Abstract
Human microbiome variation is linked to the incidence, prevalence, and mortality of many diseases and associates with race and ethnicity in the United States. However, the age at which microbiome variability emerges between these groups remains a central gap in knowledge. Here, we identify that gut microbiome variation associated with race and ethnicity arises after 3 months of age and persists through childhood. One-third of the bacterial taxa that vary across caregiver-identified racial categories in children are taxa reported to also vary between adults. Machine learning modeling of childhood microbiomes from 8 cohort studies (2,756 samples from 729 children) distinguishes racial and ethnic categories with 87% accuracy. Importantly, predictive genera are also among the top 30 most important taxa when childhood microbiomes are used to predict adult self-identified race and ethnicity. Our results highlight a critical developmental window at or shortly after 3 months of age when social and environmental factors drive race and ethnicity-associated microbiome variation and may contribute to adult health and health disparities.
Race is not associated with gut microbiome variation in newborns, but is evident in older infants (>3 months of age) and children.
Introduction
Two major goals of the human microbiome sciences include increasing the representation of undersampled groups in microbiome datasets [1–3] and understanding the tempo by which inequitable experiences, intergenerational inequality, and structural racism impact microbiome variation and health outcomes [4–8]. Early-life social and environmental exposures can have large and lasting effects on child development and adult health, and perturbations to the gut microbiome may be important to future disease risk [9–19]. In the United States, adult gut microbiome diversity correlates with self-identified race and ethnicity [1,3]. However, socioeconomic status (SES)—neighborhood deprivation index, individual and parental education, or household income—is both correlated with adult gut microbiome diversity and is associated with race and ethnicity [20–24]. We emphasize that race and ethnicity are proxies for inequitable exposure to social and environmental determinants of health due to structural racism [6–8,25,26]. When human microbiome differences arise during development and whether or not distinguishing gut taxa overlap between childhood and adulthood are key questions that have implications for long-term effects of early life experiences, including structural racism, on microbiome variation.
To identify the developmental window when microbiome variation emerges, how long it persists during childhood, and which distinguishing taxa overlap between children and adults, we combined 8 gut microbiome composition datasets from 2,756 samples spanning 729 children between birth and 12 years of age throughout the US (S1 Table). We used caregiver-identified race (Asian/Pacific Islander, Black, White) and ethnicity (Hispanic, non-Hispanic) to capture complex interactions of multiple biosocial factors that influence gut microbiome composition, even though race and ethnicity are not biological categories that directly influence microbiome variation [5–7,26]. We used a diverse dataset of childhood microbiome samples to identify features of the gut microbiome that are potential markers of the inequitable experiences underlying health disparities. We selected samples from multiple 16S rRNA gene sequencing studies that represent a higher diversity of children than is commonly present in large analyses of the gut microbiome [1–3]. In the present study, 17.2% of samples were from non-White individuals, and 14.3% of samples were from Hispanic individuals. While the majority of samples from Hispanic individuals are from Hispanic White children, some Hispanic Black children are present in the dataset.
Results
Microbiome variation emerges at or shortly after 3 months of age
Subject explained the greatest proportion of variation, consistent with other studies of the gut microbiome (S1 Fig). As age had the second strongest association with gut microbiome composition of the variables tested (Figs 1 and S1–S9 and S2–S4 Tables), we stratified samples by age and analyzed each age category separately while controlling for study differences to disentangle when in development race and ethnicity-associated microbiome variation originates. Delivery route and infant diet were not included in the age-stratified analysis, as they covaried with race and ethnicity (S10 and S11 Figs and S5 Table).
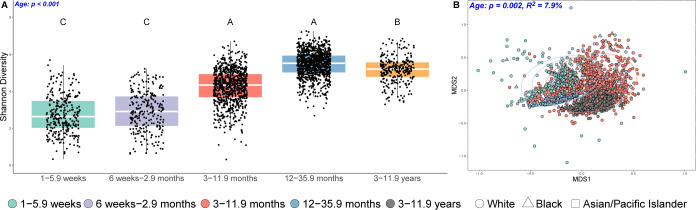
Fig 1. Age structures variation in the gut microbiome.
(A) Boxplots show increases in Shannon diversity with age, and (B) nonmetric multidimensional scaling (NMDS) plots show a significant association of age with weighted UniFrac distances. Colors and 95% confidence ellipses denote age, and shape denotes race. Blue text in the panels highlights significant p-values. Data underlying this figure can be found in S1 and S2 Data.
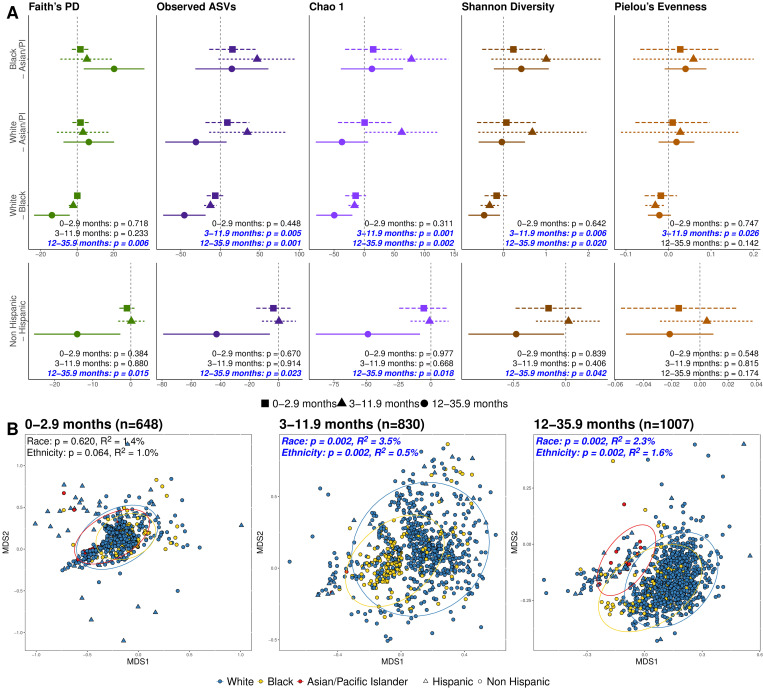
Notably, race and ethnicity did not significantly vary with gut microbiome alpha diversity (within-individual diversity) or beta diversity (between-individual diversity) in the early weeks and months of life, including the first week, 1 to 5.9 weeks, and 6 weeks to 2.9 months (permutational multivariate analysis of variance (PERMANOVA), all p > 0.05) (Figs 2, S2, S12, and S13 and S2 Table). However, at 3 to 11.9 and 12 to 35.9 months, gut microbiome composition based on UniFrac distances varied slightly but significantly by both race and ethnicity (PERMANOVA, all p < 0.05) (Figs 2B, S2, S12, and S13 and S2 Table). Additionally, most measures of alpha diversity varied across racial categories at 3 to 11.9 months and across both racial and ethnic categories at 12 to 35.9 months (LME, p < 0.05) (Fig 2A and S4 Table). Pairwise comparisons confirmed that Black individuals had higher within-sample diversity than White individuals at 3 to 11.9 and 12 to 35.9 months for at least one of the 5 measures of diversity (Fig 2A and S4 Table) [27]. While higher alpha diversity is consistently associated with better cardiometabolic health and lower incidence of inflammatory disease in adults [28–30], studies have found mixed results in children. For example, studies of associations between alpha diversity and risk of allergic disease have found negative [31], positive [32], and no [33] association. From 3 to 11.9 years, race associated with gut microbiome composition using only unweighted UniFrac distances (PERMANOVA, all p < 0.05) (S12 and S13 Figs and S2 Table). Collectively, these results reveal that race and ethnicity associate with microbial diversity after 3 months of age, and, notably, this variation persists through childhood years.
