Abstract
Objective:
To study the effects of ovulation induction on mouse postnatal health, with a focus on growth pattern and glucose tolerance. To study the effect of ovulation induction on DNA methylation, we took advantage of the agouti viable yellow (Avy) mouse.
Design:
Animal study.
Setting:
University Setting.
Animals:
Agouti viable yellow (Avy) mice on a C57BL/6 background.
Intervention(s):
Avy female mice were either allowed to mate spontaneously (control group, C) or after superovulation with 5 IU of PMSG and hCG (ovulation induction group, OI).
Main Outcome Measure(s):
Birth parameters and postnatal growth of the offspring were followed up to 29 weeks of age. Body composition analysis was performed by EchoMRI; fasting insulin, intraperitoneal glucose tolerance tests, and glucose-stimulated insulin secretion by beta cells were assessed to study glucose metabolism.
Result(s):
Mice born to superovulated dams had lower survival rates, shorter anogenital distances, and shorter crown-rump lengths. Female mice generated by OI weighed less at birth, whereas male mice generated by OI had lower weight gain and had reduced lean mass. Glucose parameters, including islet functions, did not differ between the groups. No difference in agouti coat color was noted between the groups.
Conclusion(s):
Ovulation induction resulted in mice having increased morphometric differences at birth and male mice showing reduced weight gain but no difference in glucose tolerance or agouti coat color.
Keywords: Agouti mice, DNA methylation, DOHaD, glucose metabolism, superovulation
Ovulation induction (OI) is routinely performed in animals and humans to increase the number of ovulated oocytes and treat patients with infertility. Although both oral and injectable medications are available, the use of injectable medication is prevalent in the context of assisted reproductive technologies (ARTs), such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
Overall, both OI and embryo culture are highly successful, resulting in significant improvement in live births (1). However, a number of potential adverse obstetrical and postnatal health outcomes have been described (2, 3). For example, increased risks of hypertension and altered glucose metabolism have been found in mice and humans after the combined use of OI and embryo manipulation (4, 5). These outcomes are believed to be an adaptive response of embryos and fetuses to the suboptimal conditions encountered during development: the organism adapts to the altered environment by reprogramming its genome and its future growth. According to the Developmental Origins of Health and Disease hypothesis, this reprogramming may result in survival, but it may predispose the offspring to chronic diseases (6).
Although considerable research has been conducted on the long-term health outcomes of offspring conceived by ART, few studies have examined the effects of gonadotropin stimulation alone on offspring health. Chao et al. (7) showed that repeated superovulation resulted in declined oocyte quality in mice. Ertzeid et al.(8), in experiments injecting superovulated or control embryos into superovulated or control uteri, showed that superovulation affects both the endometrium and the oocytes. Further, Mann et al. (9, 10) showed that gonadotropins alter imprinting marks in oocytes and embryos, suggesting that DNA methylation changes can follow superovulation.
Given these prior studies, we aimed to investigate whether superovulation alone affects long-term health in mice. Further, to study the effect of OI on DNA methylation, we took advantage of the agouti viable yellow (Avy) mouse (11). These mice carry the Avy allele, an epigenetically sensitive locus that can be modified by environmental factors. Methylation at CpG sites in the intracisternal type A particle within the agouti locus determines agouti gene expression. If the cytosine bases within the long terminal repeat of this element are heavily methylated, the agouti allele will be transcriptionally repressed, resulting in a pseudo-agouti (i.e., brown-colored) mouse; if they are unmethylated, the agouti allele will be expressed, resulting in patterns of yellow fur expression correlated with methylation levels. Mice expressing the agouti protein have a propensity for obesity and the development of diabetes with age (12). Agouti mice have been used to show that in vitro culture (13) or exposure to bisphenol A during pregnancy (14) alters the coat color ratio and hence the epigenetic profile of the offspring. In particular, using this mouse model offers two major advantages. First, by simply assessing coat color, we will know whether superovulation changes the DNA methylation status of the agouti locus. Second, if the incidences of diabetes and obesity are increased in yellow agouti mice, it could be inferred that the process of superovulation has an additional metabolically adverse effect on individuals.
This study was therefore designed to investigate the effects of OI on mouse postnatal health, with a focus on growth pattern and glucose tolerance.
MATERIALS AND METHODS
Animals
Agouti viable yellow (Avy) mice on a C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, Maine), and were used in all experiments. Female (Aa/a) C57BL/6 mice (age 6–12 weeks) were mated with male (Avy/a) C57BL/6 mice carrying one agouti viable yellow (Avy) allele as described (11).
The control group (C) was generated by mating a female with a fertile male.
The experimental ovulation induction group (OI) was generated by stimulating female mice with 5 IU of pregnant mare serum gonadotropins and 48 hours later with 5 IU of human chorionic gonadotropin before mating. The female mice were then allowed to mate with fertile males.
All mice were maintained according to institutional regulations and National Institutes of Health guidelines. The mice were housed on a 12-hour light: 12-hour dark regimen. The mice were fed a standard chow diet (PicoLab #5058) ad libitum and had free access to drinking water. All experiments were approved by the University of California, San Francisco Institutional Animal Care and Use Committee. For coat color, we examined 30 control litters (n = 198 pups, of which 130 were followed postnatally) and 24 superovulated litters (n = 111 pups). Table 1 shows the color distribution of the experimental mice.
TABLE 1.
Agouti color distribution of experimental mice.
| Control | Superovulated | |||
|---|---|---|---|---|
| Agouti color | Male | Female | Male | Female |
| 0 | 53 | 55 | 36 | 23 |
| 1 | 12 | 5 | 8 | 5 |
| 2 or 3 | 19 | 20 | 9 | 17 |
| 4 or 5 | 23 | 11 | 5 | 8 |
Harner. Superovulation alters growth pattern in mice. Fertil Steril Sci 2021.
Ethical Approval
The study was approved by the Institutional Review Board of the University of California San Francisco.
Morphometric Data and Growth
Developmental parameters (weight, crown-rump length [CRL], and anogenital distance [AGD]) were measured at birth (P0) with calipers, as we have done before (15). On postnatal day one (P1), the number of live and dead mice and their weight were recorded. Sex was assessed at P7. On P1, newborn pups were individually marked with different colored sharpies, and toe markings were applied on P7. Weight was measured weekly until 29 weeks of age.
Assignment of Coat Color and Group Distribution (Zero to Five)
Coat color was assigned by the same observer (R.H.) at P21 as recommended (11). Coat color was assessed on a 0–5 scale, with nonagouti mice labeled 0 and agouti mice spanning a range from 1 to 5. Mice labeled 1 exhibited almost no agouti expression, whereas those labeled 5 exhibited agouti expression across most or the entire coat.
