Abstract
Background and Objectives
Exit strategies such as de-escalations have not been evaluated for rituximab in patients with neuromyelitis optica spectrum disorder (NMOSD). We hypothesized that they are associated with disease reactivations and aimed to estimate this risk.
Methods
We describe a case series of real-world de-escalations from the French NMOSD registry (NOMADMUS). All patients met the 2015 International Panel for NMO Diagnosis (IPND) diagnostic criteria for NMOSD. A computerized screening of the registry extracted patients with rituximab de-escalations and at least 12 months of subsequent follow-up. We searched for 7 de-escalation regimens: scheduled discontinuations or switches to an oral treatment after single infusion cycles, scheduled discontinuations or switches to an oral treatment after periodic infusions, de-escalations before pregnancies, de-escalations after tolerance issues, and increased infusion intervals. Rituximab discontinuations motivated by inefficacy or for unknown purposes were excluded. The primary outcome was the absolute risk of NMOSD reactivation (one or more relapses) at 12 months. AQP4+ and AQP4− serotypes were analyzed separately.
Results
We identified 137 rituximab de-escalations between 2006 and 2019 that corresponded to a predefined group: 13 discontinuations after a single infusion cycle, 6 switches to an oral treatment after a single infusion cycle, 9 discontinuations after periodic infusions, 5 switches to an oral treatment after periodic infusions, 4 de-escalations before pregnancies, 9 de-escalations after tolerance issues, and 91 increased infusion intervals. No group remained relapse-free over the whole de-escalation follow-up (mean: 3.2 years; range: 0.79–9.5), except pregnancies in AQP+ patients. In all groups combined and within 12 months, reactivations occurred after 11/119 de-escalations in patients with AQP4+ NMOSD (9.2%, 95% CI [4.7–15.9]), from 0.69 to 10.0 months, and in 5/18 de-escalations in patients with AQP4− NMOSD (27.8%, 95% CI [9.7–53.5]), from 1.1 to 9.9 months.
Discussion
There is a risk of NMOSD reactivation whatever the rituximab de-escalation regimen.
Trial Registration Information
Registered on ClinicalTrials.gov: NCT02850705.
Classification of Evidence
This study provides Class IV evidence that de-escalation of rituximab increases the probability of disease reactivation.
The therapeutic management of patients treated with immunoactive drugs in the long term raises the question of exit strategies such as “de-escalation.”1 De-escalation seeks to match the potency of treatments with decreasing disease activity. It may consist of the discontinuation of treatment, a switch to a less active treatment, or a decreased posology, for example, by increasing infusion intervals. Neuromyelitis optica spectrum disorder (NMOSD) is a severe chronic autoimmune disease of the CNS associated with aquaporin 4-IgG (AQP4-IgG) antibodies. NMOSD is associated with an attack-driven disability. Relapses are more damaging than in multiple sclerosis (MS) and may cause irreversible disability, despite acute treatments with corticosteroids or plasma exchanges.2-4 The long-term maintenance of relapse-preventive immunoactive treatments is therefore mandatory.
At the time of our study, rituximab (anti-CD20) was the mainstay treatment in NMOSD. It has proven its efficacy in numerous real-world studies and in randomized clinical trials (RCTs) against azathioprine5 and placebo.6 Several regimens of rituximab exist. In most cases, rituximab is maintained with reinfusions either periodically every 6 months7 or with personalized infusion intervals based on the result of CD19+ CD27+ memory B-cell repopulation monitoring.8,9 Less classically, the regimen may also be a single infusion cycle induction, followed either by treatment discontinuation or by a switch to an oral immunosuppressive drug. B-cell repopulation after rituximab infusions tends to become slower as the treatment duration increases.10 This suggests a potential induction effect of rituximab on NMOSD activity, which could be sustained during a de-escalation.
De-escalations must be considered in various situations as the benefit-risk ratio of immunosuppression evolves over the follow-up of a patient. Maintained rituximab treatments induce a progressive secondary hypogammaglobulinemia.11 It may be sustained for years after rituximab discontinuation and may require immunoglobulin replacement therapies in profound symptomatic cases.12 Secondary lymphopenia and neutropenia have also been described.13 Thus, rituximab-treated patients have an increased risk of opportunistic infections, especially as the treatment duration increases, as the patient grows older, and as the disability worsens.13,14 Pandemics, such as COVID-19, may require preventive decreases of immunosuppression.15,16 The humoral response to vaccinations is decreased by B-cell–depleting therapies.17 Rituximab is incompatible with pregnancy. Finally, rituximab requires hospital-based infusions, which may be perceived as inconvenient by patients who have been doing well for a long time.
In MS, de-escalations expose patients to a moderate increase of the annualized relapse rate (ARR).18 However, inflammatory rebounds have been reported after discontinuation of natalizumab19,20 and fingolimod.21-23 Anti-CD20 seems to be safer in this regard.24-26 However, data about de-escalations in NMOSD are lacking. Only one case series has studied this question in NMOSD so far,27 reporting that 82% of patients suffered a relapse at a median interval of 6 months. Our primary research question was whether de-escalations of rituximab are associated with NMOSD reactivations. In this registry-based study, our aim was to describe real-world de-escalations and estimate the risk of reactivation in the various de-escalation regimens.