Fig 2. Microbiome variation emerges at or shortly after 3 months of age.
(A) Dot and whisker plots show estimates for Tukey pairwise comparisons in the alpha diversity linear mixed effects models. Dots indicate the estimated difference in alpha diversity when accounting for other covariates in the model, whiskers denote 95% confidence intervals, and the dashed line indicates zero or no difference. Comparisons with whiskers that do not cross zero indicate a significant difference in alpha diversity between those 2 categories. Colors in the dot whisker plots denote alpha diversity metric, and dot shape and line type denote age category. (B) NMDS plots show weighted UniFrac distances between by race and ethnicity at 0–2.9 months, 3–11.9 months, and 12–35.9 months. Colors and 95% confidence ellipses in the NMDS plots denote race, and shape denotes ethnicity. Blue text in the panels highlights significant p-values. NMDS plots for additional age categories and unweighted UniFrac distances can be found in the Supporting information (S12 and S13 Figs). Data underlying this figure can be found in S1, S2, and S4 Data.
Child gut microbiome variation recapitulates that of adults
To identify differentially abundant taxa, we used analysis of compositions of microbiomes with bias correction (ANCOM-BC) for each variable of interest across all age categories. Age was included as a factor in the models, and numerous taxa were differentially abundant across age categories (S6–S9 Tables). The abundances of several taxa significantly were associated with race and/or ethnicity in all samples combined (S5–S9 Tables), including several that varied in abundance between age categories (S14 and S15 Figs). Taxa positively associated with breastfeeding (Bifidobacterium, Lactobacillus, and Staphylococcus) [34,35] were significantly negatively correlated with age, as expected (S14 and S15 Figs and S9 Table). These taxa were differentially abundant between racial or ethnic categories, likely due to differences in rates of breastfeeding across these groups (S10 and S11 Figs and S5 Table). Delivery route also differed between racial and ethnic categories—vaginal delivery was more likely than expected in White, Asian/Pacific Islander, and non-Hispanic children and less likely than expected in Black and Hispanic children (S10 and S11 Figs and S5 Table). However, some individual species within Bacteroides, which is often more abundant in vaginally delivered children [34,35], were more enriched in Black and Hispanic children (S9 Table), contrary to our expectations.
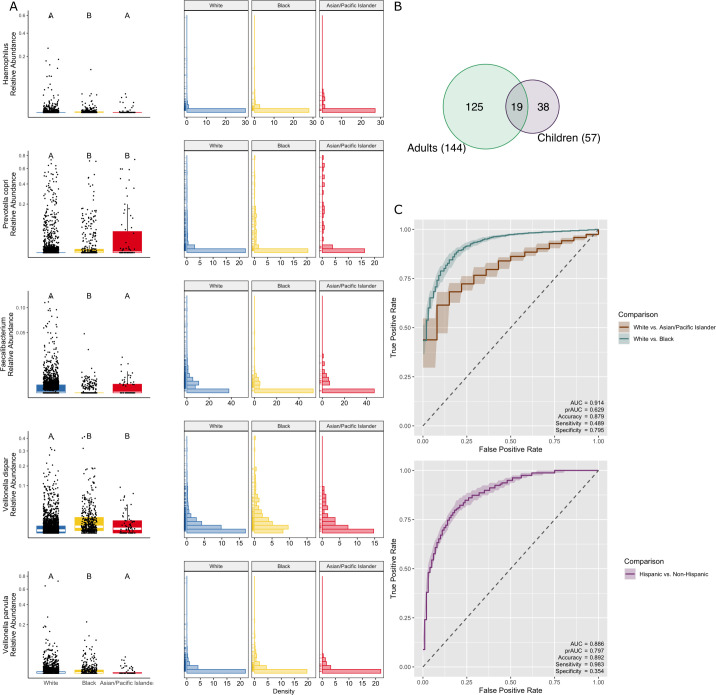
Notably, there was moderate overlap between studies for differentially abundant taxa (S10 Table). Of the 57 gut microbial taxa that varied in abundance between children of differing self-identified racial categories, 19 were previously identified as differentially abundant between Black and White adult individuals in a recent controlled study of gut microbiome variation [3] (Fig 3A and S9 Table). Four of the 19 overlapping taxa were higher in abundance in both Black children and adults compared with White children and adults, and 4 of the overlapping taxa were lower in abundance in both Black children and adults. The remaining 11 overlapping taxa were either differentially abundant between either Asian/Pacific Islander children and Black children or Asian/Pacific Islander children and White children, or the direction of effect differed between Black and White adults and children. Among the 8 taxa that overlapped and had the same effect in children and adults, Haemophilus spp. and Prevotella copri are higher in abundance in both Black children and adults compared to White individuals (Haemophilus spp.: log2 fold change (log2FC)adults = 0.712, log2FCchildren = 0.739; P. copri: log2FCadults = 5.110, log2FCchildren = 2.513) (ANCOM-BC, all q < 0.05) (Fig 3C). These taxa have been associated with an increased risk of autoimmune and allergic diseases, asthma, and obesity across humans in Europe and North America [28,36–39]. Faecalibacterium, which is generally considered to be protective against inflammation [33,40], is lower in abundance in Black children and adults compared to White individuals (log2FCadults = −1.356, log2FCchildren = −0.230) (ANCOM-BC, q < 0.05) (Fig 3C). Conversely, Veillonella, which is associated with a decreased risk of asthma and allergic disease [33,36], is consistently lower in abundance in White children (Veillonella dispar: log2FCadults = 1.295, log2FCchildren = 0.550; Veillonella parvula: log2FCadults = 3.321, log2FCchildren = 1.010) (ANCOM-BC, both q < 0.05) (Fig 3C). Thus, we are finding higher relative abundances of at least one taxon that is positively associated with health in Asian/Pacific Islander, Black, and White children, highlighting the complexity of linking the relative abundance of individual gut microbial taxa to health as a whole. We do note, however, that several of the 19 differentially abundant taxa that overlap between adults and children (S8 Table) have also been found to be associated with SES and unfavorable social and environmental exposures [10,23,41,42].