To assess whether superovulation had a unique effect on metabolism on the basis of coat color, we collapsed the five coat color groups into four subgroups: coat color 0, coat color 1, coat color 2 or 3, and coat color 4 or 5 (Supplemental Figs. 1–5, available online) as done by Rosenfeld (16). This was done because of the relatively low number of animals present in subgroups 2 to 5 and to achieve statistical significance.
Body Composition Analysis
To assess body composition, EchoMRI (Echo Medical System, Houston, TX) was performed at 8, 16, 21, and 29 weeks of age, as described (17). Briefly, the mice were placed into a thin-walled plastic cylinder, with a cylindrical plastic insert added to limit movement. Then the mice were placed into a magnetic resonance imagining machine (EchoMRI-3in1) and were briefly subjected to a low-intensity electromagnetic field to measure fat, lean mass, and free water.
Glucose-Stimulated Insulin Secretion
Glucose-stimulated insulin secretion (GSIS) was performed as we have done before (18). Briefly, islets of Langerhans were isolated from the surrounding exocrine pancreas in mice aged 29 weeks by the University of California San Francisco Islet Production Core Facility according to standard procedures (19). The pancreases were distended with 3 mL of cold M199 medium (Gibco BRL) containing 1.5 mg/dL collagenase (type P; Boehringer Mannheim), excised, and incubated in a 37°C stationary bath. The islets were separated by density gradients (Histopaque-1077; Sigma), hand-picked under a stereomicroscope, and pooled. Islets in groups of 200 to 500 cells were cultured free-floating for 24 hours in 5 mL of RPMI 1640 medium supplemented with L-glutamine and benzylpenicillin (100 U/mL) and exposed to basal and stimulatory concentrations of glucose (2.8 and 28 mM), and the supernatant was collected for insulin determination. Insulin levels were normalized to total islet DNA.
Insulin Measurement
Mice at 13 and 29 weeks of age had their insulin tested (20). After the mice had fasted for 6 hours, blood was collected from the tail veins, serum separated, and stored at −80°C until analyzed. Then, 10 μL of serum was assayed after the Mouse Insulin ELISA kit protocol (Mercodia 10–1247-01).
Intraperitoneal Glucose Tolerance Test
The intraperitoneal glucose tolerance test was performed at 13 and 29 weeks of age, as we have done before (15). The mice were fasted for 6 hours, and then a blood sample was collected from the tail vein. Glucose was measured with a hand-held glucometer (Roche Diagnostics). At time zero, after the initial glucose measurement, the mice underwent intraperitoneal injection of a bolus of glucose (2 mg/g). Glucose levels were then measured at 15, 30, 60, and 120 minutes after injection. Food was immediately returned to the mice after the last measurement.
Statistics
GraphPad Prism version 9.0.2 (GraphPad Software, San Diego, CA) was used for all analyses. All data are presented as means ± SD unless otherwise specified. For birth-related data, the statistical unit of comparison was the litter (21), whereas for metabolic data collected throughout growth, the individual mouse was the statistical unit.
For parametric data and comparisons between the two groups, a two-tailed Student’s t-test was used. The frequency of agouti coat color between the groups was calculated by chi-squared analysis. For multiple comparisons, one-way analysis of variance with Tukey post hoc correction was used. A P-value <.05 was considered to indicate statistical significance. Sample size calculation was on the basis of the results of our prior study comparing fasting glucose in IVF vs. in vivo-conceived mice (18). Given that IVF mice had higher fasting glucose at 29 weeks of age (IVF, 192 mg/dL vs. flushed blastocyst, 59 mg/dL; P<.05), we assumed that to find a difference in fasting glucose of 33 mg/dL with a P<.05 and power of 0.8, we needed a sample size of 13 mice per group. We further made the assumption that obese agouti 4 or 5 mice would have shown a higher glucose difference (50 mg). To find a difference in fasting glucose of 50 mg/dL with a P<.05 and power of 0.8, we needed a sample size of six mice per group.
RESULTS
Newborns of Superovulated Dams Have Lower Survival and Morphological Differences at Birth
At birth, litter size (Fig. 1A) was similar between the groups. However, on postnatal day 1, a higher number of pups in the OI group did not survive (on average one pup per litter) compared with the control group (on average, one pup did not survive every two litters, P< .05; Fig. 1B). Mice that did not survive had lower weight (OI = 1.12 g; C = 1.21 g) than surviving pups (OI = 1.29 g, P< .05; C = 1.38 g, P< .05). The sex ratio (OI = 0.5; C = 0.6; not significant [NS]) was similar between the groups. At birth, female pups from superovulated dams had significantly lower weight (OI = 1.21 g; C = 1.38 g; P<.05; Fig. 2A). Male mouse weight was similar between the groups (OI = 1.28 g; C = 1.37 g; NS). Male and female pups born to superovulated dams had shorter CRL (P< .05, Fig. 2B) and shorter AGD (P< .05, Fig. 2C) than naturally conceived pups.
FIGURE 1.
