased as [MgATP] increased for the untreated and Danicamtiv-treated myocardial strips (Figure 5A; Table S5). krel was slower for the Danicamtiv-treated strips at [MgATP] >0.5 mmol/L. Fits to Eq. 1 suggests that slowed cross-bridge detachment stems from a combination of slower cross-bridge MgADP release rate (k-ADP) and faster cross-bridge MgATP-binding rate (k+ATP) in the presence of Danicamtiv (Figure 5B and 5C). Because the rate-limiting step of the cross-bridge cycle under load is MgADP dissociation from strongly bound myosin cross bridges in demembranated myocardial strips, it was the slower k-ADP that primarily drove the differences in cross-bridge kinetics between the untreated and Danicamtiv-treated strips. As seen in Figure 5, k+ATP was significantly faster than k-ADP, and with k+ATP being a second-order nucleotide binding process, the [MgATP] needs to be very low for k+ATP to affect cross-bridge detachment rates. Figure 5D shows that the MgATP concentration at half-maximal detachment rate ([MgATP]50) was much lower for the Danicamtiv-treated strips. This suggests that Danicamtiv-treated preparations required less MgATP to reach their maximal cross-bridge detachment rate. Using the same approach, we found a similar relationship between krel and [MgATP] in the presence of 1 µm OM. OM resulted in the same magnitude of k-ADP inhibition and decreased [MgATP]50 as Danicamtiv, suggesting these small molecules affect similar steps of the cross-bridge cycle.
Figure 5.
One-micromolar Danicamtiv (Dani) and Omecamtiv Mecarbil (OM) increased ATP binding and decreased ADP release in activated porcine cardiac muscle. A, The cross-bridge detachment rates (krel) increased with increasing [MgATP] for the untreated, Dani-treated and OM-treated myocardial strips. Treatment with both Dani and OM decreased krel at [MgATP] >0.5 mmol/L (B). Dani and OM decreased ADP release rate (B, k-ADP) while increasing ATP binding (C, k+ATP). MgATP concentration at half-maximal detachment rate ([MgATP]50) was lower for both Dani-treated and OM-treated strips (D). Data are from 9 tissue preparations per condition.
Danicamtiv Recovered Abnormal Tension in a Rodent DCM Model
Since Danicamtiv is under investigation for the treatment of patients with genetic cardiomyopathy, we tested its ability to recover the force deficit in a thin filament DCM mouse model with the characteristic progressive dilation and hypocontractility of human genetic DCM. Permeabilized muscle preparations showed a rightward shift in the force versus pCa relationship in the I61Q cTnC DCM model.13 Additionally, the I61Q cTnC mutant decreased Fmax and the Hill coefficient. As seen in Figure S8, 1 µm Danicamtiv was able to shift the force versus pCa curves to the left for both control and I61Q cTnC mice with an increase in pCa50 (5.58±0.01 versus 5.72±0.02 for control, 5.35±0.04 versus 5.47±0.03 for I61Q cTnC). This increase in pCa50 was significant versus no drug regardless of genotype. Similar to the results in pig cardiac tissue, we found a decrease in the Hill coefficient and maximal ktr but with no increase in maximal force (Figure S8; Table S3).