Fig 3. Child gut microbiome variation recapitulates that of adults.
(A) Boxplots showing the relative abundance of select taxa identified as differentially abundant using ANCOM in the current study that overlap with taxa identified as differentially abundant in adults [3]. All boxplots show the median and interquartile range (IQR), and whiskers extend to 1.5*IQR. Relative abundances for boxplots and histograms are square root transformed. (B) Venn diagram showing overlapping taxa that are differentially abundant in the gut microbiome between Black individuals and White individuals in the present study in children and in previously published work in adults. (C) Receiver operating characteristic (ROC) curves for a random forest model classifying race and ethnicity metadata based on the gut microbiome. Shading represents a 50% confidence interval around the median. Overall model accuracy for race and ethnicity was >87% (the percentage of samples correctly classified as Asian/Pacific Islander, Black, or White and Hispanic and non-Hispanic). Data underlying this figure can be found in S5, S6, and S7 Data and S9 Table.
To detect differentially abundant taxa within each age category, we used generalized linear mixed models with a negative binomial distribution (ANCOM-BC requires more samples per group than we had within each age category). However, few taxa were identified as differentially abundant within each age category (S6–S9 Tables). No phyla or families were differentially abundant between racial and ethnic categories within any age category, and only one genus differed between White and Asian/Pacific Islander children (S6–S9 Tables). Of the 6 species that differed in abundance between racial categories and 4 species that differed in abundance between ethnic categories, none were found in more than one age group (S9 Table). Coprococcus, one of the differentially abundant taxa within a specific age group (12 to 35.9 months), was more abundant in non-Hispanic children and has been previously associated both with obesity and a high-fiber diet [43]. The other differentially abundant taxa within specific age groups did not have clear links to health-related outcomes in the literature. Overall, taxa with age-associated variation did not systematically vary by race or ethnicity.
We next used a machine learning approach to identify additional characteristics of the microbiome that may be markers of inequitable exposure to social and environmental determinants of health. A random forest classifier based on the abundance of genera spanning all childhood samples distinguished Black versus White versus Asian/Pacific Islander categories and Hispanic versus non-Hispanic categories with 87% accuracy. Notably, 13 amplicon sequence variants (ASVs) among the top 30 most important genera that increased classification accuracy in the model (S16 and S17 Figs and S11 Table) are taxa identified as differentially abundant between self-identified racial categories in both children in the current study and adults in previous work [3] (Fig 3B and S9 Table). For race, we used a 3-part model, and model performance estimated as area under the curve (AUC; values above 0.5 indicate the classifier is performing better than chance) was 0.914 (Fig 3B). For ethnicity, we used a binary model, and AUC was 0.886 (Fig 3B).
Additionally, we used the childhood microbiome data in a random forest model to assess if childhood microbiome variation predicts that of healthy adults in the American Gut Project (AGP) dataset. As expected, compositional data from children did not reliably distinguish adults of differing racial categories (S18 and S19 Figs), with an AUC of 0.570. Twenty-six of the top 30 taxa identified as important microbiome characteristics in the model using data from children to predict adult metadata were also identified as important taxa in the random forest model that only used data from children (S16 and S19 Figs). However, the taxa with the highest importance differed with respect to the magnitude and direction of the differences between adults and children (S20 Fig).
Specifically, Enterobacteriaceae and Prevotella are highly important in child–child models but are of modest importance in child–adult models (S16 and S19 Figs), and their relative abundances are lowest in White children but highest in White adults (S20 Fig). Other studies have similarly found that specific taxa can be used to differentiate the gut microbiome of groups of people but that the direction of effect can differ between adults and children. Prevotella was highly important in both adult and child random forest models used to detect taxa that distinguish the gut microbiome across geographic regions, but the direction of the differences in relative abundance differed [44]. In children, Prevotella was more abundant in the US, but Prevotella was more abundant in adults outside of the US [44]. Alistipes was found to be protective against irritable bowel syndrome (IBS) in adults, but predictive of IBS in children [45].
In contrast, other taxa have a similar direction of effect in both children and adults. Ruminococcus is specifically important in the child–adult models, likely due to similar variation in abundance between racial categories in both children and adults (S20 Fig). Higher abundances of Ruminococcus are linked with an increased risk of colorectal cancer [46], a disease for which there is a known racial health disparity [47,48]; however, we find that Ruminococcus is most abundant in White individuals, a group whose colorectal cancer risk is lower than that of Black individuals but higher than that of Asian/Pacific Islander individuals. Race-associated variation in the relative abundance of Ruminococcus across adult guts is not universal, is likely due to a subset of Ruminococcus species, and may interact with other factors such as stress or BMI [1,49]. Thus, it is difficult to know how or if the differences observed in the microbiome here contribute directly to health disparities.
Discussion
Race and ethnicity associate with gut microbiome composition and diversity beginning at 3 months of age, indicative of a narrow window of time (at or shortly after 3 months) and tempo when this variation emerges. Specifically, we found both race and ethnicity account for small but statistically significant proportions of the variation in gut microbiome composition, multiple taxa were differentially abundant between self-reported racial and ethnic categories, several of which were previously identified as differentially abundant in adults [3], and a random forest classifier reliably distinguishes caregiver-identified race and ethnicity. Notably, our findings do not support race- or ethnicity-associated variation appearing at birth or shortly after, when mother-to-infant and other mechanisms of vertical microbial transmission are expected to be strongest [50,51]. None of the differentially abundant taxa identified in the current study are known to be vaginally acquired by infants, and only 2 species are known to be vertically transmitted from the mother [51]. Instead, external factors are most likely shaping race- and ethnicity-associated microbiome variation at or shortly after 3 months. Our results highlight the impetus to increase the diversity of individuals included in studies in the microbiome sciences [1–3] and support the call for studies investigating how structural racism and other structural inequities affect microbiome variation and health [4–7].
