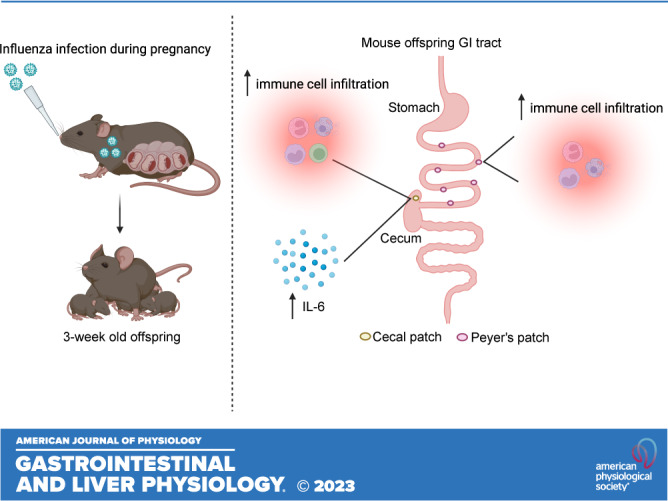
Keywords: cecal patch, inflammation, influenza A virus, offspring development, Peyer’s patches
Abstract
Maternal influenza A virus (IAV) infection during pregnancy can affect offspring immune programming and development. Offspring born from influenza-infected mothers are at increased risk of neurodevelopmental disorders and have impaired respiratory mucosal immunity against pathogens. The gut-associated lymphoid tissue (GALT) represents a large proportion of the immune system in the body and plays an important role in gastrointestinal (GI) homeostasis. This includes immune modulation to antigens derived from food or microbes, gut microbiota composition, and gut-brain axis signaling. Therefore, in this study, we investigated the effect of maternal IAV infection on mucosal immunity of the GI tract in the offspring. There were no major anatomical changes to the gastrointestinal tract of offspring born to influenza-infected dams. In contrast, maternal IAV did affect the mucosal immunity of offspring, showing regional differences in immune cell profiles within distinct GALT. Neutrophils, monocytes/macrophages, CD4+ and CD8+ T cells infiltration was increased in the cecal patch offspring from IAV-infected dams. In the Peyer’s patches, only activated CD4+ T cells were increased in IAV offspring. IL-6 gene expression was also elevated in the cecal patch but not in the Peyer’s patches of IAV offspring. These findings suggest that maternal IAV infection perturbs homeostatic mucosal immunity in the offspring gastrointestinal tract. This could have profound ramifications on the gut-brain axis and mucosal immunity in the lungs leading to increased susceptibility to respiratory infections and neurological disorders in the offspring later in life.
NEW & NOTEWORTHY Influenza A virus (IAV) infection during pregnancy is associated with changes in gut-associated lymphoid tissue (GALT) in the offspring in a region-dependent manner. Neutrophils and monocytes/macrophages were elevated in the cecal patch of offspring from infected dams. This increase in innate immune cell infiltration was not observed in the Peyer’s patches. T cells were also elevated in the cecal patch but not in the Peyer’s patches.
INTRODUCTION
Influenza A virus (IAV) infection during pregnancy is not only associated with serious maternal complications and mortality in pregnant women but there are also increased risk of adverse fetal outcomes including preterm birth, low birthweight, and even infant mortality (1). However, there are limited studies that have examined whether offspring that survive, delivered at term gestation, and have normal weight range will develop serious adverse health outcomes later in life. Previous epidemiological and preclinical animal studies have reported impaired antiviral immunity against respiratory viral infections and neurological disorders in adult offspring born to IAV-infected mothers (2, 3). Unlike most viruses, IAV does not directly infect the placenta or fetus (4). From preclinical studies, it is postulated that these adverse effects on the offspring are due to IAV-induced maternal immune activation and a “vascular storm” event that consists of IAV dissemination and profound inflammation and oxidative stress in the aorta resulting in vascular dysfunction, impaired placental blood flow, and a hypoxic intrauterine environment for the developing fetus (4). Exposure to intrauterine hypoxia can damage the integrity of the intestinal barrier in the offspring, exacerbate immune responses to bacterial infection in the gut and trigger necrotizing enterocolitis (NEC) development (5–7). In neonatal rats, intrauterine hypoxia can also alter the composition of colonizing gut microbiota (8), which can increase the susceptibility of NEC in premature infants (9).
Gut-associated lymphoid tissue (GALT) is a prominent component of mucosal immunity in the gut, representing a large proportion of the immune system in the body (10). It is composed of aggregated lymphoid follicles in the small intestine (Peyer’s patches) and the cecum, and appendix (cecal patch) in rodents and humans, respectively. The Peyer’s patch and cecal patch are involved in maintaining homeostatic balance between immunological tolerance to innocuous food and commensal microbial antigens without compromising the ability to mount an inflammatory response to invading microorganisms. Studies have identified GALT to play an important role in maintaining gut health as well as mediating the cross talk of the gut-brain axis by regulating inflammation, neuroimmune interactions, and gut microbiota composition (11). More recently, changes in the gut microbiome have been shown to influence the susceptibility and severity to IAV and Streptococcus pneumoniae respiratory infections (12–14). Therefore, dysregulated GALT function may not only trigger inflammation and dysfunction of the gastrointestinal (GI) tract but could also have wider implications in neurodevelopmental disorders and respiratory health.
