Abstract
Rodent husbandry requires careful consideration of environmental factors that may impact colony performance and subsequent physiological studies. Of note, recent reports have suggested corncob bedding may affect a broad range of organ systems. As corncob bedding may contain digestible hemicelluloses, trace sugars, and fiber, we hypothesized that corncob bedding impacts overnight fasting blood glucose and murine vascular function. Here, we compared mice housed on corncob bedding, which were then fasted overnight on either corncob or ALPHA-dri bedding, a virgin paper pulp cellulose alternative. Male and female mice were used from two noninduced, endothelial-specific conditional knockout strains [Cadherin 5-cre/ERT2, floxed hemoglobin-α1 (Hba1fl/fl) or Cadherin 5-cre/ERT2, floxed cytochrome-B5 reductase 3 (CyB5R3fl/fl)] on a C57BL/6J genetic background. After fasting overnight, initial fasting blood glucose was measured, and mice were anesthetized with isoflurane for measurement of blood perfusion via laser speckle contrast analysis using a PeriMed PeriCam PSI NR system. After a 15-min equilibration, the mice were injected intraperitoneally with the α1-adrenergic receptor agonist, phenylephrine (5 mg/kg), or saline, and monitored for changes in blood perfusion. After a 15-min response period, blood glucose was remeasured postprocedure. In both strains, mice fasted on corncob bedding had higher blood glucose than the pulp cellulose group. In the CyB5R3fl/fl strain, mice housed on corncob bedding displayed a significant reduction in phenylephrine-mediated change in perfusion. In the Hba1fl/fl strain, phenylephrine-induced change in perfusion was not different in the corncob group. This work suggests that corncob bedding, in part due to its ingestion by mice, could impact vascular measurements and fasting blood glucose. To promote scientific rigor and improve reproducibility, bedding type should be routinely included in published methods.
NEW & NOTEWORTHY This study demonstrates real-time measurement of changes in perfusion to pharmacological treatment using laser speckle contrast analysis. Furthermore, this investigation revealed that fasting mice overnight on corncob bedding has differential effects on vascular function and that there was increased fasting blood glucose in mice fasted on corncob bedding compared with paper pulp cellulose bedding. This highlights the impact that bedding type can have on outcomes in vascular and metabolic research and reinforces the need for thorough and robust reporting of animal husbandry practices.
Keywords: ARRIVE, blood glucose, corncob bedding, mouse husbandry, vascular function
INTRODUCTION
Every facet of animal husbandry has the potential to alter outcomes in research that uses model organisms (1, 2). Although researchers are trained to identify and minimize variables in their experimental design, the disclosure of seemingly routine factors (i.e., housing conditions) can often be overlooked. If details related to animal husbandry, such as feed, bedding, light cycle, temperature, etc. are not stated in the methods, variation between vivarium facilities may inadvertently contribute to the lack of reproducibility of published studies. In line with a recent publication (3), following an institutional move, our group identified bedding as a key variable impacting several of our physiological measurements. Since there is not a single modus operandi of husbandry between animal facilities, and laboratories routinely relocate, the impact subtle environmental factors have on animal research is becoming more evident. As journals strive for rigor, inclusion of comprehensive animal husbandry practices in the methods section, such as bedding used, should be standard practice. This allows researchers to be more conscientious of factors that may underlie inconsistent results in the literature and between laboratories.
For example, it has been well established over recent years that bedding type used in rodent studies can impact physiology across many organ systems (3–12). Depending on institutional vivarium policy, the type of bedding available may include woodchips/shavings from trees such as aspen, processed paper, and/or ground corncob, as well as others (13). The chemical composition and physical properties of these common bedding types differ significantly. Thus, when exposed to different bedding materials, there is a potential for mice to exhibit varying physiological responses, especially if consumption of bedding occurs (9).
Corncob bedding has been a common rodent cage bedding for decades because of its reported absorbent qualities, low cost, and abundance in certain regions (14). In more recent years, however, its use has been reevaluated because of the biological effects caused by housing on and/or ingestion of the corncob bedding. Corncob bedding impacts the microbiome and metabolic profiles of gastrointestinal bacteria in calorie-restricted mice (9) and reduces feed conversion and weight gain in mice on a high-fat diet (12). Recently, it was shown in Dahl salt-sensitive hypertensive rats that corncob bedding reduces blood pressure, markers of inflammation, and kidney damage (3), suggesting that bedding is a confounding factor in these hypertension studies. In addition, housing on corncob bedding reduces the expression of estrogen receptor-α in the mouse brain (11), which may contribute to altered reproductive behavior and reduced litter size (11, 15, 16). Similar sentiments regarding corncob bedding arise when considering the well-being and expression of natural behavior in rodents (15, 17). Rodents avoid corncob when given the option and display a preference for alternative bedding types (17–19). Mice on corncob show reduced nest building (15), an indicator of general well-being (20). In addition, corncob bedding reduces slow-wave sleep in rats, which is indicative of reduced sleep quality (6). Thus, there is sufficient evidence to support that corncob bedding can influence rodent research end points.