The race- and ethnicity-associated differences in the gut microbiome likely reflect differences in environmental and social factors [6–8,25,26]. In the US, there are clear racial and ethnic disparities in health that are tied to differences in these same factors—psychosocial stressors, socioeconomic differences, culture, diet and access to food, access to healthcare and education, interactions with the built environment, and environmental pollutants [6,25,49,52,53]. These factors are important social and environmental determinants of health that have tangible impacts through the modification of human physiology [52,53]. In addition, there is evidence that the developmental trajectory of the gut microbiome is associated with immune system development, metabolic programming, antibiotic resistance, and risk of asthma, allergic, and autoimmune disease [17,33,36,54–60]. Thus, variation in social and environmental determinants of health that is associated with race and ethnicity may not only shape microbiome variation and impact health but also contribute to health disparities [6,7,20,25]. The tempo and types of factors contributing significantly to race- and ethnicity-associated gut microbiome variation are a priority for research.
Previous studies have identified race- and ethnicity-associated variation in the gut microbiome of children [27,61–64], though they did not pinpoint when in development variation appears and the association is not consistent across studies [36,41,65–73]. In particular, previous work demonstrated that sociodemographic factors related to rates of exposure to stress, access to grocery stores and healthcare, and environmental exposure risk are correlated with race-associated variation in the gut microbiome and that the effect of some of these factors, such as household income, are stronger in infants compared with neonates [27]. Due to the limitations of available metadata for all studies, we were not able to include all factors known to be important in our analysis, such as antibiotic exposure [10,27,74,75], environmental microbial exposures [27,34,56,76], childhood diet [54,70], and various measures of maternal health during pregnancy [9,27,54,63,66,72,77–79]. Many of the studies did not measure potentially important factors that are associated with race and ethnicity, including SES, discrimination or stress, and detrimental environmental exposures. Factors that are known to impact gut microbiome composition and were included in our models—age, sex, delivery route, and infant diet—were not independent of race and/or ethnicity (S10 and S11 Figs and S5 Table). While our study included a relatively high proportions of non-Hispanic Black and Hispanic White children, our inferences were limited by low numbers of Asian American/Pacific Islander children. The datasets used in the current study did not have a sufficient number of Middle Eastern, Native American, and Alaskan Native children to include those individuals in the analysis.
Self-identified race and ethnicity are complex concepts and have limitations. Self-identification varies over time, may not be reflected by predetermined categories used in surveys, and may not capture all aspects of race and ethnicity [80–82]. An additional limitation is that the majority of included studies were conducted in urban areas in distinct geographic locations. The data may not be representative of children from rural areas or the entirety of the US. The results of our study are also not generalizable to other countries due to cultural variation in definitions of racial and ethnic categories. These limitations highlight the necessity of future efforts to recruit a far greater diversity of participants for understanding human microbiome diversity [1–3].
During the first 3 months of age, typically high inter- and intraindividual variability in the infant gut microbiome may contribute to the effect of race and ethnicity, in addition to other maternal, environmental, and social factors that associate with the gut microbiome during this developmental period [35,83,84]. Additionally, the rapid development and marked variation in abundance of microbial taxa within and between individuals continues for at least the first year of life [34,85,86]. Differences in social exposures through childcare, dietary variation due to differential rates of breastfeeding and methods of starting solid food, and environmental exposures through time spent in green spaces may be especially impactful starting at 3 months of age and continuing throughout the first year [9–19,87,88]. Many studies of early life and external factor associations with gut microbiome variation have had limited power to detect the effects of multiple factors, finding few or inconsistent relationships between early life determinants and gut microbiome diversity and composition [10,17,76]. Our findings underscore the need for well-powered, longitudinal studies of diverse cohorts that comprehensively assess all internal and external factors known to affect the developmental trajectory of the microbiome [5–7,25,89–92]. Other studies have found that the development of the gut microbiome appears to be particularly sensitive to environmental factors and early life events during the first 3 years of life [14,34,93,94]. Additional work is now needed to assess if social and environmental determinants of health begin to influence variation in the microbiome at or near 3 months of age in a way that is potentially important for understanding health disparities in adults, providing a relatively narrow window of time in which to identify potentially impactful factors.
Materials and methods
Eight datasets with 16S rRNA sequencing data and available race and ethnicity metadata were used in this study [27,66,67,70,72,95,96] (S21 Fig and S1 Table). Individuals between birth and 12 years of age, living in the US, with a caregiver-reported race of Black, White, or Asian/Pacific Islander, and with a caregiver-reported ethnicity of Hispanic or non-Hispanic were included in the analysis. Individuals were not selected based on a known disease phenotype (e.g., type 1 diabetes). Study was included in all models as strata to control for the effects of different study parameters, and individual identity was included as a factor in all models to assess the impact of individual differences on microbiome communities. While sequencing method, primer choice, and sequencing depth did have a significant association with microbial community composition when included in models, including study as strata removed the effect of these study-specific parameters (S2 Table). As some of the included studies had multiple participants from the same family, we also tested if individual identity or family had a larger effect size. In all cases, individual identity explained a larger proportion of the variation than family (S2 Table).
Sequence analyses were carried out in QIIME2 (v.2021.4) [97]. Each study was individually imported into QIIME, and the DADA2 algorithm was used to denoise each study separately to allow us to use appropriate trimming and truncation parameters for each dataset. Feature tables and representative sequences from all studies were then merged using the fragment insertion method [98] to control for differences in amplification and sequencing methodologies between studies. The merged table was filtered to remove sequences absent from the insertion tree. Taxonomy was assigned using a Naïve-Bayesian classifier trained on the Greengenes 13_8 99% OTU full-length 16S rRNA gene sequence database. Mitochondria and chloroplast sequences were filtered from the merged feature table prior to downstream analysis.