The aim of this study was to assess the effect of gestational IAV infection on gut anatomy and the mucosal immune profile of prepubescent juvenile offspring. In this study, 3-wk-old (postnatal day 21) offspring born from IAV-infected dams showed no anatomical changes in their GI tract when compared with offspring from uninfected dams. However, cellular analysis of GALT of offspring born to IAV-infected dams showed a significant influx of neutrophils, monocytes/macrophages, CD4 and CD8 T cells in the cecal patch and not in Peyer’s patches. Also, offspring exposed to gestational IAV infection had increased inflammation in the cecal patch, whereas no such change was observed in the Peyer’s patch. Herein, to our knowledge, our study is the first to characterize region-specific immunological changes in the GALT of offspring exposed to gestational IAV infection.
MATERIALS AND METHODS
Animal Ethics
This study was approved by the Royal Melbourne Institute of Technology University (RMIT) Animal Ethics Committee (AEC No. 1801, 24336) and as specified by the guidelines of the Australian Code of Practice for the Care of Experimental Animals and National Health and Medical Research Council of Australia (NHMRC).
Virus
Mouse-adapted HKx31 influenza virus (H3N2 strain) was grown in 10-day-old embryonated chicken eggs and quantified by standard plaque assay procedure using Madin-Darby canine kidney (MDCK) cells. HKx31 IAV virus is a recombinant influenza strain that contains the hemagglutinin and neuraminidase glycoproteins from the H3N2 virus with the internal genes from the PR8 H1N1 virus.
Influenza A Virus Inoculation of Mice
Time-mated 8- to 12-wk-old C57BL6 female mice at embryonic day 11 (E11) were obtained from the Animal Resource Centre (ARC) Western Australia, Australia. At embryonic day 12 (E12) dams were intranasally inoculated with HKx31 virus at 103 plaque-forming units (PFUs) or mock-infected with PBS (controls) and housed individually in individually ventilated cages (IVC) cages with daily monitoring until delivery. Dams were housed in a regular 12-h dark/light cycle and provided with a standard mouse chow diet with no additional enrichment and access to water ad libitum.
Flow Cytometry
Dissected cecal and Peyer’s patches from offspring were placed in 1 mL of ice-cold PBS until ready for processing. Tissues were then transferred into Hanks’ buffer containing 0.1% liberase (Sigma Aldrich) and mechanically digested with scissors for 1 min. The tissues were then incubated at 37°C on a shaking platform (750 rpm) for 30 min. The cell suspension was passed through a 70-μm strainer and followed with 20 mL of ice-cold FACS buffer (2.5% FBS in PBS). The samples were then centrifuged at 400 g for 5 min at 4°C. The supernatant was discarded, and the cell pellet was resuspended in FACS buffer for antibody staining. Cell viability was assessed using the Live/Dead Fixable Aqua Dead Cell Stain Kit as per the manufacturer’s instructions (Invitrogen). Cells were preincubated on ice for 10 min with Fc block antibody and then stained for 25 min on ice with following surface antibodies at 1:200 dilution from BioLegend: CD45-Alexa Fluor 700 (clone 30-F11), CD3-APC (clone 145-2C11), CD8-PerCP-Cy5.5 (clone 53.6.7), CD4-BV605 (clone RM4-5), Ly6C-FITC (clone HK1.4), Ly6G-APC-Cy7 (clone 1A8), CD11b-BV421 (clone M1/70), and CD69-BV650 (clone BM8). Cells were fixed and permeabilized for intracellular staining with FoxP3-PE antibody at 1:1,000 dilution (clone FJK-16s; eBiosciences) for 15 min at room temperature. Flow cytometry was performed using a FACS Fortessa (BD Biosciences) and data were analyzed using Flow Jo software. The gating strategy for immune cell populations is shown in Supplemental Fig. S1.
RNA Extraction and qPCR
Cecal and Peyer’s patches were homogenized using steel beads on the TissueLyserLT (Qiagen) in 1 mL of TRIzol reagent (Thermo Fisher Scientific, Scoresby, VIC, Australia). Total RNA was extracted from the homogenate using the RNeasy Mini kit (Qiagen). The High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used as per the manufacturer’s instruction to convert 1 µg RNA into cDNA. Expression of housekeeping gene RPS18 (Assay ID: Mm02601777_g1) and cytokine genes IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), TNF-α (Mm00443258_m1), IFN-β (Mm00439552_s1), IFN-γ (Mm01168134_m1), and CD69 (Mm01183378_m1) was evaluated using predesigned TaqMan primers (Thermo Fischer Scientific) and the TaqMan Universal PCR Master Mix (Applied Biosystems). Quantitation of gene expression was performed using the comparative threshold cycle (Ct) method (15), with target gene expression normalized against the housekeeping gene RPS18.