Methods
Study Population
We conducted a retrospective case series study based on the French NMOSD registry NOMADMUS28 (ClinicalTrials.gov Identifier: NCT02850705). Nationwide NMOSD case detection is possible as the French MS/NMOSD Observatory network (Observatoire français de la sclérose en plaques) covers all major neuroimmunology units in France. Within the network, testing for AQP4-IgG and myelin oligodendrocyte glycoprotein antibodies (MOG-IgG) is centralized in Lyon. NOMADMUS data are collected as initial reports by clinicians for all suspected cases and are then checked and completed retrospectively by clinical research assistants at the corresponding French MS/NMOSD Observatory center. Data were extracted on June 15, 2020, with right censoring on June 15, 2019, to ensure that data had been completed. We included the seropositive (AQP4+) and seronegative (AQP4−) patients for AQP4-IgG who met the 2015 International Panel for NMO Diagnosis (IPND) diagnostic criteria for NMOSD.29 They had one or more therapeutic de-escalations during their follow-up, as detected by computerized screening (see below). We focused our analysis on rituximab because it is the most prescribed current treatment in the data set. MOG-IgG–seropositive patients were excluded as myelin oligodendrocyte glycoprotein antibody disease (MOGAD) is now considered to be a distinct disease, based on clinical, radiologic, and pathophysiologic considerations.30,31
Data Processing and De-escalation Detection
We processed the data using R (version 4.1.3). An algorithm screened the registry for de-escalations, defined as a complete treatment discontinuation, a switch from rituximab to an oral treatment, or an increased interval between 2 infusions (Figure 1). Individual patients could have multiple de-escalations during their follow-up. Rituximab discontinuations were considered “complete” in the absence of a switch in the following 6 months + a margin of 20 days and no further rituximab infusion. Infusion intervals were considered increased from 6 months + a margin of 20 days to 14 months (corresponding to the skipping of a periodic infusion with a 2-month margin). In the case of further rituximab infusions beyond 14 months, the index de-escalations were considered as “complete” discontinuations and the further infusions were analyzed as new rituximab therapeutic sequences. For simplicity, we classified methotrexate as an oral treatment, although it may also be administered by the subcutaneous route. A quality control procedure excluded cases with inconsistent follow-up dates and whose follow-up after the de-escalation baseline was less than 1 year. The results of this processing were plotted as timelines and checked visually by the investigators for every patient (eFigures 1–4, links.lww.com/WNL/C872, links.lww.com/WNL/C873, links.lww.com/WNL/C874, links.lww.com/WNL/C875).
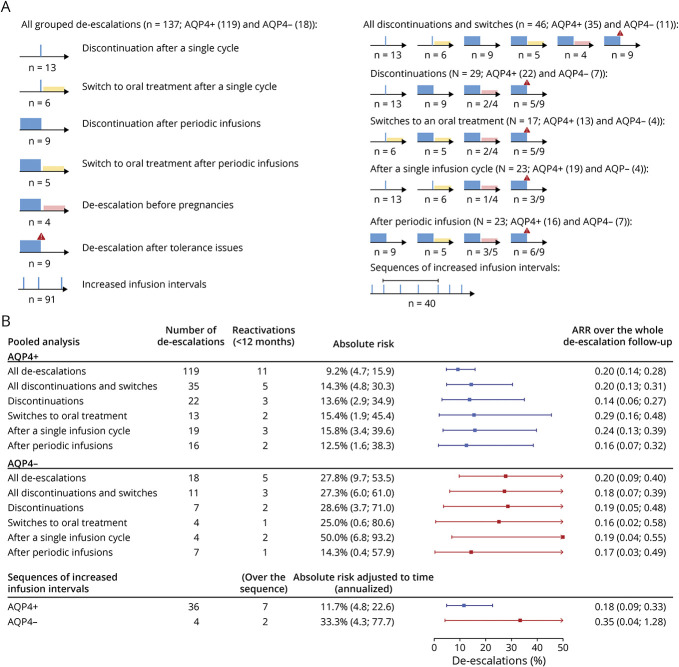
Figure 1. Flowchart.
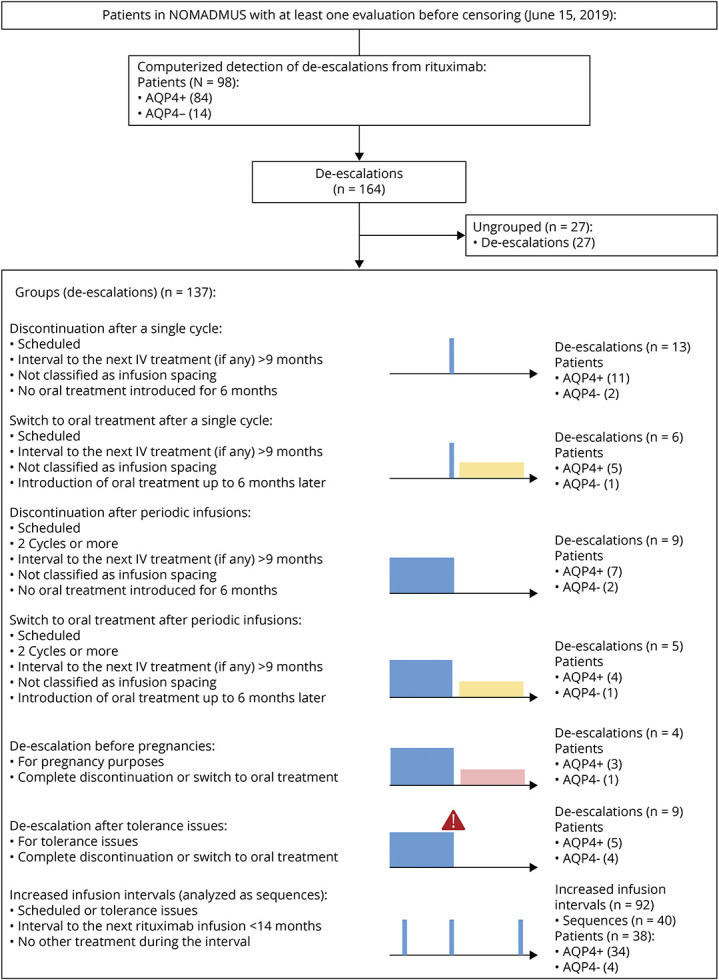
Patients with de-escalations of rituximab were identified through a computerized screening among patients in the NOMADMUS registry. De-escalations were grouped into 7 regimens defined in the lower part of the figure. Colors in the lower part: blue for rituximab, yellow for oral treatments, and pink for pregnancies. AQP4+ = antiaquaporin 4-IgG antibodies.
Data Analysis
We addressed the heterogeneity of real-world rituximab regimens by classifying the de-escalations into 7 groups (Figure 1): (1) complete discontinuations scheduled after a single infusion cycle, (2) switches to an oral treatment scheduled after a single infusion cycle, (3) discontinuations scheduled after periodic infusions (at least 2 cycles), (4) switches to an oral treatment scheduled after periodic infusions (at least 2 cycles), (5) de-escalations before pregnancies, (6) de-escalations after tolerance issues, and (7) sequences of successive increased infusion intervals. Scheduled de-escalations excluded discontinuations motivated by tolerance issues, inefficacy, or pregnancies. The de-escalations before pregnancies or after tolerance issues could be either discontinuations or switches. The tolerance issues could be general, local, or biological. The discontinuations for inefficacy or for unknown purposes remained ungrouped because the reason for the treatment change was either different than a de-escalation strategy or uncertain. Increased infusion intervals represent a gradual de-escalation, but each new interval was counted as a de-escalation by the screening algorithm whatever its length. Therefore, we described and analyzed this regimen separately by considering the sequences of successive increased infusion intervals as observation units.
