Abstract
Background
Coagulopathy and massive bleeding are common complications of patients with Stanford type A acute aortic dissection repair, and patients with these complications require many transfusions. Autologous platelet-rich plasma (PRP) is widely used to reduce the need for blood products. In the present study, we aimed to investigate the effects of PRP on blood conservation and the postoperative conditions of patients who underwent aortic arch replacement.
Methods
Patients with aortic dissection undergoing aortic arch replacement were included initially application In all, 837 patients were divided into the PRP and non-PRP groups according to PRP use, whereupon a propensity score match was performed. The data analyzed included patient basic information, intraoperative information, postoperative biochemical examinations, and CTA reports.
Results
In total, 610 patients were finally included (305 patients per group). Groups were well balanced after matching. Compared to the non-PRP group, less cryoprecipitate was transfused in the PRP group (10.0 [7.5, 11.0] vs. 10.0 [10.0, 11.5], P = 0.021), while no differences were found in packed RBC, FFP, and platelets between the two groups. Also, the surgery variables showed no differences. After surgery, patients in the PRP group showed higher postoperative serum albumin (36.43±4.20 vs. 35.39±4.40 g/L, P = 0.004) and total protein levels (59.38±6.25 vs. 58.06±7.19 g/L, P = 0.019) than the non-PRP group, but no significant differences in the levels of ALT, AST, Scr, and BUN. CTA reports showed that the proportion of patients with pleural effusion was lower in the PRP group (76.66% vs. 83.99%, OR = 1.59, 95% CI: 1.04–2.45, P = 0.028), while the proportions of pericardial effusion were not significantly different.
Conclusions
PRP application in aortic arch replacement surgery reduced the transfusion of cryoprecipitate, increased the postoperative serum albumin and total protein levels, and reduced the incidence of pleural effusion. No effect of PRP application was found on other postoperative blood indicators and CTA reports.
Introduction
Stanford type A acute aortic dissection (AAD) is a relatively rare but catastrophic vascular disease. It is defined as disruption of the medial layer provoked by intramural bleeding, resulting in separation of the aortic wall layers and subsequent formation of a true lumen and a false lumen with or without communication. Patients without treatment die at a rate of 1%–2% per hour on the first day, and within 48 hours almost 50% of patients present clinical symptoms, such as rupture, pericardial tamponade, valvular malfunction, and stroke [1, 2]. Treatment is challenging; conservative therapies showed markedly worse long-term outcomes than surgeries, while surgeries are complicated and result in high incidences of perioperative mortality (25%) and neurological complications (18%) [3]. Meanwhile, some anti-coagulation therapies might influence the severity and the outcomes of these patients [4–6].
Massive bleeding is a common and worrisome perioperative complication that requires compensative transfusion of blood products. Transfusion causes adverse effects on the body dependent on the amount transfused and increases the burden of health care resources. In this regard, various blood conservation methods have been explored in clinical practice, such as platelet-rich plasma (PRP) transfusion, blood salvaging systems, cell-saver techniques, and a minimized pump prime [7]. PRP is mainly applied in cardiovascular surgery for blood conservation. Harke was the first to attempt to re-transfuse platelets in extracorporeal circulation [8]. In the present retrospective clinical cohort study, we aimed to investigate (i) the effects of PRP on blood conservation and (ii) other effects on the postoperative conditions of patients who underwent aortic arch replacement.