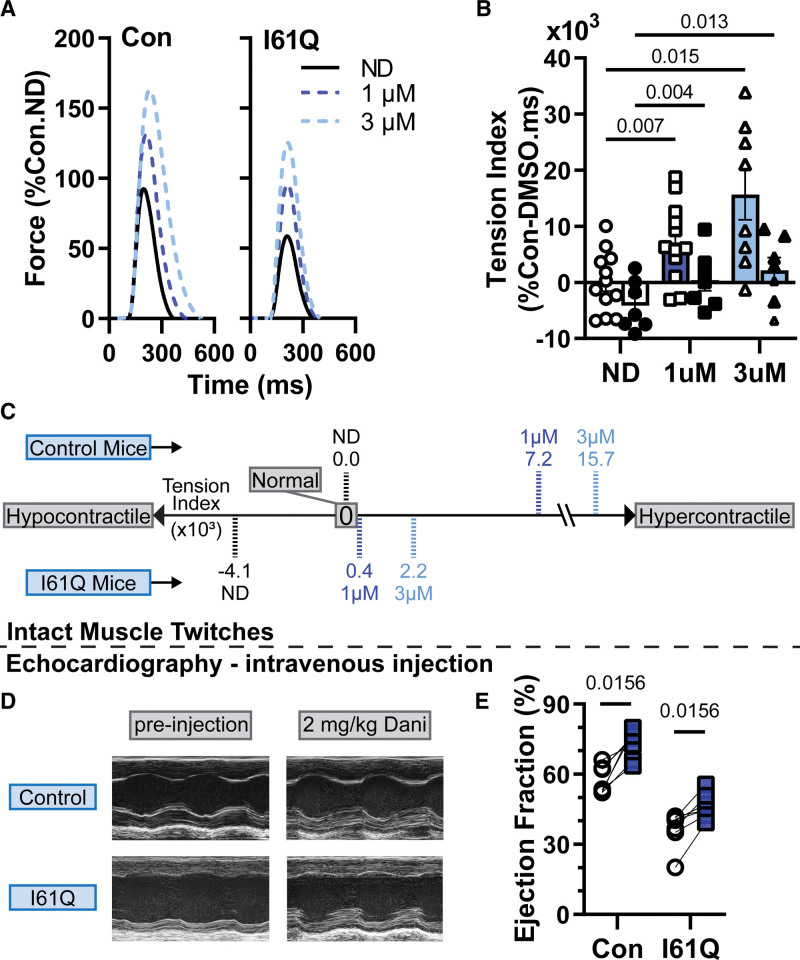
Our intact trabecula measurements showed a decrease in peak tension (Tp) and tension index in the I61Q cTnC mice (Figure 6; Table S4) as previously reported.13,40 In the presence of Danicamtiv, Tp increased in both control and DCM. In control mice, 1 and 3 µmol/L Danicamtiv resulted in ≈63% and 131% increase in Tp, while the increase is ≈87% and ≈148%, respectively in I61Q cTnC trabecula. While the time to peak is unchanged in the presence of Danicamtiv, the time to 50% and 90% relaxation is increased in the presence of Danicamtiv (Figure S9; Table S4). We have previously shown that the tension index, the area under the twitch curve that is subtracted from a healthy twitch, is predictive of cardiomyopathy phenotype.13,14 While the tension index increased with Danicamtiv in a dose-dependent manner, the increase was greater in the control mice. This is mostly due to the more pronounced relaxation change in the control tissue. Using mixed-effects analysis, the interaction between genotype and Danicamtiv is statistically significant, suggesting a different treatment response in control versus I61Q cTnC tissue. These results suggest that the underlying mechanism of cardiomyopathy might need to be considered when dosing a medication such as Danicamtiv. We tested in vivo efficacy of Danicamtiv by intravenous injection of 2 mg/kg and saw a significant increase in ejection fraction in both controls (58.1±2.5 versus 74.7±2.0) and DCM (36.6±2.9 versus 46.3±2.0) hearts 10 minutes post-treatment (Figure 6; Table S6). There was a decrease in heart rate post-treatment, which was likely due to sedation. Treatment with vehicle (DMSO) did not significantly increase ejection fraction or heart rate (Table S7).
Figure 6.
Danicamtiv (Dani) mitigated the contractile abnormalities in a rodent dilated cardiomyopathy model. A, Average twitch force-time traces (in % control-ND Tp) of intact papillary muscle trabeculae from control (left) and I61Q cTnC (cardiac troponin C; right) mice stimulated at 1 Hz. Dani increased Tp (A) and tension index (B and C) in control and I61Q cTnC twitches in a dose-dependent manner. C, Intravenous injection of 2 mg/kg Dani resulted in significant increase in ejection in both control and I61Q cTnC hearts 10-minute post-injection. Data are from 15 control mice and 11 I61Q mice for intact twitches and 7 mice for in each echo group.