Outcomes
Disease reactivations were defined as the occurrence of one or more relapses during a given period at risk. For discontinuations and switches, the outcome was the absolute risk of disease reactivation at 12 months after the de-escalation. For sequences of successive increased infusion intervals, the outcome was the absolute risk of disease reactivation during the sequence. We annualized it to make both outcomes comparable. As secondary outcome, we estimated the ARR during the whole de-escalation follow-up (i.e., from the de-escalation baseline to the introduction of another IV treatment or censoring).
Statistical Analysis
Patients with AQP4+ and AQP4− NMOSD were analyzed separately to account for differences in their general therapeutic responses.32,33 Proportions of reactivating patients were considered as binomial and were presented with their estimated 95% CI using the Clopper and Pearson method.34,35 ARRs were presented with their estimated 95% CI using a Poisson regression. First, we analyzed each de-escalation regimen separately. We refrained from comparing the outcomes statistically between the groups because they were not randomly allocated. We also refrained from doing a multivariate inferential analysis because of the small numbers in each group. Second, we performed a pooled analysis on group combinations, including the combination of the 7 groups as “all de-escalations” (analyzing each increased infusion interval as a de-escalation). The proportions of reactivation between the subgroups were not compared statistically because pooling all the de-escalations together introduced a significant amount of dependency in the data. The low number of de-escalations per patient (mostly 1 per patient) prevented the convergence of models adjusting for patient effects. Third, we performed an exploratory subgroup analysis over the combination of all de-escalations. We defined subgroups of dichotomized variables that we considered clinically relevant when planning a de-escalation: age, disease duration, cumulated number of relapses, time since the last relapse, and last Expanded Disability Status Scale (EDSS). The dichotomization threshold was set to the variable's median over the whole AQP4+ and AQP4− pool of de-escalations. We analyzed the time to NMOSD reactivation over the whole de-escalation follow-up using a multivariate Cox proportional-hazards model adjusting for the five variables using the “survival” and “survminer” R packages. Missing irreversible EDSS was imputed using the multiple imputation method of the “mice” R package.
Data Availability and Ethical Statement
Pseudonymized data of this study will be available on reasonable written request from any qualified investigator. R codes are provided in supplemental material (eAppendices 1–8, links.lww.com/WNL/C864, links.lww.com/WNL/C865, links.lww.com/WNL/C866, links.lww.com/WNL/C867, links.lww.com/WNL/C868, links.lww.com/WNL/C869, links.lww.com/WNL/C870, links.lww.com/WNL/C871). All patients gave their informed consent, and data collection was approved by the national ethical authority (Commission Nationale de l'Informatique et des Libertés, Registration No. 914066v2).
Results
Characteristics of the Population and De-escalations
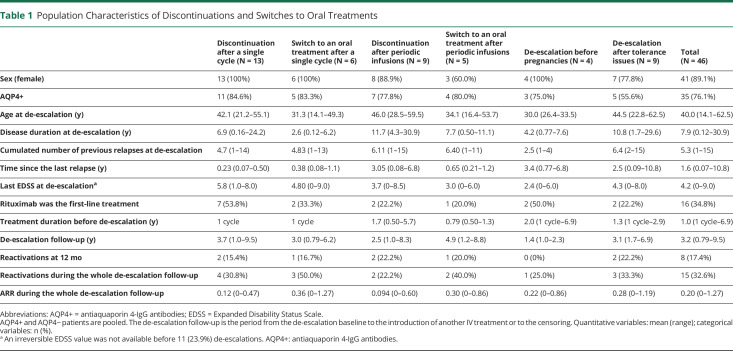
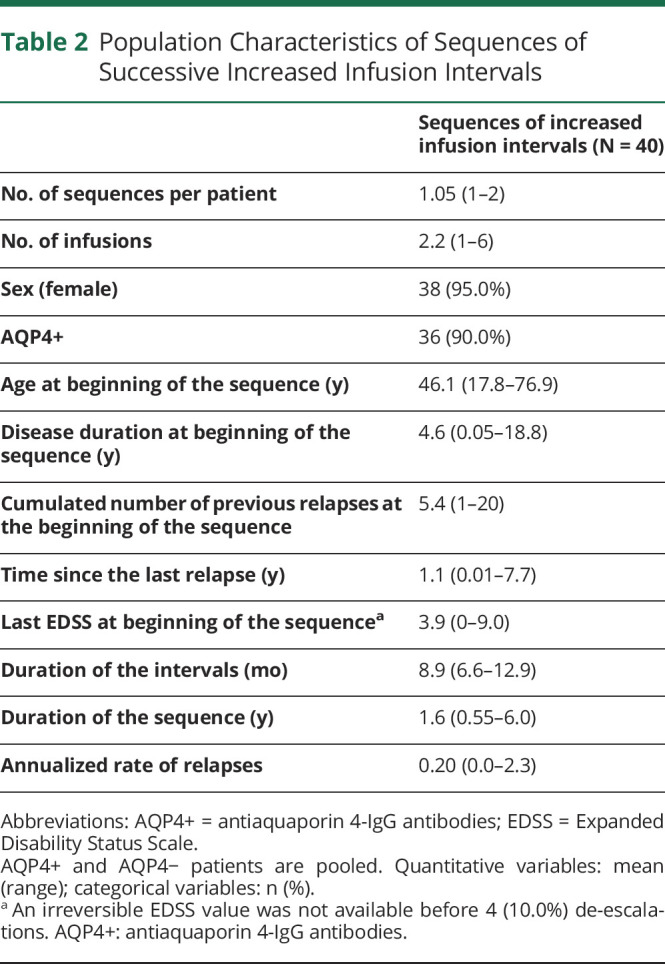
The computerized screening of the NOMADMUS registry identified 164 real-world rituximab de-escalations among 98 patients between 2006 and 2019. Among them, 137 corresponded to a predefined group (Figure 1) and occurred in 78 patients. Most of these patients were female (n = 71; 91.0%), and most were AQP4+ (n = 65; 83.3%). The mean age at disease onset was 36.6 years (min: 7.4; max: 69.1; 12.8% younger than 18 years). We describe the characteristics of the patients at the time of de-escalation in each group in Tables 1 and 2 (AQP4+ and AQP4− patients pooled). All de-escalations among a given group were independent (one de-escalation in the group per patient), except for 2 patients with 2 distinct sequences of successive increased infusion intervals during their follow-ups. Five patients had 2 or 3 de-escalations in different groups (eTable 1, links.lww.com/WNL/C876). Although all de-escalations had at least 12 months of follow-up, the de-escalation follow-up (i.e., the follow-up after the de-escalation baseline until the next IV treatment or censoring) lasted between 0.76 and 9.5 years (Table 1).
