Abstract
Fibrosing colonopathy is a unique pathology characterized by long segment stricture, usually of the ileocecal region. Historically, it is most commonly described in patients with cystic fibrosis (CF). Fibrosing colonopathy is felt to be secondary to excessive doses of exogenous lipase medication. This condition is rarely seen in the last decade. In this case presentation, fibrosing colonopathy was identified in a patient with the lysosomal storage disorder of cystinosis. Fibrosing colonopathy has not previously been described in patients with cystinosis. The patient was found to have fibrosing colonopathy after perforation of the colon during a colonoscopy for bloody diarrhea. This case report aims to draw attention to a noteworthy case of fibrosing colonopathy in a patient who does not have cystic fibrosis, but rather cystinosis.
Keywords: fibrosing colonopathy, cystinosis, cystic fibrosis, methacrylic acid, procysbi
INTRODUCTION
Fibrosing colonopathy is a rare colon condition which was first described in 1994 by Smith (1). It is characterized by fusiform long-segment stenosis of the colon, typically of the ascending colon, that can lead to colonic obstruction. Patients with fibrosing colonopathy may experience nausea, abdominal pain, bloody diarrhea, vomiting, and poor weight gain. Histologic findings in the affected colon include a cobblestone appearance, submucosal fibrosis, thickening of the muscularis propria, and chronic mucosal inflammation (2). Imaging can show loss of haustration, shortening, and diffuse narrowing of the colonic lumen with relative rectal sparing (3). Although the pathophysiology for the development of fibrosing colonopathy is not fully understood, it is well correlated with cystic fibrosis (CF). Smith further theorized that the fibrosing colon develops as a result of taking exogenous pancreatic enzymes (4). It was then specifically correlated to a dose-dependent oral intake of exogenous lipase supplementation (5).
This case of fibrosing colonopathy is of particular interest because it is one of few reported in the literature in a patient without CF. Moreover, this is the only known report of a patient with cystinosis. Cystinosis is a congenital, autosomal recessive, lysosomal storage disorder caused by cystinosin gene mutation which codes for the carrier protein, cystinosin, that transports cystine out of a lysosome (6). The resultant accumulation crystallizes and disrupts the proper function of many organs. Patients with cystinosis often have developmental deficiencies, such as stunted growth, rickets, vision problems, and renal insufficiency which may progress to renal failure. Because there is no known cure for cystinosis, treatments center around reducing intracellular cysteine buildup via cysteamine enzyme supplementation.
CASE REPORT
We present a 6-year-old male with a medical history of cystinosis diagnosed initially in 2017. Due to his stage 2 chronic kidney disease, his pediatric nephrology team prescribed Procysbi (cysteamine bitartrate) 7 capsules twice each day, a 75 mg total dosage. Cysteamine bitartrate is compounded as delayed-release granules, enterically coated with high doses of methacrylic acid, to prevent immediate release in an acidic stomach environment. Before the development of cysteamine bitartrate, it was necessary to take medications in greater doses and up to 6 times daily.
At age 5 years, the patient developed a new onset of hematochezia and crampy abdominal pain. He had a several-year history of nausea, intermittent vomiting, and abdominal distention that was felt to be related to the side effects of his medication. Blood work and stool studies were conducted (Table 1) and he was scheduled for diagnostic upper endoscopy and colonoscopy.
TABLE 1.
Gastroenterology ordered labs (February 2021)
| Labs | Results |
| HB (hemoglobin) | 12.0 g/dL (normal is 11.0–14.5) |
| ESR (erythrocyte sedimentation rate) | 10 mm/hr (normal is ≤10.0) |
| Platelets | 499 k/mm3 (normal is 130–450) |
| Calprotectin (stool) | 504 mcg/g (normal is 130–450) |
| Giardia | Negative |
| C difficile toxin A/B | Negative |
| Rotavirus | Negative |
| Lipase | 34 IU/L (normal is ≤34.0) |
| CRP | 39.9 mg/L (normal is 0.0–3.0) |
| Tissue antibody IgG | Negative |
| Tissue antibody IgA | Negative |
CRP = C-Reactive Protein; ESR = erythrocyte sedimentation rate.