Materials and methods
Study design, patient population and data collection
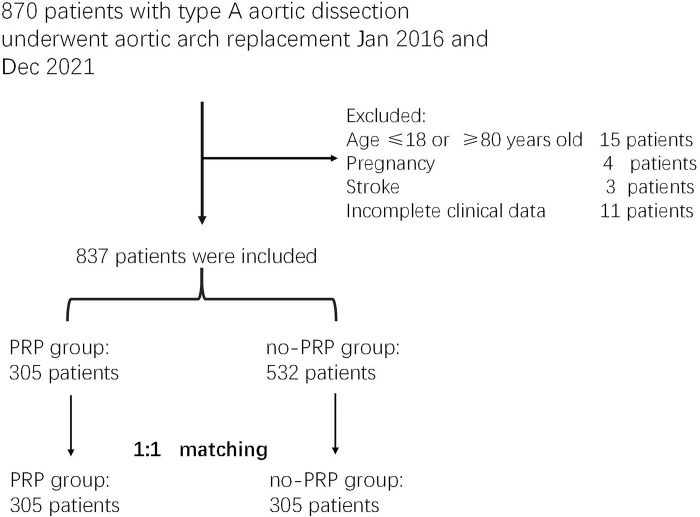
This was a retrospective cohort study approved by the Internal Review Board of the Second Xiangya Hospital, Central South University, Changsha, Hunan Province, China (Ethical code: 2023EECRK001). The need for informed consent was waived because of the retrospective nature of the study. Patients with Stanford type A AAD were eligible if they were between 18 and 80 years of age and suitable for emergency surgery. A total of 870 consecutive patient records were collected between January 2016 and December 2021. Exclusion criteria were as follows: age below 18 or above 80 years, pregnancy, stroke, and incomplete data (Fig 1). Patients were divided into two groups based on PRP application during operation.
Fig 1. Study flow diagram.
Anesthesia routine
After routine anesthesia and intubation, general anesthesia was maintained with intravenous sufentanil, propofol, and neuromuscular blockade drugs. ECG, pulse oxygen saturation, left upper limb and lower limb arterial blood pressure, central venous pressure, nasopharyngeal and bladder temperature, and urine volume were monitored during the operation.
PRP harvest technique
After anesthesia induction, whole blood (about 15–20 mL/kg) was collected via the right internal jugular vein using an autologous transfusion system (Cell saver elite harmonics corporation, USA). Ringer’s lactate and colloidal solution were injected into the peripheral vein for acute normovolemic hemodilution. The collected whole blood was divided into RBC and PRP by a machine. The whole process was completed before systemic heparinization. The isolated PRP was placed in a platelet oscillator with a frequency of 60 rpm at room temperature and transfused back into the patient after neutralizing heparin with protamin. Ringer’s lactate and colloidal solution were used to maintain the intravascular volume and hemodynamic stability during the PRP harvest, as well as continuous intravenous infusion of noradrenaline or dopamine. No cases of hemodynamic instability were noted during PRP collection. Isolated red blood cells can be transfused back to the patient at any time.
Transfusion practice
The amount of allogeneic blood transfusion was determined by the surgeon, anesthesiologist, and perfusionist based on clinical parameters and TEG. Hemoglobin was maintained at approximately ≥7 g/dL during CPB and ≥8 g/dL after CPB and after the operation. Meanwhile, the amount of cryoprecipitate, FFP, and platelets was transfused during the operation according to the maximum amplitude, reaction time, and angle of the TEG.
Statistical analysis
Patients with AAD underwent aortic arch replacement would receive different treatments according to their postoperative conditions. Therefore, we primarily analyzed the first results of the biochemical examination of blood and the CTA report to eliminate confounding factors of the consequence treatments.
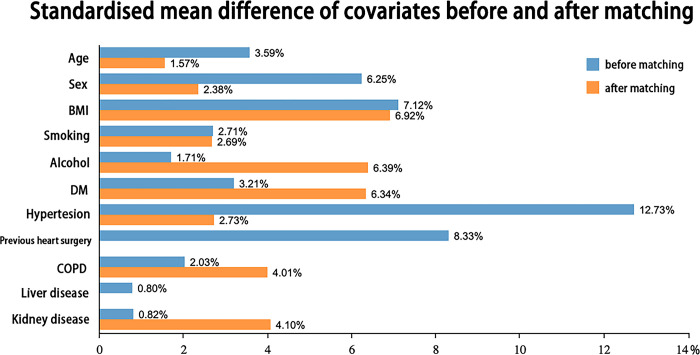
Propensity score matching was performed to reduce the risk of confounding effects between the PRP and non-PRP groups. Patients were 1:1 matched according to covariates including sex, age, BMI, hypertension, smoking, alcohol consumption, and other factors (Table 1) using a logistic regression model with the k-nearest neighbor algorithm. After matching, an absolute standardized difference of <0.1 was considered as well balanced.