In the current study, we provide evidence that corncob bedding may impact fasting blood glucose (FBG) and vascular function in mice. We directly compared corncob bedding with a virgin paper pulp cellulose bedding, which exhibits similar absorbent qualities and relatively low ammonia accumulation (14, 21). By assaying FBG and blood perfusion via laser speckle contrast analysis (LASCA), we show corncob bedding causes increased FBG and a strain-specific effect on adrenergic-mediated change in perfusion when compared with mice fasted on a paper pulp cellulose alternative. These results are in alignment with recent literature (3, 8), indicating corncob bedding is not a biologically inert material. Therefore, careful consideration should be taken before its use in rodent studies depending on the physiological or metabolic measurement of interest.
MATERIALS AND METHODS
Animal Housing and Management
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center (Omaha, NE). Mice were housed in ∼12 × 6.5 × 5.75-in. Super Mouse 750 Zyfone cages on ventilated racks (Lab Products, LLC.) in an environmentally controlled room (Temp: 70–75°F, 21.11–23.89°C; humidity: 30–70%) in the University of Nebraska Medical Center’s vivarium. Animal density did not exceed five mice per cage. The photoperiod was set to “on” at 5:00 AM, “off” at 7:00 PM. A 14-h:10-h day/night schedule was used because of similar practice by The Jackson Laboratory. Housing cage bedding consisted of 1/4 inch grade corncob substrate (Envigo, 7097). Mice were fed Teklad 7012 diet (Envigo) and provided hyperchlorinated reverse osmosis water in pouches produced by a Hydropac AWS-2500 pouch machine (Avidity Science). Food and water were provided ad libitum. Cages also contained paper nest packets for enrichment (Enviropak, WF Fisher and Son). Cage changes occurred in the morning, once every other week. Sterile conditions when handling mice during cage change were maintained using Clidox-S (Pharmacal) and 70% ethanol in a ducted biosafety hood.
Strain Information
The mouse strains used in this study consisted of two inbred lines of congenic mice (C57BL/6J background, RRID:IMSR_JAX:000664); strain 1) possessed loxP-flanked cytochrome-B5 reductase 3 (simplified here as CyB5R3fl/fl) and strain 2) possessed loxP-flanked hemoglobin α1 (simplified here as Hba1fl/fl). Both CyB5R3fl/fl and Hba1fl/fl strains were mixed populations of mice positive or negative for tamoxifen inducible endothelial-specific Cre-recombinase {cadherin-5 promoter-Cre estrogen ligand-binding domain [C57BL/6-Tg(Cdh5-cre/ERT2)1Rha, RRID: IMSR_EM:14891]} (22). The design and use of these gene cassettes have been previously described (23, 24). Although endothelial-specific knockout of these proteins via Cdh5-cre/ERT2 can be induced by tamoxifen treatment, the mice in this study were never treated with tamoxifen and thus were considered background controls within their respective strains. Throughout the article, CyB5R3fl/fl mice are indicated by pink icons, Hba1fl/fl mice are indicated by purple icons. Icons of Cre+ mice are solid, whereas Cre− mice are open fill. Male mice are denoted as squares, female mice are denoted as triangles. All mice were aged between 161 and 191 days (∼23–27 wk).
Fasting Blood Glucose Measurement
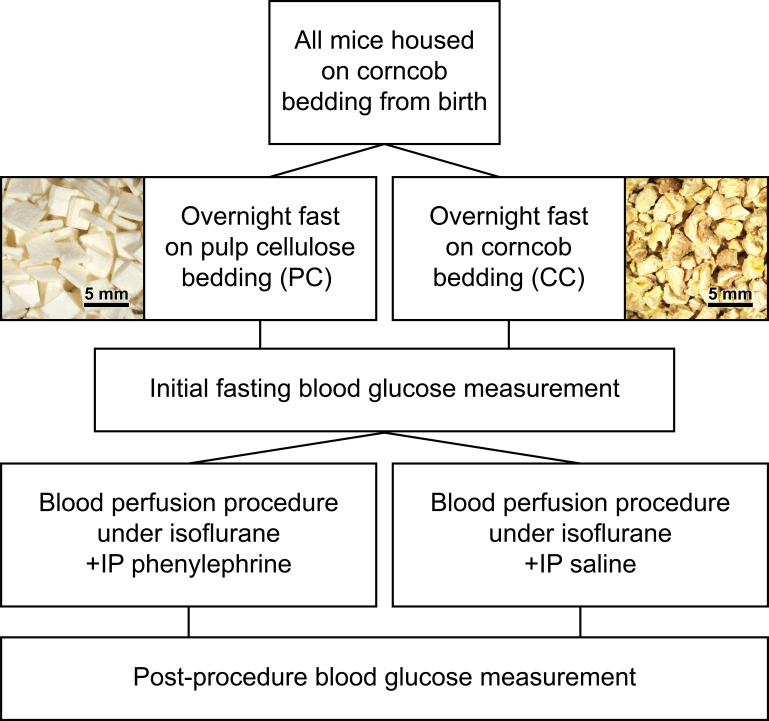
All mice in the current study were born and housed on corncob bedding. For bedding comparisons, mice were transferred to cages containing either ∼125 g ALPHA-dri (Shepherd Specialty Papers) or ∼140 g corncob bedding, for a single overnight fast (feed removed for ∼15–16) to ensure depletion of glucose stores (Fig. 1; 25). An initial FBG measurement was taken via tail vein nick of a restrained mouse without anesthetization, using a Contour Next glucometer (Ascensia Diabetes Care) with Contour Next measurement strips (26). After completion of the LASCA blood perfusion protocol, mice were euthanized with 4% isoflurane followed by cervical dislocation, then a second blood glucose measurement was made postprocedure at the time of euthanasia via blood drawn from the heart.
