Abstract
Background and Objectives
Hemispheric surgery effectively treats unihemispheric pediatric drug-resistant epilepsy (DRE) by resecting and/or disconnecting the epileptic hemisphere. Modifications to the original anatomic hemispherectomy have generated multiple functionally equivalent, disconnective techniques for performing hemispheric surgery, termed functional hemispherotomy. While a myriad of hemispherotomy variants exist, all of them can be categorized according to the anatomic plane they are performed in, which includes vertical approaches at or near the interhemispheric fissure and lateral approaches at or near the Sylvian fissure. This meta-analysis of individual patient data (IPD) aimed to compare seizure outcomes and complications between the hemispherotomy approaches to better characterize their relative efficacy and safety in the modern neurosurgical treatment of pediatric DRE, given emerging evidence that outcomes may differ between them.
Methods
CINAHL, Embase, PubMed, and Web of Science were searched from inception to September 9, 2020, for studies reporting IPD from pediatric patients with DRE who underwent hemispheric surgery. Outcomes of interest were seizure freedom at last follow-up, time-to-seizure recurrence, and complications including hydrocephalus, infection, and mortality. The χ2 test compared the frequency of seizure freedom and complications. Multivariable mixed-effects Cox regression controlling for predictors of seizure outcome was performed on propensity score–matched patients to compare time-to-seizure recurrence between approaches. Kaplan-Meier curves were made to visualize differences in time-to-seizure recurrence.
Results
Fifty-five studies reporting on 686 unique pediatric patients treated with hemispheric surgery were included for meta-analysis. Among the hemispherotomy subgroup, vertical approaches resulted in a greater proportion of seizure free patients (81.2% vs 70.7%, p = 0.014) than lateral approaches. While there were no differences in complications, lateral hemispherotomy had higher rates of revision hemispheric surgery due to incomplete disconnection and/or recurrent seizures than vertical hemispherotomy (16.3% vs 1.2%, p < 0.001). After propensity score matching, vertical hemispherotomy approaches independently conferred longer time-to-seizure recurrence than lateral hemispherotomy approaches (hazard ratio 0.44, 95% CI 0.19–0.98).
Discussion
Among functional hemispherotomy techniques, vertical hemispherotomy approaches confer more durable seizure freedom than lateral approaches without compromising safety. Future prospective studies are required to definitively determine whether vertical approaches are indeed superior and how it should influence clinical guidelines for performing hemispheric surgery.
Epilepsy is the most prevalent chronic neurologic condition in the pediatric population, affecting approximately 1/150 children during the first 10 years of life.1 Approximately 17%–35% of these children are refractory to medical treatment and live with persistent seizures that disrupt cognitive development and quality of life (QOL).2,3 Surgery is the mainstay of treatment for drug-resistant epilepsy (DRE), with approximately 80% of surgical patients experiencing seizure freedom and better QOL than those with sustained medical therapy.4 For patients whose DRE etiology is unihemispheric or diffusely multilobar, hemispheric surgery, a procedure that disconnects and/or removes the epileptic hemisphere, has become an effective and safe treatment since its first use for epilepsy halfway through the 20th century.5,6
The evolution of hemispheric surgery for DRE originated with the anatomic hemispherectomy, a technique that resects the entire epileptic hemisphere. It achieved good seizure control but fell out of favor due to high rates of delayed complications including superficial cerebral hemosiderosis and hydrocephalus.7,8 Anatomic hemispherectomy has since undergone modifications that minimize resection to prevent complications. This was first accomplished through a “functionally equivalent” but “anatomically subtotal” hemispherectomy by Rasmussen who retained the frontal and occipital lobes but disconnected them from the corpus callosum and brainstem.9 The success of functional hemispherectomy by Rasmussen in mitigating complications catalyzed a paradigm shift where disconnection is emphasized in lieu of tissue removal.10 Several variations of accomplishing hemispheric disconnection with minimal tissue removal, now termed hemispherotomy, have since been developed and include the vertical parasagittal approach by Delalande et al.,11 the lateral periinsular approach by Villemure and Mascott,12 and the lateral transsylvian approach by Schramm et al.13 Hemispherotomy variants are first line for hemispheric surgery due to comparable seizure outcomes but decreased morbidity.5 While hemispherotomy approaches all include corpus callosotomy, frontobasal, insular, mesial temporal and corona radiata disconnection, fundamental differences intrinsic to the anatomic plane they are performed in (lateral or vertical) exist, thereby allowing them to be categorized accordingly. In lateral approaches, transventricular white matter disconnection occurs through the Sylvian fissure and includes notable removal of mesiotemporal structures including the amygdala, hippocampus, and uncus, whereas dissection is accomplished through the parasagittal plane in vertical approaches with retention of mesiotemporal structures but disconnection at the forniceal columns and floor of the ventricular trigone.7
As seen by the rich history of hemispheric surgery, a wide array of techniques exists; however, it remains unclear whether a superior approach exists, especially between the 2 contemporary hemispherotomy approaches. The comparison of lateral and vertical hemispherotomies originated in 2019 through a multicenter cohort that found no differences in seizure outcome.14 No consensus has been reached, with a study-level meta-analysis also concluding no superiority but a post hoc analysis of a larger, international multi-institutional cohort suggesting that vertical approaches confer more durable seizure freedom.15-17 The meta-analysis was limited by its inability to control for follow-up duration, heterogeneity across studies, and known predictors of seizure outcome, whereas the post hoc study was limited by unequal sample sizes between the approaches and lack of complication data. Thus, the primary objective of this systematic review was to collect high-quality, individual patient data (IPD) to overcome the aforementioned limitations and elucidate whether a superior hemispherotomy approach exists regarding efficacy and safety. Given the paradigm shift from resective to disconnective techniques, our secondary objective was to evaluate whether the transition is justified by comparing seizure outcomes and complications and characterize indications and considerations for older resective techniques, notably anatomic hemispherectomy, in contemporary neurosurgical practice.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study is reported according to PRISMA guidelines (eMethods, links.lww.com/WNL/C829).18 The study protocol was not registered a priori. No funding was received. Institutional review board approval and informed consent were waived, given the public, deidentified nature of our data.