Alpha and beta diversity indices were calculated in QIIME and exported for statistical analysis in R [99]. Linear mixed effects models as implemented in the lme4 package [100] were used to detect significant associations between race, ethnicity, age, sex, delivery route, and infant diet on multiple measures of within-sample diversity (Faith’s PD, observed ASVs, Chao 1, Shannon diversity, and Pielou’s evenness). Study and individual identity were included as random effects in all linear models to control for the effects of different study parameters and repeatedly sampling individuals. PERMANOVA, as implemented in the vegan package [101], was used to examine associations between race, ethnicity, age, sex, delivery route, and infant diet on unweighted and weighted UniFrac distances (example model: WeightedUniFrac ~ Race + Ethnicity + Age + Sex + Delivery route + Infant diet + SubjectID, strata = Study). Study was included as the strata in the PERMANOVA models to constrain permutations within each study and control for study-specific methodological differences in sample collection and processing. For both the alpha and beta diversity analyses, we additionally examined the effect of sequencing technology, primer set, and sequencing depth (S2 and S4 Tables) (S7–S9 and S21 Figs). Analysis of composition of microbiomes was used to identify differentially abundant phyla, families, genera, and species across all samples using the ANCOM-BC package [102]. Generalized linear models using a negative binomial distribution were used to detect differentially abundant phyla, families, genera, and species within each age category using the glmmTMB package [103]. Random forest classification was performed using the mikropml package [104] in R. A total of 100 training/test data splits were used for each model, and 5-fold cross-validation was repeated 100 times for each of the 100 training/test data splits using the default settings of the run_ml() command. Median AUC, precision recall AUC (prAUC), accuracy, sensitivity, and specificity are reported for each model.
Supporting information
(DOCX)
Nonmetric multidimensional scaling plots showing the effect of race on weighted (A) and unweighted (B) UniFrac distances in all samples combined. Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Data underlying this figure can be found in S2 and S3 Data.
(EPS)
Nonmetric multidimensional scaling plots showing the effect of sex on weighted (A) and unweighted (B) UniFrac distances. Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Nonmetric multidimensional scaling plots showing the effect of delivery mode on weighted (A) and unweighted (B) UniFrac distances. Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Low depth is <20,000 reads, medium depth is 20,000–49,999 reads, and high depth is ≥50,000 reads. Data underlying this figure can be found in S2 and S3 Data.
(TIF)
Data underlying this figure can be found in S5 Table.
(EPS)
Data underlying this figure can be found in S5 Table.
(EPS)
Data underlying this figure can be found in S2 and S3 Data.
(EPS)
Data underlying this figure can be found in S2 and S3 Data.
(EPS)
Taxa that are differentially abundant across age categories according to ANCOM-BC results and were identified as differentially abundant between racial categories are included. Data underlying this figure can be found in S8 Data.
(EPS)
Taxa that are differentially abundant across age categories according to ANCOM-BC results and were identified as differentially abundant between ethnicity categories are included. Data underlying this figure can be found in S9 Data.
(EPS)
Dots denote the median importance, and whiskers denote 95% confidence intervals. Data underlying this figure can be found in S10 Data.
(TIFF)
Relative abundances across White (blue), Black (yellow), and Asian/Pacific Islander (red) children of the 13 taxa identified as (1) important features in the random forest model; (2) differentially abundant in the ANCOM analysis; and (3) differentially abundant in a previous study of adult gut microbiomes. All boxplots show the median and interquartile range (IQR), and whiskers extend to 1.5*IQR. Relative abundances for boxplots are square root transformed. Data underlying this figure can be found in S11 Data.
(EPS)
Shading represents a 50% confidence interval around the median. Data underlying this figure can be found in S12 Data.
(TIFF)
Dots denote the median importance, and whiskers denote 95% confidence intervals. Data underlying this figure can be found in S13 Data.
(TIFF)
Enterobacteriaceae and Prevotella (A and B) were highly important in the child–child models and Ruminococcus (C) was highly important in the child–adult models. All boxplots show the median and interquartile range (IQR), and whiskers extend to 1.5*IQR. Relative abundances for boxplots are square root transformed. Data underlying this figure can be found in S14 Data.
(EPS)
Data underlying this figure can be found in S15 Data.
(EPS)
Data underlying this figure can be found in S1 Data.
(TIF)
Data underlying this figure can be found in S1 Data.
(TIF)
Data underlying this figure can be found in S1 Data.
(TIF)
Data underlying this figure can be found in S1 Data.
(TIF)
Data underlying this figure can be found in S1 Data.
(TIF)
Data underlying this figure can be found in S1 Data.
(TIF)
(XLSX)
(XLSX)
(XLSX)
Model statistics are reported on the table on the left, and pairwise comparison statistics are presented in the table on the right for variables that were significant.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Both child–child and child–adult models are listed.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Tukey contrasts were performed using the multcomp package in R after running linear mixed effects models using the lme4 package in R. The values below are from the summary output of those contrasts.
(XLSX)
(XLSX)
(XLSX)
These data were used to construct the ROC curve in Fig 3C.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
These data were used to construct the ROC curve in S18 Fig.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
Computational resources were supported by the Vanderbilt Microbiome Innovation Center. This work was conducted in part using the resources of the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University.
Abbreviations
- AGP
American Gut Project
- ANCOM-BC
analysis of compositions of microbiomes with bias correction
- ASV
amplicon sequence variant
- AUC
area under the curve
- IBS
irritable bowel syndrome
- PERMANOVA
permutational multivariate analysis of variance
- prAUC
precision recall AUC
- SES
socioeconomic status
Data Availability
Sequencing data and metadata included in this study was downloaded from NCBI’s Sequence Read Archive (accessions: PRJNA322554, PRJEB11697, and PRJEB13896), QIITA (studies 11129 and 10894), and FigShare (https://doi.org/10.6084/m9.figshare.7011272.v3). Additional sequencing data and metadata for included studies are available as outlined in the original publications, which are listed in S1 Table. All data necessary to reproduce main text and supplementary figures are included in S1–S15 Data files. Code for all analyses can be found on GitHub (https://github.com/BordensteinLaboratory/Childhood_micro_metaanalysis) and are archived on Zenodo (https://doi.org/10.5281/zenodo.8063024).