Table 1. Preoperative variables in the non-PRP and PRP groups.
| Preoperative variable | non-PRP (n = 305) | PRP (n = 305) | Standardized mean differences | P-value |
|---|---|---|---|---|
| Age | 49.95±11.96 | 49.78±10.53 | 1.57% | 0.846 |
| Sex | 240 (78.69%) | 237 (77.70%) | 2.38% | 0.769 |
| BMI | 26.37±4.53 | 26.06±4.45 | 6.92% | 0.393 |
| Current smoker | 119 (39.02%) | 115 (37.70%) | 2.69% | 0.740 |
| Alcohol consumption | 89 (29.18%) | 98 (32.13%) | 6.39% | 0.430 |
| Diabetes | 9 (2.95%) | 6 (1.97%) | 6.34% | 0.434 |
| Hypertension | 198 (64.92%) | 194 (63.61%) | 2.69% | 0.740 |
| Previous cardiac surgery | 8 (2.62%) | 8 (2.62%) | 0 | 1 |
| COPD | 18 (5.90%) | 21 (6.89%) | 4.01% | 0.620 |
| Liver diseases | 1 (0.33%) | 1 (0.33%) | 0 | 1 |
| Kidney diseases | 7 (2.30%) | 9 (2.95%) | 4.10% | 0.613 |
Normally distributed data, including basic patient information, are presented as mean ± standard deviation and were analyzed using an unpaired t-test. Non-normally distributed data, including surgery duration, perioperative transfusions, and length of hospital stay, are presented as median with interquartile range and were analyzed with the Mann–Whitney U test. Categorical data, including the incidences of pleural effusion, are presented as numbers and percentages and were analyzed using the chi-squared test, and odds ratios (OR) with 95% confidence intervals (CIs) were calculated. Two-sided P-values of less than 0.05 were considered statistically significant. All analyses were performed with SPSS 24.0 (IBM, Armonk, NY, USA) and the Python statistical package (https://scipy.org).
Results
Baseline characteristics
After matching, a total of 610 patients with Stanford type A AAD were included. All standardized mean differences of covariates were less than 0.1 and simultaneously respective P-values were more than 0.05, indicating the patients in the two groups were well balanced (Fig 2, Table 1). The mean amount of PRP collected was 800 [665, 950] ml in the PRP group.
Fig 2. Standardized mean difference before and after matching.
Intraoperative data
As shown in Table 2, no significant differences in terms of duration of operation, CPB, aortic cross-clamping, and DHCA were observed between the two groups (P > 0.05). As for the transfusion of blood products, cryoprecipitate transfusion (u) was 10.0 [10.0, 11.5] in the PRP group, compared to 10.0 [7.5, 11.0] in the non-PRP group (P = 0.021). The transfusions of cell saver, packed RBC, FFP, platelets, and human albumin showed no significant differences between the two groups (P > 0.05). In arch surgery, the proportion of patients who underwent total arch or hemiarch replacement with or without CABG showed no statistical differences (P > 0.05) (Table 2).
Table 2. Perioperative variables in the non-PRP and PRP groups.