Search Strategy
CINAHL, Embase, PubMed, and Web of Science were systematically searched from inception to September 9, 2020, for articles that report outcomes for individual pediatric patients who underwent hemispheric surgery for DRE. The search strategy was designed using permutations of the following terms: “hemispherectomy/hemispherotomy,” “epilepsy,” and “outcomes.” The complete search strategy and its results are detailed in the eMethods (links.lww.com/WNL/C829).
Study Selection
Queried articles were reviewed for inclusion by 3 authors (J.C., W.B.H., K.J.W.) in the Covidence systematic review software (Veritas Health Innovation). Duplicate articles were consolidated. Titles and abstracts of unique articles were screened for relevance. Relevant articles underwent a full-text review using the eligibility criteria described further. Screening and full-text review were performed independently by 2 authors.
Study Eligibility Criteria
Inclusion and exclusion criteria were established a priori. A study was eligible for data extraction if all the following applied: (1) used case-control, cohort, or randomized controlled trial study design; (2) >80% of the study cohort is pediatric patients with DRE (age at surgery, younger than 21 years), (3) report, in English, individual seizure outcomes and/or surgical complications of patients who underwent hemispheric surgery and type of hemispheric surgery each patient underwent.
Studies were excluded if any of the following applied: (1) is a case report, meta-analysis, or review article, (2) <80% of cohort are pediatric patients with DRE, or (3) does not report seizure outcomes and complications after hemispheric surgery or type of hemispheric surgery performed for individual patients. Studies where individual data were reported only for hemispheric surgeries with specific outcomes such as failed and/or revision cases, patients with remarkable seizure outcomes, and palliative surgeries, for example, were also excluded to prevent introducing biased outcomes for or against an approach.
Data Extraction
IPD was abstracted by 2 authors (J.C., K.J.W.). Each patient had the following datapoints extracted when available: sex, age at seizure onset and surgery, seizure semiology, epilepsy etiology, EEG, MRI and PET findings, type and side of hemispheric surgery, complications including hydrocephalus, need for CSF-shunting, infection, and death, seizure recurrence/freedom at last follow-up, time-to-seizure recurrence, if applicable, and last follow-up, need for revision hemispheric surgery, and cognitive development, as defined by the authors, or total, verbal, and performance IQ. All corresponding authors of included studies were contacted for missing data. IPD from different studies but identical institutions were compared to identify duplicates. When duplicates were found, the record with longer follow-up was retained, while the other was removed.19 Individual patients were removed if they did not undergo hemispheric surgery or if the hemispheric surgery type, outcomes, or follow-up time were still missing. Time-to-seizure recurrence was estimated as half the follow-up duration if not provided.19 Hemispheric surgery types include anatomic hemispherectomy, Rasmussen functional hemispherectomy, and hemispherotomy, with the latter 2 also categorized as functional hemispherectomy. For hemispherotomies, the approach (lateral or vertical) was recorded, whenever possible. Endoscopic-assisted hemispherotomies were excluded. Classification of hemispheric surgery type and approach was confirmed by both senior authors with technical expertise (A.G.W., A.F.).
Statistical Analysis
All statistical analyses were performed in RStudio (version 1.2.1335; RStudio, Inc., Boston, MA). Continuous variables were summarized with median values and interquartile ranges and compared through the Mann-Whitney U test. Categorical variables were reported using frequencies and proportions and compared through the χ2 test. Kaplan-Meier curves with log-rank test were constructed to illustrate differences in time-to-seizure recurrence. A 2-tailed p value <0.05 was the threshold for statistical significance. Multiple imputation by chained equations was performed to handle missing data in variables needed to calculate the Hemispherectomy Outcome Prediction Scale (HOPS) score, which includes age at seizure onset, generalized seizure semiology, stroke etiology, and PET findings.20,21 Multiple imputation was performed only when <40% of the aforementioned data variables were missing.22 The HOPS score is a validated metric that uses clinical attributes with prognostic value to estimate seizure freedom likelihood in patients undergoing hemispherectomy.21 The HOPS variables were used to perform propensity score matching to mitigate exposure selection bias and generate comparable subgroups for regression analysis.23 Given limitations of propensity score matching on small samples, many-to-one matching was performed as needed to obtain a minimum sample of 200 patients while not exceeding a 5:1 ratio to avoid increasing bias in the treatment effect.24-26 Multivariable mixed-effects Cox regression, with the study that the patient originated from as the random-effects variable and the surgical technique and HOPS score as covariates, was performed to determine whether different hemispherectomy types and approaches were independently associated with differences in time-to-seizure recurrence. A Gaussian distribution was assumed for the random-effects variable. The HOPS score was controlled for as a fixed-effects variable to further minimize confounding effects of baseline differences in characteristics associated with seizure outcome, which are present due to our nonrandomized study design. Estimated parameters and standard errors from regression analysis were combined through the Rubin rule.27 Hazard ratios (HRs) and 95% CIs were calculated to summarize regression results.