Funding Statement
The WHEALS study was supported by P01 AI089473-01 from the National Institutes of Health (Bethesda, MD) (ARS). The MARC-35 study was supported by UG3/UH3 OD-023253 from the National Institutes of Health (Bethesda, MD) (CAC and KH). The Early Growth and Development Study microbiome data was supported by UH3 OD023389, P50GM098911, R01 DA035062 from the National Institutes of Health (Bethesda, MD), and a Faculty Alumni Award from the College of Education, University of Oregon (LDL and CC). EKM was supported by the Vanderbilt Microbiome Innovation Center. SRB was supported by the One Health Microbiome Center in the Huck Institutes at The Pennsylvania State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16(18):e2006842. doi: 10.1371/journal.pbio.2006842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdill RJ, Adamowicz EM, Blekhman R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 2022. Feb 15;20(2):e3001536. doi: 10.1371/journal.pbio.3001536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Markowitz RHG, Brooks AW, Mallott EK, Leigh BA, Olszewski T, et al. Individuality and ethnicity eclipse a short-term dietary intervention in shaping microbiomes and viromes. PLoS Biol. 2022. Aug 23;20(8):e3001758. doi: 10.1371/journal.pbio.3001758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Wolfe TJ, Arefin MR, Benezra A, Rebolleda Gómez M. Chasing Ghosts: Race, Racism, and the Future of Microbiome Research. mSystems. 6(5):e00604–21. doi: 10.1128/mSystems.00604-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benezra A. Race in the microbiome. Sci Technol Hum Values. 2020. Sep 1;45(5):877–902. [Google Scholar]
- 6.Byrd DA, Carson TL, Williams F, Vogtmann E. Elucidating the role of the gastrointestinal microbiota in racial and ethnic health disparities. Genome Biol. 2020. Aug 3;21(1):192. doi: 10.1186/s13059-020-02117-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozik AJ. Frameshift—a vision for human microbiome research. mSphere. 2020. Oct 28;5(5):e00944–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishaq SL, Rapp M, Byerly R, McClellan LS, O’Boyle MR, Nykanen A, et al. Framing the discussion of microorganisms as a facet of social equity in human health. PLoS Biol. 2019. Nov 26;17(11):e3000536. doi: 10.1371/journal.pbio.3000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, et al. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021. Jan 1;13(1):1–30. doi: 10.1080/19490976.2021.1897210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gschwendtner S, Kang H, Thiering E, Kublik S, Fösel B, Schulz H, et al. Early life determinants induce sustainable changes in the gut microbiome of six-year-old children. Sci Rep. 2019;9:12675. doi: 10.1038/s41598-019-49160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–421. doi: 10.1038/nature13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallès Y, Francino MP. Air pollution, early life microbiome, and development. Curr Envir Health Rpt. 2018. Dec 1;5(4):512–21. doi: 10.1007/s40572-018-0215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The Intestinal Microbiome in Early Life: Health and Disease. Front Immunol. 2014;5(5):427. doi: 10.3389/fimmu.2014.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stinson LF. Establishment of the early-life microbiome: a DOHaD perspective. J Dev Orig Health Dis. 2020. Jun;11(3):201–210. doi: 10.1017/S2040174419000588 [DOI] [PubMed] [Google Scholar]
- 16.Montoya-Williams D, Lemas DJ, Spiryda L, Patel K, Carney OO, Neu J, et al. The Neonatal Microbiome and Its Partial Role in Mediating the Association between Birth by Cesarean Section and Adverse Pediatric Outcomes. Neonatology. 2018;114(2):103–111. doi: 10.1159/000487102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sbihi H, Boutin RCT, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy. 2019;74(11):2103–2115. doi: 10.1111/all.13812 [DOI] [PubMed] [Google Scholar]
- 18.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016. Jul;22(7):713–722. doi: 10.1038/nm.4142 [DOI] [PubMed] [Google Scholar]
- 19.Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J Clin Med. 2021. Jan 25;10(3):459. doi: 10.3390/jcm10030459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amato KR, Arrieta MC, Azad MB, Bailey MT, Broussard JL, Bruggeling CE, et al. The human gut microbiome and health inequities. Proc Natl Acad Sci U S A. 2021. Jun 22 [cited 2021 Jun 14];118(25). Available from: http://www.pnas.org/content/118/25/e2017947118 doi: 10.1073/pnas.2017947118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowyer R, Jackson M, Le Roy C, Ni Lochlainn M, Spector T, Dowd J, et al. Socioeconomic status and the gut microbiome: A TwinsUK cohort study. Microorganisms. 2019;7(1):17. doi: 10.3390/microorganisms7010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison CA, Taren D. How poverty affects diet to shape the microbiota and chronic disease. Nat Rev Immunol. 2018. Apr;18(4):279–287. doi: 10.1038/nri.2017.121 [DOI] [PubMed] [Google Scholar]
- 23.Lewis CR, Bonham KS, McCann SH, Volpe AR, D’Sa V, Naymik M, et al. Family SES Is Associated with the Gut Microbiome in Infants and Children. Microorganisms. 2021. Aug;9(8):1608. doi: 10.3390/microorganisms9081608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GE, Engen PA, Gillevet PM, Shaikh M, Sikaroodi M, Forsyth CB, et al. Lower neighborhood socioeconomic status associated with reduced diversity of the colonic microbiota in healthy adults. PLoS ONE. 2016;11(2):e0148952. doi: 10.1371/journal.pone.0148952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Findley K, Williams DR, Grice EA, Bonham VL. Health disparities and the microbiome. Trends Microbiol. 2016. Nov 1;24(11):847–850. doi: 10.1016/j.tim.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortenberry JD. The uses of race and ethnicity in human microbiome research. Trends Microbiol. 2013. Apr 1;21(4):165–166. doi: 10.1016/j.tim.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 27.Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. 2016. Aug 25;6(1):31775. doi: 10.1038/srep31775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanislawski MA, Dabelea D, Lange LA, Wagner BD, Lozupone CA. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes. 2019. Jul 1;5(1):18. doi: 10.1038/s41522-019-0091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes JD, Chen C Knox NC, Marrie R-A, El-Gabalawy H, de Kievit T, et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases—does a common dysbiosis exist? Microbiome. 2018;6:221. doi: 10.1186/s40168-018-0603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moroishi Y, Gui J, Hoen AG, Morrison HG, Baker ER, Nadeau KC, et al. The relationship between the gut microbiome and the risk of respiratory infections among newborns. Commun Med. 2022. Jul 14;2(1):1–8. doi: 10.1038/s43856-022-00152-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850. doi: 10.1111/cea.12253 [DOI] [PubMed] [Google Scholar]
- 33.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015. Sep 30;7(307):307ra152–307ra152. doi: 10.1126/scitranslmed.aab2271 [DOI] [PubMed] [Google Scholar]
