Abstract
Tularemia and Q fever are endemic diseases in Iran; however, little information is available on the prevalence of the causative agents, Coxiella burnetii and Francisella tularensis, in Iranian ticks. This study investigated C. burnetii and F. tularensis among hard ticks in this country. We collected ticks from livestock and other mammals in Guilan, Mazandaran, Golestan (northern Iran), Kurdistan (western Iran), and West Azerbaijan (northwestern Iran) provinces. Genomic DNA from collected ticks was extracted and screened for C. burnetii and F. tularensis using Real-time PCR. A total of 4,197 ticks (belonging to 12 different species) were collected, and Ixodes ricinus (46.4%), Rhipicephalus turanicus (25%), and Rhipicephalus sanguineus sensu lato (19.1%) were the most collected species. Of 708 pooled tick samples, 11.3% and 7.20% were positive for C. burnetii and F. tularensis, respectively. The genus of Rhipicephalus had the highest (18.3%) C. burnetii infection among the collected tick pools (P<0.001). Furthermore, the most positive pools for F. tularensis belonged to Haemaphysalis spp. (44.4%). Kurdistan had the most significant percentage of C. burnetii-infected ticks (92.5%), and there was a meaningful relationship between the provinces and the infection (P< 0.001). The ticks from Golestan exhibited the highest F. tularensis infection rate (10. 9%), and the infection showed no significant relationship with the provinces (P = 0.19). Ticks collected from grasslands had a higher Coxiella burnetii infection rate than those collected from animals (39.4% vs. 7.9%; p<0.01). However, ticks collected from animal surfaces had a slightly higher rate of Francisella tularensis infection than those collected from grasslands (7.6% vs. 3.9%; p = 0.24). Here, we demonstrated the presence of both pathogens in the north (Guilan, Mazandaran, and Golestan provinces), the west (Kurdistan province), and the northwest (West Azerbaijan province) of Iran. The public health system should pay particular attention to tick bites in veterinary medicine and humans.
Introduction
Zoonotic diseases have an increasing impact on global public health. More than 60% of the emerging infectious diseases in humans are zoonotic, and more than 70% have wildlife origin [1]. These infectious agents can be transmitted to humans through direct contact, aerosol inhalation, arthropod bites, and contaminated food and water consumption. Arthropods transmit about 25% of emerging zoonotic infectious diseases. Vector-borne diseases are considered a severe threat to human and animal health. The transmission of vector-borne diseases between humans and animals depends on a complex network of the interaction of different factors. These diseases mainly occur when the vectors, hosts, appropriate weather conditions, pathogens, and human populations exist at the same time [2–4].
Ticks are among the most significant vectors of arthropod-borne illnesses globally, capable of transmitting a broad range of infectious pathogens to people and animals. The epidemiology and ecology of tick-borne diseases are influenced by dynamic interactions between living and non-living factors. These include the biological characteristics of ticks and the associated pathogens, climate change, or changes related to human activities such as globalization, urbanization, travel, land-use change, habitat improvement, economics, politics, and demographic changes [5]. The awareness of the effects of tick-borne diseases is constantly increasing [6, 7]. Among tick-borne diseases, infections caused by Coxiella burnetii and Francisella tularensis have been reported in most regions of the world in recent years [8, 9].
Coxiella burnetii is a small gram-negative obligate intracellular highly infectious bacterium that causes Q fever. This pathogen is a particularly significant danger to people working with animals, such as slaughterhouse workers, farmers, or veterinarians, because of the rapid aerosol dispersal, survival in harsh environmental conditions, low infectious dosage, and high infectivity. It can be said that it is known as an occupational disease [8, 10, 11]. Domestic livestock is the main reservoir of C. burnetii. Q fever in animals is generally asymptomatic, and in pregnant domestic animals (cattel, sheep, and goats), it is associated with pneumonia and reproductive disorders such as abortion, stillbirth, placenta infection, uterine infection and infertility [12]. In the infectious cycle of C. burnetii, humans are considered accidental hosts for this zoonotic pathogen [13]. The main transmission route to humans is inhaling aerosols and dust particles contaminated with C. burnetii [14]. Tick bites, direct contact, consumption of raw milk and contaminated dairy products, blood transfusions, and sexual transmission are alternative routes for transmitting bacteria to individuals [15]. The manifestation of clinical features of Q fever in humans varies from asymptomatic to acute Q fever, chronic Q fever, and chronic fatigue syndrome. In humans, persistent acute and asymptomatic C. burnetii infections can proceed to a severe chronic form associated with endocarditis in 5–6% of cases [16]. The occurrence of Q fever endocarditis, if untreated, brings a significant mortality rate of up to 60% [17]. C. burnetii infection is widespread worldwide, with thousands of human clinical cases and positive animal and environmental cases documented yearly. According to reports in recent years, Q fever is considered an endemic zoonotic illness in Iran [18].
Coxiella burnetii is an endemic disease in Iran, and human cases of Q fever endocarditis have been identified in this country [19, 20]. In other studies, this pathogen has been identified in different parts of Iran in milk and abortion samples. In a systematic study, the prevalence of Q fever in cattel, goat, and sheep milk was reported as 15.1%, 7.8%, and 3.8%, respectively [21]. The prevalence of C. burnetii in abortion samples of domestic animals was reported as 24.7% in a study in different parts of Iran [22]. In another survey on slaughterhouse workers in Kerman province, considered risk groups for this disease, Q fever antibody was detected in 68% of the workers [23].
Francisella tularensis is a bacterium that causes tularemia, a highly infectious organism for humans and many animals, commonly in the Northern Hemisphere [9, 24]. This pathogenic agent comprises three subspecies, tularensis, holarctica, and mediastica. The infection by F. tularensis subsp. tularensis and F. tularensis subsp. holarctica can lead to tularemia in humans. Francisella tularensis has various animal reservoirs, including vertebrates and invertebrates [25]. Tularemia is a vector-borne infection transmitted to humans via the bites of infective ticks and fleas. Arthropods may acquire infection from infected animals and contaminated environmental water. Inhalation of contaminated aerosols, direct contact with the animal reservoir, arthropod (tick or deer-fly) bites, and consumption of contaminated water are the primary modes of transmission of tularemia infection to humans [9]. Tularemia clinical features can vary from asymptomatic to severe cases leading to human death [24]. The first human clinical case of tularemia in Iran was reported in Kurdistan, West of Iran 1981 [26]. In recent years, positive serological and molecular cases in rodents and humans have been reported from different regions of Iran, indicating the endemicity of this disease in Iran [27–32].
Considering the limited data on the C. burnetii and F. tularensis infections among ticks collected from vegetation, livestock, and small mammals in Iran, the present study aimed to investigate the status of infections with these two pathogenic agents among ticks and spleen samples of small mammals in the north, west, and northwest of Iran.
Material and methods
Ethics approval and consent to participate
The study complied with the Research Ethical Committee (REC) guidelines for experimental and clinical studies at the Pasteur Institute of Iran (IR.PII.REC.1395.29). Tick and spleen samples were collected from small mammals according to the REC protocol at the Pasteur Institute of Iran.