Quality Appraisal and Sensitivity Analysis
The quality and risk of bias was evaluated by 2 authors (J.C., K.J.W.) using the Newcastle-Ottawa Scale (NOS).28 The NOS generates a score out of 9 based on cohort selection, comparability of participants, and adequacy of outcome metrics to determine whether a study is poor (0–3), fair (4–6), or good (7+) quality. Because time-to-seizure recurrence was estimated from follow-up time when not provided, sensitivity analyses were performed by removing patients with an estimated time-to-seizure recurrence and repeating analyses on the subgroup without estimated time-to-seizure recurrence to determine whether the estimation influenced the treatment effect.
Data Availability
Datasets from this study will be made available by the corresponding author on reasonable request.
Results
Study and Patient Selection
The study selection process is detailed in Figure 1. From 1959 initial citations, IPD for 1253 patients was sought from 62 articles. However, 7 articles ultimately did not contribute IPD because they failed to report the hemispherectomy type (n = 3),29-31 follow-up time (n = 3),32-34 or both35 and were removed. Eighty-three patients from 8 articlese1,e3,e24,e26,e30,e36,e37,e52 were duplicates from other studiese4,e26,e28,e30,e31,e39,e53 and removed. The final cohort included 686 unique pediatric patients who underwent a specified hemispheric surgery procedure for DRE and had reported seizure outcomes and follow-up duration.
Figure 1. PRISMA IPDMA Flow Diagram of Search Results and Study Selection.
IPD = individual patient data; IPDMA = IPD meta-analysis; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study Characteristics
Characteristics of the included studies, stratified by which continent their affiliated institution is located in, are summarized in Table 1. Included studies were published between 1996 and 2020, spanning 5 continents (North America (n = 23),e1-e23 Europe (n = 16),e24-e39 Asia (n = 12),e40-e51 South America (n = 3),e52-e54 and Oceania (n = 1)e55) and 36 institutions. Hemispherotomy was the most popular procedure, with all but 2 institutions (94.4%) reporting its utilization. North and South American institutions used only lateral hemispherotomy, while European and Asian institutions used both lateral and vertical approaches. European institutions had the greatest seizure freedom rate at the last follow-up (76.8%) and longest average follow-up.
Table 1.
Summary Characteristics of Studies Included in the Individual Patient Data Meta-analysis Categorized by the Continental Location of Each Study's Institution
According to the NOS, most studies were of “fair” quality (80%), with a mean score of 5.5 across all studies (eTable 1, links.lww.com/WNL/C829). Nine (16.4%) were of “good” quality while 2 were “poor.” When studies had a high risk of bias, it was generally because their cohort lacked diversity among DRE etiologies often due to a bias for or study focus on a specific pathology. European studies had a higher quality because they reported using multiple techniques more frequently, thus improving their data's generalizability.
Cohort Characteristics
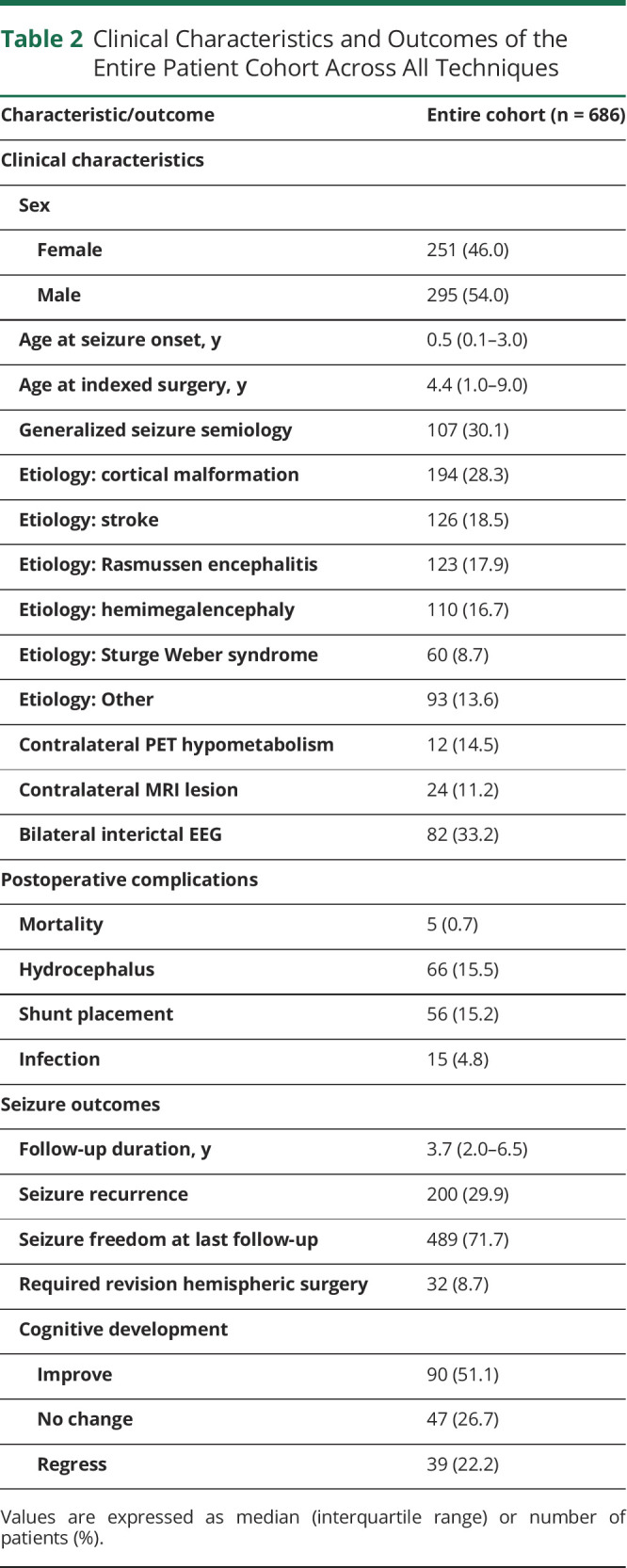
Clinical characteristics and outcomes of the entire cohort are reported in Table 2. The median age of surgery was 4.4 years. The most common etiology was cortical malformation (28.3%), followed by stroke (18.5%), Rasmussen encephalitis (17.9%), and hemimegalencephaly (16.7%). Mortality occurred in 5 (0.7%) cases, while hydrocephalus and shunting for CSF diversion represented 15.5% and 15.2% of the cohort, respectively. Patients were followed up for a median of 3.7 years. During follow-up, 200 (29.9%) had seizure recurrence. At the last follow-up, 489 (71.7%) were seizure-free, and 137 (77.8%) had stable or improved cognition. Fourteen (2.1%) had seizure recurrence postoperatively but were able to achieve seizure freedom by their last follow-up.