Statistical Analysis
Graphs were generated using GraphPad Prism software version 9 (San Diego, CA). All data were expressed as means ± SE. Comparisons between the two groups were performed using Student’s t tests. Comparisons examining sex differences were performed using two-way ANOVA. For parameters that showed no sex interactions, data from males and females were pooled for combined analysis using Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Gestational Influenza Infection Does Not Cause Gross Anatomical Changes in the Offspring GI Tract
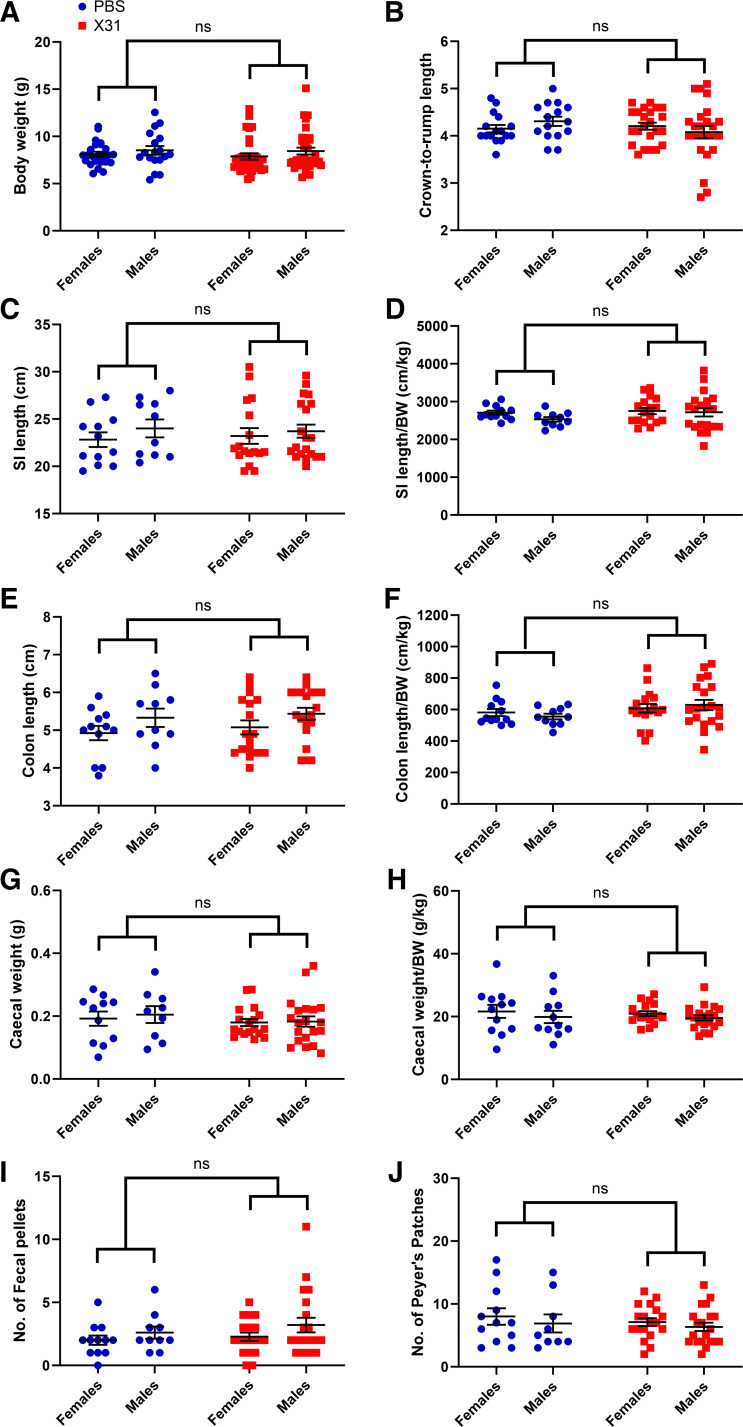
Pregnant dams were infected with a low dose of the moderately pathogenic IAV strain HKx31 at E12 gestation. Analysis for changes in the gross anatomy of the GI tract was performed on juvenile offspring (3-wk old). Offspring born from IAV-infected dams when compared with offspring from PBS-mock-infected dams, did not show any differences in body weight nor any anatomical changes in the GI tract, including small intestine length, colon length, cecal weight, or the number of formed fecal pellets in the gut at the time of tissue harvesting (Fig. 1).
Figure 1.
Body weight and gastrointestinal (GI) tract anatomy of 3-wk-old mice offspring. The body weights (n = 24–32 females/group; n = 18–33 males/group) (A), crown-to-rump lengths (B), small intestine (SI) lengths (C), small intestine (SI) length/body weight (D), colon lengths (E), colon lengths/body weight (F), cecal weights (G), cecal weigh/body weight (H), number of fecal pellets (I), and number of Peyer’s patches (J) of 3-wk-old offspring born to influenza A virus (IAV)-infected (X31) or PBS-mock-infected dams. Body weights and crown-to-rump lengths were collected from n = 24 PBS females, n = 32 IAV (X31) females, n = 18 PBS males and n = 33 IAV (X31) males. All other GI assessments were collected from n = 12 PBS females, 17 IAV (X31) females, n = 10 PBS males, and n = 20 IAV (X31) males. Two-way ANOVA analysis identified no sex interaction. ns, not significant.