Study area
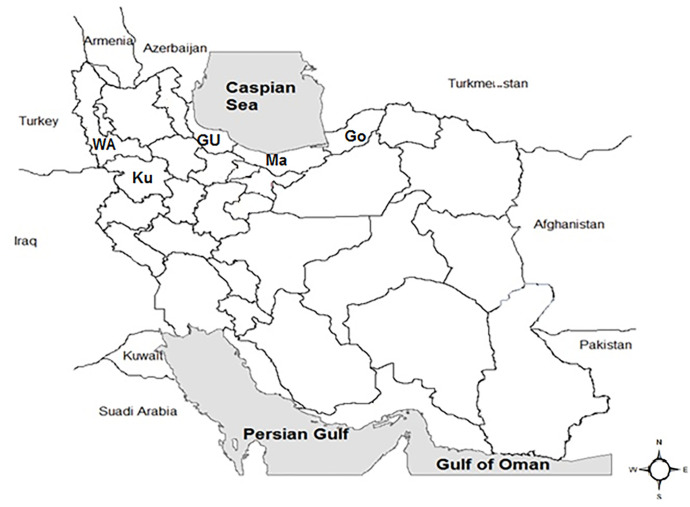
The study area in Iran included Guilan, Mazandaran, and Golestan provinces in the north, Kurdistan in the west, and West Azerbaijan in the northwest (Fig 1). The north of Iran extends from the distance south of the Caspian Sea to the north of the Alborz mountains. The area of the north of the country is 58,167 Km2, and its population is more than 10 million people. This region has a moderate and humid climate, and agriculture and livestock farming are practised in the rural areas. The province of Kurdistan is in the west of Iran and adjacent to the country of Iraq. This province occupies 29,500 Km2 and has a population of ~2 million. The climate of this region is hot and humid Mediterranean, and it is one of the important agriculture and animal husbandry zones. West Azerbaijan province is located in the northwest of Iran and borders with Turkey. The area of this province is 3,700 Km2, and its population is about 4 million people. This province is mountainous, with many rivers suitable for agriculture and animal husbandry. This province has mild weather in spring and summer and cold and snowy in winter.
Fig 1. Provinces in which ticks were collected in this study.
Abbreviations: Guilan (Gu), Mazandaran (Ma), Golestan (Go), Kurdistan (Ku), and West Azerbaijan (WA).
Sample collection
The sampling in this study was conducted from 23 October to 6 November 2017 and from 8 to 22 January 2018. The ticks from domestic livestock (sheep, goats, cattels, camels, horses, dogs, and donkeys) and other mammals (rodents and hedgehogs) were collected. Besides, the blanketing method was used to collect ticks from grasslands. The collected ticks were identified based on available morphological keys [33], labelled, and stored in 70% alcohol at 4°C until DNA extraction.
Small mammal trapping
Hand-made wooden 25×15×15 cm3 life traps were used to entrap small mammals. This type of trap is suitable for capturing all small mammals in the study area. The traps were placed in plains, and dates, lettuce, and cucumber were used as bait. The spleen samples of the trapped small mammals were collected after identifying them with morphological keys [34] and kept at -20° C until DNA extraction.
DNA extraction from ticks
After the identification of collected ticks, the ticks were pooled for DNA extraction. 4,197 collected ticks were pooled based on the same tick’s species, the same collected locations, the same host, the same tick sex, and the growth stage of ticks. Finally, the pools of ticks included 1 to 22 ticks based on the above criteria and 708 pools were prepared. The 708 pools were first homogenized in liquid nitrogen and sterile PBS, and the DNA was extracted by the potassium acetate method recommended by Rodríguez et al. [35]. Amount of 500 μl of lysis buffer (0.1 M TRIS-HCl, 0.05 M EDTA, 0.2 M sucrose, and 0.5% SDS) with 10 mL proteinase K were added to the homogenized specimens and incubated at 56°C overnight. Then, 120 μl 5 M sodium acetate was added to the specimens and kept on ice for 10 min. The suspensions were centrifuged at 12,000 ×g for 10 min, and the supernatant was recovered. For precipitation of DNA, 35 μl of 4 M sodium acetate and 1 ml of pure ethanol were added to specimens, mixed well, and kept on ice for 10 min. The samples were centrifuged at 12,000 ×g for 20 min, and the supernatant was discarded. The precipitates were washed with 500 μl of 70% ethanol, and the remaining alcohol was allowed to dry completely at room temperature. Finally, completely-dried precipitates were dissolved in 200 μl of elution buffer (1 molar Tris-HCl, 1 molar EDTA) and kept at -20°C until the analysis.
DNA extraction from spleen samples of small mammals
DNA extraction of small mammals (hedgehogs, shrews, and rodents) spleen samples was performed using a commercial High Pure PCR Template Preparation kit (Roche, Germany). Approximately 200 μl of lysis buffer and 40 μl of proteinase K were added to 25–50 mg samples and incubated at 55°C overnight. Then, 200 μl of binding buffer was added to each sample and incubated at 70°C for 25 min. Following adding 100 μl of isopropanol to the extraction columns, the suspensions were centrifuged at 8,000 ×g for one minute. Then, 500 μl of inhibitor buffer was added to each column and centrifuged for one minute at 8,000 ×g. In the next stage, 500 μl of wash buffer was added to each column, centrifuged at 12,000 ×g for one min, and then the wash buffer step was repeated. Finally, 120 μl of elution buffer solution was added to the samples and DNAs were extracted. Extracted DNAs were kept at—20°C until the molecular test.
Detection of Coxiella burnetii and Francisella tularensis
Extracted DNA from ticks and the spleen of small mammals were screened for C. burnetii and F. tularensis by a real-time PCR using the specific primers and the probe specific for the gene IS1111 and ISftu2 genes respectively. The probes were marked with 6-Carboxyfluorescein (6-FAM) fluorescent dye as a reporter dye and TAMRA as a quencher. The 20 μl reactions contained 10 μl commercial 2x RealQ Plus Master Mix (Ampliqon, Denmark), 900 nmol of forward primers (5’-AAAACGGATAAAAAGAGTCTGTGGTT-3’ for C. burnetii and 5’-TTGGTAGATCAGTTGGTAGGATAACC-3’ for F. tularensis), 900 nmol reverse primers (5’-CCACACAAGCGCGATTCAT-3’ for C. burnetii and 5’- TGAGTTTTATCCTCTGACAACAATATTTC-3’ for F. tularensis), 200 nmol of the probes (5’-6-FAM-AAAGCACTCATTGAGCGCCGCG-TAMRA-3’ for C. burnetii and 5’-6-FAM-AAAATCCATGCTATGACTGATGCTTTAGGTAATCCA- TAMRA-3’ for F. tularensis), and 4 μl template DNA and distilled water to the final volume. Coxiella burnetii strain Nine Mile RSA493 and F. tularensis subsp. holarctica NCTC 10857 was used as a positive control for the detection of C. burnetii and F. tularensis in real-time PCR tests, respectively. Also, distilled water was used as the negative control. The amplification was performed in a Corbett 6000 Rotor-Gene system thermocycler (Corbett, Victoria, Australia) programmed for 10 min at 95°C and 45 cycles at 95°C for 15 sec and 60°C for 60 seconds. The reading in each cycle was performed in the green spectrum at 60°C. Rotor-Gene Q Series Software was used for analyzed the real-time PCR results.
Statistical analysis
The data were analyzed by SPSS software (version 16). Chi-squared, Fisher exact, and logistic regression tests were used to compare the variables. A P-value <0.05 was considered statistically significant. Statistical analysis explored the correlation between the incidence of examined diseases and factors like tick species, collection province, and tick-animal host relationship.
Results
Tick identification
A total of 4,197 ticks were collected in the present study. Among the collected ticks, 56.1% (n = 2356) belonged to Mazandaran province, 34.7% (n = 1456) to Golestan province, 4.4% (n = 187) to Guilan province, 2.7% (n = 115) to Kurdistan province, and 1.9% (n = 83) to West Azerbaijan province. Among the ticks, 36.4% (n = 1530) were male, 62.5% (n = 2624) were female, and 1% (n = 43) were nymphs.
Twelve tick species belonging to 4 different genera (Ixodes, Haemaphysalis, Hyalomma, and Rhipicephalus) were identified, and I. ricinus (46.4%), Rh. turanicus (25%), Rh. sanguineus sensu lato (19.1%), Hy. marginatum (3.4%), and Rh. bursa (2.8%) species were the most collected ticks (Table 1).