- 34.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–588. doi: 10.1038/s41586-018-0617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor G, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy. 2018;73(1):145–152. doi: 10.1111/all.13232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Mathis D, editor. Elife. 2013. Nov 5;2:e01202. doi: 10.7554/eLife.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015. Feb 1;91(2):1–9. doi: 10.1093/femsec/fiu002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016. Oct;22(10):1187–1191. doi: 10.1038/nm.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008. Oct 28;105(43):16731–16736. doi: 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flannery JE, Stagaman K, Burns AR, Hickey RJ, Roos LE, Giuliano RJ, et al. Gut Feelings Begin in Childhood: the Gut Metagenome Correlates with Early Environment, Caregiving, and Behavior. mBio. 2020. Feb 25;11(1):e02780–19. doi: 10.1128/mBio.02780-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun. 2011. Mar 1;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Usyk M, Vázquez-Baeza Y, Chen GC, Isasi CR, Williams-Nguyen JS, et al. Microbial co-occurrence complicates associations of gut microbiome with US immigration, dietary intake and obesity. Genome Biol. 2021. Dec 10;22(1):336. doi: 10.1186/s13059-021-02559-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–228. doi: 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology. 2019. Jul 1;157(1):97–108. doi: 10.1053/j.gastro.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 46.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, et al. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS ONE. 2011. Jan 27;6(1):e16393. doi: 10.1371/journal.pone.0016393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ollberding NJ, Nomura AMY, Wilkens LR, Henderson BE, Kolonel LN. Racial/Ethnic Differences in Colorectal Cancer Risk: The Multiethnic Cohort Study. Int J Cancer. 2011. Oct 15;129(8):1899–1906. doi: 10.1002/ijc.25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carethers JM. Chapter Six—Racial and ethnic disparities in colorectal cancer incidence and mortality. In: Berger FG, Boland CR, editors. Advances in Cancer Research [Internet]. Academic Press; 2021. [cited 2023 May 9]. p. 197–229. (Novel Approaches to Colorectal Cancer; vol. 151). Available from: https://www.sciencedirect.com/science/article/pii/S0065230X21000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carson TL, Wang F, Cui X, Jackson BE, Van Der Pol WJ, Lefkowitz EJ, et al. Associations between Race, Perceived Psychological Stress, and the Gut Microbiota in a Sample of Generally Healthy Black and White Women: A Pilot Study on the Role of Race and Perceived Psychological Stress. Psychosom Med. 2018. Sep;80(7):640–648. doi: 10.1097/PSY.0000000000000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korpela K, Costea P, Coelho LP, Kandels-Lewis S, Willemsen G, Boomsma DI, et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018;28:561–568. doi: 10.1101/gr.233940.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018. Jul 11;24(1):133–145.e5. doi: 10.1016/j.chom.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40(1):105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams DR, Sternthal M. Understanding Racial-ethnic Disparities in Health: Sociological Contributions. J Health Soc Behav. 2010. Mar 1;51(1_suppl):S15–S27. doi: 10.1177/0022146510383838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong H, Penders J, Shi Z, Ren H, Cai K, Fang C, et al. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school-age children. Microbiome. 2019;7(1):2. doi: 10.1186/s40168-018-0608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busi SB, de Nies L, Habier J, Wampach L, Fritz JV, Heintz-Buschart A, et al. Persistence of birth mode-dependent effects on gut microbiome composition, immune system stimulation and antimicrobial resistance during the first year of life. ISME Commun. 2021. Mar 26;1:8. doi: 10.1038/s43705-021-00003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Depner M, Taft DH, Kirjavainen PV, Kalanetra KM, Karvonen AM, Peschel S, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med. 2020. Nov 2;26:1766–1775. doi: 10.1038/s41591-020-1095-x [DOI] [PubMed] [Google Scholar]
- 57.Calatayud M, Koren O, Collado MC. Maternal Microbiome and Metabolic Health Program Microbiome Development and Health of the Offspring. Trends Endocrinol Metab. 2019;30:735–744. doi: 10.1016/j.tem.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 58.Lebeaux RM, Coker MO, Dade EF, Palys TJ, Morrison HG, Ross BD, et al. The infant gut resistome is associated with E. coli and early-life exposures. BMC Microbiol. 2021. Jul 2;21(1):201. doi: 10.1186/s12866-021-02129-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivares M, Walker AW, Capilla A, Benítez-Páez A, Palau F, Parkhill J, et al. Gut microbiota trajectory in early life may predict development of celiac disease. Microbiome. 2018;6:36. doi: 10.1186/s40168-018-0415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. , The human gut microbiome of early onset type 1 diabetes in the TEDDY study. Nature. 2018;562(7728):589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta TA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015. Aug 26;3(1):36. doi: 10.1186/s40168-015-0101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3–6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol. 2017. Feb 1;139(2):482–491.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stearns JC, Zulyniak MA, de Souza RJ, Campbell NC, Fontes M, Shaikh M, et al. Ethnic and diet-related differences in the healthy infant microbiome. Genome Med. 2017. Mar 29;9(1):32. doi: 10.1186/s13073-017-0421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balakrishnan B, Selvaraju V, Chen J, Ayine P, Yang L, Ramesh Babu J, et al. Ethnic variability associating gut and oral microbiome with obesity in children. Gut Microbes. 2021. Jan 1;13(1):1882926. doi: 10.1080/19490976.2021.1882926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI, Warner BB, Dantas G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med. 2018;24:1822–1829. doi: 10.1038/s41591-018-0216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. doi: 10.1186/s13073-016-0330-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cioffi CC, Tavalire HF, Neiderhiser JM, Bohannan B, Leve LD. History of breastfeeding but not mode of delivery shapes the gut microbiome in childhood. PLoS ONE. 2020. Jul 2;15(7):e0235223. doi: 10.1371/journal.pone.0235223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galley JD, Bailey M, Dush CK, Schoppe-Sullivan S, Christian LM. Maternal Obesity Is Associated with Alterations in the Gut Microbiome in Toddlers. PLoS ONE. 2014. Nov 19;9(11):e113026. doi: 10.1371/journal.pone.0113026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grier A, McDavid A, Wang B, Qui X, Java J, Bandyopadhyay S, et al. Neonate gut and respiratory microbiota: coordinated development through time and space. Microbiome. 