DISCUSSION
We set out to perform a first detailed analysis of the mechanism of action of the myosin activator Danicamtiv. There are several key findings that emerged from this study. First, we showed that treatment with Danicamtiv restructured the thick filament of resting muscle to a more ON configuration, where myosin heads are positioned closer to actin. This increased the number of myosin heads available to contribute to contraction, a result that is consistent with more myosin motors binding at lower calcium concentrations. It also suggests reduced reliance on calcium-mediated cooperativity of myosin binding, hence an increase in calcium sensitivity with a decreased Hill coefficient. Second, we showed that Danicamtiv slowed cross-bridge turnover and subsequently a decreased rate of myofibril relaxation and prolonged thin filament deactivation. This prolonged relaxation manifested in intact cardiac muscle, which had slower twitch relaxation kinetics. Third, we provided evidence to show that the decrease in myosin ADP release rate is the mechanism for decreased cross-bridge turnover and slower relaxation. Fourth, we showed that Danicamtiv improved the tissue and the organ level hypocontractility in a genetic DCM model.
Danicamtiv Restructures Myosin in an Analogous Manner to Other Myosin Activators
Myosin activation is a novel way to treat HF. OM, the first-in-class myosin activator, recently completed a phase 3 clinical trial for the treatment of systolic HF.9 Studies using fluorescent probes have demonstrated that OM stabilizes the ON state of the thick filament resulting in more myosin motors available for binding to the thin filament.10 Our x-ray diffraction studies also showed partial thick filament activation with Danicamtiv under resting conditions. This activation under resting conditions can be expected to result in more myosin motors binding at lower calcium concentrations with less reliance on cooperativity. Results of our sinusoidal length-perturbation analysis further support that Danicamtiv increases cross-bridge binding and promotes an increase in the ratio of ON to OFF cross-bridge populations. Similar changes in myosin structure under resting conditions are induced by 2-dATP (deoxy ATP), a nucleotide known to increase force and calcium sensitivity in rodent, dog, and human cardiac myocardium,22,41,42 which correlate well with the active force.30
Danicamtiv Inhibits Cross-Bridge Cycling Rate, Prolonging Thin Filament Deactivation and Relaxation in Myofibrils
The myofibril kinetics measurements suggest that Danicamtiv prolongs thin filament activation, as indicated by the longer tREL(slow) and that this is due to a decreased rate of cross-bridge detachment as measured by kREL(slow). A similar mechanism of action involving prolonged actomyosin attachment resulting in cooperative thin filament activation has been proposed as a mechanism for OM.7,43 The inhibitor-like mechanism is further supported by decreased ATP turnover in HMM with increasing concentrations of Danicamtiv, which was also observed for OM (Figure S5). While both Danicamtiv and OM inhibit maximal force at high drug concentrations, OM inhibits to a greater extent and at a lower concentration (Figure S4). The duration of the slow phase of relaxation (tREL(slow)) is known to be affected by changes in troponin complex calcium affinity,19 so we measured the effect of Danicamtiv on troponin function. Danicamtiv did not change Ca2+ binding to cTnC as measured by steady-state fluorescence spectroscopy (Figure S10). Therefore, the decreased cross-bridge detachment rate is likely explained by the decreased ADP release rate, and this is supported by myofibril relaxation measurements in the presence of elevated ADP, which mimic relaxation kinetic changes seen with Danicamtiv under conditions of load.