| Variable | non-PRP (n = 305) | PRP (n = 305) | P-value |
|---|---|---|---|
| Cryoprecipitate (u) | 10.0 (10.0, 11.5) | 10.0 (7.5, 11.0) | 0.021 |
| Cell saver (ml) | 1800 (1800, 2000) | 1800 (1600, 2000) | 0.493 |
| Packed RBC (u) | 4.0 (2.0, 6.0) | 3.75 (2.0, 6.0) | 0.348 |
| FFP (ml) | 350 (200, 400) | 350 (200, 400) | 0.993 |
| Platelets (u) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 0.171 |
| 20% Human albumin (ml) | 100 (100, 100) | 100 (100, 100) | 0.348 |
| Surgery duration (min) | 503 (395, 592) | 481 (383, 596) | 0.998 |
| Bypass time (min) | 190 (117, 277) | 206 (122, 297) | 0.156 |
| Aortic cross-clamp time (min) | 96 (55, 147) | 105 (72, 147) | 0.276 |
| Circulatory arrest time (min) | 21 (16, 38) | 23 (17, 37) | 0.862 |
| Total arch replacement | 262 (85.90%) | 274 (89.84%) | 0.136 |
| Hemiarch replacement | 43 (14.10%) | 31 (10.16%) | 0.136 |
| CABG | 21 (6.89%) | 26 (8.52%) | 0.448 |
Postoperative outcomes
Postoperative biochemical tests showed that the serum total protein level in the PRP group was significantly higher than in the non-PRP group (59.38±6.25 vs. 58.06±7.19, P = 0.019), and the serum albumin level was significantly higher in the PRP group than in the non-PRP group (36.43±4.20 vs. 35.39±4.40, P = 0.004). However, no significant differences in AST, ALT, TBIL, Scr, BUN, INR, APTT, and PT were found between the two groups (P > 0.05) (Table 3).
Table 3. Postoperative biochemical indicators in the non-PRP and PRP groups.
| Postoperative clinical variable | non-PRP (n = 305) | PRP (n = 305) | P-value |
|---|---|---|---|
| HB (g/L) | 99.79±14.11 | 99.79±13.72 | 0.996 |
| WBC (109/L) | 11.94±3.67 | 12.23±3.64 | 0.342 |
| PLT (109/L) | 144.18±74.88 | 136.82±71.70 | 0.223 |
| ALT (U/L) | 88.27±109.29 | 96.03±107.24 | 0.404 |
| AST (U/L) | 95.03±116.43 | 105.19±112.07 | 0.302 |
| TBIL (μM) | 27.47±25.15 | 29.72±39.96 | 0.422 |
| Total protein (g/L) | 58.06±7.19 | 59.38±6.25 | 0.019 |
| Albumin (g/L) | 35.39±4.40 | 36.43±4.20 | 0.004 |
| APTT (s) | 50.82±9.46 | 50.97±16.53 | 0.977 |
| PT (s) | 17.24±4.64 | 17.31±4.58 | 0.968 |
| INR | 1.29±0.57 | 1.33±0.42 | 0.771 |
| Scr (μM) | 114.06±78.92 | 126.71±110.26 | 0.114 |
| BUN (mM) | 10.57±2.84 | 11.05±2.94 | 0.075 |
| Hospital stay (days) | 19 (15.0, 24.0) | 18.5 (15, 23) | 0.860 |
Based on postoperative CTA reports, the proportion of patients with pleural effusion was 76.66% in the PRP group and 83.99% in the non-PRP group (OR = 1.59, 95% CI: 1.04–2.45, P = 0.028); the proportion of patients with pericardial effusion was not significantly different (71.43% in the PRP group vs. 70.11% in the non-PRP group, P = 0.729) (Table 4).
Table 4. Postoperative variables in the non-PRP and PRP groups from CTA reports.
| Postoperative CTA | non-PRP (n = 281) | PRP (n = 287) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Pericardial effusion | 197 (70.11%) | 205 (71.43%) | 0.93 (0.65, 1.34) | 0.729 |
| Pleural effusion | 236 (83.99%) | 220 (76.66%) | 1.59 (1.04, 2.45) | 0.028 |
Discussion
Platelet-rich plasma (PRP) is autologous serum containing not only high concentrations of platelets, but also abundant growth factors and cytokines, such as transforming growth factor-β (TGF-β), epidermal and vascular endothelial growth factors (EGF and VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and insulin-like growth factor-1 (IGF-1). PRP is widely used, with applications ranging from orthopedic procedures and sports injuries to urology surgery and cardiac surgery [9].