Table 2.
Clinical Characteristics and Outcomes of the Entire Patient Cohort Across All Techniques
Hemispherotomy Subgroup Characteristics and Regression Analysis
Characteristics and outcomes of the hemispherotomy subgroup stratified by approach are summarized in Table 3. Lateral approaches were performed in 287 cases, while vertical approaches were used for 193. Overall, the approaches were comparable across sex, age at seizure onset and surgery, seizure semiology, EEG findings and imaging. However, vertical approaches were performed more frequently for DRE attributed to strokes (23.8% vs 16.0%, p = 0.044), while lateral approaches were used more frequently for uncommon etiologies classified as “other” (19.9% vs 7.3%, p < 0.001), which includes encephalitis, hemorrhage, trauma, and various syndromes. There were no differences in complications; however, patients treated with vertical approaches had lower seizure recurrence rates (20.9% vs 30.3%, p = 0.030) and higher seizure freedom rates at the last follow-up (81.2% vs 70.7%, p = 0.014). The number needed to treat (NNT) with vertical hemispherotomy to achieve an additional case of seizure freedom is 9.5. Vertical hemispherotomy required fewer revision operations (1.2% vs 16.3%, p < 0.001) despite a longer follow-up (4.0 vs 3.8 years, p = 0.029). Postoperative cognitive development was similar between approaches, with most improving or maintaining their preoperative baseline.
Table 3.
Clinical Characteristics and Outcomes of the Hemispherotomy Cohort Stratified by Lateral vs Vertical Approaches
The multivariable mixed-effects Cox regression for hemispherotomy patients is detailed in Table 4. After 1:1 propensity matching and multiple imputation of 19.4% of the data, vertical approaches were independently associated with longer duration of postoperative seizure freedom than lateral approaches (HR 0.44, 95% CI 0.19–0.98). The proportion of patients with DRE due to stroke after propensity matching in the vertical and lateral cohorts was 24.2% and 21.3%, respectively. The standardized mean difference (SMD) across all variables used in propensity score matching between vertical and lateral cohorts before and after matching is illustrated in the Love plot in eFigure 1 (links.lww.com/WNL/C829). All variables had an SMD <0.10 after matching. Kaplan-Meier analysis comparing techniques was consistent with regression modeling and demonstrated longer time-to-seizure recurrence (log-rank p = 0.005) with vertical approaches (Figure 2).
Table 4.
Multivariable Mixed-Effects Cox Regression Analysis With Propensity Score Matching on the Hemispherotomy Cohort While Controlling for Hemispherotomy Technique and HOPS Score to Identify Predictors of Time to Seizure Recurrence

Figure 2. Comparison of Kaplan-Meier Curves Depicting the Seizure Freedom Functions for Patients Treated With Lateral Hemispherotomy and Vertical Hemispherotomy.
Hemispherectomy Subgroup Characteristics and Regression Analysis
Characteristics and outcomes of the cohort stratified by anatomic and functional hemispherectomies are reported in Table 5. The anatomic group was younger at seizure onset (0.3 vs 0.6 years, p = 0.007) and surgery (1.3 vs 5.0 years, p < 0.001). The anatomic group had more cases with hemimegalencephaly (42.6% vs 14.1%, p < 0.001) but were comparable across other etiologies, seizure semiology, and imaging and EEG findings. Anatomic cases had higher postoperative hydrocephalus (45.2% vs 12.2%, p < 0.001) and CSF-shunting (50.0% vs 11.2%, p < 0.001) rates but were comparable in all other complications. While anatomic cases had a longer follow-up (9.6 vs 3.5 years, p < 0.001), rates of seizure recurrence (24.6% vs 30.4%, p = 0.427) and freedom (78.7% vs 71.0%, p = 0.262) were comparable. No differences in time-to-seizure recurrence were observed on Cox regression after 1:4 propensity matching and multiple imputation of 19% of the data (HR 2.89, 95% CI 0.88–9.56).
Table 5.
Clinical Characteristics and Outcomes of Entire Patient Cohort Stratified by Anatomic vs Functional Approaches
Sensitivity Analysis
To assess the validity of our findings, multivariable Cox regression was reperformed after removing patients with estimated time-to-seizure recurrence. Results are summarized in eTable 2 (links.lww.com/WNL/C829). Recomparison of lateral and vertical hemispherotomies was concordant with initial analyses, with vertical hemispherotomy independently conferring longer seizure freedom in Cox (HR 0.09, 95% CI 0.01–0.97) and Kaplan-Meier analyses (log-rank p = 0.003) (eFigure 2). Sensitivity analysis comparing anatomic and functional hemispherectomies was not possible due to a limited sample of anatomic hemispherectomy patients (n = 18).