Table 1. The tick species collected from the studied provinces in 2017–2018.
| Genus | Species | Mazandaran N (%) | Golestan N (%) | Guilan N (%) | Kurdistan N (%) | West-Azerbaijan N (%) | Total N (%) |
|---|---|---|---|---|---|---|---|
| Ixodes | I. ricinus | 1805 (76.6) | 6 (0.4) | 140 (74.9) | - | - | 1951 (46.4) |
| Haemaphysalis | Ha. concinna | 6 (0.2) | - | 1 (0.5) | - | - | 7 (0.2) |
| Ha. Inermis | 3 (0.1) | - | 1 (0.5) | - | - | 4 (0.1) | |
| Ha. punctata | - | - | 1 (0.5) | - | - | 1 (0.02) | |
| Hyalomma | Hy. anatolicum | - | 40 (2.7) | - | - | 4 (4.8) | 44 (1.00) |
| Hy. marginatum | 56 (2.4) | 65 (4.5) | 16 (8.6) | - | 6 (7.2) | 143 (3.4) | |
| Hy. dromedarii | - | 18 (1.2) | - | - | - | 18 (0.4) | |
| Hyalomma spp. | - | - | - | - | 3 (3.6) | 3 (0.1) | |
| Rhipicephalus | Rh. bursa | 48 (2.0) | 1 (0.1) | - | - | 70 (84.3) | 119 (2.8) |
| Rh. sanguineus | 343 (14.5) | 343 (23.6) | 1 (0.5) | 115 (100.0) | - | 802 (19.1) | |
| Rh. turanicus | 61 (2.6) | 983 (67.5) | 9 (4.8) | - | - | 1053 (25.1) | |
| Rh. annulatus | 34 (1.4) | - | 18 (9.6) | - | - | 52 (1.2) | |
| Total | 2356 (100) | 1456 (100) | 187 (100) | 115 (100) | 83 (100) | 4197 (100) | |
Of the collected ticks, 45.46% (n = 1908) were collected from cattel, 34.7% (n = 1458) from sheep, 9.33% (n = 384) from goats, 0.11% (n = 5) from horses, 0.04% (n = 2) from donkeys, 3.0% (n = 128) from camels, 1.19% (n = 50) from dogs, and 1.1% (n = 47) from hedgehogs.
Coxiella burnetii detection
Of the 708 pooled tick samples, 80 (11.3%) were positive for C. burnetii. The prevalence of C. burnetii in Rhipicephalus (18.3%) was significantly higher compared to other ticks genera (P<0.001). Among tick species, the highest prevalence belonged to Rh. sanguineus sensu lato (26.1%) and Rh. turanicus (15.2%) (Table 2). About 13.8% of positive pools belonged to Golestan province, 5.82% to Mazandaran province, 92.5% to Kurdistan province, and 11% to West Azerbaijan province. There was a significant relationship between C. burnetii infection and the provinces (P<0.001). The highest positive pools were from Sanandaj county, in Kurdistan province, i.e., all 14 pools from this area were positive. Bandar Torkaman county showed the highest number of positive pools in Golestan province; out of 56 pools, 13 were reported positive. Amol County in Mazandaran province showed the highest positive rate, with 10 out of 78 pools being positive. In Guilan province, no ticks were positive for C. burnetii (Table 3). The highest number of positive pools according to the hosts from which the ticks were collected belonged to Rh. turanicus and Rh. sanguineus collected from sheep in Golestan province, where 17 (25%) and 8 (19%) pools out of 80 pools were reported positive. Also, it should be mentioned that 92.6% (N = 25) of Rh. sanguineus collected from the grassland in Kurdistan province were infected with this pathogen (Table 4).
Table 2. Coxiella burnetii and Francisella tularensis infections among ticks in Iran (2017–2018).
| Genus | Species | Number of tested pools | Number of positive pools for F. tularensis in species (%) | Number of positive pools for F. tularensis per genus (%) | p-value | Number of positive pools for C. burnetii (%) | Number of positive pools for C. burnetii per genus (%) | p-value |
|---|---|---|---|---|---|---|---|---|
| Ixodes | I. ricinus | 259 | 7 (2.7) | 7(2.7) | <0.001 | 8 (3.1) | 8 (3.1) | <0.001 |
| Haemaphysalis | Ha. concinna | 4 | 0 (0) | 4(44.4) | 0 (0) | 0 (0) | ||
| Ha. inermis | 4 | 4 (100) | 0 (0) | |||||
| Ha. punctata | 1 | 0 (0) | 0 (0) | |||||
| Hyalomma | Hy. anatolicum | 13 | 1 (7.7) | 4(5.8) | 1 (7.7) | 4 (5.8) | ||
| Hy. marginatum | 49 | 3 (6.1) | 3 (6.1) | |||||
| Hy. dromedarii | 4 | 0 (0) | 0 (0) | |||||
| Hyalomma spp. | 3 | 0 (0) | 0 (0) | |||||
| Rhipicephalus | Rh. bursa | 45 | 2 (4.4) | 36(9.70) | 3 (6.7) | 68 (18.3) | ||
| Rh. sanguineus | 172 | 18 (10.5) | 45 (26.2) | |||||
| Rh. turanicus | 131 | 15 (11.4) | 20 (15.3) | |||||
| Rh. annulatus | 23 | 1 (4.3) | 0 (0) | |||||
| Total | 708 | 51 (7.2) | 80 (11.3) | |||||
Table 3. Prevalence of Coxiella burnetii and Francisella tularensis in ticks based on localities.
| Counties | Provinces | Number of pools tested | Number of positive pools for C. burnetii per genus (%) | Number of positive pools for F. tularensis per genus |
|---|---|---|---|---|
| (%) | ||||
| Golestan | Aq Qala | 67 | 0 (0) | 7 (10.4) |
| Bandar Torkaman | 56 | 13 (23.2) | 5 (8.9) | |
| Aliabad | 8 | 1 (12.5) | 2 (25) | |
| Gorgan | 39 | 5 (12.8) | 5 (12.8) | |
| Gomishan | 8 | 0 (0) | 1 (12.5) | |
| Aliabad-e-Katul | 23 | 9 (39.1) | 2 (8.7) | |
| Gonbad Kavus | 1 | 0(0) | 0 (0) | |
| Total | 202 | 28 (13.8) | 22 (10. 9) | |
| Mazandaran | Nur | 62 | 4 (6.45) | 5 (8) |
| Amol | 78 | 10 (12.8) | 8 (10.3) | |
| Babol | 55 | 0 (0) | 1 (1.8) | |
| Sari | 71 | 5 (7.04) | 3 (4.2) | |
| Qaemshahr | 55 | 2 (3.6) | 5 (9) | |
| Mahmudabad | 16 | 1 (6.2) | 0 (0) | |
| Savadkuh | 41 | 0 (0) | 1 (2.4) | |
| Total | 378 | 22 (5.8) | 23 (6.1) | |
| Guilan | Talesh | 46 | 0 (0) | 3 (6.5) |
| Masal | 5 | 0 (0) | 0 (0) | |
| Rudsar | 1 | 0 (0) | 0 (0) | |
| Lahijan | 6 | 0 (0) | 0 (0) | |
| Total | 58 | 0 (0) | 3 (5.2) | |
| Kurdistan | Sanandaj | 14 | 14 (100) | 1 (7.1) |
| Divandarreh | 9 | 8 (88.8) | 0 (0) | |
| Mariwan | 4 | 3 (75) | 0 (0) | |
| Total | 27 | 25 (92.5) | 1 (3.7) | |
| West-Azerbaijan | Showt | 43 | 5 (11.6) | 2 (4.6) |
| Total | 43 | 5 (11.6) | 2 (4.6) |
Table 4. Prevalence of Coxiella burnetii and Francisella tularensis in ticks according to the host and provinces studied.