Gestational IAV Infection Is Associated with Increased Influx of Neutrophils, Monocytes, and CD4+ and CD8+ T Cell into the Offspring Cecal Patch
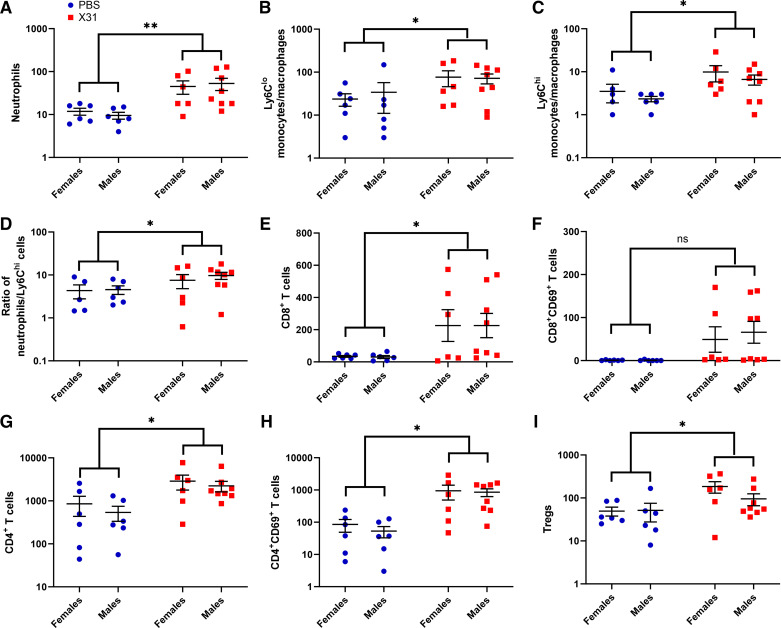
Flow cytometric analysis of the cecal patch identified an increased number of neutrophils, “patrolling” Ly6Clo macrophages/monocytes, and “proinflammatory” Ly6Chi macrophages/monocytes in IAV offspring when compared with PBS offspring (Fig. 2, A–C). IAV offspring also had an increased ratio of neutrophils to Ly6Chi macrophage/monocytes in the cecal patch compared with PBS offspring (Fig. 2D). CD4+ and CD8+ T cells were also significantly increased in the cecal patch of IAV offspring (Fig. 2, E and G). IAV offspring showed elevated activation of CD4+ T cells in the cecal patch but not CD8+ T cell activation when compared with PBS offspring (Fig. 2, F and H). We also observed elevated CD4+ regulatory T cells (Tregs; FoxP3+) numbers in the cecal patch of IAV offspring compared with PBS offspring (Fig. 2I). The changes in the immune cell profile within the cecal patch were not dependent on offspring sex.
Figure 2.
Gestational influenza A virus (IAV) infection increases immune cell infiltration into the cecal patches of 3-wk-old offspring. Neutrophils (A), patrolling Ly6Clo monocytes/macrophages (B), proinflammatory Ly6Chi monocytes/macrophages (C), neutrophil-to-Ly6Chi ratio (D), CD8+ T cells (E), CD69+-activated CD8+ T cells (F), CD4+ T cells (G), CD69+-activated CD4+ T cells (H), and CD4+ regulatory T cells (Tregs) (I) present in the cecal patches of 3-wk-old offspring born from IAV-infected (X31) or PBS mock-infected dams were quantified by flow cytometry (n = 6 or 7 animals/group). Two-way ANOVA analysis identified no sex interaction and thus male and female offspring were subsequently combined for Student’s t test analysis. *P < 0.05; **P < 0.01.
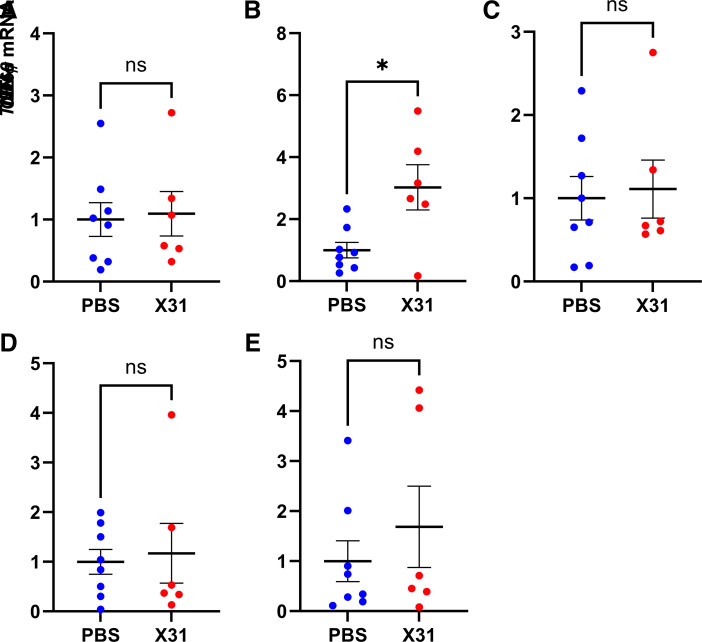
Gene expression of proinflammatory mediators, such as IL-1β, IL-6, TNF-α, IFN-γ, and the pan-immune cell activation marker CD69, was assessed in the cecal patch of IAV offspring (Fig. 3). Given that there were no sex differences in immune cell profiling of the offspring cecal patch, male and female samples were combined for qPCR analysis. Only IL-6 mRNA expression was significantly upregulated in the cecal patch of IAV offspring (Fig. 3B). CD69 mRNA expression showed a trend for an increase in the IAV offspring; however, this did not reach statistical significance (P = 0.430, Fig. 3E).
Figure 3.