Discussion
This is an IPD meta-analysis (IPDMA) and rigorous comparison of hemispheric surgery techniques for pediatric DRE. Our primary objective was to compare hemispherotomy approaches. After propensity score matching and controlling for predictors of seizure outcome, we showed that vertical approaches are independently associated with more durable seizure freedom than lateral approaches.
Contrary to the study-level meta-analysis by Cossu et al.15 that showed no difference in outcomes between hemispherotomy approaches, our IPDMA found that patients treated with vertical approaches had less seizure recurrence requiring revision surgery, higher seizure freedom rates at the last follow-up, and longer time-to-seizure recurrence than lateral approaches. Our findings agree with the post hoc study from our group that demonstrated long-term seizure freedom advantages for vertical hemispherotomy when compared with lateral approaches. The superiority of vertical approaches is especially evident when comparing seizure freedom rates from Kaplan-Meier curves. In the sensitivity analysis of this study, 5-year and 10-year seizure freedom rates were 90.7% and 85.5%, respectively, for vertical approaches, which were significantly higher than 76.6% and 59.0% for lateral approaches. These rates are consistent with those observed in the study conducted by Fallah et al.,16 which observed 5-year and 10-year seizure freedom rates of 85.5% for vertical approaches and 72.1% and 50.6% for lateral approaches, respectively. A letter by Bourdillon et al.17 responding to the post hoc study used preliminary data to support the findings favoring vertical hemispherotomy and reported a 10-year seizure freedom rate of 78.3% for 317 patients who underwent vertical hemispherotomy. However, the lack of significant differences across complications in our hemispherotomy cohort concurs with that in the study conducted by Cossu et al., suggesting that both approaches are equally safe.
The longer time-to-seizure recurrence associated with vertical hemispherotomy coupled with rates of seizure freedom that overlap with hemispherotomy cohorts from other studies with long-term follow-up is highly suggestive of a clinical benefit when using vertical approaches over lateral approaches. While Cossu et al. concluded otherwise, our analysis overcame their limitation of heterogeneity across follow-up and cohort demographics and increased confidence that the observed difference is attributed to technique by performing propensity score matching to create comparable cohorts. Propensity score matching is a statistical technique that attempts to approximate a randomized trial design by balancing potentially confounding variables across cohorts being compared. If performed properly, propensity score matching reduces the biases inherent in retrospective analyses and strengthens the likelihood that results are due to qualities of the intervention, not the cohort. SMD is the standard for evaluating the balance of variables between matched groups.36 The SMDs after matching across all variables used in propensity score matching between the vertical and lateral cohorts were <0.10, which is the threshold for declaring balance.36,37 This is notable because at baseline, the vertical group has significantly more patients with DRE due to stroke, which is known to respond more favorably to hemispherotomy.21 Thus, by creating balanced cohorts, we can conclude that better outcomes with vertical approaches are more likely to be explained by the technique and not because the group had more patients who were more likely to respond to surgery. Furthermore, we controlled for predictors of seizure outcome in a time-to-event analysis unlike Cossu et al. and overcame limitations of the post hoc HOPS analysis by accruing a larger sample of vertical approaches and complication data. These elements improved our ability to make conclusions on differences in efficacy between approaches and their clinical implications.
It remains unclear why vertical approaches have superior efficacy relative to lateral approaches because explanatory data for why seizure recurrence occurred (e.g., postoperative imaging) was unavailable. However, it has been hypothesized that a complete disconnection is more feasible through vertical approaches.16 While increased completeness of disconnection in vertical approaches has not been formally demonstrated, we were able to indirectly substantiate this hypothesis by collecting IPD on revision hemispheric surgery, which is commonly indicated by an incomplete disconnection and thus a reasonable surrogate. On analysis, we found that lateral approaches had significantly higher rates of revision surgery than vertical approaches. While this could be due to differences in clinical practice whereby surgeons who perform lateral approaches are more amenable to exploratory surgery for recurrent seizures, it also suggests that vertical approaches inherently enable a more thorough disconnection. One possible source of incomplete disconnection is the frontobasal disconnection.38 In lateral approaches, the sphenoid ridge may be erroneously used as a landmark to guide the frontobasal disconnection with the junction of A1 and A2 being the posterior limit, which creates the possibility of leaving the posterior third of the fronto-orbital cortex and its residual frontobasal connections intact.39 This differs from vertical approaches, which first resect the posterior gyrus rectus. This step may portend improved visualization and operator room for visual confirmation of desired disconnections, including the amygdala, anterior temporal lobe, and frontal lobe.11 An alternate source of variability may arise from differences in access and visualization of the hemisphere's central core consisting of the extreme external and internal capsules, claustrum, lentiform and caudate nucleus, and thalamus, as proposed by Wen et al.39 In lateral approaches, the central core is accessed indirectly through the insula and lateral ventricle with potential obstruction by the surrounding frontal, parietal, and temporal opercula. Conversely, incisions around the central core in vertical approaches are directly realized and thus more accurately assessed for completeness.11,39 Differences in the intraoperative management of the insular cortex are also noteworthy because residual insular tissue is highly correlated with persistent postoperative seizures.40 Interruption of associative neuronal fibers through a perithalamic incision vertically extending from the trigone to the most anterior part of the temporal horn in vertical approaches mirror anatomic hemispherectomy and enable complete separation of the insular cortex. Conversely, lateral approaches require insular resection, often in a semiblind piecemeal fashion, or leave the insula unresected, both of which increase the likelihood of retaining epileptogenic insular tissue.11 The perithalamic incision that disconnects the basal ganglia in vertical approaches may also isolate dysplastic neurons that failed to migrate from the subventricular zone to the cortex in congenital malformation cases.11,41,42