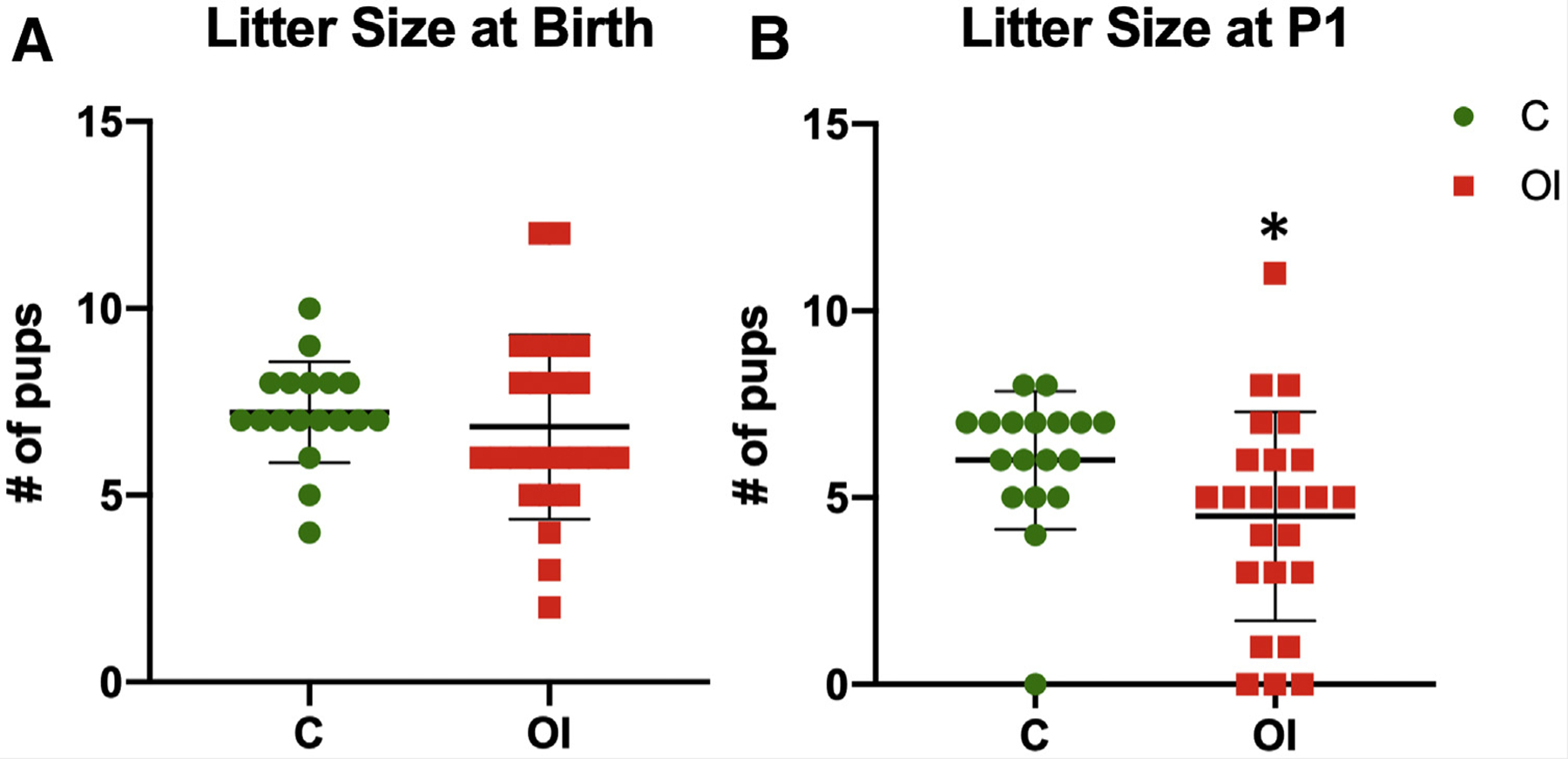
Litter size. Although the two groups had similar numbers of pups at birth, fewer of the superovulated group survived after 24 hours. (A) Litter size is similar between naturally conceived litters (7.2 pups) and superovulated litters (6.8 pups, NS [not significant]). (B) However, fewer superovulated pups survived (4.5 pups) compared with naturally conceived pups (6 pups, P<.05). C = control group; OI = ovulation induction group.
FIGURE 2.
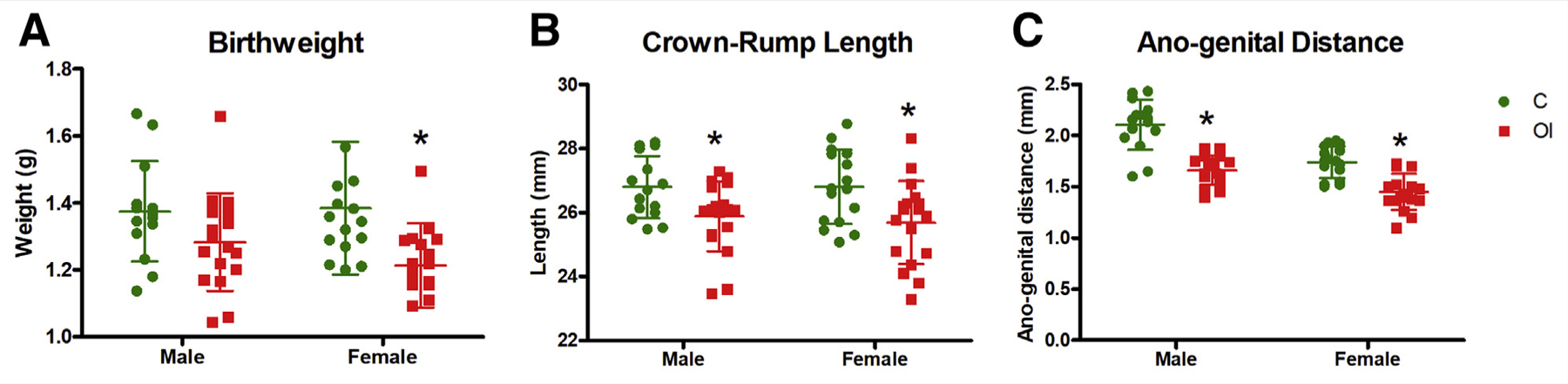
Birth parameters. Superovulated pups trend toward being smaller and shorter at birth, indicating potential health complications in superovulated pregnancies. (A) There is no difference in male birth weight (C = 1.38 g, OI = 1.28 g, P = .097), but superovulated females have lower birth weight than offspring of naturally mated mice (C = 1.39 g, OI = 1.21 g, P<.05). (B) Superovulated males (C = 26.8 mm, OI = 25.9 mm, P = .017) and females (C = 26.8 mm, OI = 25.7 mm, P<.05) have shorter crown-rump length. (C) Superovulated males (C = 2.11 mm, OI = 1.66 mm, P <.01) and females (C = 1.74 mm, OI = 1.45 mm, P<.01) have shorter anogenital distances at birth. C = control group; OI = ovulation induction group.
Male Offspring of Superovulated Mothers Have Reduced Weight Gain Because of Reduced Lean Mass
Male offspring of superovulated mothers had lower weight gain than controls (Fig. 3A), whereas females only showed lower weight at 29 weeks of age (Fig. 3B). Body composition analysis showed that the reduced weight was secondary to a decrease in lean mass; in males, this was found to occur at every time point measured (8, 16, 21, and 29 weeks; P<.05; Fig. 4A–D); in females, it occurred only at 29 weeks (P<.05; Fig. 4D). Fat mass was equal between the groups (Fig. 4E–H).
FIGURE 3.