Gestational influenza A virus (IAV) infection increased IL-6 gene expression in the cecal patches of 3-wk-old offspring. IL-1β (A), IL-6 (B), TNF-α (C), IFN-γ (D), and CD69 mRNA expression (E) in the cecal patches of offspring born from IAV-infected (X31) or PBS mock-infected dams was assessed by qPCR (n = 6–8 animals/group). Student’s t test analysis. *P < 0.05. ns, Not significant.
Gestational Influenza Induces Increased Activation of CD4+ T Cells in Peyer’s Patches
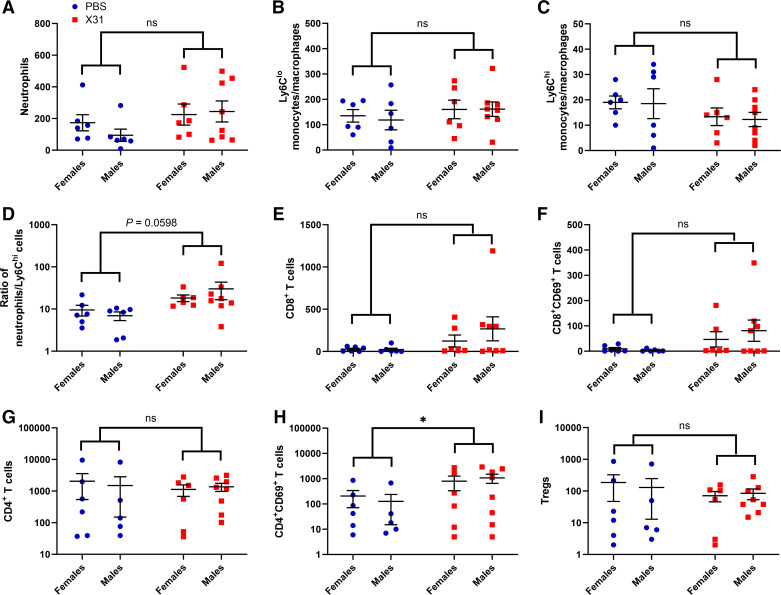
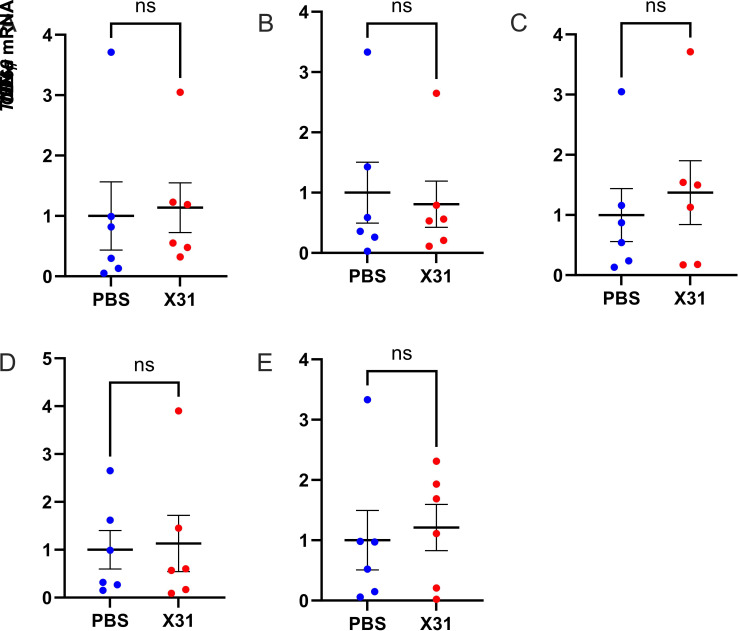
Analysis of the immune cell profile of the offspring Peyer’s patches did not show any sex differences in innate immune cell populations (Fig. 4). There was no change in absolute cell numbers and ratios between neutrophils and Ly6C macrophages/monocytes in the Peyer’s patches of IAV offspring (Fig. 4, A–D). The total number of CD4+ T cells, CD8+ T cells, and Tregs in the Peyer’s patches also did not change in offspring from IAV-infected dams (Fig. 4, E, G, and I). However, activation of CD4+ T cells was significantly increased in IAV offspring (Fig. 5H). Gene expression analysis of Peyer’s patch tissue did not show any changes in IL-1β, IL-6, TNF-α, and IFN-γ expression between IAV and PBS offspring (Fig. 5).
Figure 4.
Gestational influenza A virus (IAV) infection increases immune cell infiltration into the Peyer’s patches of 3-wk-old offspring. Neutrophils (A), patrolling Ly6Clo monocytes/macrophages (B), proinflammatory Ly6Chi monocytes/macrophages (C), neutrophil-to-Ly6Chi ratio (D), CD8+ T cells (E), CD69+-activated CD8+ T cells (F), CD4+ T cells (G), CD69+-activated CD4+ T cells (H), and CD4+ regulatory T cells (Tregs) (I) present in the Peyer’s patches of 3-wk-old offspring born from IAV-infected (X31) or PBS mock-infected dams were quantified by flow cytometry (n = 6 or 7 animals/group). Two-way ANOVA analysis identified no interaction sex and thus male and female offspring were subsequently combined for Student’s t test analysis. *P < 0.05. ns, not significant.
Figure 5.