The mounting independent evidence that suggests superior efficacy with vertical approaches creates a clinical dilemma for how epilepsy neurosurgeons should perform hemispherotomies moving forward.16,17 Traditionally, an epilepsy neurosurgeon trains in 1 approach, each possessing a unique learning curve that does not directly translate to executing the other without additional training. Thus, it may be impractical and ill-advised to expect pediatric epilepsy senior neurosurgeons who have established their practice performing lateral approaches to suddenly transition to vertical approaches, given that high QOL and patient satisfaction is currently attainable with lateral hemispherotomy and the NNT with vertical hemispherotomy (9.5) is on the border of what experts consider a practice-redefining NNT (<10).43,44 However, our findings suggest that undifferentiated neurosurgery trainees should consider learning both lateral and vertical approaches to be prepared to accommodate any future recommendations if further investigation determines that there is definitive evidence to perform one approach over the other. At the very least, even if future studies determine that neither approach is inferior, training in both approaches still has potential to further improve patient outcomes and pioneer the evolution of how hemispheric surgery is performed by allowing surgeons to select which hemispherotomy to perform according to the patient's specific neuroanatomy and leverage the different advantages of each approach. This recommendation does not come without challenges, given the geographic trends for hemispherotomy approaches, as highlighted by our study. However, it is becoming more feasible to learn new techniques in this era with published technical descriptions, surgical videos, and multidisciplinary international conferences on the rise.45,46 Randomized expertise-based prospective studies are required to definitively determine whether our recommendation is substantiated.16
Regarding our analysis of anatomic vs functional approaches, the statistically insignificant difference in seizure outcomes and a higher rate of hydrocephalus after anatomic hemispherectomy is consistent with prior reports.5 While our study did not demonstrate new knowledge regarding seizure outcomes and complications of anatomic hemispherectomy, our analysis shows that it still has a niche in this modern era dominated by functional approaches. In our cohort, anatomic hemispherectomy was predominantly used to treat developmental epilepsy etiologies, specifically hemimegalencephaly, and younger patients. Hemimegalencephaly is among the most technically difficult etiologies to treat with hemispheric surgery, possessing the lowest seizure freedom rate among all conditions.47 This was seen in our functional cohort, with 60.2% of cases with hemimegalencephaly seizure-free, whereas 80.8% of cases with anatomic hemimegalencephaly were seizure-free. Functional hemispherectomy may be severely limited by anatomical distortions of the malformed hemisphere that preclude safe and complete disconnection.48 Furthermore, 26.9% of cases with functional hemimegalencephaly required revision surgery, whereas no anatomic cases needed reoperation. Given this, anatomic hemispherectomy may also be preferable for families who hope to avoid multiple surgeries. Overall, anatomic hemispherectomy still has an important role in treating DRE because it may be used to treat etiologies such as hemimegalencephaly and younger children more effectively or mitigate the risk of requiring multiple operations. However, given the tradeoff of increased hydrocephalus risk, consideration of patient/family preferences and values is essential when anatomic hemispherectomy may be indicated.
Our study had several notable strengths and limitations that we offset to the best of our ability. Heterogeneity in patient characteristics and follow-up are limitations of not only prior studies on this topic but also traditional meta-analyses and nonrandomized cohort studies in general. We minimized these factors by propensity score matching to generate comparable cohorts, controlling for HOPS score to assess the effect of technique independent of other prognostic factors and performing Cox regression to evaluate outcomes on a time-to-event basis, which appropriately weighs a seizure-free event relative to follow-up duration. Our analysis was unable to control for the expertise of each surgeon; thus, it is possible that once surgeons overcome the learning curve for either technique, the outcomes between them become comparable. However, we attempted to mitigate this factor by creating a mixed-effects model with the source study as a random-effects variable to account for potential differences between authors.
Explanations for the differential effect between techniques were explored and substantiated to a certain degree through differences in revision hemispherectomy rates. This was a limitation of the post hoc study that we overcame. Furthermore, this is the first meta-analysis that evaluated cognitive outcomes in conjunction with surgical technique. However, given the anecdotal nature of hypotheses highlighted in our discussion, the exact mechanism benefitting vertical approaches remains unclear. Studies geared towards understanding patient selection differences, post-operative structural imaging as well as tractography for each approach are necessary to understand the reason behind differences in seizure freedom. In addition, while we collected cognitive outcomes, the data were not granular and summarized the broad spectrum of cognition. Thus, our conclusions require further investigation to determine whether deficits not captured by IQ manifest for specific approaches.
As with all meta-analyses, this IPDMA was subject to traditional limitations of potentially not identifying relevant studies because of screening errors or inappropriate indexing. In addition, data abstraction is susceptible to biases of the abstractor and misinterpretation. One notable example and limitation is our estimation of time-to-seizure recurrence using follow-up duration when unavailable. While this should not have introduced systematic bias between cohorts because it provides a standardized and reasonable estimate to all applicable patients, we still performed a sensitivity analysis using only verified data, which reaffirmed our initial conclusions and the fact that no systematic bias should have resulted from the assumption. Furthermore, we believe that our rigorous inclusion/exclusion criteria for patients and studies were able to identify data points genuinely capable of answering our proposed question. This is observed in our large number of excluded patients (n = 567) and the overall high quality of included studies according to NOS. However, future studies should ensure that their cohort spans the diverse spectrum of epilepsy etiologies because that was a notable source of bias across many included studies.