Figure 1.
Approach to testing the impact of bedding on fasting blood glucose and vascular function. All mice were housed on corncob bedding (CC) from birth and were then fasted overnight (∼15–16 h) before experimental study on either corncob bedding or pulp cellulose (PC). An initial fasting blood glucose measurement was taken. Corncob and pulp cellulose-fasted mice were then anesthetized with isoflurane, and blood perfusion was measured basally and in response to intraperitoneally (IP) injection of either phenylephrine or saline. Following the procedure, mice were euthanized by 4% isoflurane and cervical dislocation; then, a postprocedure measurement of fasting blood glucose was made via blood drawn from the heart.
Laser Speckle Contrast Analysis
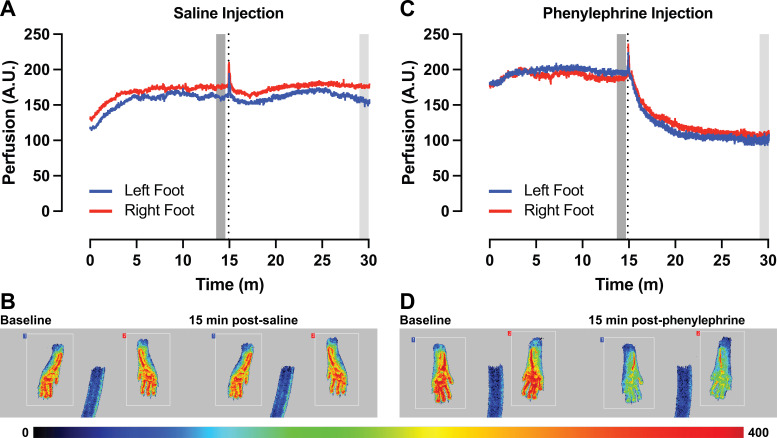
Blood perfusion was measured in arbitrary units via LASCA using a PeriCam PSI NR system (PeriMed). Before observation of blood perfusion in mice, anesthesia was induced using 4% isoflurane (Piramal Critical Care) and maintained at 2% with 2 L/min flow of oxygen throughout the experiment. Anesthesia was facilitated using a Vetaflex 5 veterinary anesthesia machine (Pitman-Moore). Once anesthetized, mice were placed on a heating pad maintained by a TCS-100 controller (iWorx) and monitored via RET-3 rectal probe using a BAT-12 microprobe thermometer (Physitemp) to maintain temperature between ∼37.0 and 37.2°C. After a 15-min equilibration to stabilize temperature and establish baseline perfusion, mice were injected intraperitoneally with either 5 mg/kg phenylephrine, an α1-adrenergic receptor agonist (P6126-5G, Sigma-Aldrich), or saline as a vehicle control (Fig. 2). Phenylephrine was dissolved in 0.9% saline (Baxter) under sterile conditions at a stock concentration of 1 mg/mL. After a 15-min response period to phenylephrine, the recordings were ended, and mice were euthanized as described earlier. To account for baseline perfusion, vasoconstriction to phenylephrine was calculated as percent change in perfusion between a 1-min average at the 15-min baseline timepoint (Bavg) and a 1-min average at the 15-min postphenylephrine response timepoint (Ravg) (Fig. 2). Thus, a negative percent change in perfusion corresponds to vasoconstriction. The same calculation was used following saline exposure. The percent change in perfusion calculation normalizes the response, allowing for comparisons between treatments (e.g., saline, phenylephrine).
Figure 2.
Use of laser speckle contrast analysis (LASCA) for measurement of blood perfusion and vasoreactivity. A: representative trace of blood perfusion in arbitrary units (AU) before and after intraperitoneal injection of saline (vertical dotted line). B: representative images of perfusion before (left) and 15 min after (right) intraperitoneal injection of saline. C: representative trace of blood perfusion before and after intraperitoneal injection of phenylephrine (PE, vertical dotted line). D: representative images of perfusion before (left) and 15 min after (right) intraperitoneal injection of PE. Pseudo-colored scale bar represents level of perfusion with higher values in red and lower values in blue. Blue lines denote left foot, and red lines denote the right foot, which correspond to the feet and regions of interest in the representative images. Dark gray bar denotes region selected for average baseline perfusion (Bavg). Light gray bar denotes region selected for average perfusion response 15 min postinjection (Ravg).