Gestational influenza A virus (IAV) infection had no effect on proinflammatory gene expression in the Peyer’s patches of 3-wk-old offspring. IL-1β (A), IL-6 (B), TNF-α (C), IFN-γ (D), and CD69 mRNA expression (E) in the cecal patches of offspring born from IAV-infected (X31) or PBS mock-infected dams was assessed by qPCR (n = 6 or 7 animals/group). Student’s t test analysis. ns, not significant.
DISCUSSION
IAV infection during pregnancy has been shown to significantly alter the offspring’s immune response to pathogens and behavior later in life (2, 3). In this study, offspring born from IAV-infected dams showed no differences in body weight or crown-to-rump length at 3 wk of age. Analysis of the GI tract from IAV offspring revealed no changes in the lengths, cecal weights, and number of formed fecal pellets or Peyer’s patches when compared with offspring born from PBS mock-infected dams. Despite normal macroscopic anatomical characteristics of the GI tract of IAV offspring, our model identified several changes in the immune profile of the GALT of 3-wk-old IAV offspring.
IAV infection of nonpregnant mice can indirectly alter the intestinal microbiota composition to cause intestinal inflammation and injury via CD4+ T helper 17 (Th17) cell-dependent inflammation (16). Conversely, other studies have shown that IAV-infected dams have reduced colon length and dysregulated expression of immune and antimicrobial genes in the gut despite an absence of overt intestinal injury (17), suggesting that IAV infection can adversely impact certain aspects of GI health in mice. There is also emerging evidence in humans and preclinical studies showing that the maternal gut microbiota plays a fundamental role in developing early life immune competence, with studies showing increased risk of allergic disease and asthma (18) and altered immune cell profiles in infants (19). Taken together, these studies implicate gestational IAV infection in the alteration of maternal gut microbiota and long-term consequences to offspring mucosal immunity.
IAV-induced maternal immune activation during pregnancy has been linked to atypical neurodevelopment in the offspring (3). However, given that IAV does not cross the placenta to directly infect the fetus, the precise mechanisms that contribute to disrupted offspring neurodevelopment during maternal immune activation remain unknown. GI dysfunction is a common comorbidity in individuals with neurodevelopmental disorders including autism. It is currently unknown whether GALT dysfunction is a comorbidity of autism spectrum disorders (ASD). Cecal dysfunction however has been reported in the Neuroligin-3R451C mouse model of autism, with increased infiltration of activated macrophages in the cecal patch (20). Here, we demonstrate that gestational IAV infection increased the infiltration of neutrophils, Ly6Chi and Ly6Clo monocytes/macrophages and increased the numbers of CD4+ and CD8+ T cells in the cecal patch of 3-wk-old offspring. In contrast, only activated CD4+ T cell numbers were increased in the Peyer’s patches of 3-wk-old offspring.
Excessive activation of neutrophils has been associated with chronic inflammation of the gut and ulcerative colitis severity (21). Neutrophils are a major cellular source of reactive oxygen species (ROS) that play an important role in pathogen clearance. Aberrant production of ROS however can have detrimental effects by promoting persistent inflammation and damage to intestinal tissue (22). Neutrophil infiltration was significantly upregulated in the cecal patch of IAV offspring. Although not assessed in this study, increased neutrophil infiltration may indicate an increased oxidative stress environment within these lymphoid tissues in the absence of an active infection and thus contribute to the perturbation of normal GALT function.
In mice, monocyte-derived macrophages can be classed into two major subsets, Ly6Chi and Ly6Clo. Tissue-resident macrophages involved in homeostatic activities including clearance of apoptotic cells and tissue immune surveillance (23) express Ly6Clo and a “patrolling” phenotype, whereas Ly6Chi macrophages are not involved in tissue homeostasis and rarely reside in tissues except in response to inflammation (24). Ly6Chi macrophages express cytokines that can further exacerbate inflammation and have been implicated in the progression of autoimmune disease (24) and in triggering the recruitment of circulating proinflammatory Ly6Chi monocytes precursors during colitis (25). In this study, we observed that gestational IAV infection increased infiltration of Ly6Clo and Ly6Chi monocytes/macrophages that may affect macrophage dynamics and tissue homeostasis in the cecal patch of IAV offspring. Other studies have also reported that tissue-resident intestinal macrophages can suppress intestinal colitis onset by downregulating neutrophil infiltration in the gut (26). In our study, however, although macrophage populations were increased in cecal patch tissues, this was not associated with reduced neutrophil infiltration that may be suggestive of impaired macrophage function to inhibit inflammation. Although not assessed in this study, macrophage function has been shown to be impaired by gestational IAV infection. Alveolar macrophages from offspring born from IAV-infected dams have impaired capacity to clear pathogens later in life (2). Thus, a dysregulated profile of macrophages and neutrophils in GALT could lead to persistent low-grade inflammation and increase offspring susceptibility to GI dysfunction later in life. Further studies are required to determine whether offspring exposed to gestational IAV infection also have dysfunctional macrophage activity in the gut.