Hemispheric surgery is an effective treatment for pediatric DRE that has undergone many modifications since it was introduced. Among hemispherotomy variants, vertical approaches have greater seizure freedom rates and duration than lateral approaches but equivalent safety. Future, well-designed, expertise-based prospective studies are required to definitively determine whether, and why, superiority exists between approaches to further inform the clinical practice of hemispherotomy.
Glossary
- DRE
drug-resistant epilepsy
- HOPS
Hemispherectomy Outcome Prediction Scale
- IPD
individual patient data
- IPDMA
IPD meta-analysis
- NNT
number needed to treat
- NOS
Newcastle-Ottawa Scale
- QOL
quality of life
- SMD
standardized mean difference.
Appendix. Authors

Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Aaberg KM, Gunnes N, Bakken IJ, et al. Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics. 2017;139(5):e20163908. doi: 10.1542/peds.2016-3908 [DOI] [PubMed] [Google Scholar]
- 2.Sultana B, Panzini MA, Veilleux Carpentier A, et al. Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology. 2021;96(17):805-817. doi: 10.1212/wnl.0000000000011839 [DOI] [PubMed] [Google Scholar]
- 3.Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia. 2000;41(4):426-431. doi: 10.1111/j.1528-1157.2000.tb00184.x [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639-1647. doi: 10.1056/nejmoa1615335 [DOI] [PubMed] [Google Scholar]
- 5.Griessenauer CJ, Salam S, Hendrix P, et al. Hemispherectomy for treatment of refractory epilepsy in the pediatric age group: a systematic review. J Neurosurg Pediatr. 2015;15(1):34-44. doi: 10.3171/2014.10.peds14155 [DOI] [PubMed] [Google Scholar]
- 6.Krynauw RA. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13(4):243-267. doi: 10.1136/jnnp.13.4.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahuleyan B, Robinson S, Nair AR, Sivanandapanicker JL, Cohen AR. Anatomic hemispherectomy: historical perspective. World Neurosurg. 2013;80(3-4):396-398. doi: 10.1016/j.wneu.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 8.Oppenheimer DR, Griffith HB. Persistent intracranial bleeding as a complication of hemispherectomy. J Neurol Neurosurg Psychiatry. 1966;29(3):229-240. doi: 10.1136/jnnp.29.3.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10(2):71-78. doi: 10.1017/s0317167100044668 [DOI] [PubMed] [Google Scholar]
- 10.Tinuper P, Andermann F, Villemure JG, Rasmussen TB, Quesney LF. Functional hemispherectomy for treatment of epilepsy associated with hemiplegia: rationale, indications, results, and comparison with callosotomy. Ann Neurol. 1988;24(1):27-34. doi: 10.1002/ana.410240107 [DOI] [PubMed] [Google Scholar]
- 11.Delalande O, Bulteau C, Dellatolas G, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Oper Neurosurg. 2007;60(2):ONS19-ONS32; discussion ONS32. doi: 10.1227/01.neu.0000249246.48299.12 [DOI] [PubMed] [Google Scholar]
- 12.Villemure JG, Mascott CR. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37(5):975-981. doi: 10.1097/00006123-199511000-00018 [DOI] [PubMed] [Google Scholar]
- 13.Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49(4):891-901. doi: 10.1097/00006123-200110000-00021 [DOI] [PubMed] [Google Scholar]
- 14.de Palma L, Pietrafusa N, Gozzo F, et al. Outcome after hemispherotomy in patients with intractable epilepsy: comparison of techniques in the Italian experience. Epilepsy Behav. 2019;93:22-28. doi: 10.1016/j.yebeh.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 15.Cossu M, Nichelatti M, De Benedictis A, Rizzi M; Commission for Epilepsy Surgery of the Italian League Against Epilepsy LICE. Lateral versus vertical hemispheric disconnection for epilepsy: a systematic review and meta-analysis. J Neurosurg. 2021;2021:1-11. doi: 10.3171/2021.5.JNS21949 [DOI] [PubMed] [Google Scholar]
- 16.Fallah A, Lewis E, Ibrahim GM, et al. Comparison of the real-world effectiveness of vertical versus lateral functional hemispherotomy techniques for pediatric drug-resistant epilepsy: a post hoc analysis of the HOPS study. Epilepsia. 2021;62(11):2707-2718. doi: 10.1111/epi.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourdillon P, Bulteau C, Dorfmuller G, Ferrand-Sorbets S. Vertical hemispherotomy for drug-resistant epilepsy: toward confirmation of the HOPS study. Epilepsia. 2021;62(12):3150-3151. doi: 10.1111/epi.17111 [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(9):2018-2030. doi: 10.1093/brain/awh221 [DOI] [PubMed] [Google Scholar]
- 20.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1-67. [Google Scholar]
- 21.Weil AG, Lewis EC, Ibrahim GM, et al. Hemispherectomy Outcome Prediction Scale: development and validation of a seizure freedom prediction tool. Epilepsia. 2021;62(5):1064-1073. doi: 10.1111/epi.16861 [DOI] [PubMed] [Google Scholar]
- 22.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials: a practical guide with flowcharts. Bmc Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8). doi: 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 24.Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med. 2012;31(7):681-697. doi: 10.1002/sim.4168 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172(9):1092-1097. doi: 10.1093/aje/kwq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shadish WR. Propensity score analysis: promise, reality and irrational exuberance. J Exp Criminol. 2013;9(2):129-144. doi: 10.1007/s11292-012-9166-8 [DOI] [Google Scholar]