Danicamtiv Corrects Abnormal Contraction in a Genetic DCM Mouse Model
Using a rodent model of sarcomeric genetic DCM with decreased thin filament activation, we demonstrated that Danicamtiv could normalize the decreased calcium sensitivity of contraction in I61Q cTnC mice. At the lowest calcium levels of our F-pCa curve, there is a full recovery of the force deficit to levels of control myocardium. However, the maximum force is still significantly lower in the Danicamtiv-treated I61Q cTnC trabeculae. The decreased ktr in mice suggested that slowing of cross-bridge kinetics was present in hearts with both MYH6 (myosin heavy chain 6; mice) and MYH7 (myosin heavy chain 7; pig). Intact trabecula experiments demonstrated that tension increased in both control and I61Q cTnC mice after treatment with Danicamtiv. Control mice consistently achieved a higher peak force and tension index, a summative measure encompassing peak tension and kinetics of both activation and relaxation, compared with I61Q. Control mice also expressed greater sensitivity to Danicamtiv treatment with significantly greater time to 50% and 90% relaxation compared with I61Q. An explanation for the difference in relaxation is that Danicamtiv normalizes the duration of thin filament deactivation in the I61Q cTnC, which are abnormal without treatment. However, in the control mice, the thin filament stays on much longer, resulting in prolonged relaxation. Most of the DCM mice achieved a positive tension index at a dose of 3 μM, which is higher than the reported maximum serum concentrations.44 Our in vivo treatment with Danicamtiv resulted in about a 27% increase in ejection fraction in both control and I61Q hearts. While the cardiac function of the I61Q hearts improved, it did not fully recover to the untreated control levels, which matches the twitch and tension index data.
Our study has some limitations. Our mouse model of genetic DCM is based on an engineered mutation in cTnC and not a known disease-causing mutation. It is also worth noting that MYH6 is the dominant form of cardiac myosin in mice, while humans express the MYH7 variant. It is possible that our genetic DCM results could be different in organisms with MYH7 as the dominant myosin. Intact twitch measurements were only performed in mice, which expresses the fast form of myosin. Our studies have not assessed the relative affinities of Danicamtiv for different cardiac myosin isoforms; however, we found comparable results in mouse and pig, which have different predominant isoforms.
In conclusion, we use a variety of tools to demonstrate the biophysical mechanisms of how Danicamtiv works to increase force and calcium sensitivity. This methodological framework can be used to connect information from mechanical measurements to understand how myosin recruitment or the cross-bridge cycle affect cardiac myofibril activation and relaxation. The initial discovery of Danicamtiv was based on increased myofibril ATPase activity. Our studies support a more complex process than a simple increase in myosin cycling rate. The inhibition of ADP release leads to slower relaxation kinetics of myofibrils and consequently slower relaxation in intact tissue. In addition, myosin heads are more primed to interact with actin at low levels of calcium, resulting in increased calcium sensitivity and higher contractile forces at levels of calcium the sarcomere experiences during the cardiac cycle. This increase in force production and widening of twitch duration contribute to the increased tension index and augmenting cardiac function.In addition to providing the mechanism of action, our data may help to identify subsets of patients that may derive the most benefit from Danicamtiv. For example, there could be a different response in sarcomeric versus nonsarcomeric DCM or thin filament versus thick filament variants. Genetic variants are being recognized as an increasing cause of HF and DCM. Myosin activators may provide a treatment that addresses the causative hypocontractile phenotype. This motivated a current clinical trial of patients with genetic DCM for treatment with Danicamtiv. However, there is lack of data about the utility of this medication in genetic cardiomyopathy preclinical models. Our study shows that Danicamtiv increased cardiac function in rodent genetic DCM hearts. Future studies will need to look at a diverse group of variants and extend the studies to humans with genetic DCM.
ARTICLE INFORMATION
Acknowledgments
The authors acknowledge Mr Darron Marzolf who provided fresh pig muscle. This project used resources from the University of Washington Center for Translational Muscle Research supported by National Institutes of Health (NIH) grant P30AR074990. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by the Argonne National Laboratory under Contract DE-AC02-06CH11357. BioCAT is supported by NIH grant P30 GM138395. We acknowledge Dr David Mack for providing access to BioRENDER.