Antigen-presenting cells such as macrophages and dendritic cells present antigens to GALT residing T cells. These T cells must differentiate between harmful pathogens from non-self antigens from food or commensal microbes. Activation of T cells by proinflammatory cytokines can trigger inappropriate immune responses that can lead to excessive gut inflammation and dysfunction. Assessment of GALT inflammation in the offspring suggests the presence of persistent inflammation in 3-wk-old offspring born from IAV-infected dams. We observed significantly elevated IL-6 gene expression in the cecal patch. IL-6 drives acute and chronic inflammation with increased IL-6 levels in patients with chronic inflammatory diseases including inflammatory bowel disease (27). In addition, IL-6 promotes T cell survival by increasing proliferation and limiting apoptosis during inflammation (28, 29). IL-6 also drives cytokine production in primed or memory CD4+ T cells (30), and in synergy with IL-1β and IL-23 can initiate Th17 cell differentiation (31). IL-6 is also an upstream regulator of IL-22 production in T cells, natural killer cells, and neutrophils (32). In humans, IL-22 is upregulated in diseases associated with increased permeability of the epithelial barrier in the gut, including celiac and inflammatory bowel diseases (33, 34). Although not examined in this study, future investigation of IL-22 levels in the gut could provide information about the epithelial tight junction integrity of IAV offspring gut. Another limitation of this study was that we did not investigate potential sex differences in cytokine expression in the Peyer’s patches or cecal patches of the offspring. There is evidence to support that gut microbiota compositions differ with biological sex that may contribute to sex differences in the immune system (35). Future assessment of sex-segregated cytokine and immune cell profiles in IAV offspring gut may reveal sex-dependent differences in the inflammatory profiles in the offspring.
Compared with Peyer’s patch tissue, the role of the cecal patch in gut immunity and health is relatively unknown. Our study has demonstrated long-term alterations in the immune cell profiles within the Peyer’s patches and cecal patch tissues in offspring exposed to maternal influenza. In the absence of infection, elevated IL-6 expression in the cecal patch could underlie cecal dysfunction in the offspring. Short-chain fatty acids are produced as metabolites from bacterial fermentation occurring in the cecum (36) and are thought to be critical in host immunometabolism and the gut-brain axis (37). The cecum is also thought to be a microbial reservoir that can replenish commensal bacteria following inflammation-induced diarrhea (38). Immune cell profiles within the cecal patch could also influence the composition of commensal microbes in the gut (38). A recent study identified that Proteobacteria and Deferribacteres were predominantly localized in the cecal crypts, whereas Firmicutes are mostly localized in the cecal lumen in mice (36). Thus, modifications to cecal patch immunity in offspring exposed to gestational IAV infection could not only impact microbial sensing in the cecal patch but also affect the composition of cecal bacterial populations, short-chain fatty acid production, and potentially influence offspring brain physiology and behavior.
In conclusion, gestational IAV infection contributes to changes in offspring mucosal immunity, with effects extending beyond impaired immune response to respiratory pathogens later in life (2), as well as perturbing intestinal immunity via immune cell population changes and inflammation in the GALT. Our findings demonstrate a regional-specific susceptibility of GALT in the offspring to gestational IAV infection with persistent IL-6 expression in the cecal patch, a phenomenon that is absent in Peyer’s patches. Further investigation is needed to assess whether the cecal microbiome is altered in IAV offspring, how such changes might affect the cross talk within the gut-brain axis and potential contributions to gestational IAV-induced neurological disorders in the offspring.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.25439/rmt.23589699.v1.
GRANTS
This work was supported by the Australian National Health and Medical Research Council Grant 2002948 (to S. Liong and S. Selemidis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L., E.L.H.-Y., and S.S. conceived and designed research; S.L., M.A.M., M.M., and F.L. performed experiments; S.L. analyzed data; S.L., E.L.H.-Y., and S.S. interpreted results of experiments; S.L. prepared figures; S.L. drafted manuscript; S.L., M.A.M., M.M., F.L., E.L.H.-Y., and S.S. edited and revised manuscript; S.L., E.L.H.-Y., and S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract was created with BioRender.