Table 1.
Population Characteristics of Discontinuations and Switches to Oral Treatments
Table 2.
Population Characteristics of Sequences of Successive Increased Infusion Intervals
Combined Group Analysis
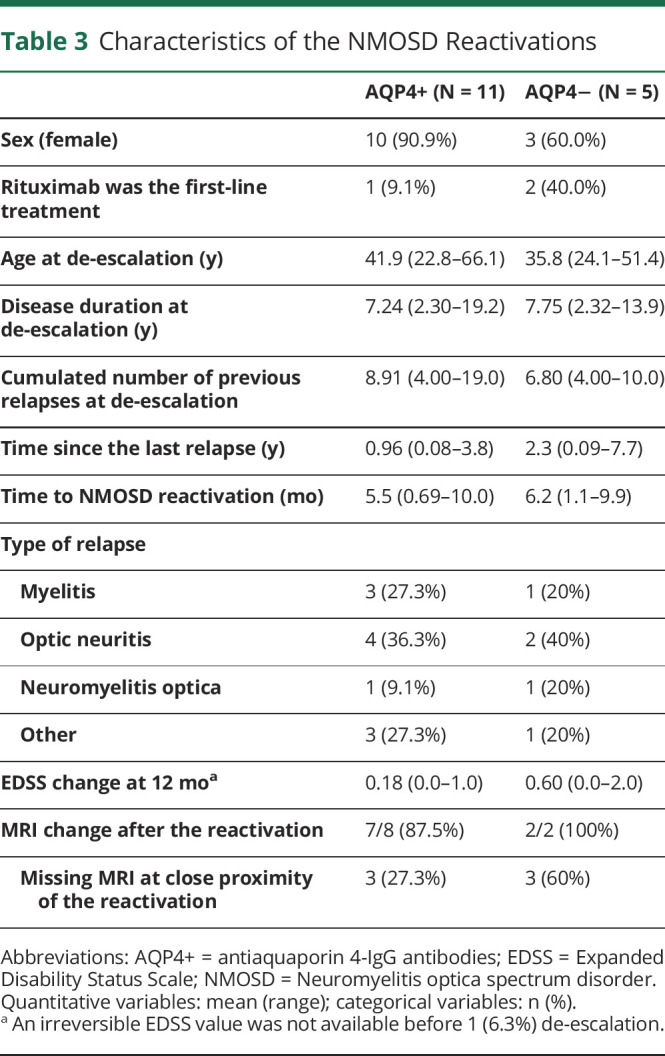
We first describe the combined group analysis, as depicted in Figure 2. Over the whole set of 137 de-escalations, NMOSD reactivations at 12 months occurred after 11 de-escalations in patients with AQP4+ NMOSD (9.2% 95% CI [4.7–15.9]) and after 5 de-escalations in patients with AQP4− NMOSD (27.8% 95% CI [9.7–53.5]). Details about the reactivations are presented in Table 3. The sequences of increased infusion intervals lasted from 1 to 6 successive infusions over 0.55–6.0 years. The intervals ranged from 6.6 to 12.9 months. Most sequences were followed by further rituximab infusions at periodic intervals (eFigure 4, links.lww.com/WNL/C875). In patients with AQP4+ NMOSD, the risk was higher when considering only discontinuations and switches (14.3%; 95% CI [4.8–30.3]). It was similar whatever the combination of de-escalation groups from 11.7% (during sequences of increased infusion intervals) to 15.8% (after single infusion cycles). The ARR over the whole de-escalation follow-up was estimated to be 0.20 95% CI (0.14–0.28). In patients with AQP4− NMOSD, the risk estimates were higher but less precise and are therefore not detailed in the text of this report.
Figure 2. Absolute Risk of Disease Reactivation Among Different Combinations of De-escalation Groups.
(A) Analysis plan of all the combinations of the groups defined in the flowchart with their respective icons. (B) Combined analysis. The forest plot shows the estimated risks of NMOSD reactivation at 12 months and their 95% CIs in AQP4+ patients (blue) and in AQP4− patients (red). For comparability with the other groups, the risk of reactivation during a sequence of increased infusion intervals was adjusted to its duration, yielding annualized risks of reactivation. ARR = annualized relapse rate; AQP4+ = antiaquaporin 4-IgG antibodies.
Table 3.
Characteristics of the NMOSD Reactivations
Group Analysis
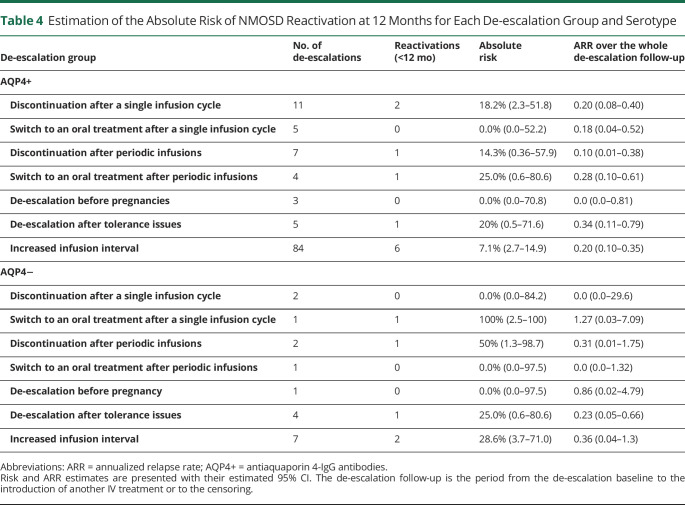
We then describe the analysis of each de-escalation group (Table 4). Most de-escalations were increased infusion intervals (84/119 in patients with AQP4+ NMOSD and 7/18 in patients with AQP4− NMOSD). Table 4 presents their outcome by taking each increased interval as an observation unit. They are presented as sequences in Table 2 and analyzed as such in Figure 2. In the other groups, the number of de-escalations was low (max: 11 for discontinuations after single infusion cycles). Most de-escalations after single infusions were close to the last relapse (range of time since the last relapse: 0.07–1.1 years), which is consistent with induction strategies (Table 1). The sequences of periodic infusions lasted from 0.5 to 5.6 years. Among the 9 de-escalations after tolerance issues, 5 were motivated by general adverse events, 1 by a local adverse event, and 3 by biological adverse events. In patients with AQP4+ NMOSD, the absolute risk of reactivation at 12 months comprised between 0.0% (for switches after a single infusion cycle and pregnancies) and 25.0% (for switches after periodic infusions). However, the large 95% CI ranges hinder numerical comparisons. Over the whole de-escalation follow-up, no group remained relapse-free, except pregnancies. One patient with AQP4− NMOSD relapsed more than 12 months after a de-escalation for pregnancy, which likely occurred during the postpartum period (eFigure 3, links.lww.com/WNL/C874). We encourage the reader to refer to the individual timelines, available in the supplementary materials (eFigures 1–4, links.lww.com/WNL/C872, links.lww.com/WNL/C873, links.lww.com/WNL/C875), for a description of the individual patient histories.