Statistics
All statistics were performed using Prism 9 (GraphPad). Student’s t tests were used assuming Gaussian distribution. One-way ANOVA was used assuming Gaussian distribution, and Šídák test was used for multiple comparisons. A value of P < 0.05 was considered statistically significant. Data are presented as means ± SE.
RESULTS
Housing on Corncob Bedding Results in Higher Fasting Blood Glucose Levels before and after Phenylephrine Exposure
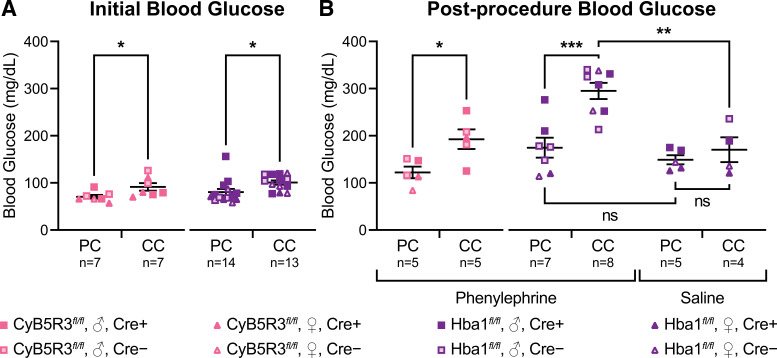
Following the overnight fast (Fig. 1), mice housed on corncob exhibited higher initial FBG levels compared with pulp cellulose in both CyB5R3fl/fl and Hba1fl/fl strains (Fig. 3A). These mice then underwent one of two blood perfusion procedures (phenylephrine or saline exposure) under isoflurane anesthesia, followed by a postprocedure blood glucose measurement. Acute isoflurane exposure is known to cause insulin dysregulation and increases in blood glucose (27–29). Regardless of bedding type or experimental procedure, we observed that postprocedure blood glucose values were consistently higher than the initial FBG (Table 1). In addition, postprocedure blood glucose measurements after phenylephrine exposure were significantly greater in both strains when fasted on corncob (Fig. 3B, left and middle). To determine the contribution of isoflurane anesthetic versus phenylephrine to the observed corncob-mediated postprocedure blood glucose increase, we measured blood glucose in Hba1fl/fl mice following a saline vehicle control (Fig. 2, A and B). In the saline vehicle control cohort, postprocedure blood glucose was not significantly different between mice fasted on pulp cellulose and corncob (Fig. 3B, right). Furthermore, postprocedure blood glucose in Hba1fl/fl mice fasted on pulp cellulose bedding was not significantly different when exposed to either phenylephrine or saline. Hba1fl/fl mice fasted on corncob bedding and exposed to phenylephrine showed exacerbated increases in blood glucose compared with pulp cellulose/phenylephrine and corncob/saline groups. This suggests that corncob bedding increases blood glucose in an α1-adrenergic-dependent manner.
Figure 3.
Initial fasting and postphenylephrine blood glucose are elevated with corncob bedding. A: initial fasting blood glucose was higher in CyB5R3fl/fl and Hba1fl/fl mice housed overnight on corncob bedding (CC) vs. pulp cellulose (PC). B: following the laser speckle contrast analysis (LASCA) procedure, postprocedure fasting blood glucose measurements were taken. Postphenylephrine blood glucose was higher in CyB5R3fl/fl and Hba1fl/fl mice housed overnight on corncob bedding vs. pulp cellulose. Mice treated with saline vehicle control did not show a CC-dependent increase in blood glucose. Mouse strain, sex, and Cre status are indicated. None of these animals was treated with tamoxifen and thus are background strain controls. Student’s t test or ANOVA with Šídák multiple comparisons test: *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Table 1.
Summary data for blood glucose and change in blood perfusion measurements
| Initial Fasting Blood Glucose, mg/dL |
Postisoflurane/Phenylephrine Blood Glucose, mg/dL |
Postisoflurane/Saline Blood Glucose, mg/dL |
Phenylephrine Perfusion Change, % |
Saline Perfusion Change, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | n | ||||||
| CyB5R3fl/fl | ||||||||||
| Pulp cellulose | 70.57 ± 4.07 | 7 | 122.20 ± 12.30 | 5 | N/A | −42.14 ± 2.59 | 5 | N/A | ||
| Corncob | 91.43 ± 7.94 | 7 | 192.60 ± 17.53 | 5 | N/A | −30.19 ± 2.39 | 5 | N/A | ||
| Hba1fl/fl | ||||||||||
| Pulp cellulose | 80.36 ± 6.73 | 14 | 174.57 ± 21.22 | 7 | 149.20 ± 9.79 | 5 | −40.84 ± 3.16 | 7 | 1.31 ± 2.27 | 5 |
| Corncob | 100.77 ± 4.46 | 13 | 295.00 ± 17.20 | 8 | 170.50 ± 26.26 | 4 | −38.58 ± 3.93 | 8 | −1.62 ± 2.79 | 4 |
Values are means ± SE; n, number of mice. N/A, not applicable.