Sources of Funding
This work was supported by NIH Grants R01HL157169 (F. Moussavi-Harami), R01HL128368 (M. Regnier), RM1GM131981 (M. Regnier), R01HL142624 (J. Davis), R01HL149164 (B.C.W. Tanner), and American Heart Association Collaborative Sciences Award (F. Moussavi-Harami and J. Davis).
Disclosures
None.
Supplemental Material
Supplemental Methods
Tables S1–S7
Figures S1–S10
Supplementary Material
Nonstandard Abbreviations and Acronyms
- cTnC
- cardiac troponin C
- DCM
- dilated cardiomyopathy
- HF
- heart failure
- OM
- Omecamtiv Mecarbil
- pCa
- negative log of calcium concentration
- Pi
- inorganic phosphate
K.B. Kooiker and S. Mohran contributed equally.
For Sources of Funding and Disclosures, see page 441.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.123.322629.
REFERENCES
- 1.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 2.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074 [DOI] [PubMed] [Google Scholar]
- 3.Maack C, Eschenhagen T, Hamdani N, Heinzel FR, Lyon AR, Manstein DJ, Metzger J, Papp Z, Tocchetti CG, Yilmaz MB, et al. Treatments targeting inotropy. Eur Heart J. 2019;40:3626–3644. doi: 10.1093/eurheartj/ehy600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teerlink JR, Metra M, Zaca V, Sabbah HN, Cotter G, Gheorghiade M, Cas LD. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail Rev. 2009;14:243–253. doi: 10.1007/s10741-009-9153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy L, Kovacs A, Bodi B, Pasztor ET, Fulop GA, Toth A, Edes I, Papp Z. The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br J Pharmacol. 2015;172:4506–4518. doi: 10.1111/bph.13235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woody MS, Greenberg MJ, Barua B, Winkelmann DA, Goldman YE, Ostap EM. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun. 2018;9:3838. doi: 10.1038/s41467-018-06193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohde JA, Thomas DD, Muretta JM. Heart failure drug changes the mechanoenzymology of the cardiac myosin powerstroke. Proc Natl Acad Sci U S A. 2017;114:E1796–E1804. doi: 10.1073/pnas.1611698114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sorensen T, et al. ; GALACTIC-HF Investigators. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–116. doi: 10.1056/NEJMoa2025797 [DOI] [PubMed] [Google Scholar]
- 10.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Biering-Sorensen T, Bohm M, Bonderman D, Fang JC, et al. ; GALACTIC-HF Investigators. Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC-HF. J Am Coll Cardiol. 2021;78:97–108. doi: 10.1016/j.jacc.2021.04.065 [DOI] [PubMed] [Google Scholar]
- 11.Voors AA, Tamby JF, Cleland JG, Koren M, Forgosh LB, Gupta D, Lund LH, Camacho A, Karra R, Swart HP, et al. Effects of danicamtiv, a novel cardiac myosin activator, in heart failure with reduced ejection fraction: experimental data and clinical results from a phase 2a trial. Eur J Heart Fail. 2020;22:1649–1658. doi: 10.1002/ejhf.1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ráduly AP, Sárkány F, Kovács MB, Bernát B, Juhász B, Szilvássy Z, Porszász R, Horváth B, Szentandrássy N, Nánási P, et al. The novel cardiac myosin activator danicamtiv improves cardiac systolic function at the expense of diastolic dysfunction in vitro and in vivo: implications for clinical applications. Int J Mol Sci . 2022;24:446. doi: 10.3390/ijms24010446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, et al. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell. 2016;165:1147–1159. doi: 10.1016/j.cell.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers JD, Kooiker KB, Mason AB, Teitgen AE, Flint GV, Tardiff JC, Schwartz SD, McCulloch AD, Regnier M, Davis J, et al. Modulating the tension-time integral of the cardiac twitch prevents dilated cardiomyopathy in murine hearts. JCI Insight. 