Most recent studies demonstrated that PRP application in AAD surgery is an effective way for blood conservation in the perioperative period. In whole blood exposed to the CPB circuit, the platelets are activated, and coagulation factors are consumed during CPB. The use of PRP can maintain normal platelet function, preserve plasma volume, and ultimately reduce the transfusion volume [10]. But whether it can decrease postoperative complications and improve short-term outcomes is under debate. Some studies showed that PRP can decrease the length of ICU stay [11, 12], decrease the mean number of ventilator days [11–13] and the incidence of tracheostomy [11, 13], and decrease the length of hospital stay [12, 14], while another study showed that PRP application might increase the risk of postoperative acute kidney injury, without decreasing the length of hospital stay or in-hospital mortality [10].
Consistent with the abovementioned studies, we found that the incidence of perioperative cryoprecipitate transfusion was reduced in the PRP group compared to the non-PRP group. Unexpectedly, we observed no significant differences in other blood product transfusions between the two groups, including packed red blood cells and fresh frozen plasma. The differences from previous studies may result from different blood transfusion standards and programs in the different centers. Additionally, we made the following observations.
1) Increased serum albumin and total protein levels in the first postoperative examination
Serum albumin levels are decreased after cardiac surgery due to surgical injury, blood loss, systematic inflammation, hypermetabolism, and so on [15, 16]. Serum albumin levels serve as a marker of the host response to a severe operative insult [17]. In patients with low serum albumin levels, complication and mortality rates after CABG or other cardiac surgeries are high [15, 18, 19]. As for patients with AAD, a recent study showed that albumin levels are associated with a high risk of in-hospital mortality [16].
In PRP which is re-transfused to the body, the albumin concentration is nearly the same as in blood and therefore PRP application can increase serum albumin. Additionally, high concentrations of platelets and growth factor in PRP (TGF-β, VEGF) may also play a key role by stimulating epithelial and endothelial cell regeneration, boost angiogenesis and collagen deposition, and consequently accelerate the healing process [20, 21]. Better wound healing of surgical incisions and tears can reduce serum albumin leakage or exudation to the extravascular space. At the same time, various growth factors in PRP were proven to exert some modulatory effects on acute and chronic inflammation [20]. For example, TGF-β was reported to prevent excessive leukocyte recruitment at the lesion sites [22]. and HGF seems to have a crucial anti-inflammatory function by inhibiting NF-κB signaling [23]. By regulating and inhibiting excessive inflammation, PRP can reduce systematic protein consumption and thus slow down albumin catabolism in the perioperative period. Additionally, some other growth factors (such as IGF-1) in PRP were reported to promote protein synthesis and increase serum albumin and total protein levels in some liver diseases or injuries [24–26]. Interestingly, we found that serum total protein showed the same trends as serum albumin. Taken together, albumin in PRP, reduced protein degradation, reduced protein catabolism, and increased protein production may also contribute to the increase in serum albumin levels.
2) The proportion of patients with pleural effusion is reduced
Pleural effusion demonstrated by serial CT is a common finding in patients with AAD (87.5%) [27]. It is a common complication of cardiac surgery, and it is associated with postoperative mortality and significant resource consumption [28]. The most common conditions that result in effusion are cardiac failure, pneumonia, and increased pleural membrane permeability [29].
In the present study, the proportion of patients with pleural effusion in the first postoperative CTA report was significantly lower in the PRP group than in the non-PRP group. This may be explained as follows. Better surgical wound healing and higher plasma colloid oncotic pressure due to increased serum albumin levels can reduce fluid leakage or exudation into the pleural space. Furthermore, recent studies demonstrated that PRP attenuated these cardiac pathological changes by exerting anti-inflammatory effects and promoting cardiomyocyte repair in high-dose isoproterenol-induced cardiotoxicity and LPS-induced cardiac injury rat models [30, 31]. Mishra found that PRP-treated mice had a greater left ventricular ejection fraction after undergoing ischemia and ischemia–reperfusion than PBS controls [32]. Furthermore, after acute myocardial infarction for 8 weeks, PRP was reported to structurally and functionally improve the injured heart muscle in pigs [33]. Therefore, PRP may mediate the repair and regeneration of cells in the early stage of cardiac injury and thus improve cardiac output, which may play a key role in decreasing the incidence of postoperative pleural effusion.