Table 4.
Estimation of the Absolute Risk of NMOSD Reactivation at 12 Months for Each De-escalation Group and Serotype
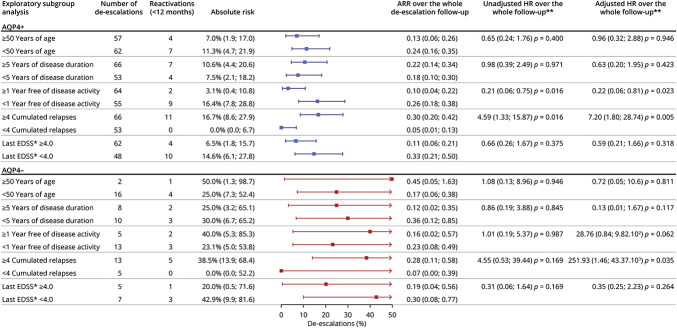
Exploratory Subgroup Analysis
Finally, to get an exploratory insight into the contexts most frequently associated with NMOSD reactivations after a de-escalation, we performed a subgroup analysis over the whole set of 137 de-escalations (Figure 3). Each increased infusion interval was analyzed as an observation unit. No reactivation occurred in patients who had less than 4 previous relapses in their histories. Otherwise, the greatest contrast in patients with AQP4+ NMOSD was between de-escalations close to and those at a distance from NMOSD activity: 3.1% 95% CI (0.4–10.8) more than 1 year after the last relapse vs 16.4% 95% CI (7.8–28.8) less than 1 year after the last relapse. In the survival analysis, both variables were independently associated with the time to reactivation (adjusted HR of the count of previous relapses: 7.20 95% CI [1.80–28.74] p = 0.005) (adjusted HR of the time since NMOSD activity: 0.22 95% CI [0.06–0.81] p = 0.023).
Figure 3. Exploratory Subgroup Analysis of the Pooled De-escalations.
Age, disease duration, time since disease activity, the cumulated number of relapses, and the last EDSS were dichotomized according to their medians. Each increased infusion interval was analyzed as an observation unit. The forest plot shows the estimated absolute risks of reactivation at 12 months and their 95% CIs in AQP4+ patients (blue) and in AQP4− patients (red). *An irreversible EDSS value was not available before 15 (10.9%) de-escalations. **The hazard ratios were computed using a Cox proportional-hazards model adjusting for the five variables. Missing EDSS were imputed. ARR = annualized relapse rate; AQP4+ = antiaquaporin 4-IgG antibodies; EDSS = Expanded Disability Status Scale.
Classification of Evidence
This study provides Class IV evidence that de-escalation of rituximab increases the probability of disease reactivation.
Discussion
This study addressed therapeutic de-escalation in a large cohort of patients with NMOSD. NOMADMUS is one of the largest NMOSD registries for this rare disease, with a nationwide coverage of patient recruitment. This enabled us to detect 164 real-world de-escalations of rituximab and gain insight into 137 of them in several regimen scenarios. We preferred to estimate the risk of disease reactivation as primary outcome rather than the ARR. In our opinion, it better reflects the clinical considerations in the context of a de-escalation in NMOSD because the disability is driven by severe attacks.36 Nonetheless, we estimated the ARR as a secondary outcome for ease of comparison with the literature. Across the studies, the proportions of relapse-free patients are not reported over consistent follow-up durations. Our primary results are thus qualitative. Reactivations occurred in most of the de-escalation groups at 12 months and in all group combinations, in both patients with AQP4+ and patients with AQP4− NMOSD. Although less precise, the risk estimates were higher in AQP4− NMOSD. In our exploratory subgroup analysis (Figure 3), great age and long disease durations were associated with similar risks of reactivation. De-escalations tended to be more successful after prolonged relapse-free periods (1 year or more) and higher EDSS (4.0 or more). A high number of cumulated relapses were the characteristic most associated with reactivations because all reactivations occurred in patients with a history of at least 4 relapses. Over the whole de-escalation follow-up, a high number of cumulated relapses and short relapse-free periods were significantly statistically associated with shorter times to reactivation. In comparison, a recent case series reported 17 patients with immunosuppressive treatment discontinuations, including 2 treated with rituximab27; it found 82% of NMOSD reactivations after a median interval of 6 months.
The quantitative results of our study need to be interpreted in the light of the efficacy of rituximab maintenance in the literature. Because de-escalations in our study occurred after relatively short treatment durations (mean: 1.0 year range [1 cycle, 6.9 years]; Table 1), our analysis population was not representative of long-term rituximab maintenance. A background risk before the de-escalation baseline could therefore not be estimated reliably. However, the efficacy of rituximab has already been estimated in the same source population over longer periods. Our group estimated the ARR as 0.1 (SD: 0.2), when used as first-line therapy,37 and as 0.4 (SD: 0.5), when used as second-line therapy.38 In the rest of the literature, the ARR under rituximab has been estimated at around 0.1, ranging from 0.06,39 to 0.21.5 The highest estimation is from a 12-month RCT pooling patients with AQP4+ and AQP4− NMOSD.5 Patients with MOGAD were most likely included as AQP4− patients because the screening of anti-MOG antibodies was not available at that time. In general, the ARR estimate under rituximab is higher than that of patients with AQP4+ NMOSD (0.25–0.79).40 However, in the reports of the long-term efficacy of rituximab, the individual timelines suggest a better long-term efficacy of rituximab compared with its short-term efficacy during the first years of treatment.37,41-44 This would be consistent with an induction effect and with the possibility of spacing rituximab infusions even more as the treatment duration increases.10 In our study, the ARR after the discontinuations and switches (0.20, 95% CI [0.13–0.31] in AQP4+ patients and 0.18, 95% CI [0.09–0.40], in AQP4− patients) were a little higher than the ARR under rituximab maintenance in the literature, or at best, similar to the short-term efficacy of rituximab. There was no dramatic rebound that reported in MS after the discontinuations of natalizumab19,20 or fingolimod.21-23 Taken together, the results of our real-world study suggest that de-escalations from rituximab yield a moderate increase of NMOSD activity compared with what could be expected under a long-term maintenance.