| Host | Tick species | Number tested (% positive) for Coxiella burnetii | Number tested (%positive) for Francisella tularensis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maz. N (%) | Gol. N (%) | Guil. N (%) | Kurd. N (%) | Azer. N (%) | Total N (%) | Maz. N (%) | Gol. N (%) | Guil. N (%) | Kurd. N (%) | Azer. N (%) | Total N (%) | ||
| Cattel | I. ricinus | 179 (1.7) | 0(0) | 32 (0) | 0(0) | 0(0) | 211 (1.4) | 179 (1.2) | 0(0) | 32 (3.1) | 0 (0) | 0 (0) | 211 (1.4) |
| Hy. Marginatum | 4 (0) | 6 (0) | 4 (0) | 0(0) | 0(0) | 14 (0) | 4 (25) | 6 (0) | 4 (0) | 0 (0) | 0 (0) | 14 (7.1) | |
| Rh. Turanicus | 4 (0) | 5 (0) | 1 (0) | 0(0) | 0(0) | 10 (0) | 4 (0) | 5 (20) | 1 (0) | 0 (0) | 0 (0) | 10 (10) | |
| Ha. Inermis | 1 (0) | 0(0) | 1 (0) | 0(0) | 0(0) | 2 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 2 (100) | |
| Ha. Concinna | 3 (0) | 0(0) | 1 (0) | 0(0) | 0(0) | 4 (0) | 3 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 4 (0) | |
| Rh. Annulatus | 9 (0) | 0(0) | 14 (0) | 0(0) | 0(0) | 23 (0) | 9 (0) | 0 (0) | 14 (7.1) | 0 (0) | 0 (0) | 23 (4.3) | |
| Rh. Sanguineus | 5 (20) | 5 (0) | 1 (0) | 0(0) | 0(0) | 11 (9) | 5 (40) | 5 (20) | 0 (0) | 0 (0) | 0 (0) | 11 (27.3) | |
| Hy. Anatolicum | 0(0) | 1 (100) | 0(0) | 0(0) | 0(0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |
| Total | 205 (1.9) | 17 (5.9) | 54 (0) | 0(0) | 0(0) | 276 (1.8) | 205 (2.9) | 17 (17.6) | 54 (5.5) | 0 (0) | 0 (0) | 276 (4.3) | |
| Camel | Hy. Anatolicum | 0 (0) | 9 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (0) | 0 (0) | 9 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (0) |
| Hy. Marginatum | 0 (0) | 8 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (0) | 0 (0) | 8 (12.5) | 0 (0) | 0 (0) | 0 (0) | 8 (12.5) | |
| Rh. Sanguineus | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | |
| Rh. Turanicus | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) | 0 (0) | 4 (25) | 0 (0) | 0 (0) | 0 (0) | 4 (25) | |
| Hy. Dromedarii | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) | |
| Total | 0 (0) | 26 (0) | 0 (0) | 0 (0) | 0 (0) | 26 (0) | 0 (0) | 26 (7.7) | 0 (0) | 0 (0) | 0 (0) | 26 (7.7) | |
| Dog | Rh. Sanguineus | 0 (0) | 3 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0) | 0 (0) | 3 (33.3) | 0 (0) | 0 (0) | 0 (0) | 3 (33.3) |
| Rh. Turanicus | 5 (0) | 9 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (0) | 5 (20) | 9 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (7.1) | |
| I. ricinus | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1(0) | |
| Total | 6 (0) | 12 (0) | 0 (0) | 0 (0) | 0 (0) | 18 (0) | 6 (16.6) | 12 (8.3) | 0 (0) | 0 (0) | 0 (0) | 18 (11.1) | |
| Donkey | Rh. Sanguineus | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Total | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | |
| Goat | Ha. Punctata | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) |
| Rh. Sanguineus | 31 (16.1) | 14 (0) | 0 (0) | 0 (0) | 0 (0) | 45 (11.1) | 31 (9.7) | 14 (7.1) | 0 (0) | 0 (0) | 0 (0) | 45 (8.9) | |
| Rh. Turanicus | 4 (0) | 15 (6.7) | 0 (0) | 0 (0) | 0 (0) | 19 (5.3) | 4 (25) | 15 (6.7) | 0 (0) | 0 (0) | 0 (0) | 19 (10.5) | |
| I. ricinus | 10 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (20) | 10 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (20) | |
| Hy. Marginatum | 9 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (0) | 9 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (0) | |
| Rh. Bursa | 6 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (0) | 6 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (0) | |
| Ha. Inermis | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | |
| Total | 62 (11.3) | 29 (3.4) | 1 (0) | 0 (0) | 0 (0) | 92 (8.7) | 62 (12.9) | 29 (6.9) | 1 (0) | 0 (0) | 0 (0) | 92 (10.9) | |
| Sheep | Rh. Sanguineus | 42 (14.3) | 42 (19) | 0 (0) | 0 (0) | 0 (0) | 84 (16.7) | 42 (7.1) | 42 (14.3) | 0 (0) | 0 (0) | 0 (0) | 84 (11) |
| Rh. Turanicus | 12 (8.3) | 68 (25) | 0 (0) | 0 (0) | 0 (0) | 80 (22.5) | 12 (25) | 68 (10.3) | 0 (0) | 0 (0) | 0 (0) | 80 (12.5) | |
| I. ricinus | 36 (8.3_) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 37 (8.1) | 36 (2.8) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 37 (5.4) | |
| Rh. Bursa | 4 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (0) | 4 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (0) | |
| Hy. Marginatum | 8 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (12.5) | 8 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8(12.5) | |
| Total | 102 (10.8) | 112 (22.3) | 0 (0) | 0 (0) | 0 (0) | 214 (16.8) | 102 (7.8) | 112 (12.5) | 0 (0) | 0 (0) | 0 (0) | 214 (10.3) | |
| Hedgehog | Rh. Turanicus | 0 (0) | 4 (25) | 0 (0) | 0 (0) | 0 (0) | 4 (25) | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) |
| Total | 0 (0) | 4 (25) | 0 (0) | 0 (0) | 0 (0) | 4 (25) | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) | |
| Horse | Hy. Marginatum | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Total | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | |
| All animals | I. ricinus | 226 (3.5) | 1 (0) | 32 (0) | 0 (0) | 0 (0) | 259 (3.1) | 226 (2.2) | 1 (100) | 32 (3.1) | 0 (0) | 0 (0) | 259 (2.7) |
| Ha. Concinna | 3 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 4 (0) | 3(0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 4 (0) | |
| Ha. Inermis | 3 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 4 (0) | 3 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 4 (100) | |
| Ha. Punctata | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) | |
| Hy. Anatolicum | 0 (0) | 10 (10) | 0 (0) | 0 (0) | 0 (0) | 10 (10) | 0 (0) | 10 (10) | 0 (0) | 0 (0) | 0 (0) | 10 (10) | |
| Hy. Marginatum | 21 (4.8) | 15 (0) | 4 (0) | 0 (0) | 0 (0) | 40 (2.5) | 21 (9.5) | 15 (6.7) | 4 (0) | 0 (0) | 0 (0) | 40 (7.5) | |
| Hy. Dromedarii | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) | 0 (0) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (0) | |
| Hyalomma spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Rh. Bursa | 10 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 11 (0) | 10 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 11 (0) | |
| Rh. Sanguineus | 78 (15.3) | 66 (12.1) | 1 (0) | 0 (0) | 0 (0) | 145 (13.8) | 78 (12.3) | 66 (13.6) | 1 (0) | 0 (0) | 0 (0) | 145 (11.7) | |
| Rh. Turanicus | 24 (4.1) | 105 (18.1) | 1 (0) | 0 (0) | 0 (0) | 131 (15.3) | 24 (20.8) | 105 (9.5) | 1 (0) | 0 (0) | 0 (0) | 131 (11.4) | |
| Rh. Annulatus | 9 (0) | 0 (0) | 14 (0) | 0 (0) | 0 (0) | 23 (0) | 9 (0) | 0 (0) | 14 (7.1) | 0 (0) | 0 (0) | 23 (4.3) | |
| Total | 375 (5.9) | 202 (13.9) | 55 (0) | 0 (0) | 0 (0) | 632 (7.9) | 375 (6.1) | 202 (10.9) | 55 (5.4) | 0 (0) | 0 (0) | 632 (7.6) | |
| Grassland | Rh. Sanguineus | 0 (0) | 0 (0) | 0 (0) | 27 (92.6) | 0 (0) | 27 (92.6) | 0 (0) | 0 (0) | 0 (0) | 27 (3.7) | 0 (0) | 27 (3.7) |
| Rh. Bursa | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 32 (9.4) | 34 (8.8) | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 32 (6.3) | 34 (5.9) | |
| Hyalomma spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0) | 3 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0) | 3 (0) | |
| Hy. Marginatum | 1 (0) | 0 (0) | 3 (0) | 0 (0) | 5 (40) | 9 (22.2) | 1 (0) | 0 (0) | 3 (0) | 0 (0) | 5 (0) | 9 (0) | |
| Hy. Anatolicum | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0) | 3 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0) | 3 (0) | |
| Total | 3 (0) | 0 (0) | 3 (0) | 27/ (92.6) | 43 (11.6) | 76 (39.4) | 3 (0) | 0 (0) | 3 (0) | 27 (3.7) | 43 (4.7) | 76 (3.9) | |
The rate of C. burnetii infection in ticks was influenced by the type of host animal, with the highest infection rates observed in collected ticks from the body surface of sheep (16.8%), goats (8.7%), and cattel (1.8%) (p<0.01). Ticks collected from grasslands were significantly more infected with C. burnetii compared to those collected from animal surfaces (39.4% vs. 7.9%; p<0.01) (Table 4).