Corncob Bedding Differentially Effects Adrenergic Responses between Strains
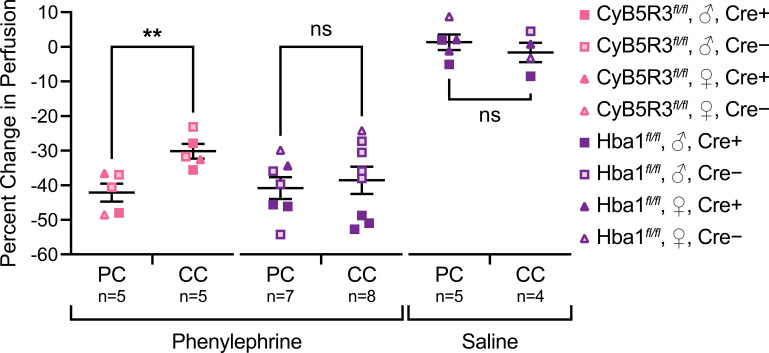
Quantifying phenylephrine-mediated change in perfusion in vivo revealed strain-specific physiological effects of corncob bedding (Table 1). CyB5R3fl/fl mice exhibited a significant reduction in the percent change in perfusion to corncob bedding (Fig. 4, left) that was not observed in Hba1fl/fl mice (Fig. 4, middle). The observed change in perfusion was due to phenylephrine as there was negligible change in perfusion in mice given a saline vehicle control (Fig. 4, right; Supplemental Videos 1 and 2, see https://doi.org/10.6084/m9.figshare.22311202.v1 and https://doi.org/10.6084/m9.figshare.22311925.v1, respectively).
Figure 4.
Corncob bedding has strain-specific effects on adrenergic-mediated changes in perfusion. When compared with pulp cellulose (PC), corncob bedding (CC) reduced the response to phenylephrine in CyB5R3fl/fl mice (left) but did not have that effect in Hba1fl/fl mice (middle). Injection of the saline vehicle control had negligible impact on blood perfusion (right). Mouse strain, sex, and Cre status are indicated. None of these animals was treated with tamoxifen and thus are background strain controls. Student’s t test: **P < 0.01; ns, not significant.
DISCUSSION
Although animal models are essential to research, they come with the added challenge of having numerous variables that need to be carefully identified and controlled. Even though there are many ways to mitigate potentially confounding variables, the possibility remains that differences in animal husbandry practices across institutions may have unintended effects. In this report, we further reinforce this concept by demonstrating that corncob bedding can influence vascular physiology and blood glucose.
In the current study, mice that had been born and raised on corncob bedding were fasted for a single night on either corncob or pulp cellulose bedding before experiments. Fasting blood glucose was significantly lower in mice fasted overnight on pulp cellulose bedding compared with corncob, which agrees with a recent report comparing corncob to hardwood bedding (8). When examining additional published studies reporting overnight FBG in mice, we found that bedding type was typically not mentioned. The mean overnight FBG values in these studies could generally be separated into two groups of ∼75 mg/dL (25, 27) and ∼100–110 mg/dL (30–33). When comparing those values to our pulp cellulose FBG of ∼70–80 mg/dL and corncob FGB of ∼90–100 mg/dL, it may be that reports of overnight FBG values ≥100 mg/dL were on corncob bedding or were otherwise incompletely fasted. Since corncob can contain trace sugars and potentially digestible hemicelluloses and fiber (34, 35), it would be expected that ingestion of corncob bedding by fasting rodents would increase FBG.
Blood glucose was similar between saline-exposed mice, regardless of bedding type, and pulp cellulose-fasted mice exposed to phenylephrine (Fig. 3). However, blood glucose was significantly increased in phenylephrine-exposed mice fasted on corncob. Phenylephrine stimulates immediate glucose production from liver glycogen stores and suppresses glycogen synthase (36, 37). Therefore, the observation that α1-adrenergic stimulation liberated a larger pool of glucose in corncob-fasted mice suggests that ingestion of corncob partially replenished glycogen stores in the absence of feed. Essentially, the corncob bedding was acting as an alternative source of nutrition.
We made the novel observation that CyB5R3fl/fl mice fasted on pulp cellulose were more reactive to phenylephrine compared with corncob bedding which showed a dampened α1-adrenergic-mediated percent change in perfusion. Recently, Dasinger et al. (3) observed corncob bedding reduces hypertension in Dahl salt-sensitive rats, which they hypothesized may be due to changes in the microbiome. In the current study, it is striking the impact of a one-night fast on pulp cellulose had on α1-adrenergic-mediated responses. Evidence supports that the microbiome can rapidly change within hours of an intervention (38) and a recent review examines the potential link between microbiome changes and hypertension (39). Thus, our findings that overnight fasting on corncob bedding dampens α-adrenergic receptor-mediated changes in perfusion may be attributed to acute alterations in the microbiome and presents a possible mechanism to explain the observations by Dasinger et al. (3). With these data in mind, we conclude that corncob bedding may impact vascular function and thus it is critical to disclose bedding use in publications.