Significance of bolding is labs outside of normal parameters.
Upper endoscopy was noted to be visually normal and colonoscopy showed a visually normal rectum but the sigmoid and descending colon showed areas of erythema and cobblestoning with mild friability. There were no areas of ulceration, active bleeding, or obvious stricture. Unfortunately, during inspection of this area, the colonoscope was noted to be extraluminal, concerning iatrogenic perforation.
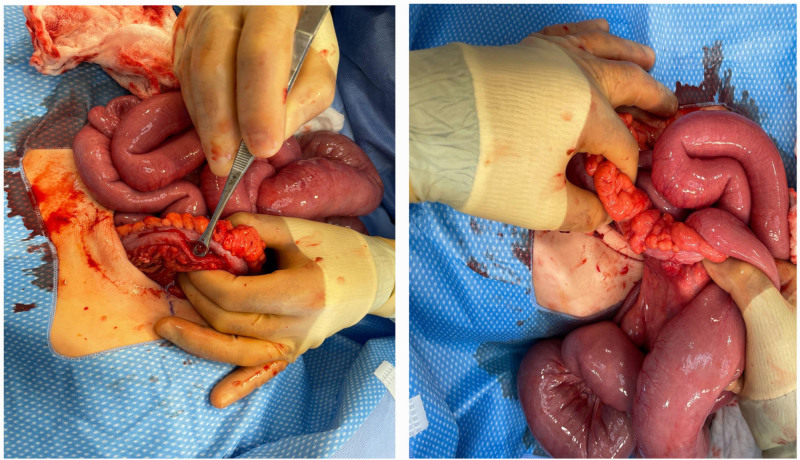
On emergent surgical exploration, a 2 cm longitudinal tear was identified in the antimesenteric border of the mid-transverse colon as seen in Fig. 1. Additionally, the proximal transverse colon to the ileocecal valve was thickened and constricted, and the distal ileum chronically dilated, consistent with a high-grade obstruction (Fig. 1). Due to questionable involvement of the remaining distal colon, a decision was made to perform a diverting end ileostomy, extended right hemicolectomy, and long Hartman’s pouch over a primary anastomosis. The patient’s obstruction was resolved and he was discharged home in stable condition on postoperative day 10.
FIGURE 1.
Transverse colon perforation (left) and creeping fat and thickened stenotic ascending colon with dilated small bowel (right).
Pathology confirmed a diagnosis of fibrosing colonopathy. Specifically, the colon demonstrated diffuse submucosal dense fibrosis and thickening of the muscularis propria with eosinophilic mucosal inflammation. Neither classic inflammatory bowel disease, granulomas, nor cystine crystals were noted.
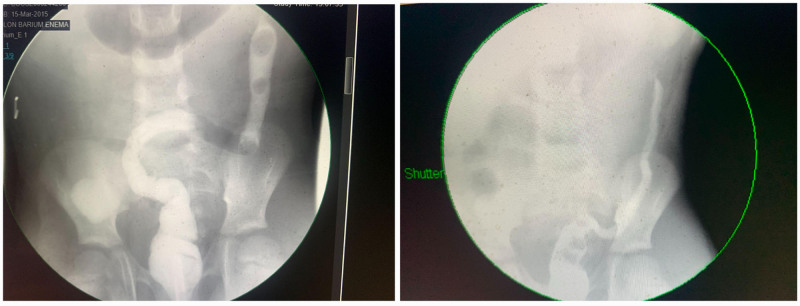
A barium contrast enema (Fig. 2) obtained 6 weeks and 16 weeks postoperatively showed a stricture at 10–12 cm from the anus. Upon repeat colonoscopy, the strictured segment appeared nodular, with erythema, and friability. Biopsies demonstrated mild chronic active colitis with focal cryptitis and crypt branching.
FIGURE 2.