Francisella tularensis detection
Out of 708 pools, 51 (7.2%) were reported positive for F. tularensis. Based on the test results, the genus Haemaphysalis had the highest F. tularensis infection among the collected ticks, and 4 out of 9 pools were reported positive for F. tularensis. There was a significant relationship between F. tularensis infection and the ticks genus (P<0.001); the genera Haemaphysalis (44.4%) and Rhipicephalus (9.7%) had the highest F. tularensis infection. Among the tick species, the highest prevalence belonged to H. inermis (100%), Rh. turanicus (11.4%), and Rh. sanguineus sensu lato (10.4%) (Table 2). Based on the results, 10.89% of positive pools belonged to Golestan Province, 6.1% to Mazandaran Province, 5.2% to Guilan Province, 3.7% to Kurdistan Province, and 4.6% to West Azerbaijan Province, respectively. However, there was no significant relationship between F. tularensis and different provinces (P = 0.19). The highest positive pools of F. tularensis were reported in Gorgan county of Golestan province, and 5 out of 39 pools were positive. In Mazandaran province, the highest positive pool was seen in Amol County, where 8 out of 78 pools were positive. In Talesh County of Guilan province, 3 out of 46 pools were positive. In Sanandaj county of Kurdistan province, 1 out of 14 pools was positive, and 2 out of 43 pools in West Azerbaijan province were positive (Table 3).
Ticks collected from the body surface of dogs (11.1%), goats (10.9%), and sheep (10.3%) had the highest infection rates for F. tularensis, but there was no statistically significant difference between the level of Francisella infection and the type of host animal (p = 0.28). The rate of F. tularensis infection in ticks collected from grasslands (3.9%) did not differ significantly from those collected from animal surfaces (7.6%) (p = 0.24).
Coxiella burnetii and Francisella. tularensis in animals
Of 135 spleen DNAs belonging to small mammals (Table 5), none exhibited C. burnetii or F. tularensis infections.
Table 5. Geographic distribution of the included small mammals in the present study.
| Species/ Province | Mazandaran N (%) | Guilan N (%) | Golestan N (%) | Kurdistan N (%) | West-Azerbaijan N (%) | Total |
|---|---|---|---|---|---|---|
| Apodemus hyrcanicus | 2 (11.1) | 2 (16.6) | - | 0(0) | 0(0) | 4 (3.0) |
| Mus musculus | 2 (11.1) | 4 (33.3) | 1 (7.14) | 0(0) | 0(0) | 7 (5.2) |
| Rattus norvegicus | 13 (72.2) | 0(0) | 0(0) | 0(0) | 0(0) | 13 (9.6) |
| Rattus rattus | 1 (5.5) | 4 (33.3) | 0(0) | 0(0) | 0(0) | 5 (3.7) |
| Crocidura caspica | 0(0) | 1 (8.3) | 0(0) | 0(0) | 0(0) | 1 (0.7) |
| Microtus obscurus | 0(0) | 1 (8.3) | 0(0) | 0(0) | 0(0) | 1 (0.7) |
| Nesokia indica | 0(0) | 0(0) | 6 (42.8) | 0(0) | 0(0) | 6 (4.4) |
| Erinaceus concolor | 0(0) | 0(0) | 3 (21.4) | 0(0) | 0(0) | 3 (2.2) |
| Apodemus uralensis | 0(0) | 0(0) | 1 (7.14) | 0(0) | 0(0) | 1 (0.7) |
| Crocidura suaveolens | 0(0) | 0(0) | 1 (7.14) | 0(0) | 0(0) | 1 (0.7) |
| Microtus paradoxus | 0(0) | 0(0) | 2 (14.2) | 0(0) | 0(0) | 2 (1.48) |
| Apodemus witherbyi | 0(0) | 0(0) | 0(0) | 22 (26.8) | 0(0) | 22 (16.3) |
| Apodemus ponticus | 0(0) | 0(0) | 0(0) | 1 (1.2) | 0(0) | 1 (0.7) |
| Arvicola persicus | 0(0) | 0(0) | 0(0) | 8 (9.7) | 0(0) | 8 (5.9) |
| Nothocricetulus migratorius | 0(0) | 0(0) | 0(0) | 2 (2.4) | 0(0) | 2 (1.5) |
| Meriones persicus | 0(0) | 0(0) | 0(0) | 5 (6.1) | 1 (11.1) | 6 (4.4) |
| Meriones tristrami | 0(0) | 0(0) | 0(0) | 1 (1.2) | 0(0) | 1 (0.7) |
| Microtus schidlovskii | 0(0) | 0(0) | 0(0) | 2 (2.4) | 0(0) | 2 (1.5) |
| Microtus qazvinensis | 0(0) | 0(0) | 0(0) | 4 (4.9) | 0(0) | 4 (3.0) |
| Mus macedonicus | 0(0) | 0(0) | 0(0) | 2 (2.4) | 0(0) | 2 (3.0) |
| Microtus qazvinensis | 0(0) | 0(0) | 0(0) | 35 (42.7) | 0(0) | 35 (25.9) |
| Meriones vinogradovi | 0(0) | 0(0) | 0(0) | 0(0) | 7 (77.7) | 7 (5.2) |
| Microtus socialis | 0(0) | 0(0) | 0(0) | 0(0) | 1 (11.1) | 1 (0.7) |
| Total | 18 (100) | 12 (100) | 14 (100) | 82 (100) | 9 (100) | 135 (100) |
Discussion
The present study investigated the hard ticks from three Northern provinces (Mazandaran, Golestan, and Guilan) and the northwestern and western provinces of Iran (Kurdistan, West Azerbaijan) for C. burnetii and F. tularensis. Our real-time PCR showed that 11.3% of tick pools were positive for C. burnetii and 7.2% for F. tularensis, confirming the circulation of both pathogens via the ticks in the regions.
Due to the spread of tick-borne diseases, including the two pathogens in the present study in the Middle East, more studies should be conducted. Also, the possibility of human bites by ticks as vectors of these diseases and the detection of human cases infected with these two pathogens in Iran [19, 20] requires a broad approach to determine the presence of these bacteria in ticks and animals (mammals).
In this study, C. burnetii DNA was identified in collected ticks from Northern provinces (except for Guilan), Kurdistan, and West Azerbaijan provinces, and the highest positive pools belonged to Kurdistan province. Domestic ruminants are considered the main reservoirs for C. burnetii, but it seems that ticks may also be involved in the cycle of transmission and maintenance of this pathogen [36]. The first tick species in which C. burnetii was identified belonged to Dermacentor andersoni. Coxiella burnetii is acquired by arthropods during the hematophagy process; however, not all ticks that feed on infected animals become infected with this pathogen. In general, the acquisition of C. burnetii is harmless for ticks, in contrast to Coxiella-like endosymbionts, they are non-infectious for vertebrates, but they can be problematic for ticks, and this issue can be a major concern for conducting epidemiological studies to identify C. burnetii in ticks [37]. Based on the studies, it seems that ticks are not necessary for the transmission of C. burnetii to livestock, but among vertebrates, including rodents and wild birds, they probably play an important role. According to some studies, ticks seem to excrete a significant amount of these bacteria along with their faeces when they defecate, and inhalants of these infected particles by domestic animals can infect them [8, 38, 39]. Also, humans, while shearers, may get Q fever as a result of inhaling the particles of this bacterium [8]. The role of ticks in the transmission cycle of this bacterium has been discussed in many studies [40, 41]. DNA of C. burnetii has been detected in 40 species of ticks [8]. Furthermore, the presence of DNA of C. burnetii in Ixodes, Hyalomma, and Rhipicephalus ticks in the present study indicates the presence of this bacterium in a wide range of tick genera and shows the role of ticks in the epidemiology of Q fever [42, 43].