The strain-specific difference in the α-adrenergic response is somewhat surprising, as these mice are on a similar C57BL/6J background and used the same Cre transgenic mouse line [C57BL/6-Tg(Cdh5-cre/ERT2)1Rha] (22). As with any congenic, genetically modified mouse line, the two strains used in the current study differ around the loxP-flanked gene targets (40), which may contribute to the differential effects observed. As the mice in this study were never treated with tamoxifen, the target genes should have remained intact. Despite these data coming from transgenic mouse strains, as opposed to unmodified C57BL/6J mice, the impact of corncob bedding is still important to report. Transgenic mouse strains are often unavoidable in research, and the Cdh5-cre/ERT2 strain is commonly used in the vascular field (24, 41–45). The current data suggest that corncob bedding may limit the ability to compare between different mouse strains. This could be especially vital if transgenic strains are shared between institutions, as different husbandry practices could impact the data generated.
Increasing Rigor through Reporting of Animal Husbandry
This report serves as an example, among many (3, 8, 12), why transparency of research animal husbandry practices is necessary for reproducibility and interdisciplinary application of observations. In mouse models alone, there is a wealth of evidence supporting that common husbandry elements can alter murine biology in many organ systems. These common husbandry elements include, but are not limited to, bedding type and volume (4, 7), housing temperature (46), cage size and density (47, 48), enrichment opportunities (49, 50), commercial food formulation (51), photoperiod (52), and disinfectant type (53–56). All of these examples demonstrate the possibility that critical variables between experiments could go unidentified because of a lack of descriptive methodology. With these reports and others in mind, it is imperative that researchers report comprehensive animal husbandry information for their studies, in addition to animal strain, sex, and age. This would include but is not limited to: 1) cage size, type, and manufacturer; 2) animal density; 3) bedding type, manufacturer, and volume; 4) vivarium temperature range; 5) photoperiod; 6) diet formulation and manufacturer; 7) enrichment materials provided; 8) disinfection protocols, products used, and frequency of cage changes.
Although journals may already require part of this information, especially in fields where the effects of these variables are well established, the inclusion of an abundance of seemingly routine details will only improve translation of studies across disciplines, institutions, and generations, as well as reproducibility of results. It may be that different fields have established standards that are largely shared word of mouth, such as appropriate beddings for metabolic experiments. Despite this, it cannot be assumed that every investigator is aware of these practices, and researchers outside a given field will not be aware of trade standards unless it is routinely documented. Thus, our data here and recent reports (8, 12) suggest that corncob should be avoided for metabolic and/or fasting studies.
Several agencies are increasing efforts to improve documentation of methods, including the National Institutes of Health, which recently posted a notice recommending the use of Animal Research: Reporting of In Vivo Experiments (ARRIVE) “Essential 10” guidelines for vertebrate and cephalopod research (Notice No.: NOT-OD-23-057). Although not part of the Essential 10, it should be emphasized that housing and husbandry are currently number 15 on the ARRIVE guidelines. As greater attention is placed on the impact husbandry practices can have on research outcomes, it is likely that agencies and journals will soon insist on reporting such information. Although variation in animal husbandry practices may not have significant impacts on all strains or fields, simply providing the information and being aware of the possibility could save animals, time, labor, and funding on troubleshooting failed protocols or conflicting results. Inclusion of comprehensive animal husbandry practices will undoubtedly contribute to improved rigor and reproducibility of research.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Video 1: https://doi.org/10.6084/m9.figshare.22311202.v1.
Supplemental Video 2: https://doi.org/10.6084/m9.figshare.22311925.v1.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL155618 (to B.E.I., A.C.S., and P.B.), HL161177 (to A.C.S.), HL088554 (to B.E.I.), and HL137112 (to B.E.I.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M.S. and P.B. conceived and designed research; T.M.S. and P.B. performed experiments; T.M.S. and P.B. analyzed data; T.M.S. and P.B. interpreted results of experiments; T.M.S. prepared figures; T.M.S. drafted manuscript; T.M.S., B.E.I., A.C.S., and P.B. edited and revised manuscript; T.M.S., B.E.I., A.C.S., and P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The abstract was accepted for presentation at the 2023 American Physiology Summit. Special thanks to Ray Mitchell for figure presentation and creative input. Additional thanks to the Vascular Research Program Writing Workgroup within University of Nebraska Medical Center’s Center for Heart and Vascular Research.