Contrast enema comparison, 6 weeks post op (March 19) (left) vs 16 weeks post op (July 15) (right).
DISCUSSION
Our patient with vague gastrointestinal symptoms with hematochezia was found to have fibrosing colonopathy after exploratory laparotomy. Fibrosing colonopathy is strongly associated with CF and consumption of high-dose oral pancreatic lipase enzymes.
However, this explanation may not be complete. Initially, Lowensfels et. al strongly suggested a dose-dependent phenomenon and that the daily dose of lipase remains below 10,000 units (5). Importantly, many of these oral exogenous pancreatic lipase supplements are encased by a protective coating containing methacrylic acid to ensure the slow release of the lipase. It has been postulated that the methacrylic acid copolymer coating of the per os lipase is a confounder (7). Further studies suggest that the enteric coating polymer for the lipase, methacrylic acid (Eudragit L 30 D-55), is a major contributor to the development of fibrosing colonopathy (8). After the medications for CF management were changed the incidence has decreased drastically.
In 2013, cysteamine bitartrate was introduced into the US drug market with a promising bioprofile of twice-a-day dosing with fewer surveillance periods. During its food drug administration testing phase, a correlation of long-segment ileocolonic strictures was noted but has never been published. The pathophysiology of this association has not been explained. Notably, cysteamine bitartrate contains methacrylic acid (Eudragit L 30 D-55), the same compound found in the protective delay-release coating of high-dose lipase enzymes taken by many CF patients who developed fibrosing colonopathy.
This case report raises the question of whether there is a causal association between methacrylic acid and fibrosing colonopathy. Of note, this enzyme is used in other medications such as delayed-release bupropion, DR-aspirin, DR-Omeprazole, Mesalamine, and DR-81mg aspirin without a described case of fibrosing colonopathy. However, the development of a fibrous colon could be dose-dependent and related to the frequency or the quantity of methacrylic acid ingested. Physicians should be aware of morbidity evolving when a patient develops vague abdominal and gastrointestinal obstructive symptoms while taking medications formulated with methacrylic acid. Additionally, more investigation should be performed into a treatment regimen to avoid potentially harmful consequences in children.
Written consent was obtained and signed for the publication of this case report. Informed Consent for publication of the details of this case was provided by the patient’s mother, legal guardian.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Smyth RL, Van Velzen D, Smyth AR, et al. Strictures of ascending colon in cystic fibrosis and high-strength pancreatic enzymes. Lancet. 1994;343:85–86. [DOI] [PubMed] [Google Scholar]
- 2.Pawel BR, de Chadarévian JP, Franco ME, et al. The pathology of fibrosing colonopathy of cystic fibrosis: a study of 12 cases and review of the literature. Hum Pathol. 1997;28:395–399. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzenberg SJ, Wielinski CL, Shamieh I, et al. Cystic fibrosis–associated colitis and fibrosing colonopathy. J Pediatr. 1995;127:565–570. [DOI] [PubMed] [Google Scholar]
- 4.Smyth RL, Ashby D, O’Hea U, et al. Fibrosing colonopathy in cystic fibrosis: results of a case-control study. The Lancet. 1995;346:1247–1251. [DOI] [PubMed] [Google Scholar]
- 5.FitzSimmons SC, Burkhart GA, Borowitz D, et al. High-Dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283–1289. [DOI] [PubMed] [Google Scholar]
- 6.Elmonem MA, Veys KR, Soliman NA, et al. Cystinosis: a review. Orphanet J Rare. 2016;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott P, Bakowski MT. Pathogenesis of fibrosing colonopathy: the role of methacrylic acid copolymer. Pharmacoepidemiol Drug Saf. 1999;8:377–384. [DOI] [PubMed] [Google Scholar]
- 8.Van Velzen D, Ball LM, Dezfulian AR, et al. Comparative and experimental pathology of fibrosing colonopathy. Postgrad Med J. 1996;72:S39–48; discussion S49. [PubMed] [Google Scholar]