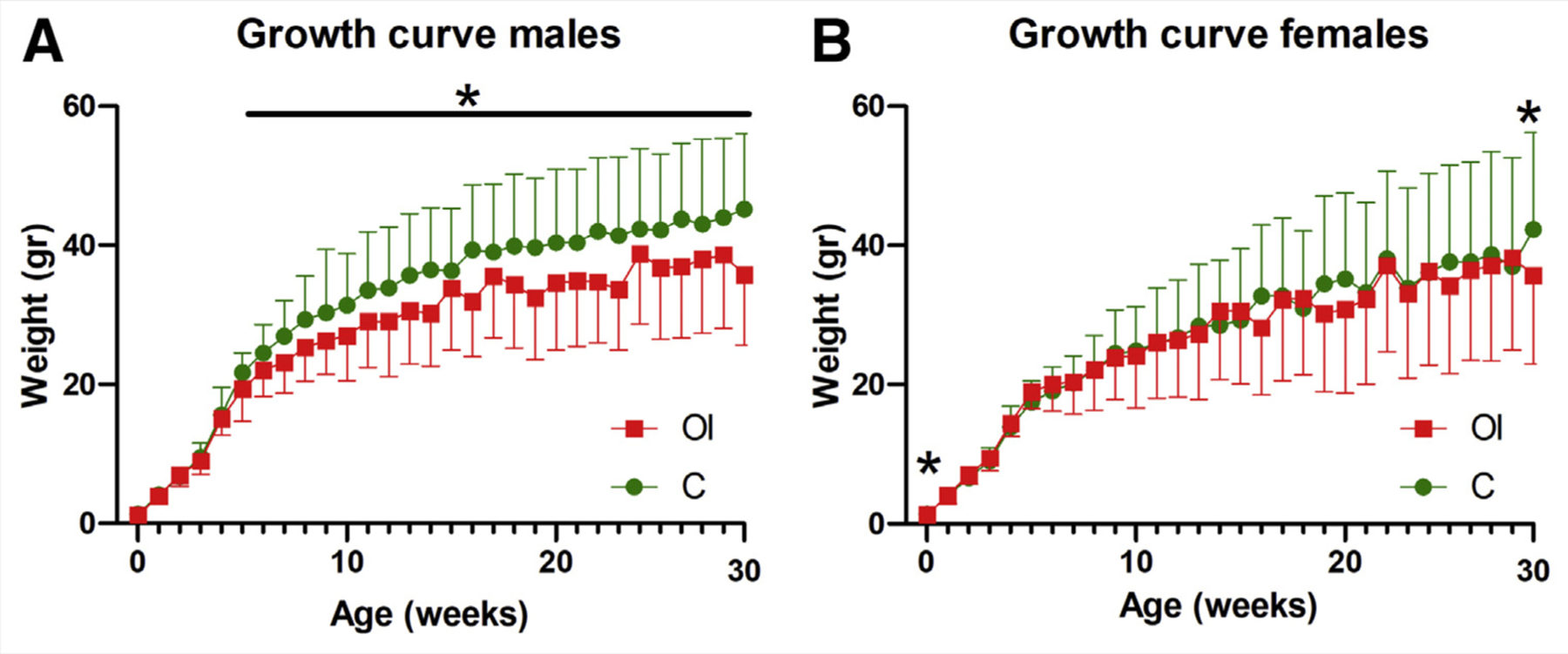
Growth curve. (A) Males born to superovulated mothers have lower weight (P<.05) starting at 5 weeks of age. (B) Females born to superovulated mothers have lower weight only at birth and at 29 weeks of age. C = control group; OI = ovulation induction group.
FIGURE 4.
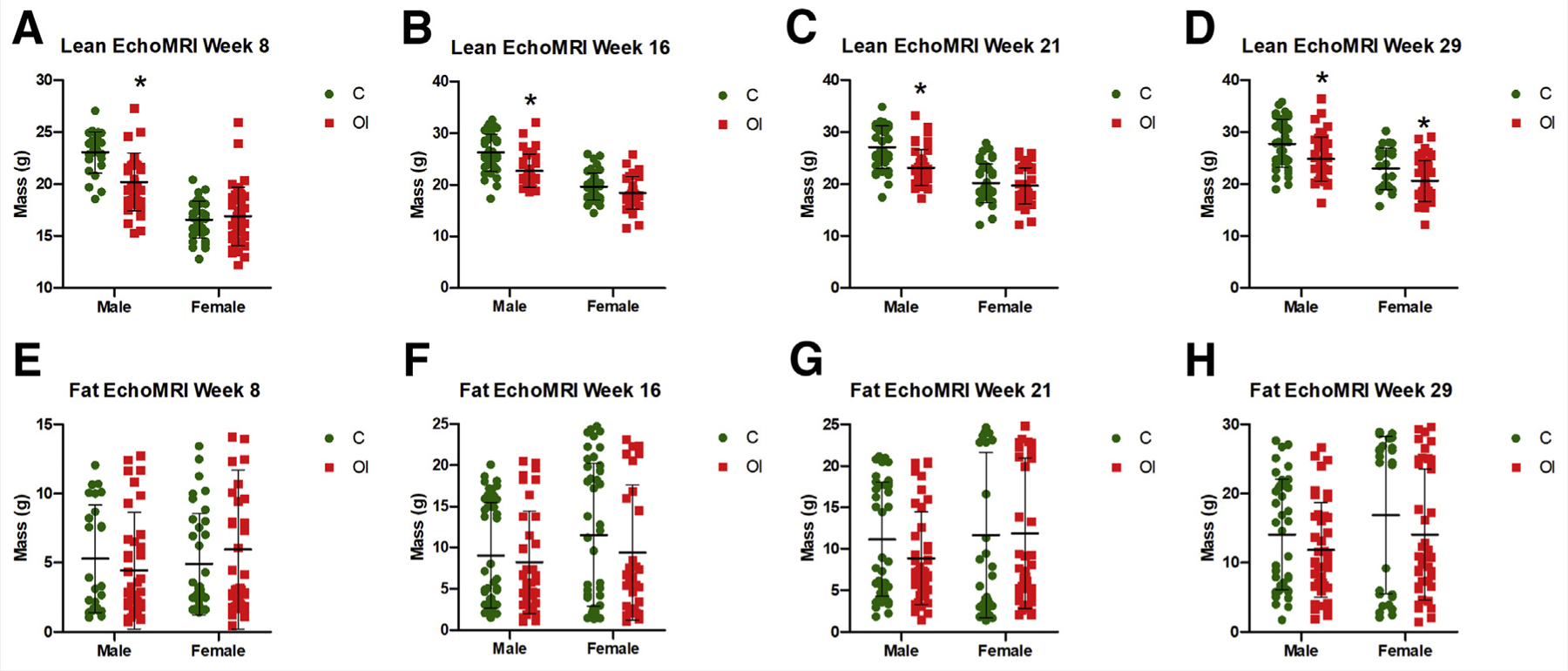
Body composition. Superovulated male mice have lower lean mass at (A) 8 weeks (C = 23.05 g, OI = 20.19 g, P<.01), (B) 16 weeks (C = 26.24 g, OI = 22.76 g, P<.01), (C) 21 weeks (C = 27.07 g, OI = 23.13 g, P<.01), and (D) 29 weeks (C = 27.79 g, OI = 24.84 g, P <.01). Female mice have lower lean mass at 29 weeks only (C = 22.94 g, OI = 20.57 g, P<.05). Fat mass (E–H) does not differ between the groups. C = control group; OI = ovulation induction group.