- 27.Rubin D. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2004. [Google Scholar]
- 28.Wells GA, Shea B, O'Connel D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa Hospital Research Institute; 2016. [Google Scholar]
- 29.Doring S, Cross H, Boyd S, Harkness W, Neville B. The significance of bilateral EEG abnormalities before and after hemispherectomy in children with unilateral major hemisphere lesions. Epilepsy Res. 1999;34(1):65-73. doi: 10.1016/s0920-1211(98)00101-6 [DOI] [PubMed] [Google Scholar]
- 30.van Empelen R, Jennekens-Schinkel A, Gorter JW, Volman MJM, van Nieuwenhuizen O, Helders PJM. Epilepsy surgery does not harm motor performance of children and adolescents. Brain. 2005;128(7):1536-1545. doi: 10.1093/brain/awh499 [DOI] [PubMed] [Google Scholar]
- 31.van Empelen R, Jennekens-Schinkel A, van Rijen PC, Helders PJ, van Nieuwenhuizen O. Health-related quality of life and self-perceived competence of children assessed before and up to two years after epilepsy surgery. Epilepsia. 2005;46(2):258-271. doi: 10.1111/j.0013-9580.2005.27304.x [DOI] [PubMed] [Google Scholar]
- 32.Flack S, Ojemann J, Haberkern C. Cerebral hemispherectomy in infants and young children. Pediatr Anesth. 2008;18(10):967-973. doi: 10.1111/j.1460-9592.2008.02713.x [DOI] [PubMed] [Google Scholar]
- 33.Scavarda D, Major P, Lortie A, Mercier C, Carmant L. Periinsular hemispherotomy in children with stroke-induced refractory epilepsy. J Neurosurg Pediatr. 2009;3(2):115-120. doi: 10.3171/2008.11.peds08218 [DOI] [PubMed] [Google Scholar]
- 34.Kasasbeh A, Hwang EC, Steger-May K, et al. Association of magnetic resonance imaging identification of mesial temporal sclerosis with pathological diagnosis and surgical outcomes in children following epilepsy surgery. J Neurosurg Pediatr. 2012;9(5):552-561. doi: 10.3171/2012.1.peds11447 [DOI] [PubMed] [Google Scholar]
- 35.Loddenkemper T, Holland KD, Stanford LD, Kotagal P, Bingaman W, Wyllie E. Developmental outcome after epilepsy surgery in infancy. Pediatrics. 2007;119(5):930-935. doi: 10.1542/peds.2006-2530 [DOI] [PubMed] [Google Scholar]
- 36.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8):S84-S90.e1. doi: 10.1016/j.jclinepi.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Kim HJ, Lonjon G, Zhu Y, written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7(1):16. doi: 10.21037/atm.2018.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Guan Y, Liu C, et al. Treatment for patients with recurrent intractable epilepsy after primary hemispherectomy. Epilepsy Res. 2018;139:137-142. doi: 10.1016/j.eplepsyres.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 39.Wen HT, Rhoton AL, Marino R. Anatomical landmarks for hemispherotomy and their clinical application. J Neurosurg. 2004;101(5):747-755. doi: 10.3171/jns.2004.101.5.0747 [DOI] [PubMed] [Google Scholar]
- 40.Cats EA, Kho KH, van Nieuwenhuizen O, van Veelen CWM, Gosselaar PH, van Rijen PC. Seizure freedom after functional hemispherectomy and a possible role for the insular cortex: the Dutch experience. J Neurosurg Pediatr. 2007;107(4):275-280. doi: 10.3171/ped.2007.107.4.275 [DOI] [PubMed] [Google Scholar]
- 41.Dorfer C, Czech T, Dressler A, et al. Vertical perithalamic hemispherotomy: a single-center experience in 40 pediatric patients with epilepsy. Epilepsia. 2013;54(11):1905-1912. doi: 10.1111/epi.12394 [DOI] [PubMed] [Google Scholar]
- 42.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703-716. doi: 10.1016/s0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- 43.Verdinelli C, Olsson I, Edelvik A, Hallbook T, Rydenhag B, Malmgren K. A long-term patient perspective after hemispherotomy: a population based study. Seizure. 2015;30:76-82. doi: 10.1016/j.seizure.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 44.Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407-411. doi: 10.1111/ijcp.12142 [DOI] [PubMed] [Google Scholar]
- 45.Baumgartner JE, Blount JP, Blauwblomme T, Chandra PS. Technical descriptions of four hemispherectomy approaches: from the Pediatric Epilepsy Surgery Meeting at Gothenburg 2014. Epilepsia. 2017;58:46-55. doi: 10.1111/epi.13679 [DOI] [PubMed] [Google Scholar]
- 46.Wagner K, Vaz-Guimaraes F, Camstra K, Lam S. Endoscope-assisted hemispherotomy: translation of technique from cadaveric anatomical feasibility study to clinical implementation. J Neurosurg Pediatr. 2019;23(2):178-186. doi: 10.3171/2018.8.peds18349 [DOI] [PubMed] [Google Scholar]
- 47.Kossoff EH, Vining EP, Pillas DJ, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology. 2003;61(7):887-890. doi: 10.1212/01.wnl.0000090107.04681.5b [DOI] [PubMed] [Google Scholar]
- 48.Di Rocco C, Battaglia D, Pietrini D, Piastra M, Massimi L. Hemimegalencephaly: clinical implications and surgical treatment. Childs Nerv Syst. 2006;22(8):852-866. doi: 10.1007/s00381-006-0149-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets from this study will be made available by the corresponding author on reasonable request.