In addition to the lack of an estimated background risk, our study has several limitations related to the retrospective, real-world, registry-based study design. (1) In the case of periodic infusions, the de-escalations occurred after relatively short treatment durations (Table 1). They are likely not representative of de-escalations after several years of treatment maintenance, which remained an anecdotal scenario here. (2) Rituximab regimens were not standardized as the therapeutic management of NMOSD was not codified during the extracted period of follow-up. Some guidelines have been proposed for the use of rituximab at the end of the extracted period.7 To some extent, rituximab treatments were subject to retrospective strategic interpretations. We addressed this issue by adopting a descriptive rather than an epidemiologic approach by grouping de-escalations into numerous, but homogeneous, regimens and eventually pooling them in a controlled fashion for the combined analysis. (3) A consequence of this approach was to have a small number of patients in most regimen groups. This resulted in a poor statistical power for the 95% CI estimates. Therefore, we refrained from multivariate statistical analysis and patient-matching techniques. (4) The comparability of the patients between the regimen groups was limited, thereby making between-group comparisons only indicative. (5) Relapses were not retrospectively assessed for the purpose of excluding any flare-ups. This was circumvented by considering disease reactivations (1 or more relapses) as our primary outcome, rather than the ARR. Therefore, de-escalation clinical trials are needed to compare standardized de-escalation regimens prospectively.
The immunophenotyping is also limited in the data collected in the registry. (1) Longitudinal AQP4-IgG titer and B-cell subpopulation monitoring (CD19 and CD27 counts) are known predictors of NMOSD reactivation8,9,45 but are not recorded in the NOMADMUS database. (2) The analysis of the 4 de-escalations related to pregnancy in our study is limited because few details of the pregnancy were recorded in the database. Pregnancies in patients with NMOSD have been extensively analyzed elsewhere.46,47 As in our study, the pregnancy period is associated with a lower disease activity but is followed by a rebound of activity during the postpartum period, which is the most likely interpretation of the reactivation at 13.5 months in our patient 33. (3) Eculizumab, inebilizumab, and satralizumab are recently approved drugs for NMOSD whose RCTs were only published during the last years of our follow-up period.32,33,48 These drugs will only be assessable after years of use. (4) De-escalations required by the COVID-19 pandemic could not be analyzed either because data were extracted just after the first “wave” and a right censoring of 1 year was required for data quality purposes. Therefore, our de-escalation set reflects spontaneous, real-world scenarios rather than de-escalations constrained by the pandemic.
Aging is often taken into consideration when planning a therapeutic de-escalation in neuroinflammatory diseases. Contrary to MS,1 the inflammatory activity of NMOSD is also important at great ages at onset.49 Although recent data on late-onset NMOSD have been reported,50,51 data about aging in patients with early-onset NMOSD are scarce in the literature. In our study, the risk of reactivation before and beyond 50 years was similar. Therefore, clinicians should remain cautious even for patients at advanced ages. On the other hand, this population is also more vulnerable to opportunistic infections and to the risk of cancer, either through a cumulative cytotoxicity or through a reduced level of immunosurveillance. It is therefore the population that will most likely be affected by eventual sustained rituximab-induced secondary immunodeficiencies. In summary, besides any biological monitoring, a close follow-up is needed after the de-escalation to treat any reactivation as early as possible.4
Therapeutic de-escalations of rituximab in patients with NMOSD are associated with a risk of disease reactivation at 12 months whatever the de-escalation regimen. This risk seems to be a moderate increase of NMOSD activity, compared with what could be expected under long-term maintenance of rituximab therapy. De-escalation clinical trials are needed to confirm these findings. B-cell repopulation monitoring is a promising approach to assess the propensity of NMOSD to reactivate. The risk of disease reactivation after discontinuations of the recently approved NMOSD drugs remains to be assessed.
Acknowledgment
The authors acknowledge all the researchers and clinical research assistants involved in Observatoire Français de la Sclérose en Plaques (OFSEP), and especially Fabien Rollot, Romain Casey, and those involved in its branch dedicated to NMOSD. This work was supported by a grant from the French State through the “Agence Nationale de la Recherche,” within the framework of the “Investments for the Future” program, under the reference ANR-10-COHO-002 Observatoire Français de la Sclérose en plaques (OFSEP).
Glossary
- AQP4-IgG
aquaporin 4-IgG
- EDSS
Expanded Disability Status Scale
- MOGAD
myelin oligodendrocyte glycoprotein antibody disease
- MOG-IgG
myelin oligodendrocyte glycoprotein antibodies
- MS
multiple sclerosis
- NMOSD
neuromyelitis optica spectrum disorder
- RCTs
randomized clinical trials
Appendix 1. Authors

Appendix 2. Co-investigators

Footnotes
Editorial, page 153
Class of Evidence: NPub.org/coe
Study Funding
This work was supported by a grant from the French State through the “Agence Nationale de la Recherche,” within the framework of the “Investments for the Future” program, under the reference ANR-10-COHO-002 Observatoire Français de la Sclérose en plaques (OFSEP).