At sacrifice, in addition to lower weight in pups of superovulated mothers, there were other noticeable phenotypic differences, with the only exception that male offspring of superovulated mothers had significantly shorter AGD (OI = 10.02 mm, C = 13.02 mm, P<.05). In particular, heart weight (male OI = 181.1 g, C = 177.4 g, NS; female OI = 191.5 g, C = 180.3 g, NS) and CRL (male OI = 10.02 mm, C = 13.02 mm, NS; female OI = 5.13 mm, C = 4.62 mm, NS) did not differ between the groups.
Superovulated and Control Offspring Have Similar Glucose Sensitivity
Fasting glucose was similar between the groups at 13 and 21 weeks (Fig. 5A and B, respectively). Only female offspring of superovulated dams exhibited lower fasting glucose at 29 weeks (females OI = 189.6 mg/dL, C = 207.6 mg/dL, P<.05; males OI = 198.7 mg/dL, C = 204.2 mg/dL, NS; Fig. 5C). Fasting insulin levels did not differ between the groups at 13 weeks (Fig. 5D) but were lower only in male offspring of superovulated mothers at 29 weeks (OI = 2.51 μg/L, C = 6.99 μg/L, P<.001; Fig. 5E). Given that we had observed alteration in GSIS in mice conceived by IVF (18), we measured GSIS at sacrifice in a subgroup of control and superovulated offspring and found no differences (Fig. 5F).
FIGURE 5.
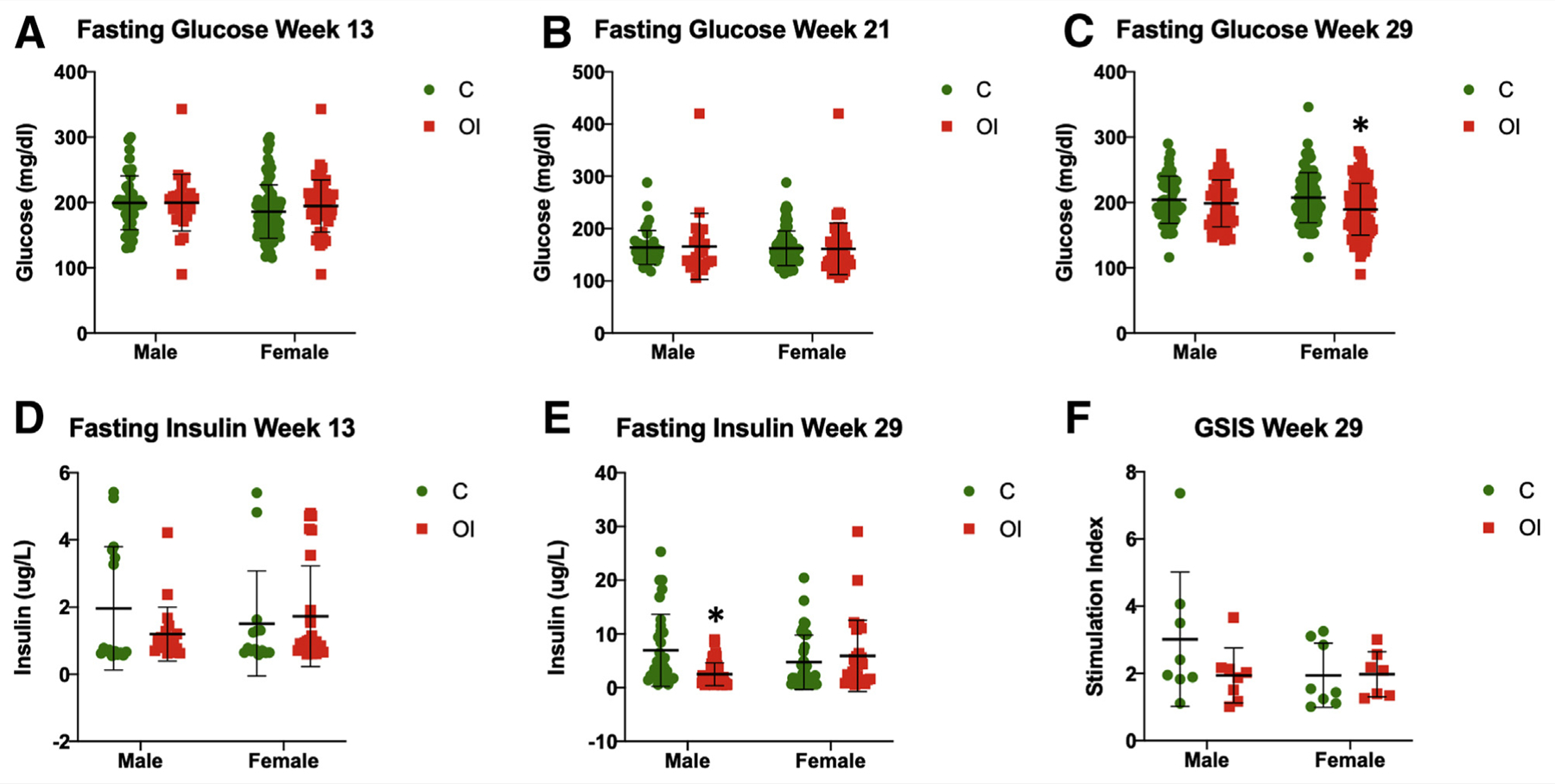
Glucose metabolism—fasting glucose and insulin levels. There are no observable differences between the groups in fasting glucose levels at 13 weeks (A) and 21 weeks (B). At 29 weeks, only superovulated female mice exhibit lower fasting glucose (C = 207.6 mg/dL, OI = 189.6 mg/dL, P<.01) (C). Fasting insulin does not differ at 13 weeks (D), whereas only superovulated males have lower fasting glucose at 29 weeks (E): males C = 6.99 μg/L, OI = 2.51 μg/L, P<.01; females C = 4.75 μg/L, OI = 5.99 μg/L, NS). (F) Glucose-stimulated insulin secretion does not differ between the groups. C = control group; OI = ovulation induction group.
Glucose tolerance tests at 13 and 29 weeks did not show differences between the groups in either male or female offspring, with the only exception that 13-week-old OI male mice had significantly lower glucose at 120 minutes (Fig. 6).
FIGURE 6.