However, there were no differences in the proportion of patients with pericardial effusion between the two groups, though the association between pericardial and pleural effusion has been previously reported in studies primarily investigating pericardial effusion [28].
3) There were no differences in other postoperative outcomes between the PRP and non-PRP groups
Although the mean levels of Scr and BUN were higher in the PRP group than in the non-PRP group, no statistically significant differences were found. These results contrast with those of Tong, who showed that patients in the PRP group showed higher Scr and lactic acid levels, which were associated with a higher incidence of acute kidney injury on postoperative day 1–3 [10]. The diversion may come from different transfusion amounts, anesthesia routines, or operation details. Simultaneously, the results showed no differences in AST and ALT levels, which serve as markers of acute liver injury, between the two groups.
Conclusion
Because of high platelet concentrations and various growth factors, PRP application in aortic arch replacement surgery reduced the transfusion of cryoprecipitate, increased serum albumin and total protein levels, and reduced the incidence of pleural effusion. No effects of PRP application on other postoperative blood biochemical indicators and CTA reports were found.
Limitations
Surgery in the two groups was performed by different surgical teams, and the duration of operation, bypass time, aortic cross-clamp time, and circulatory arrest time were different. Although the surgical operations and perioperative management of the two groups were consistent, the results may still be affected. Intraoperative transfusion of allogeneic blood products is a clinical decision with a degree of subjectivity, although perioperative transfusion indications are specified. This study is a single-center retrospective study and requires a multi-center prospective study for validation.
Acknowledgments
We are grateful to Mr. Xiya Li for data collection and extraction work.
Data Availability
Data cannot be shared publicly because they contain potentially identifying patient information and the Ethics Committee has imposed restrictions. Data are available from the Ethics Committee of Second Xiangya Hospital, Central South University (contact via xyf2gcp@126.com) for researchers who meet the criteria for access to confidential data.
Funding Statement
Yaping Wang was funded by the Natural Science Foundation of Hunan Province (2020JJ4811), collected the data. Yanying Xiao was funded by Scientific Research Project of Hunan Provincial Health Commission (202104112304), designed and supervised the study.
References
- 1.Nienaber CA, Clough RE. Management of acute aortic dissection. The Lancet. 2015;385(9970):800–11. [DOI] [PubMed] [Google Scholar]
- 2.Fattouch K, Sampognaro R, Navarra E, Caruso M, Pisano C, Coppola G, et al. Long-Term Results After Repair of Type A Acute Aortic Dissection According to False Lumen Patency. The Annals of Thoracic Surgery. 2009;88(4):1244–50. doi: 10.1016/j.athoracsur.2009.06.055 [DOI] [PubMed] [Google Scholar]
- 3.2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. European Heart Journal. 2014;35(41):2873–926. [DOI] [PubMed] [Google Scholar]
- 4.Chang H, Rockman CB, Cayne NS, Veith FJ, Jacobowitz GR, Siracuse JJ, et al. Anticoagulation and antiplatelet medications do not affect aortic remodeling after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg. 2021;74(6):1833–42.e1. doi: 10.1016/j.jvs.2021.05.059 [DOI] [PubMed] [Google Scholar]
- 5.Jesse K, Meuli L, Kopp R, Reutersberg B, Stadlbauer T, Zimmermann A, et al. ORal anticoagulation risks late aortic intervention in Conservatively managed type B Aortic dissection (ORCA study). Eur J Cardiothorac Surg. 2022;62(5). doi: 10.1093/ejcts/ezac495 [DOI] [PubMed] [Google Scholar]