Disclosure
S. Demuth reports no disclosures. N. Collongues has received honoraria for consulting or presentation from Biogen Idec, Alexion, Novartis, Merck Serono, Bristol-Myers Squibb, Sanofi-Genzyme, and Roche, and is a member of the Editorial Board of the Journal de la Ligue Française contre la Sclérose en plaques. B. Audoin, X. Ayrignac, B. Bourre report no disclosures. J. Ciron has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi-Genzyme, Roche, Celgene-BMS, Alexion, none related to this study. M. Cohen has received honorarium for participating on scientific advisory boards for Ad Scientiam, Alexion, Biogen, BMS, Horizon therapeutics, Merck, Novartis, Roche, and Teva. R. Deschamps, F. Durand Dubief report no disclosures. E. Maillart reports personal fees from Alexion, Biogen, Merck, Novartis, Roche, Sanofi, and Teva, and grants from Biogen, outside the submitted work. C. Papeix reports no disclosures. A. Ruet reports personal fees and research grants from Biogen, personal fees and research grant from Roche, research grant from Genzyme, personal fees and research grant from Merck, research grant from Bayer, outside the submitted work. H. Zéphir has received consulting fees from Biogen Idec, Roche, Alexion, BMS, Sanofi, Merck, and Novartis but reports no disclosures directly related to the manuscript. R. Marignier serves on the scientific advisory board for Viela Bio, Roche, UCB, and Alexion, and has received honoraria from Biogen, Merck, and Novartis. J. de Seze reports no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Vollmer BL, Wolf AB, Sillau S, Corboy JR, Alvarez E. Evolution of disease modifying therapy benefits and risks: an argument for de-escalation as a treatment paradigm for patients with multiple sclerosis. Front Neurol. 2021;12:799138. doi. 10.3389/fneur.2021.799138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan SM, Zantek ND, Carpenter AF. Therapeutic plasma exchange in neuromyelitis optica: a case series. J Clin Apher. 2014;29(3):171-177. doi. 10.1002/jca.21304 [DOI] [PubMed] [Google Scholar]
- 3.Wang KC, Wang SJ, Lee CL, Chen SY, Tsai CP. The rescue effect of plasma exchange for neuromyelitis optica. J Clin Neurosci. 2011;18(1):43-46. doi. 10.1016/j.jocn.2010.05.030 [DOI] [PubMed] [Google Scholar]
- 4.Demuth S, Guillaume M, Bourre B, et al. Treatment regimens for neuromyelitis optica spectrum disorder attacks: a retrospective cohort study. J Neuroinflammation. 2022;19(1):62. doi. 10.1186/s12974-022-02420-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikoo Z, Badihian S, Shaygannejad V, Asgari N, Ashtari F. Comparison of the efficacy of azathioprine and rituximab in neuromyelitis optica spectrum disorder: a randomized clinical trial. J Neurol. 2017;264(9):2003-2009. doi. 10.1007/s00415-017-8590-0 [DOI] [PubMed] [Google Scholar]
- 6.Tahara M, Oeda T, Okada K, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(4):298-306. doi. 10.1016/s1474-4422(20)30066-1 [DOI] [PubMed] [Google Scholar]
- 7.Ciron J, Audoin B, Bourre B, et al. Recommendations for the use of Rituximab in neuromyelitis optica spectrum disorders. Rev Neurol (Paris). 2018;174(4):255-264. doi. 10.1016/j.neurol.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 8.Cohen M, Romero G, Bas J, et al. Monitoring CD27+ memory B-cells in neuromyelitis optica spectrum disorders patients treated with rituximab: results from a bicentric study. J Neurol Sci. 2017;373:335-338. doi. 10.1016/j.jns.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 9.Durozard P, Rico A, Boutiere C, et al. Comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann Neurol. 2020;87(2):256-266. doi. 10.1002/ana.25648 [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim Y, Kim G, et al. Less frequent rituximab retreatment maintains remission of neuromyelitis optica spectrum disorder, following long-term rituximab treatment. J Neurol Neurosurg Psychiatry. 2019;90(4):486-487. doi. 10.1136/jnnp-2018-318465 [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Park NY, Kim KH, Hyun JW, Kim HJ. Rituximab-induced hypogammaglobulinemia and risk of infection in neuromyelitis optica spectrum disorders: a 14-year real-life experience. Neurol Neuroimmunol Neuroinflammation. 2022;9(5):e1179. doi. 10.1212/nxi.0000000000001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy R, Mahévas M, Galicier L, et al. Profound symptomatic hypogammaglobulinemia: a rare late complication after rituximab treatment for immune thrombocytopenia. Report of 3 cases and systematic review of the literature. Autoimmun Rev. 2014;13(10):1055-1063. doi. 10.1016/j.autrev.2014.08.036 [DOI] [PubMed] [Google Scholar]
- 13.Vollmer BL, Wallach AI, Corboy JR, Dubovskaya K, Alvarez E, Kister I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol. 2020;7(9):1477-1487. doi. 10.1002/acn3.51136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perriguey M, Maarouf A, Stellmann JP, et al. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol Neuroimmunol Neuroinflammation. 2022;9(1):e1115. doi. 10.1212/nxi.0000000000001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison EH, Michtich K, Hersh CM. How the COVID-19 pandemic has changed multiple sclerosis clinical practice: results of a nationwide provider survey. Mult Scler Relat Disord. 2021;51:102913. doi. 10.1016/j.msard.2021.102913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870-e1885. doi. 10.1212/wnl.0000000000012753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. doi. 10.1212/wnl.0000000000010380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76. doi. 10.1016/j.jns.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 19.González-Suarez I, Rodríguez de Antonio L, Orviz A, et al. Catastrophic outcome of patients with a rebound after Natalizumab treatment discontinuation. Brain Behav. 2017;7(4):e00671. doi. 10.1002/brb3.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:175628641983780. doi. 10.1177/1756286419837809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Członkowska A, Smoliński Ł, Litwin T. Severe disease exacerbations in patients with multiple sclerosis after discontinuing fingolimod. Neurol Neurochir Pol. 2017;51(2):156-162. doi. 10.1016/j.pjnns.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 22.Fragoso YD, Adoni T, Gomes S, et al. Severe exacerbation of multiple sclerosis following withdrawal of fingolimod. Clin Drug Investig. 2019;39(9):909-913. doi. 10.1007/s40261-019-00804-6 [DOI] [PubMed] [Google Scholar]
- 23.Sacco R, Emming S, Gobbi C, Zecca C, Monticelli S. Rebound of disease activity after fingolimod withdrawal: immunological and gene expression profiling. Mult Scler Relat Disord. 2020;40:101927. doi. 10.1016/j.msard.2020.101927 [DOI] [PubMed] [Google Scholar]
- 24.Boremalm M, Sundström P, Salzer J. Discontinuation and dose reduction of rituximab in relapsing-remitting multiple sclerosis. J Neurol. 2021;268(6):2161-2168. doi. 10.1007/s00415-021-10399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juto A, Fink K, Al Nimer F, Piehl F. Interrupting rituximab treatment in relapsing-remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord. 2020;37:101468. doi. 10.1016/j.msard.2019.101468 [DOI] [PubMed] [Google Scholar]
- 26.Maarouf A, Rico A, Boutiere C, et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflammation. 2020;7(5):e825. doi. 10.1212/nxi.0000000000000825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Jang H, Park NY, et al. Discontinuation of immunosuppressive therapy in patients with neuromyelitis optica spectrum disorder with aquaporin-4 antibodies. Neurol Neuroimmunol Neuroinflammation [Online Serial]. 2021;8(2):e947. doi. 10.1212/NXI.0000000000000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ofsep.org/fr/etudes/nomadmus. Accessed August 17th, 2022.