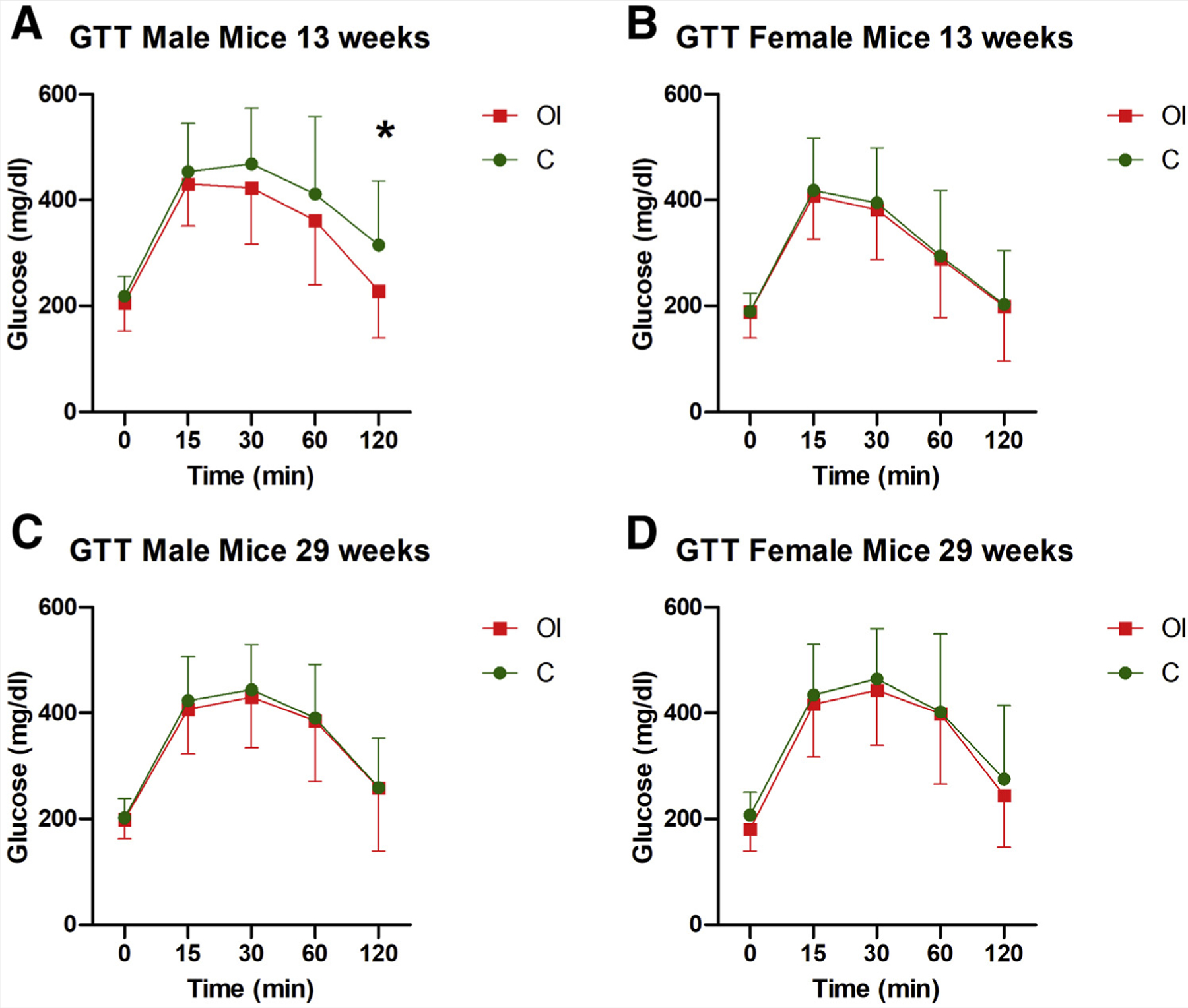
Glucose tolerance test (GTT). There are no significant differences between groups in the glucose tolerance test at 13 and 29 weeks in male (A and C, respectively) or female (B and D, respectively) mice. Only one time point (120 minutes) showed a reduced glucose level in male mice at 13 weeks (A). C = control group; OI = ovulation induction group.
Superovulation Does Not Change the Frequency of Coat Color, and Agouti Mice are Not More Sensitive to the Effects of Superovulation
On day 21, the mice were assessed for coat color. No differences in the frequency of agouti distribution were found between the groups (Fig. 7). In addition, when analyses of birth data, growth parameters, and glucose sensitivity were performed, taking into consideration the agouti color (i.e., OI agouti zero vs. C group zero; agouti 1 group vs. C agouti 1; OI agouti group 2 or 3 vs. C agouti group 2 or 3, and so on), and with the expectation to find more significant changes in mice with agouti color 4 or 5, no differences in results were noted between the groups (Supplemental Figs.1–5).
FIGURE 7.
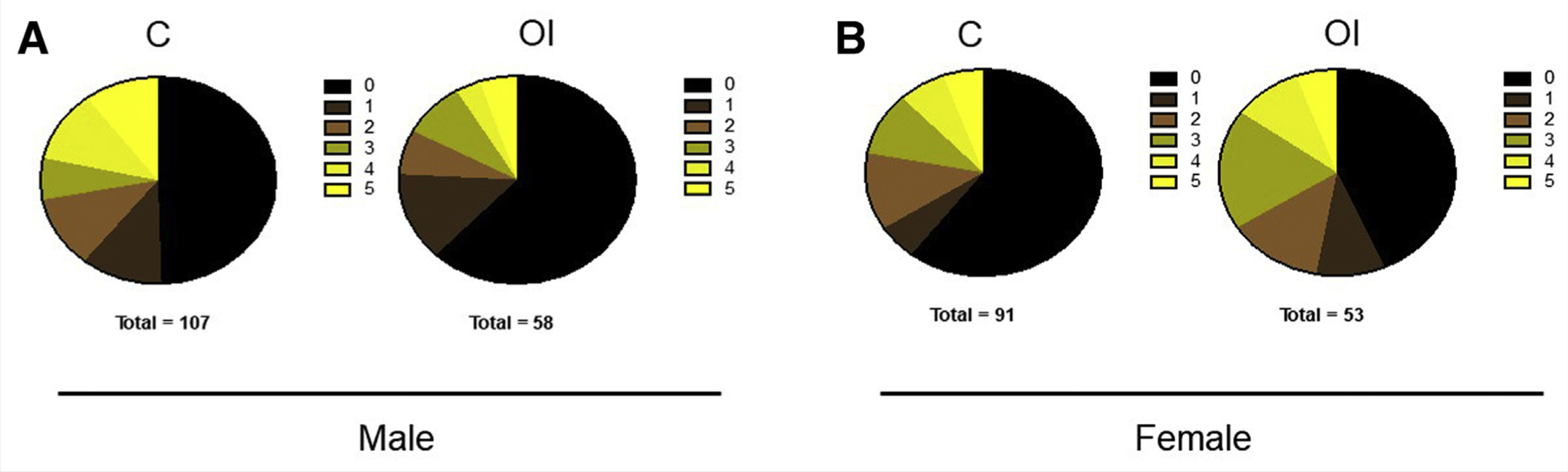
Agouti spread. No differences in Agouti coat color frequency are noted in superovulated male (A) and female (B) mice compared with the control group. C = control group; OI = ovulation induction group.