2020;5:e142446. doi: 10.1172/jci.insight.142446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, et al. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349:982–986. doi: 10.1126/science.aaa5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen S, Sewanan LR, Jacoby DL, Campbell SG. Danicamtiv enhances systolic function and frank-starling behavior at minimal diastolic cost in engineered human myocardium. J Am Heart Assoc. 2021;10:e020860. doi: 10.1161/JAHA.121.020860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell K, Anto AR, Anderson RL, Del Rio CL, Henze M. Cardiac muscle activation; the role of length dependent activation and the novel myosin activator danicamtiv. Eur Heart J. 2020;41(Suppl 2):ehaa946.3681. doi: 10.1093/ehjci/ehaa946.3681 [Google Scholar]
- 18.Wang D, McCully ME, Luo Z, McMichael J, Tu AY, Daggett V, Regnier M. Structural and functional consequences of cardiac troponin C L57Q and I61Q Ca(2+)-desensitizing variants. Arch Biochem Biophys. 2013;535:68–75. doi: 10.1016/j.abb.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreutziger KL, Piroddi N, McMichael JT, Tesi C, Poggesi C, Regnier M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J Mol Cell Cardiol. 2011;50:165–174. doi: 10.1016/j.yjmcc.2010.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner BC, Breithaupt JJ, Awinda PO. Myosin MgADP release rate decreases at longer sarcomere length to prolong myosin attachment time in skinned rat myocardium. Am J Physiol Heart Circ Physiol. 2015;309:H2087–H2097. doi: 10.1152/ajpheart.00555.2015 [DOI] [PubMed] [Google Scholar]
- 21.Racca AW, Klaiman JM, Pioner JM, Cheng Y, Beck AE, Moussavi-Harami F, Bamshad MJ, Regnier M. Contractile properties of developing human fetal cardiac muscle. J Physiol. 2016;594:437–452. doi: 10.1113/JP271290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moussavi-Harami F, Razumova MV, Racca AW, Cheng Y, Stempien-Otero A, Regnier M. 2-Deoxy adenosine triphosphate improves contraction in human end-stage heart failure. J Mol Cell Cardiol. 2015;79:256–263. doi: 10.1016/j.yjmcc.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischetti R, Stepanov S, Rosenbaum G, Barrea R, Black E, Gore D, Heurich R, Kondrashkina E, Kropf AJ, Wang S, et al. The BioCAT undulator beamline 18ID: a facility for biological non-crystalline diffraction and X-ray absorption spectroscopy at the advanced photon source. J Synchrotron Radiat. 2004;11:399–405. doi: 10.1107/S0909049504016760 [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Gong H, Kiss B, Lee EJ, Granzier H, Irving T. Thick-filament extensibility in intact skeletal muscle. Biophys J. 2018;115:1580–1588. doi: 10.1016/j.bpj.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, Henze M, Anderson RL, Gong H, Wong FL, Del Rio CL, Irving T. The super-relaxed state and length dependent activation in porcine myocardium. Circ Res. 2021;129:617–630. doi: 10.1161/CIRCRESAHA.120.318647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C, Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J Physiol. 2008;586:3683–3700. doi: 10.1113/jphysiol.2008.152181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehman SJ, Crocini C, Leinwand LA. Targeting the sarcomere in inherited cardiomyopathies. Nat Rev Cardiol. 2022;19:353–363. doi: 10.1038/s41569-022-00682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JP, Norman C, Kobayashi T, Solaro RJ, Swartz DR, Tikunova SB. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys J. 2007;92:3195–3206. doi: 10.1529/biophysj.106.095406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biesiadecki BJ, Davis JP, Ziolo MT, Janssen PML. Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophys Rev. 2014;6:273–289. doi: 10.1007/s12551-014-0143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers JD, Yuan CC, McCabe KJ, Murray JD, Childers MC, Flint GV, Moussavi-Harami F, Mohran S, Castillo R, Zuzek C, et al. Cardiac myosin activation with 2-deoxy-ATP via increased electrostatic interactions with actin. Proc Natl Acad Sci U S A. 2019;116:11502–11507. doi: 10.1073/pnas.1905028116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W, Irving TC. Small angle X-ray diffraction as a tool for structural characterization of muscle disease. Int J Mol Sci . 2022;23:3052. doi: 10.3390/ijms23063052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, Piazzesi G, Lombardi V, Irving M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 2015;528:276–279. doi: 10.1038/nature15727 [DOI] [PubMed] [Google Scholar]
- 33.Craig R, Padrón R. Structural basis of the super- and hyper-relaxed states of myosin II. J Gen Physiol. 2022;154:e202113012. doi: 10.1085/jgp.202113012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, et al. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115:E8143–E8152. doi: 10.1073/pnas.1809540115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Der Velden J, Klein LJ, Zaremba R, Boontje NM, Huybregts MA, Stooker W, Eijsman L, de Jong JW, Visser CA, Visser FC, et al. Effects of calcium, inorganic phosphate, and pH on isometric force in single skinned cardiomyocytes from donor and failing human hearts. Circulation. 2001;104:1140–1146. doi: 10.1161/hc3501.095485 [DOI] [PubMed] [Google Scholar]
- 36.Simnett SJ, Johns EC, Lipscomb S, Mulligan IP, Ashley CC. Effect of pH, phosphate, and ADP on relaxation of myocardium after photolysis of diazo 2. Am J Physiol. 1998;275:H951–H960. doi: 10.1152/ajpheart.1998.275.3.H951 [DOI] [PubMed] [Google Scholar]
- 37.Governali S, Caremani M, Gallart C, Pertici I, Stienen G, Piazzesi G, Ottenheijm C, Lombardi V, Linari M. Orthophosphate increases the efficiency of slow muscle-myosin isoform in the presence of omecamtiv mecarbil. Nat Commun. 2020;11:3405. doi: 10.1038/s41467-020-17143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA. 1985;82:658–662. doi: 10.1073/pnas.82.3.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989;412:155–180. doi: 10.1113/jphysiol.1989.sp017609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mijailovich SM, Prodanovic M, Poggesi C, Powers JD, Davis J, Geeves MA, Regnier M. The effect of variable troponin C mutation thin filament incorporation on cardiac muscle twitch contractions. J Mol Cell Cardiol. 2021;155:112–124. doi: 10.1016/j.yjmcc.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y, Hogarth KA, O’Sullivan ML, Regnier M, Pyle WG. 2-Deoxyadenosine triphosphate restores the contractile function of cardiac myofibril from adult dogs with naturally occurring dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2016;310:H80–H91. doi: 10.1152/ajpheart.00530.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res. 2000;86:1211–1217. doi: 10.1161/01.res.86.12.1211 [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi T, Oyama K, Tanaka H, Kobirumaki-Shimozawa F, Ishii S, Terui T, Ishiwata S, Fukuda N. Effects of omecamtiv mecarbil on the contractile properties of skinned porcine left atrial and ventricular muscles. Front Physiol. 2022;13:947206. doi: 10.3389/fphys.2022.947206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grillo MP, Markova S, Evanchik M, Trellu M, Moliner P, Brun P, Perreard-Dumaine A, Vicat P, Yang C, Driscoll JP, et al. Preclinical in vitro and in vivo pharmacokinetic properties of danicamtiv, a new targeted myosin activator for the treatment of dilated cardiomyopathy. Xenobiotica. 2021;51:222–238. doi: 10..1080/00498254.2020.1839982 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.