- 29.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. doi. 10.1212/wnl.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762-772. doi. 10.1016/s1474-4422(21)00218-0 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Hashimoto B, Izaki S, Oji S, Fukaura H, Nomura K. Clinical and immunological differences between MOG associated disease and anti AQP4 antibody-positive neuromyelitis optica spectrum disorders: blood-brain barrier breakdown and peripheral plasmablasts. Mult Scler Relat Disord. 2020;41:102005. doi. 10.1016/j.msard.2020.102005 [DOI] [PubMed] [Google Scholar]
- 32.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614-625. doi. 10.1056/nejmoa1900866 [DOI] [PubMed] [Google Scholar]
- 33.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114-2124. doi. 10.1056/nejmoa1901747 [DOI] [PubMed] [Google Scholar]
- 34.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404-413. doi. 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 35.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857-872. doi. [DOI] [PubMed] [Google Scholar]
- 36.Cabre P, Olindo S, Marignier R, Jeannin S, Merle H, Smadja D. Efficacy of mitoxantrone in neuromyelitis optica spectrum: clinical and neuroradiological study. J Neurol Neurosurg Psychiatry. 2013;84(5):511-516. doi. 10.1136/jnnp-2012-303121 [DOI] [PubMed] [Google Scholar]
- 37.Zéphir H, Bernard-Valnet R, Lebrun C, et al. Rituximab as first-line therapy in neuromyelitis optica: efficiency and tolerability. J Neurol. 2015;262(10):2329-2335. doi. 10.1007/s00415-015-7852-y [DOI] [PubMed] [Google Scholar]
- 38.Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler Houndmills Basingstoke Engl. 2016;22(7):955-959. doi. 10.1177/1352458515602337 [DOI] [PubMed] [Google Scholar]
- 39.Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65(11):1443-1448. [DOI] [PubMed] [Google Scholar]
- 40.Nepal G, Kharel S, Coghlan MA, Rayamajhi P, Ojha R. Safety and efficacy of rituximab for relapse prevention in myelin oligodendrocyte glycoprotein immunoglobulin G (MOG-IgG)-associated disorders (MOGAD): a systematic review and meta-analysis. J Neuroimmunol. 2022;364:577812. doi. 10.1016/j.jneuroim.2022.577812 [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Jeong IH, Hyun JW, et al. Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol. 2015;72(9):989-995. doi. 10.1001/jamaneurol.2015.1276 [DOI] [PubMed] [Google Scholar]
- 42.Kim SH, Huh SY, Lee SJ, Joung A, Kim HJ. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70(9):1110-1117. doi. 10.1001/jamaneurol.2013.3071 [DOI] [PubMed] [Google Scholar]
- 43.Jeong IH, Park B, Kim SH, Hyun JW, Joo J, Kim HJ. Comparative analysis of treatment outcomes in patients with neuromyelitis optica spectrum disorder using multifaceted endpoints. Mult Scler J. 2016;22(3):329-339. doi. 10.1177/1352458515587752 [DOI] [PubMed] [Google Scholar]
- 44.Radaelli M, Moiola L, Sangalli F, et al. Neuromyelitis optica spectrum disorders: long-term safety and efficacy of rituximab in Caucasian patients. Mult Scler Houndmills Basingstoke Engl. 2016;22(4):511-519. doi. 10.1177/1352458515594042 [DOI] [PubMed] [Google Scholar]
- 45.Akaishi T, Takahashi T, Fujihara K, et al. Risk factors of attacks in neuromyelitis optica spectrum disorders. J Neuroimmunol. 2020;343:577236. doi. 10.1016/j.jneuroim.2020.577236 [DOI] [PubMed] [Google Scholar]
- 46.Collongues N, Do Rego CA, Bourre B, et al. Pregnancy in patients with AQP4-ab, MOG-ab or double-negative neuromyelitis optica disorder. Neurology. 2021;96(15):e2006-e2015. doi. 10.1212/WNL.0000000000011744. [DOI] [PubMed] [Google Scholar]
- 47.Klawiter EC, Bove R, Elsone L, et al. High risk of postpartum relapses in neuromyelitis optica spectrum disorder. Neurology. 2017;89(22):2238-2244. doi. 10.1212/wnl.0000000000004681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet Lond Engl. 2019;394(10206):1352-1363. doi. 10.1016/s0140-6736(19)31817-3 [DOI] [PubMed] [Google Scholar]
- 49.Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain J Neurol. 2012;135(6):1834-1849. doi. 10.1093/brain/aws109 [DOI] [PubMed] [Google Scholar]
- 50.Ghadiri F, Eskandarieh S, Sahraian MA, Azimi A, Moghadasi AN. Late-onset neuromyelitis optica spectrum disorder: a case series from Iran. Rev Neurol (Paris). 2022;178(3):249-252. doi. 10.1016/j.neurol.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 51.Nakahara K, Nakane S, Nagaishi A, Narita T, Matsuo H, Ando Y. Very late onset neuromyelitis optica spectrum disorders. Eur J Neurol. 2021;28(8):2574-2581. doi. 10.1111/ene.14901 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pseudonymized data of this study will be available on reasonable written request from any qualified investigator. R codes are provided in supplemental material (eAppendices 1–8, links.lww.com/WNL/C864, links.lww.com/WNL/C865, links.lww.com/WNL/C866, links.lww.com/WNL/C867, links.lww.com/WNL/C868, links.lww.com/WNL/C869, links.lww.com/WNL/C870, links.lww.com/WNL/C871). All patients gave their informed consent, and data collection was approved by the national ethical authority (Commission Nationale de l'Informatique et des Libertés, Registration No. 914066v2).