DISCUSSION
The use of ART has increased dramatically in the last decades, and currently more than 8 million children have been conceived with fertility technologies. Although these technologies are deemed safe, it is apparent that long-term health complications have been described with the use of ART. For example, we and others have shown an increased incidence of glucose intolerance and hypertension in mouse models of ART (15, 18, 22) and in children conceived with these technologies (4, 23). Because superovulation is routinely used in the treatment of infertility, any potential ill effects secondary to superovulation alone are confounded by the concurrent use of artificial insemination and embryo culture. Given that we had found a predisposition to glucose intolerance and beta cell dysfunction in adult mice conceived with the use of superovulation and IVF, this study was designed to study the effects of superovulation with gonadotropins on growth and glucose control in the offspring.
This study had several notable findings. Offspring of superovulated mothers had reduced survival at birth and had shorter AGD and CRL. Postnatally, male mice had lower weight gain because of lower lean mass, but reassuringly, we did not find any alteration in glucose tolerance. Notably, there was no change in coat color frequency in superovulated and control mice, indicating no overt changes in DNA methylation at the agouti locus.
The first notable finding is that litter size at birth after superovulation was similar in superovulated and control mice, but fewer pups from superovulated mothers survived to postnatal day one. In particular, on P1, litters of superovulated dams lost on average one pup, whereas one pup was lost for every two litters in the control group (Fig. 1B, P<.05). To our knowledge, this is the first report of reduced postnatal survival because of superovulation.
Although counterintuitive, the similar litter size in the two groups is not surprising, since exogenous hormone stimulation is known to render the uterus less receptive (8). For example, C57Bl6 females produce an average of 25 oocytes after OI (18), whereas only 8 eggs are released during spontaneous ovulation (24).
Despite superovulation resulting in a much larger number of ovulated oocytes (25), Ertzeid has elegantly shown that embryos after superovulation have a much lower implantation rate: 10% after superovulation and 25% for control embryos (8). In addition, superovulation is known to have detrimental effects on the maturation, function, and ultrastructure of oocytes (26), resulting in delayed embryonic development in vitro and in vivo, with increased abnormal blastocyst formation, fetal growth retardation, and number of resorption sites (27).
Therefore, we can infer that although superovulation results in more eggs being released, fewer embryos are implanted, resulting in similar litter sizes on the day of birth. In addition, because nonsurviving pups have lower weight than surviving pups, it is likely that superovulation results in some pups having lower weight because of abnormal intra-uterine growth. The lower-weight mice are then culled by the mothers, because cannibalism is well described in mice and rats (28).
The second finding is that, after superovulation, only females had lower birth weight, whereas both males and females had shorter AGD and CRL. Other investigators have found that superovulation results in lower birth weight (29). The lower birth weight in females is suggestive of a sexually dimorphic effect. Indeed, there is evidence that sexually dimorphic effects occur in both humans and mice after use of ART (30). Tan showed that IVF in mice results in biased development, with fewer females being born because of impaired X chromosome inactivation (30). Our finding of a normal sex ratio suggests that superovulation alone does not play an important role in this phenomenon. In addition, the shorter CRL in superovulated mice is likely a sign of suboptimal growth in utero. In fact, CRL is often used as an indicator of gestational age and as a marker to predict future growth (31).
Because newborn AGD is affected by maternal androgen levels, the shorter AGD in superovulated pups suggests impaired fetal androgen exposure in utero (32). We can speculate that because superovulation results in a large number of corpora lutea, embryos and fetuses of superovulated dams are exposed to a different hormonal environment, which would lead to the observed reduction in AGD at birth. Indeed, there is evidence that superovulation results in altered hormonal levels. For example, in superovulated mice, vascular endothelial growth factor levels are elevated for the first 4 days after mating, whereas estrogen levels are higher only on the day of human chorionic gonadotropin trigger (33). In future studies, it will be important to measure androgen levels to determine if changes in these hormones after superovulation might explain the different AGD length.
Interestingly, at sacrifice (29 weeks), only male mice of superovulated dams maintained a shorter AGD compared with control mice. The reason for this sexually dimorphic difference is unclear. Of note, AGD in female mice has been shown to be more dynamic and to change according to the stage of the estrous cycle (34). Because shorter AGD in males is associated with impaired testis development and reduced reproductive capacity throughout life (35), it will be valuable, in future studies, to assess reproductive performance in superovulated and control male and female offspring.
The third notable finding relates to the sexually dimorphic effect on postnatal growth. Only superovulated males had lower weight gain, and this was secondary to lower lean mass. In humans, low lean mass is an inheritable genetic factor that can be altered by environmental factors, particularly sex steroids (36, 37). For example, exposure to testosterone for 24 hours in mice at P1 was associated with an increase in both lean and fat mass (38). Thus, we can speculate that the reduced weight gain in superovulated males is a consequence of the altered hormonal environment in pregnancy. It is interesting that females, however, did not exhibit differences in weight throughout their life. The only exception was at 29 weeks of age, when superovulated females additionally exhibited lower lean mass. Future studies should investigate whether female mice continue to display differences in growth and lean mass at a later age.
The fourth important finding is that superovulation did not result in changes in glucose tolerance. On the contrary, offspring of superovulated mothers tended to have higher insulin sensitivity. Given that obesity is strongly associated with insulin resistance and glucose intolerance (39) and that superovulated mice tended to have lower weight, it is likely that the increased glucose sensitivity after superovulation is secondary to the lower weight. Because our prior studies found an alteration in glucose tolerance in mouse offspring that were superovulated and conceived by IVF (15, 18), we can conclude that superovulation does not appear to be responsible for alteration in glucose control; on the contrary, it is likely that the process of embryo culture induces metabolic changes in the embryos that have long-lasting effects.
Finally, we did not find differences in the frequency of agouti coat color in superovulated mice. This is reassuring, because it indicates that superovulation does not affect DNA methylation at the agouti locus. This finding is important, because coat color was found to change after in vitro culture of embryos (13) or exposure to bisphenol A (14). Further, growth and metabolism did not differ between mice belonging to different agouti classes, indicating that superovulation did not have any additional deleterious effect on mice with a predisposition to obesity (coat color 4 or 5).
Although the agouti mouse is sensitive to environmental factors, the fact that we did not find differences in coat color after superovulation does not rule out the possibility that additional DNA methylation changes exist or that other imprinted loci might show abnormal methylation. For example, it has been shown that multiple rounds of superovulation may result in histone modifications of developing embryos (40). In addition, superovulation has been shown to alter imprinting marks in oocytes and embryos in a dose-dependent manner (10) because of abnormal methylation maintenance during preimplantation development (9).
This study has several strengths and weaknesses. The use of the agouti mouse allowed us to test whether phenotypically identifiable epigenetic changes occurred secondary to superovulation. Additionally, the relatively long and intense metabolic follow-up of superovulated mice provides some level of comfort in ruling out long-term metabolic effects secondary to superovulation.