The present study indicated a significant relationship between C. burnetii infection and the genus of Rhipicephalus. The most common positive pools belonged to Rh. sanguineus sensu lato (26.1%), our results were similar to a study on ticks of dogs in Kerman province, and the results indicated a prevalence of 12.5% of C. burnetii in Rh. sanguineus sensu lato [44]. The first study in Iran on C. burnetii infection among ticks in 2009 indicated an 8.6% C. burnetii infection in Hy. anatolicum and Rh. sanguineus, with the highest belonging to Hy. anatolicum species [45]. In a study in Iran in 2020, 13.9% of ticks collected from sheep were reported positive for C. burnetii and the highest prevalence of infection was found in D. marginatus (18.3%) and Ha. concinna (12.5%) [46]. Also, in another study in Iran, 7.4% of ticks collected from sheep and goats belonging to Rh. sanguineus and Hy. anatolicum species were reported positive for C. burnetii [47]. In Cyprus, 6.4% of Hyalomma spp. and Rh. sanguineus ticks were positive, while most positive samples belonged to the Rh. sanguineus, consistent with the results obtained in the present study [48].
Real-time PCR did not detect C. burnetii infection in the genus Haemaphysalis. In a study in Turkey, the DNA of C. burnetii was detected in Rhipicephalus and Hyalomma genus, and most cases belonged to the Rhipicephalus genus, and it was consistent with our results, while there were not any positive samples in the genus of Haemaphysalis [49]. Like other studies and the results of our study, Rhipicephalus were the most common genera infected with C. burnetii. Other studies have also identified C. burnetii in I. ricinus. For example, in a study in Slovakia and Hungary, C. burnetii was detected in I. ricinus, D. marginatus, and Ha. concinna species [42]. According to a study in Germany on I. ricinus, 1.9% of the ticks were infected with C. burnetii [50]. In another study in Poland, 15.9% of I. ricinus collected from forest areas were infected with this pathogen [51]. In this study, the DNA of C. burnetii was also detected in Ixodes (3.1%). It seems likely that I. ricinus also play a role in the transmission cycle of C. burnetii.
There are few studies on F. tularensis infection in Iran, and most studies were conducted on human disease. In neighboring countries, few studies were performed on ticks as vectors of this disease [26]. DNA of F. tularensis was detected from ticks in all provinces, and the highest positive cases belonged to the Golestan province (10. 9%). However, there was no significant correlation between positive F. tularensis in ticks and the provinces.
According to the studies, the most common tick species in which the DNA of F. tularensis was detected belong to Dermacentor, Haemaphysalis, Amblyomma, and Ixodes genera, and they are very important in the terrestrial cycle of F. tularensis [52]. The most positive samples were identified in the Haemaphysalis genus and Ha. inermis species (100%). In a study in the UAE, the DNA of F. tularensis was identified in 5.8% of Hy. dromedarii isolated from camels by the molecular method [53]. In Egypt, 4.7% of Hy. dromedarii which were collected from camels were reported positive for F. tularensis [54]. However, none of the Hy. dromedarii collected from camel was reported positive for F. tularensis in our study.
In neighboring countries of Iran, including Turkey, F. tularensis is an endemic disease. However, according to the studies conducted on ticks in different sites in Turkey, no positive samples have been reported for F. tularensis [55–57]. While in our study, the DNA of F. tularensis was identified in 4 genera of ticks (Ixodes, Haemaphysalis, Rhipicephalus, and Hyalomma) collected from domestic animals using the molecular method.
According to some studies, ticks are important biological vectors for F. tularensis, which transmit this pathogen between humans and animals through bites and can maintain this organism for a long time in nature [58, 59]. F. tularensis can be localized in the gut and hemolymph of ticks, and the number of organisms increases from the larval stage to adult ticks [60]. However, some studies have suggested the possible role of ticks as a reservoir, which requires further investigation. Also, transstadial transmission of F. tularensis has been confirmed, but transmission through the transovarial is still debated [61]. Ticks also play a crucial role as biological and mechanical vectors for F. tularensis. Their life cycle involves four stages, namely egg, larva, nymph, and adult. Ticks require blood meals during their growth stages, making them capable of transmitting the bacteria through bites [62, 63].
Based on data from epidemiological studies, rodents, and rabbits have been suggested as the main reservoirs of F. tularensis [64]. Tularemia is often fatal in animals. Studies show that a bacteremia dose of more than 108 CFU/ mL in the blood, spleen, and lung of a mic is a lethal dose. However, some rodents can survive without symptoms and transmit the infection to other rodents through ticks [52]. In a study in China to investigate F. tularensis in the spleen of rodents, 4.7% of the rodents were infected with this pathogen, but none of the samples were reported positive in our study [65].
According to a study in Turkey, the prevalence of C. burnetii and F. tularensis were reported at 40% and 22.5% in ticks, respectively. Based on the results of this study, most of the positive samples for C. burnetii were detected in questing ticks, which was consistent with our results (39.4%), while the positive samples for F. tularensis were detected in ticks collected from animals, which is not consistent with our results [66].
The number of rodents examined in this study was very limited and this was one of the limitations of our study. It is also possible that transient bacteremia in rodents prevented the detection of both pathogens investigated in this study. IS 1111 sequence is a specific transposon in C. burnetii, and it is widely used in the molecular diagnosis of infection by C. burnetii in humans and animals. Nevertheless, sometimes, similar IS 1111- homolog is also seen in endosymbionts and Coxiella-likes bacteria in ticks, and it may cause a false positive result of C. burnetii in ticks. Unfortunately, we only used IS 1111 to screen ticks in this study, and it was recommended that another specific gene be used to confirm the IS 1111-positive sample in future studies.
The positivity of ticks in the present study suggests a possibility of livestock infection, and because of the effects of these diseases on livestock, such as abortion, the possibility of human infection through livestock products, such as consuming contaminated milk, needs to be investigated. The results of this study increase our knowledge about the prevalence of these pathogens in Iran. The results also indicated that livestock ticks are infected with these pathogens, so more studies need to be conducted.
Conclusions
It seems that ticks can be a reservoir and vector of pathogenic pathogens and are responsible for the transmission of infection to domestic and wild animals and even humans. The results of this study showed the contamination of ticks in domestic animals. Considering the possibility of animal contamination by ticks, the examination of animal samples is also useful to identify the prevalence of the above pathogens, because animals can be the mediators of disease transmission to humans. Because C. burnetii and F. tularensis significantly impact public health, it is very necessary to identify the main source of their spread and manage the situation. Therefore, it is necessary to fully investigate the role of ticks in the epidemiology of diseases and prepare a suitable educational program to prevent and control the population of ticks.
Acknowledgments
We would like to express our gratitude to Mr Hamed Hanifi, Mr Seyyed Adel Hosseini, Mr Amir Hesam Neamati and Mr Sadegh Kheiri from Pasteur Institute of Iran for their support in the sampling.
Data Availability
All relevant data are within the paper.