REFERENCES
- 1. Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol 207: S3–S8, 2012. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 2. Jacobsen H, Walendy-Gnirß K, Tekin-Bubenheim N, Kouassi NM, Ben-Batalla I, Berenbrok N, Wolff M, Dos Reis VP, Zickler M, Scholl L, Gries A, Jania H, Kloetgen A, Düsedau A, Pilnitz-Stolze G, Jeridi A, Yildirim AÖ, Fuchs H, Gailus-Durner V, Stoeger C, de Angelis MH, Manuylova T, Klingel K, Culley FJ, Behrends J, Loges S, Schneider B, Krauss-Etschmann S, Openshaw P, Gabriel G. Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat Commun 12: 4957, 2021. doi: 10.1038/s41467-021-25220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23: 297–302, 2003. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liong S, Oseghale O, To EE, Brassington K, Erlich JR, Luong R, Liong F, Brooks R, Martin C, O'Toole S, Vinh A, O'Neill LAJ, Bozinovski S, Vlahos R, Papagianis PC, O'Leary JJ, Brooks DA, Selemidis S. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc Natl Acad Sci USA 117: 24964–24973, 2020. doi: 10.1073/pnas.2006905117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manresa MC, Taylor CT. Hypoxia inducible factor (HIF) hydroxylases as regulators of intestinal epithelial barrier function. Cell Mol Gastroenterol Hepatol 3: 303–315, 2017. doi: 10.1016/j.jcmgh.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haddad JJ, Harb HL. Cytokines and the regulation of hypoxia-inducible factor (HIF)-1α. Int Immunopharmacol 5: 461–483, 2005. [Erratum in Int Immunopharmacol 6: 1632, 2006]. doi: 10.1016/j.intimp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 7. Baregamian N, Rychahou PG, Hawkins HK, Evers BM, Chung DH. Phosphatidylinositol 3-kinase pathway regulates hypoxia-inducible factor-1 to protect from intestinal injury during necrotizing enterocolitis. Surgery 142: 295–302, 2007. doi: 10.1016/j.surg.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun Y, Li L, Song J, Mao W, Xiao K, Jiang C. Intrauterine hypoxia changed the colonization of the gut microbiota in newborn rats. Front Pediatr 9: 675022, 2021. doi: 10.3389/fped.2021.675022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6: e20647, 2011. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faria AM, Weiner HL. Oral tolerance. Immunol Rev 206: 232–259, 2005. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abo-Shaban T, Sharna SS, Hosie S, Lee CYQ, Balasuriya GK, McKeown SJ, Franks AE, Hill-Yardin EL. Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. J Neural Transm (Vienna) 130: 269–280, 2023. doi: 10.1007/s00702-022-02561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun 8: 1512, 2017. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA 108: 5354–5359, 2011. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman MJ, Boon ACM, Lenschow DJ, Stappenbeck TS. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 357: 498–502, 2017. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med 211: 2397–2410, 2014. [Erratum in J Exp Med 211: 2683, 2014]. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antonson AM, Kenney AD, Chen HJ, Corps KN, Yount JS, Gur TL. Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain. Brain Behav Immun 96: 28–39, 2021. doi: 10.1016/j.bbi.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao Y, Nanan R, Macia L, Tan J, Sominsky L, Quinn TP, O'Hely M, Ponsonby AL, Tang MLK, Collier F, Strickland DH, Dhar P, Brix S, Phipps S, Sly PD, Ranganathan S, Stokholm J, Kristiansen K, Gray LEK, Vuillermin P. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J Allergy Clin Immunol 148: 669–678, 2021. doi: 10.1016/j.jaci.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 19. Gao Y, O'Hely M, Quinn TP, Ponsonby A-L, Harrison LC, Frøkiær H, Tang MLK, Brix S, Kristiansen K, Burgner D, Saffery R, Ranganathan S, Collier F, Vuillermin P. Maternal gut microbiota during pregnancy and the composition of immune cells in infancy. Front Immunol 13: 986340, 2022. doi: 10.3389/fimmu.2022.986340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharna SS, Balasuriya GK, Hosie S, Nithianantharajah J, Franks AE, Hill-Yardin EL. Altered caecal neuroimmune interactions in the neuroligin-3(R451C) mouse model of autism. Front Cell Neurosci 14: 85, 2020. doi: 10.3389/fncel.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, Peyrin-Biroulet L. Comparing histological activity indexes in UC. Gut 64: 1412–1418, 2015. doi: 10.1136/gutjnl-2014-307477. [DOI] [PubMed] [Google Scholar]
- 22. Kim YJ, Kim EH, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol 27: 1004–1010, 2012. doi: 10.1111/j.1440-1746.2012.07108.x. [DOI] [PubMed] [Google Scholar]
- 23. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 14: 986–995, 2013. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li YH, Zhang Y, Pan G, Xiang LX, Luo DC, Shao JZ. Occurrences and functions of Ly6C(hi) and Ly6C(lo) macrophages in health and disease. Front Immunol 13: 901672, 2022. doi: 10.3389/fimmu.2022.901672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones GR, Bain CC, Fenton TM, Kelly A, Brown SL, Ivens AC, Travis MA, Cook PC, MacDonald AS. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol 9: 2764, 2018. doi: 10.3389/fimmu.2018.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol 80: 802–815, 2006. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 27. Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol 28: 187–196, 2005. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 28. Li B, Jones LL, Geiger TL. IL-6 promotes T cell proliferation and expansion under inflammatory conditions in association with low-level RORγt expression. J Immunol 201: 2934–2946, 2018. doi: 10.4049/jimmunol.1800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol 174: 4761–4767, 2005. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 30. Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, Jones SA, Gallimore AM. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog 4: e1000006, 2008. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30: 576–587, 2009. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med 217: e20192195, 2020. doi: 10.1084/jem.20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong M, Yeruva S, Sailer A, Nilsen SP, Turner JR. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab Invest 100: 483–490, 2020. doi: 10.1038/s41374-019-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56: 61–72, 2007. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol 61: 100912, 2021. doi: 10.1016/j.yfrne.2021.100912. [DOI] [PubMed] [Google Scholar]
- 36. Zaborin A, Penalver Bernabe B, Keskey R, Sangwan N, Hyoju S, Gottel N, Gilbert JA, Zaborina O, Alverdy JC. Spatial compartmentalization of the microbiome between the lumen and crypts is lost in the murine cecum following the process of surgery, including overnight fasting and exposure to antibiotics. mSystems 5: e00377–20, 2020. doi: 10.1128/mSystems.00377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 11: 25, 2020. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laurin M, Everett ML, Parker W. The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken) 294: 567–579, 2011. doi: 10.1002/ar.21357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.25439/rmt.23589699.v1.
Data Availability Statement
Data will be made available upon reasonable request.