- 6.Sanfilippo F, Currò JM, La Via L, Dezio V, Martucci G, Brancati S, et al. Use of nafamostat mesilate for anticoagulation during extracorporeal membrane oxygenation: A systematic review. Artif Organs. 2022;46(12):2371–81. doi: 10.1111/aor.14276 [DOI] [PubMed] [Google Scholar]
- 7.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 Update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. The Annals of Thoracic Surgery. 2011;91(3):944–82. doi: 10.1016/j.athoracsur.2010.11.078 [DOI] [PubMed] [Google Scholar]
- 8.Harke H, Tanger D, Fürst-Denzer S, Paoachrysanthou C, Bernhard A. [Effect of a preoperative separation of platelets on the postoperative blood loss subsequent to extracorporeal circulation in open heart surgery (author’s transl)]. Der Anaesthesist. 1977;26(2):64–71. [PubMed] [Google Scholar]
- 9.Gupta S, Paliczak A, Delgado D. Evidence-based indications of platelet-rich plasma therapy. Expert Rev Hematol. 2021;14(1):97–108. doi: 10.1080/17474086.2021.1860002 [DOI] [PubMed] [Google Scholar]
- 10.Tong J, Cao L, Liu L, Jin M. Impact of autologous platelet rich plasma use on postoperative acute kidney injury in type A acute aortic dissection repair: a retrospective cohort analysis. J Cardiothorac Surg. 2021;16(1):9. doi: 10.1186/s13019-020-01383-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou SF, Estrera AL, Miller CC 3rd, Ignacio C, Panthayi S, Loubser P, et al. Analysis of autologous platelet-rich plasma during ascending and transverse aortic arch surgery. Ann Thorac Surg. 2013;95(5):1525–30. doi: 10.1016/j.athoracsur.2012.09.054 [DOI] [PubMed] [Google Scholar]
- 12.Tian WZ, Er JX, Liu L, Chen QL, Han JG. Effects of Autologous Platelet Rich Plasma on Intraoperative Transfusion and Short-Term Outcomes in Total Arch Replacement (Sun’s Procedure): A Prospective, Randomized Trial. J Cardiothorac Vasc Anesth. 2019;33(8):2163–9. doi: 10.1053/j.jvca.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 13.Sandhu HK, Tanaka A, Dahotre S, Charlton-Ouw KM, Miller CC 3rd, Estrera AL, et al. Propensity and impact of autologous platelet rich plasma use in acute type A dissection. J Thorac Cardiovasc Surg. 2020;159(6):2288–97 e1. doi: 10.1016/j.jtcvs.2019.04.111 [DOI] [PubMed] [Google Scholar]
- 14.Zhou SF, Estrera AL, Loubser P, Ignacio C, Panthayi S, Miller C 3rd, et al. Autologous platelet-rich plasma reduces transfusions during ascending aortic arch repair: a prospective, randomized, controlled trial. Ann Thorac Surg. 2015;99(4):1282–90. doi: 10.1016/j.athoracsur.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 15.Berbel-Franco D, Lopez-Delgado JC, Putzu A, Esteve F, Torrado H, Farrero E, et al. The influence of postoperative albumin levels on the outcome of cardiac surgery. J Cardiothorac Surg. 2020;15(1):78. doi: 10.1186/s13019-020-01133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, Li D, Cao Y, Zhu X, Zeng Z, Tang L. Prognostic value of serum albumin for patients with acute aortic dissection: A retrospective cohort study. Medicine (Baltimore). 2019;98(6):e14486. doi: 10.1097/MD.0000000000014486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan AM, Hearty A, Prichard RS, Cunningham A, Rowley SP, Reynolds JV. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg. 2007;11(10):1355–60. doi: 10.1007/s11605-007-0223-y [DOI] [PubMed] [Google Scholar]