Funding Statement
We thank the Pasteur Institute of Iran (grant no. 617) for its financial support. We would like to express our gratitude to Mr Hamed Hanifi, Mr Adel Hosseini, and Mr Amir Hesam Neamati from Pasteur Institute of Iran for their support in the sampling. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, et al. (2008) Global trends in emerging infectious diseases. Nature 451: 990–993. doi: 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown C (2004) Emerging zoonoses and pathogens of public health significance—an overview. Revue scientifique et technique-office international des epizooties 23: 435–442. doi: 10.20506/rst.23.2.1495 [DOI] [PubMed] [Google Scholar]
- 3.Zumla A, Hui DS (2019) Emerging and reemerging infectious diseases: global overview. Infectious Disease Clinics 33: xiii–xix. doi: 10.1016/j.idc.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savić S, Vidić B, Grgić Z, Potkonjak A, Spasojevic L (2014) Emerging vector-borne diseases–incidence through vectors. Frontiers in public health 2: 267. doi: 10.3389/fpubh.2014.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt K, Dressel K, Niedrig M, Mertens M, Schüle S, et al. (2013) Public Health and Vector‐Borne Diseases–A New Concept for Risk Governance. Zoonoses and public health 60: 528–538. doi: 10.1111/zph.12045 [DOI] [PubMed] [Google Scholar]
- 6.Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E (2020) Emerging tick-borne diseases. Clinical microbiology reviews 33: e00083–00018. doi: 10.1128/CMR.00083-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, et al. (2017) Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Frontiers in cellular and infection microbiology 7: 114. doi: 10.3389/fcimb.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, et al. (2017) From Q fever to Coxiella burnetii infection: a paradigm change. Clinical microbiology reviews 30: 115–190. doi: 10.1128/CMR.00045-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hestvik G, Warns-Petit E, Smith L, Fox N, Uhlhorn H, et al. (2015) The status of tularemia in Europe in a one-health context: a review. Epidemiology & Infection 143: 2137–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melenotte C, Million M, Raoult D (2020) New insights in Coxiella burnetii infection: diagnosis and therapeutic update. Expert Review of Anti-infective Therapy 18: 75–86. doi: 10.1080/14787210.2020.1699055 [DOI] [PubMed] [Google Scholar]
- 11.España PP, Uranga A, Cillóniz C, Torres A. Q fever (Coxiella burnetii); 2020. Thieme Medical Publishers. pp. 509–521. [DOI] [PubMed] [Google Scholar]
- 12.Todkill D, Fowler T, Hawker J (2018) Estimating the incubation period of acute Q fever, a systematic review. Epidemiology & Infection 146: 665–672. doi: 10.1017/S095026881700303X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tissot-Dupont H, Raoult D (2008) Q fever. Infectious disease clinics of North America 22: 505–514. doi: 10.1016/j.idc.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Miller H, Priestley R, Kersh G (2020) Transmission of Coxiella burnetii by ingestion in mice. Epidemiology & Infection 148: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelakis E, Raoult D (2010) Q fever. Veterinary microbiology 140: 297–309. doi: 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 16.Maurin á, Raoult Df(1999) Q fever. Clinical microbiology reviews 12: 518–553. doi: 10.1128/CMR.12.4.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kampschreur LM, Delsing CE, Groenwold RH, Wegdam-Blans MC, Bleeker-Rovers CP, et al. (2014) Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. Journal of clinical microbiology 52: 1637–1643. doi: 10.1128/JCM.03221-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohabbati Mobarez A, Bagheri Amiri F, Esmaeili S (2017) Seroprevalence of Q fever among human and animal in Iran; A systematic review and meta-analysis. PLoS neglected tropical diseases 11: e0005521. doi: 10.1371/journal.pntd.0005521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaghmaie F, Esmaeili S, Francis SA, Mostafavi E (2015) Q fever endocarditis in Iran: A case report. Journal of infection and public health 8: 498–501. doi: 10.1016/j.jiph.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 20.Heydari AA, Mostafavi E, Heidari M, Latifian M, Esmaeili S (2021) Q Fever Endocarditis in Northeast Iran. Case Reports in Infectious Diseases 2021. doi: 10.1155/2021/5519164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmaeili S, Mohabati Mobarez A, Khalili M, Mostafavi E, Moradnejad P (2019) Molecular prevalence of Coxiella burnetii in milk in Iran: a systematic review and meta-analysis. Tropical animal health and production 51: 1345–1355. doi: 10.1007/s11250-019-01807-3 [DOI] [PubMed] [Google Scholar]
- 22.Mohabati Mobarez A, Khalili M, Mostafavi E, Esmaeili S (2021) Molecular detection of Coxiella burnetii infection in aborted samples of domestic ruminants in Iran. PloS one 16: e0250116. doi: 10.1371/journal.pone.0250116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalili M, Mosavi M, Diali HG, Mirza HN (2014) Serologic survey for Coxiella burnetii phase II antibodies among slaughterhouse workers in Kerman, southeast of Iran. Asian Pacific journal of tropical biomedicine 4: S209–S212. doi: 10.12980/APJTB.4.2014C1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjöstedt A (2016) Clinical manifestations and the epidemiology of tularemia. Medicina Fluminensis: Medicina Fluminensis 52: 211–216. [Google Scholar]
- 25.Telford III SR, Goethert HK (2020) Ecology of Francisella tularensis. Annual review of entomology 65: 351–372. doi: 10.1146/annurev-ento-011019-025134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zargar A, Maurin M, Mostafavi E (2015) Tularemia, a re-emerging infectious disease in Iran and neighboring countrie. Epidemiology and health 37. doi: 10.4178/epih/e2015011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esmaeili S, Rohani M, Ghasemi A, Gouya MM, Khayatzadeh S, et al. (2021) Francisella tularensis human infections in a village of northwest Iran. BMC Infectious Diseases 21: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemati M, Khalili M, Rohani M, Sadeghi B, Esmaeili S, et al. (2020) A serological and molecular study on Francisella tularensis in rodents from Hamadan province, Western Iran. Comparative immunology, microbiology and infectious diseases 68: 101379. doi: 10.1016/j.cimid.2019.101379 [DOI] [PubMed] [Google Scholar]
- 29.Esmaeili S, Ghasemi A, Naserifar R, Jalilian A, Molaeipoor L, et al. (2019) Epidemiological survey of tularemia in Ilam Province, west of Iran. BMC Infectious Diseases 19: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esmaeili S, Gooya MM, Shirzadi MR, Esfandiari B, Amiri FB, et al. (2014) Seroepidemiological survey of tularemia among different groups in western Iran. International Journal of Infectious Diseases 18: 27–31. doi: 10.1016/j.ijid.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 31.Mostafavi E, Shahraki AH, Japoni-Nejad A, Esmaeili S, Darvish J, et al. (2017) A field study of plague and tularemia in rodents, Western Iran. Vector-Borne and zoonotic diseases 17: 247–253. doi: 10.1089/vbz.2016.2053 [DOI] [PubMed] [Google Scholar]
- 32.Mostafavi E, Ghasemi A, Rohani M, Molaeipoor L, Esmaeili S, et al. (2018) Molecular survey of tularemia and plague in small mammals from Iran. Frontiers in cellular and infection microbiology 8: 215. doi: 10.3389/fcimb.2018.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker AR (2003) Ticks of domestic animals in Africa: a guide to identification of species: Bioscience Reports Edinburgh. [Google Scholar]
- 34.Darvish J, Mohammadi Z, Mahmoudi A, Siahsarvie R (2014) Faunistic and taxonomic study of Rodents from northwestern Iran. Iranian Journal of Animal Biosystematics 10: 119–136. [Google Scholar]