Among the limitations of the study, we did not check other phenotypic variables, such as hypertension or the reproductive fitness of superovulation-generated mice. Moreover, findings in the mouse model may not necessarily reflect changes in humans, given the species-specific differences.
In summary, we found that superovulation had some notable effects on newborn mice. It altered weight gain in males but did not induce profound alteration of glucose metabolism and did not alter the distribution of agouti coat color. On the basis of these observations, judicious use of superovulation medications seems prudent pending additional research.
Supplementary Material
Acknowledgments:
The investigators thank Dr. Randy Jirtle, PhD and Dr. David Skaar, PhD, who kindly donated a breeding pair of agouti mice for the initial experiments.
Supported by a National Institute of Child Health and Human Development grant (R01HD092267) to P.R. S.L.-A. was partially supported by a postdoctoral fellowship grant from The University of California Institute for Mexico and the United States (UC MEXUS) and El Consejo Nacional de Ciencia y Tecnología (CONACYT).
Footnotes
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfss-d-21-00026
REFERENCES
- 1.Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, et al. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril 2018;110:1067–80. [DOI] [PubMed] [Google Scholar]
- 2.Feuer SK, Camarano L, Rinaudo PF. ART and health: clinical outcomes and insights on molecular mechanisms from rodent studies. Mol Hum Reprod 2013;19:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duranthon V, Chavatte-Palmer P. Long term effects of ART: what do animals tell us? Mol Reprod Dev 2018;85:348–68. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Wu L, Zhao J, Wu F, Davies MJ, Wittert GA, et al. Altered glucose metabolism in mouse and humans conceived by IVF. Diabetes 2014;63:3189–98. [DOI] [PubMed] [Google Scholar]
- 5.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol 2006;46:4–14. [DOI] [PubMed] [Google Scholar]
- 7.Chao HT, Lee SY, Lee HM, Liao TL, Wei YH, Kao SH. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann N Y Acad Sci 2005;1042:148–56. [DOI] [PubMed] [Google Scholar]
- 8.Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod 2001;16:221–5. [DOI] [PubMed] [Google Scholar]
- 9.Denomme MM, Zhang L, Mann MR. Embryonic imprinting perturbations do not originate from superovulation-induced defects in DNA methylation acquisition. Fertil Steril 2011;96:734–8.e2. [DOI] [PubMed] [Google Scholar]
- 10.Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet 2010;19:36–51. [DOI] [PubMed] [Google Scholar]
- 11.Jirtle RL. The Agouti mouse: a biosensor for environmental epigenomics studies investigating the developmental origins of health and disease. Epigenomics 2014;6:447–50. [DOI] [PubMed] [Google Scholar]
- 12.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 1998;12:949–57. [PubMed] [Google Scholar]
- 13.Morgan HD, Jin XL, Li A, Whitelaw E, O’Neill C. The culture of zygotes to the blastocyst stage changes the postnatal expression of an epigentically labile allele, agouti viable yellow, in mice. Biol Reprod 2008;79:618–23. [DOI] [PubMed] [Google Scholar]
- 14.Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev 2008;66(Suppl 1):S7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod 2014;90:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld CS, Roberts RM. Maternal diet and other factors affecting offspring sex ratio: a review. Biol Reprod 2004;71:1063–70. [DOI] [PubMed] [Google Scholar]
- 17.Nixon JP, Zhang MZ, Wang CF, Kuskowski MA, Novak CM, Levine JA, et al. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity 2010;18:1652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feuer SK, Liu X, Donjacour A, Lin W, Simbulan RK, Giritharan G, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014;155:1956–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Dowd JF. The isolation and purification of rodent pancreatic islets of Langerhans. Methods Mol Biol 2009;560:37–42. [DOI] [PubMed] [Google Scholar]
- 20.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010;3:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Festing MF. Design and statistical methods in studies using animal models of development. ILAR J 2006;47:5–14. [DOI] [PubMed] [Google Scholar]
- 22.Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest 2013;123:5052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meister TA, Rimoldi SF, Soria R, von Arx R, Messerli FH, Sartori C, et al. Association of assisted reproductive technologies with arterial hypertension during adolescence. J Am Coll Cardiol 2018;72:1267–74. [DOI] [PubMed] [Google Scholar]
- 24.Hino T, Oda K, Nakamura K, Toyoda Y, Yokoyama M. Low fertility in vivo resulting from female factors causes small litter size in 129 inbred mice. Reprod Med Biol 2009;8:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler RE, Edwards RG. Induction of superovulation and pregnancy in mature mice by gonadotrophins. J Endocrinol 1957;15:374–84. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Ahn JI, Lee AR, Ko DW, Yang WS, Lee G, et al. Adverse effect of superovulation treatment on maturation, function and ultrastructural integrity of murine oocytes. Mol Cells 2017;40:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Auwera I, D’Hooghe T. Superovulation of female mice delays embryonic and fetal development. Hum Reprod 2001;16:1237–43. [DOI] [PubMed] [Google Scholar]
- 28.Lane-Petter W Cannibalism in rats and mice. Proc R Soc Med 1968;61:1295–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Weinerman R, Ord T, Bartolomei MS, Coutifaris C, Mainigi M. The superovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model. Biol Reprod 2017;97:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan K, Wang Z, Zhang Z, An L, Tian J. IVF affects embryonic development in a sex-biased manner in mice. Reproduction 2016;151:443–53. [DOI] [PubMed] [Google Scholar]
- 31.Mu J, Slevin JC, Qu D, McCormick S, Adamson SL. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod Biol Endocrinol 2008;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, et al. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl 2010;33:279–87. [DOI] [PubMed] [Google Scholar]
- 33.Mainigi M, Rosenzweig JM, Lei J, Mensah V, Thomaier L, Talbot CC Jr, et al. Peri-implantation hormonal milieu: elucidating mechanisms of adverse neurodevelopmental outcomes. Reprod Sci 2016;23:785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dusek A, Bartos L. Variation in ano-genital distance in spontaneously cycling female mice. Reprod Domest Anim 2012;47:984–7. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U, Svingen T. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxicol 2019;93:253–72. [DOI] [PubMed] [Google Scholar]
- 36.Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, et al. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res 2005;13:312–9. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol 1998;147:3–16. [DOI] [PubMed] [Google Scholar]
- 38.Jang H, Bhasin S, Guarneri T, Serra C, Schneider M, Lee MJ, et al. The effects of a single developmentally entrained pulse of testosterone in female neonatal mice on reproductive and metabolic functions in adult life. Endocrinology 2015;156:3737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang SB, Yang LL, Zhang TT, Wang Q, Yin S, Luo SM, et al. Multiple superovulations alter histone modifications in mouse early embryos. Reproduction 2019;157:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