- 18.Lee EH, Chin JH, Choi DK, Hwang BY, Choo SJ, Song JG, et al. Postoperative hypoalbuminemia is associated with outcome in patients undergoing off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25(3):462–8. doi: 10.1053/j.jvca.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 19.Montazerghaem H, Safaie N, Samiei Nezhad V. Body Mass Index or Serum Albumin Levels: Which is further Prognostic following Cardiac Surgery? J Cardiovasc Thorac Res. 2014;6(2):123–6. doi: 10.5681/jcvtr.2014.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imam SS, Al-Abbasi FA, Hosawi S, Afzal M, Nadeem MS, Ghoneim MM, et al. Role of platelet rich plasma mediated repair and regeneration of cell in early stage of cardiac injury. Regen Ther. 2022;19:144–53. doi: 10.1016/j.reth.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ra Hara G, Basu T. Platelet-rich plasma in regenerative medicine. Biomedical Research and Therapy. 2014;1(1). [Google Scholar]
- 22.Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. Journal of biological regulators and homeostatic agents. 2012;26(2 Suppl 1):35s–42s. [PubMed] [Google Scholar]
- 23.Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y. Hepatocyte Growth Factor Exerts Its Anti-Inflammatory Action by Disrupting Nuclear Factor-κB Signaling. The American Journal of Pathology. 2008;173(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel Fattah SM, Saif-Elnasr M, Soliman AF. Platelet-rich plasma as a potential therapeutic approach against lead nitrate- and/or gamma radiation-induced hepatotoxicity. Environ Sci Pollut Res Int. 2018;25(34):34460–71. doi: 10.1007/s11356-018-3366-3 [DOI] [PubMed] [Google Scholar]
- 25.Adamek A, Kasprzak A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int J Mol Sci. 2018;19(5). doi: 10.3390/ijms19051308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, et al. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43(4):630–6. doi: 10.1016/j.jhep.2005.03.025 [DOI] [PubMed] [Google Scholar]
- 27.Hata N, Tanaka K, Imaizumi T, Ohara T, Ohba T, Shinada T, et al. Clinical significance of pleural effusion in acute aortic dissection. Chest. 2002;121(3):825–30. doi: 10.1378/chest.121.3.825 [DOI] [PubMed] [Google Scholar]
- 28.Brookes JDL, Williams M, Mathew M, Yan T, Bannon P. Pleural effusion post coronary artery bypass surgery: associations and complications. J Thorac Dis. 2021;13(2):1083–9. doi: 10.21037/jtd-20-2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath EE, Anderson PB. Diagnosis of pleural effusion: a systematic approach. Am J Crit Care. 2011;20(2):119–27; quiz 28. doi: 10.4037/ajcc2011685 [DOI] [PubMed] [Google Scholar]
- 30.Yadav S, Srivastava S, Singh G. Platelet-rich plasma exhibits anti-inflammatory effect and attenuates cardiomyocyte damage by reducing NF-kappaB and enhancing VEGF expression in isoproterenol induced cardiotoxicity model. Environ Toxicol. 2022;37(4):936–53. [DOI] [PubMed] [Google Scholar]
- 31.Jiao Y, Zhang Q, Zhang J, Zha Y, Wang J, Li Y, et al. Platelet-rich plasma ameliorates lipopolysaccharide-induced cardiac injury by inflammation and ferroptosis regulation. Front Pharmacol. 2022;13:1026641. doi: 10.3389/fphar.2022.1026641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra A, Velotta J, Brinton TJ, Wang X, Chang S, Palmer O, et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc Revasc Med. 2011;12(3):158–63. doi: 10.1016/j.carrev.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 33.Vu TD, Pal SN, Ti LK, Martinez EC, Rufaihah AJ, Ling LH, et al. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: a translational approach: Vu and Pal "Myocardial Repair: PRP, Hydrogel and Supplements". Biomaterials. 2015;45:27–35. doi: 10.1016/j.biomaterials.2014.12.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because they contain potentially identifying patient information and the Ethics Committee has imposed restrictions. Data are available from the Ethics Committee of Second Xiangya Hospital, Central South University (contact via xyf2gcp@126.com) for researchers who meet the criteria for access to confidential data.