- 35.Rodríguez I, Fraga J, Noda AA, Mayet M, Duarte Y, et al. (2014) An alternative and rapid method for the extraction of nucleic acids from ixodid ticks by potassium acetate procedure. Brazilian Archives of Biology and Technology 57: 542–547. [Google Scholar]
- 36.Varela-Castro L, Zuddas C, Ortega N, Serrano E, Salinas J, et al. (2018) On the possible role of ticks in the eco-epidemiology of Coxiella burnetii in a Mediterranean ecosystem. Ticks and tick-borne diseases 9: 687–694. doi: 10.1016/j.ttbdis.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 37.Celina SS, Cerný J (2022) Coxiella burnetii in ticks, livestock, pets and wildlife: A mini-review. Frontiers in Veterinary Science 9. doi: 10.3389/fvets.2022.1068129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eklund CM, Parker R, Lackman DB, Ecklund CM, Lockman DB (1947) A case of Q fever probably contracted by exposure to ticks in nature. Public Health Reports (1896–1970): 1413–1416. [PubMed] [Google Scholar]
- 39.Smith D (1942) Studies in the Epidemiology of Q Fever. 11. Experimental Infection of the Ticks Haemaphysalis bispinosa and Ornithodorus. sc. with Rickettsia burneti. Australian Journal of Experimental Biology and Medical Science 20: 295–296. [Google Scholar]
- 40.Körner S, Makert GR, Ulbert S, Pfeffer M, Mertens-Scholz K (2021) The prevalence of Coxiella Burnetii in hard ticks in Europe and their role in Q fever transmission revisited—A systematic review. Frontiers in Veterinary Science 8: 655715. doi: 10.3389/fvets.2021.655715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yessinou RE, Katja M-S, Heinrich N, Farougou S (2022) Prevalence of Coxiella-infections in ticks-review and meta-analysis. Ticks and Tick-borne Diseases: 101926. doi: 10.1016/j.ttbdis.2022.101926 [DOI] [PubMed] [Google Scholar]
- 42.Špitalská E, Kocianová E (2003) Detection of Coxiella burnetii in ticks collected in Slovakia and Hungary. European journal of epidemiology 18: 263–266. doi: 10.1023/A:1023330222657 [DOI] [PubMed] [Google Scholar]
- 43.Toledo A, Jado I, Olmeda A, Casado-Nistal M, Gil H, et al. (2009) Detection of Coxiella burnetii in ticks collected from Central Spain. Vector-Borne and Zoonotic Diseases 9: 465–468. doi: 10.1089/vbz.2008.0070 [DOI] [PubMed] [Google Scholar]
- 44.Khalili M, Rezaei M, Akhtardanesh B, Abiri Z, Shahheidaripour S (2018) Detection of Coxiella burnetii (Gammaproteobacteria: Coxiellaceae) in ticks collected from infested dogs in Kerman, Southeast of Iran. Persian Journal of Acarology 7: 93–100. [Google Scholar]
- 45.Fard SN, Khalili M (2011) PCR-detection of Coxiella burnetii in ticks collected from sheep and goats in southeast Iran. Iranian journal of arthropod-borne diseases 5: 1. doi: 10.1099/jmm.0.021543-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahravani M, Moravedji M, Mostafavi E, Mohammadi M, Seyfi H, et al. (2022) The epidemiological survey of Coxiella burnetii in small ruminants and their ticks in western Iran. BMC Veterinary Research 18: 292. doi: 10.1186/s12917-022-03396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fard SR N, Ghashghaei O, Khalili M, Sharifi H (2016) Tick diversity and detection of Coxiella burnetii in tick of small ruminants using nested Trans PCR in Southeast Iran. Tropical Biomedicine 33: 506–511. [PubMed] [Google Scholar]
- 48.Spyridaki I, Psaroulaki A, Loukaides F, Antoniou M, Hadjichristodolou C, et al. (2002) Isolation of Coxiella burnetii by a centrifugation shell-vial assay from ticks collected in Cyprus: detection by nested polymerase chain reaction (PCR) and by PCR-restriction fragment length polymorphism analyses. The American journal of tropical medicine and hygiene 66: 86–90. doi: 10.4269/ajtmh.2002.66.86 [DOI] [PubMed] [Google Scholar]
- 49.Capin GA, Emre Z, Canpolat S, Vatansever Y, Duzgun A (2013) Detection of Coxiella burnetii from ticks by polymerase chain reaction and restriction fragment length polymorphism. Ankara Üniversitesi Veteriner Fakültesi Dergisi 60: 263–268. [Google Scholar]
- 50.Hildebrandt A, Straube E, Neubauer H, Schmoock G (2011) Coxiella burnetii and coinfections in Ixodes ricinus ticks in central Germany. Vector-Borne and Zoonotic Diseases 11: 1205–1207. doi: 10.1089/vbz.2010.0180 [DOI] [PubMed] [Google Scholar]
- 51.Szymanska-Czerwinska M, Galinska EM, Niemczuk K, Zasepa M (2013) Prevalence of Coxiella burnetii infection in foresters and ticks in south-eastern Poland and comparison of diagnostic methods. Annals of Agricultural and Environmental Medicine 20. [PubMed] [Google Scholar]
- 52.Gürcan Ş (2014) Epidemiology of tularemia. Balkan medical journal 2014; 31: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perveen N, Muzaffar SB, Al-Deeb MA (2021) Four Tick-Borne Microorganisms and Their Prevalence in Hyalomma Ticks Collected from Livestock in United Arab Emirates. Pathogens 10: 1005. doi: 10.3390/pathogens10081005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghoneim NH, Abdel-Moein KA, Zaher HM (2017) Molecular detection of Francisella spp. among ticks attached to camels in Egypt. Vector-Borne and Zoonotic Diseases 17: 384–387. doi: 10.1089/vbz.2016.2100 [DOI] [PubMed] [Google Scholar]
- 55.Duzlu O, Yildirim A, Inci A, Gumussoy KS, Ciloglu A, et al. (2016) Molecular investigation of Francisella-like endosymbiont in ticks and Francisella tularensis in ixodid ticks and mosquitoes in Turkey. Vector-Borne and Zoonotic Diseases 16: 26–32. doi: 10.1089/vbz.2015.1818 [DOI] [PubMed] [Google Scholar]
- 56.Demir S, Erkunt Alak S, Köseoğlu AE, Ün C, Nalçacı M, et al. (2020) Molecular investigation of Rickettsia spp. and Francisella tularensis in ticks from three provinces of Turkey. Experimental & applied acarology 81: 239–253. doi: 10.1007/s10493-020-00498-y [DOI] [PubMed] [Google Scholar]
- 57.Yıldırımtepe-Çaldıran F, Keskin A, Bursalı A, Tekin Ş (2021) Molecular Screening for Rickettsia, Francisella and Borrelia in Ticks Collected from the Middle Black Sea Coastline, Turkey. ACTA ZOOLOGICA BULGARICA: 125–129. [Google Scholar]
- 58.Eliasson H, Broman T, Forsman M, Bäck E (2006) Tularemia: current epidemiology and disease management. Infectious Disease Clinics 20: 289–311. doi: 10.1016/j.idc.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 59.Goodman JL, Dennis DT, Sonenshine DE (2005) Tick-borne diseases of humans: ASM press. [Google Scholar]
- 60.Petersen JM, Mead PS, Schriefer ME (2009) Francisella tularensis: an arthropod-borne pathogen. Veterinary research 40. doi: 10.1051/vetres:2008045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Genchi M, Prati P, Vicari N, Manfredini A, Sacchi L, et al. (2015) Francisella tularensis: no evidence for transovarial transmission in the tularemia tick vectors Dermacentor reticulatus and Ixodes ricinus. PLoS One 10: e0133593. doi: 10.1371/journal.pone.0133593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hopla CE (1960) The transmission of tularemia organisms by ticks in the southern states. Southern Medical Journal 53: 92–97. doi: 10.1097/00007611-196001000-00020 [DOI] [PubMed] [Google Scholar]
- 63.Parker RR, Spencer RR, Francis E (1924) Tularæmia: XI. Tularæmia Infection in Ticks of the Species Dermacentor Andersoni Stiles in the Bitterroot Valley, Mont. Public Health Reports (1896–1970): 1057–1073. [Google Scholar]
- 64.Keim P, Johansson A, Wagner DM (2007) Molecular epidemiology, evolution, and ecology of Francisella. Annals of the New York Academy of Sciences 1105: 30–66. doi: 10.1196/annals.1409.011 [DOI] [PubMed] [Google Scholar]
- 65.Zhang F, Liu W, Chu MC, He J, Duan Q, et al. (2006) Francisella tularensis in rodents, China. Emerging Infectious Diseases 12: 994. doi: 10.3201/eid1206.051324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brinkmann A, Hekimoğlu O, Dinçer E, Hagedorn P, Nitsche A, et al. (2019) A cross-sectional screening by next-generation sequencing reveals Rickettsia, Coxiella, Francisella, Borrelia, Babesia, Theileria and Hemolivia species in ticks from Anatolia. Parasites & vectors 12: 1–13. doi: 10.1186/s13071-018-3277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.