2018;6(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herman DR, Rhoades N, Mercado J, Argueta P, Lopez U, Flores GE. Dietary Habits of 2- to 9-Year-Old American Children Are Associated with Gut Microbiome Composition. J Acad Nutr Diet. 2019;120(4):517–534. doi: 10.1016/j.jand.2019.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao W, Salzwedel AP, Carlson AL, Xia K, Azcarate-Peril MA, Styner MA, et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl). 2019. May 1;236(5):1641–1651. doi: 10.1007/s00213-018-5161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson A, Fiechtner L, Roche B, Ajami NJ, Petrosino JF, Camargo CA, et al. Association of maternal gestational weight gain with the infant fecal microbiota. J Pediatr Gastroenterol Nutr. 2017. Nov;65(5):509. doi: 10.1097/MPG.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M, Differding MK, Benjamin-Neelon SE, Østbye T, Hoyo C, Mueller NT. Association of prenatal antibiotics with measures of infant adiposity and the gut microbiome. Ann Clin Microbiol Antimicrob. 2019. Jun 21;18(1):18. doi: 10.1186/s12941-019-0318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics. 2006. Aug 1;118(2):511–521. doi: 10.1542/peds.2005-2824 [DOI] [PubMed] [Google Scholar]
- 75.Yassour M, Vatanen T, Siljander H, Hämäläinen AM, Härkönen T, Ryhänen SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016. Jun 15;8(343):343ra81–343ra81. doi: 10.1126/scitranslmed.aad0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quin C, Gibson DL. Human behavior, not race or geography, is the strongest predictor of microbial succession in the gut bacteriome of infants. Gut Microbes. 2020. Sep 2;11(5):1143–1171. doi: 10.1080/19490976.2020.1736973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lundgren SN, Madan JC, Emond JA, Morrison HG, Christensen BC, Karagas MR, et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome. 2018;6:109. doi: 10.1186/s40168-018-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh SB, Madan J, Coker M, Hoen A, Baker ER, Karagas MR, et al. Does birth mode modify associations of maternal pre-pregnancy BMI and gestational weight gain with the infant gut microbiome? Int J Obes (Lond). 2020;44:23–32. doi: 10.1038/s41366-018-0273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugino KY, Paneth N, Comstock SS. Michigan cohorts to determine associations of maternal pre-pregnancy body mass index with pregnancy and infant gastrointestinal microbial communities: Late pregnancy and early infancy. PLoS ONE. 2019. Mar 18;14(3):e0213733. doi: 10.1371/journal.pone.0213733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ford CL, Harawa NT. A new conceptualization of ethnicity for social epidemiologic and health equity research. Soc Sci Med. 2010. Jul 1;71(2):251–258. doi: 10.1016/j.socscimed.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cobb RJ, Thomas CS, Laster Pirtle WN, Darity WA. Self-identified race, socially assigned skin tone, and adult physiological dysregulation: Assessing multiple dimensions of “race” in health disparities research. SSM Popul Health. 2016. Dec 1;2:595–602. doi: 10.1016/j.ssmph.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roth WD. The multiple dimensions of race. Ethn Racial Stud. 2016. Jun 20;39(8):1310–1338. [Google Scholar]
- 83.Yassour M, Jason E, Hogstrom LJ, Arthur TD, Tripathi S, Siljander H, et al. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host Microbe. 2018. 11;24(1):146–154.e4. doi: 10.1016/j.chom.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574:117–121. doi: 10.1038/s41586-019-1560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vatanen T, Plichta DR, Somani J, Münch PC, Arthur TD, Hall AB, et al. Genomic variation and strain-specific functional adaptation in the human gut microbiome during early life. Nat Microbiol. 2019;4:470–479. doi: 10.1038/s41564-018-0321-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim ES, Zhou Y, Zhao G, Bauer IK, Droit L, Ndao IM, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015. Oct;21(10):1228–1234. doi: 10.1038/nm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amir A, Erez-Granat O, Braun T, Sosnovski K, Hadar R, BenShoshan M, et al. Gut microbiome development in early childhood is affected by day care attendance. NPJ Biofilms Microbiomes. 2022. Jan 11;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Homann CM, Rossel CAJ, Dizzell S, Bervoets L, Simioni J, Li J, et al. Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts. Nutrients. 2021. Aug;13(8):2639. doi: 10.3390/nu13082639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Demmer RT. The microbiome and population health: considerations to enhance study design and data analysis in observational and interventional epidemiology. Am J Epidemiol. 2018;187(6):1291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dowd JB, Renson A. “Under the skin” and into the gut: Social epidemiology of the microbiome. Curr Epidemiol Rep. 2018. Dec;5(4):432–441. doi: 10.1007/s40471-018-0167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herd P, Palloni A, Rey F, Dowd JB. Social and population health science approaches to understand the human microbiome. Nat Hum Behav. 2018;2(11):808–815. doi: 10.1038/s41562-018-0452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishaq SL, Parada FJ, Wolf PG, Bonilla CY, Carney MA, Benezra A, et al. Introducing the Microbes and Social Equity Working Group: Considering the Microbial Components of Social, Environmental, and Health Justice. mSystems. 0(0):e00471–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol. 2017. Jan 6;13:3. doi: 10.1186/s13223-016-0173-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017. Mar;23(3):314–326. doi: 10.1038/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Planer JD, Peng Y, Kau AL, Blanton LV, Ndao IM, Tarr PI, et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016. Jun;534(7606):263–266. doi: 10.1038/nature17940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019. Aug 24;37(8):852–857. doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Janssen S, McDonald D, Gonzalez A, Navas-Molina JA, Jiang L, Xu ZZ, et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems. 3(3):e00021–18. doi: 10.1128/mSystems.00021-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Team R. R: A language and environment for statistical computing [Internet]. 2019. Available from: https://www.R-project.org/ [Google Scholar]
- 100.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, et al. lme4: Linear Mixed-Effects Models using “Eigen” and S4 [Internet]. 2020. Available from: https://CRAN.R-project.org/package=lme4 [Google Scholar]
- 101.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community ecology package [Internet]. 2019. Available from: https://cran.r-project.org/package=vegan [Google Scholar]
- 102.Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020. Jul 14;11(1):3514. doi: 10.1038/s41467-020-17041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9(2):378–400. [Google Scholar]
- 104.Topçuoğlu BD, Lapp Z, Sovacool KL, Snitkin E, Wiens J, Schloss PD. mikropml: User-Friendly R Package for Supervised Machine Learning Pipelines. J Open Source Softw. 2021;6(61):3073. doi: 10.21105/joss.03073 [DOI] [PMC free article] [PubMed] [Google Scholar]