REFERENCES
- 1. Butler-Struben HM, Kentner AC, Trainor BC. What’s wrong with my experiment?: The impact of hidden variables on neuropsychopharmacology research. Neuropsychopharmacology 47: 1285–1291, 2022. doi: 10.1038/s41386-022-01309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reinhardt V. Common husbandry-related variables in biomedical research with animals. Lab Anim 38: 213–235, 2004. doi: 10.1258/002367704323133600. [DOI] [PubMed] [Google Scholar]
- 3. Dasinger JH, Walton SD, Burns EC, Cherian-Shaw M, Abais-Battad JM, Mattson DL. Impact of bedding on Dahl salt-sensitive hypertension and renal damage. Am J Physiol Renal Physiol . 323: F666–F672, 2022. doi: 10.1152/ajprenal.00201.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hudda N, Durant JL, Nemeth A, Mann P, Petitto J, Brugge D, Nephew BC. Bedding-generated particulate matter: implications for rodent studies. Inhal Toxicol 31: 368–375, 2019. doi: 10.1080/08958378.2019.1694109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moehring F, O'Hara CL, Stucky CL. Bedding material affects mechanical thresholds, heat thresholds, and texture preference. J Pain 17: 50–64, 2016. doi: 10.1016/j.jpain.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leys LJ, McGaraughty S, Radek RJ. Rats housed on corncob bedding show less slow-wave sleep. J Am Assoc Lab Anim Sci 51: 764–768, 2012. [PMC free article] [PubMed] [Google Scholar]
- 7. Freymann J, Tsai PP, Stelzer HD, Mischke R, Hackbarth H. Impact of bedding volume on physiological and behavioural parameters in laboratory mice. Lab Anim 51: 601–612, 2017. doi: 10.1177/0023677217694400. [DOI] [PubMed] [Google Scholar]
- 8. Kondo SY, Kropik J, Wong MA. Effect of bedding substrates on blood glucose and body weight in mice. J Am Assoc Lab Anim Sci 61: 611–614, 2022. doi: 10.30802/AALAS-JAALAS-22-000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregor A, Fragner L, Trajanoski S, Li W, Sun X, Weckwerth W, König J, Duszka K. Cage bedding modifies metabolic and gut microbiota profiles in mouse studies applying dietary restriction. Sci Rep 10: 20835, 2020.doi: 10.1038/s41598-020-77831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Leu RK, Conlon MA, Bird AR, Clarke JM. Housing experimental rats in solid-based cages with digestible bedding may confound outcomes of nutritional studies. J Sci Food Agric 95: 2155–2158, 2015. doi: 10.1002/jsfa.6919. [DOI] [PubMed] [Google Scholar]
- 11. Landeros RV, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-α expression in the brain. Endocrinology 153: 949–953, 2012. doi: 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ambery AG, Tackett L, Penque BA, Hickman DL, Elmendorf JS. Effect of corncob bedding on feed conversion efficiency in a high-fat diet-induced prediabetic model in C57Bl/6J mice. Am Assoc Lab Anim Sci, 53: 449–451, 2014. [PMC free article] [PubMed] [Google Scholar]
- 13. Gonder JC, Laber K. A renewed look at laboratory rodent housing and management. ILAR J 48: 29–36, 2007. doi: 10.1093/ilar.48.1.29. [DOI] [PubMed] [Google Scholar]
- 14. Perkins SE, Lipman NS. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34: 93–98, 1995. [PubMed] [Google Scholar]
- 15. Allen PS, Lawrence J, Stasula U, Pallas BD, Freeman ZT. Effects of compressed paper bedding on mouse breeding performance and recognition of animal health concerns. J Am Assoc Lab Anim Sci 60: 28–36, 2021. doi: 10.30802/AALAS-JAALAS-20-000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patisaul HB, Dindo M, Whitten PL, Young LJ. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor-and-dependent gene expression in the brain. Endocrinology 142: 2946–2952, 2001. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- 17. Ras T, van de Ven M, Patterson-Kane EG, Nelson K. Rats’ preferences for corn versus wood-based bedding and nesting materials. Lab Anim 36: 420–425, 2002. doi: 10.1258/002367702320389080. [DOI] [PubMed] [Google Scholar]
- 18. Krohn TC, Hansen AK. Evaluation of corncob as bedding for rodents. Scand J Lab Anim Sci 35: 231–236, 2008. doi: 10.23675/sjlas.v35i4.153. [DOI] [Google Scholar]
- 19. Mulder JB. Bedding preferences of pregnant laboratory-reared mice. Behav Res Methods and Instrumentation 7: 21–22, 1975. doi: 10.3758/BF03201283. [DOI] [Google Scholar]
- 20. Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 82: 51012, 2013. doi: 10.3791/51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burn CC, Mason GJ. Absorbencies of six different rodent beddings: commercially advertised absorbencies are potentially misleading. Lab Anim 39: 68–74, 2005. doi: 10.1258/0023677052886592. [DOI] [PubMed] [Google Scholar]
- 22. Sörensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113: 5680–5688, 2009. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 23. Durgin BG, Hahn SA, Schmidt HM, Miller MP, Hafeez N, Mathar I, Freitag D, Sandner P, Straub AC. Loss of smooth muscle CYB5R3 amplifies angiotensin II-induced hypertension by increasing sGC heme oxidation. JCI Insight 4: e129183, 2019. doi: 10.1172/jci.insight.129183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keller TCS 4th, Lechauve C, Keller AS, Broseghini-Filho GB, Butcher JT, Askew Page HR, Islam A, Tan ZY, DeLalio LJ, Brooks S, Sharma P, Hong K, Xu W, Padilha AS, Ruddiman CA, Best AK, Macal E, Kim-Shapiro DB, Christ G, Yan Z, Cortese-Krott MM, Ricart K, Patel R, Bender TP, Sonkusare SK, Weiss MJ, Ackerman H, Columbus L, Isakson BE. Endothelial alpha globin is a nitrite reductase. Nat Commun 13: 6405, 2022. doi: 10.1038/s41467-022-34154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carper D, Coué M, Laurens C, Langin D, Moro C. Reappraisal of the optimal fasting time for insulin tolerance tests in mice. Mol Metab 42: 101058, 2020. doi: 10.1016/j.molmet.2020.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morley LA, Gomez TH, Goldman JL, Flores R, Robinson MA. Accuracy of 5 point-of-care glucometers in C57BL/6J mice. J Am Assoc Lab Anim Sci 57: 44–50, 2018. [PMC free article] [PubMed] [Google Scholar]
- 27. Pomplun D, Möhlig M, Spranger J, Pfeiffer AFH, Ristow M. Elevation of blood glucose following anaesthetic treatment in C57Bl/6 mice. Horm Metab Res 36: 67–69, 2004. doi: 10.1055/s-2004-814104. [DOI] [PubMed] [Google Scholar]
- 28. Desborough JP, Jones PM, Persaud SJ, Landon MJ, Howell SL. Isoflurane inhibits insulin secretion from isolated rat pancreatic islets of Langerhans. Br J Anaesth 71: 873–876, 1993. doi: 10.1093/bja/71.6.873. [DOI] [PubMed] [Google Scholar]
- 29. Diltoer M, Camu F. Glucose homeostasis and insulin secretion during isoflurane anesthesia in humans. Anesthesiology 68: 880–886, 1988. doi: 10.1097/00000542-198806000-00008. [DOI] [PubMed] [Google Scholar]
- 30. Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Physiol 295: E1323–E1332, 2008. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 31. Hosoda Y, Okahara F, Mori T, Deguchi J, Ota N, Osaki N, Shimotoyodome A. Dietary steamed wheat bran increases postprandial fat oxidation in association with a reduced blood glucose-dependent insulinotropic polypeptide response in mice. Food Nutr Res 61: 1361778, 2017. doi: 10.1080/16546628.2017.1361778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han BG, Hao CM, Tchekneva EE, Wang YY, Lee CA, Ebrahim B, Harris RC, Kern TS, Wasserman DH, Breyer MD, Qi Z. Markers of glycemic control in the mouse: comparisons of 6-h-and overnight-fasted blood glucoses to Hb A1c. Am J Physiol Endocrinol Physiol 295: E981–E986, 2008. doi: 10.1152/ajpendo.90283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayala JE, Bracy DP, Mcguinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 34. Silva J. D, Oliveira R. D, Neto A. D S, Pimentel VC, Santos A. D A D. Extraction, addition and characterization of hemicelluloses from corn cobs to development of paper properties. Procedia Materials Sci 8: 793–801, 2015. doi: 10.1016/j.mspro.2015.04.137. [DOI] [Google Scholar]
- 35. Schwietzke S, Kim Y, Ximenes E, Mosier N, Ladisch M. Ethanol production from maize. In: Biotechnology in Agriculture and Forestry, edited by Kriz AL, Larkins BA.. Berlin: Springer International Publishing, 2009, Vol 63, p. 347–364. [Google Scholar]
- 36. González-Manchón C, Saz JM, Ayuso MS, Parrilla R. Characterization of the α-adrenergic stimulation of hepatic respiration. Arch Biochem Biophys 265: 258–266, 1988. doi: 10.1016/0003-9861(88)90126-9. [DOI] [PubMed] [Google Scholar]
- 37. Hutson NJ, Brumley FT, Assimacopoulos FD, Harper SC, Exton JH. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem 251: 5200–5208, 1976. doi: 10.1016/S0021-9258(17)33147-2. [DOI] [PubMed] [Google Scholar]
- 38. Salomon JD, Qiu H, Feng D, Owens J, Khailova L, Osorio Lujan S, Iguidbashian J, Chhonker YS, Murry DJ, Riethoven JJ, Lindsey ML, Singh AB, Davidson JA. Piglet cardiopulmonary bypass induces intestinal dysbiosis and barrier dysfunction associated with systemic inflammation. Dis Model Mech 16: dmm049742, 2023. doi: 10.1242/dmm.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zubcevic J, Richards EM, Yang T, Kim S, Sumners C, Pepine CJ, Raizada MK. Impaired autonomic nervous system-microbiome circuit in hypertension a premise for hypertension therapy. Circ Res 125: 104–116, 2019. doi: 10.1161/CIRCRESAHA.119.313965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silver LM. Mouse Genetics: Concepts and Applications. New York: Oxford University Press, 1995. [Google Scholar]
- 41. Zhang C, Lai MB, Pedler MG, Johnson V, Adams RH, Petrash JM, Chen Z, Junge HJ. Endothelial cell–specific inactivation of TSPAN12 (Tetraspanin 12) reveals pathological consequences of barrier defects in an otherwise intact vasculature. Arterioscler Thromb Vasc Biol 38: 2691–2705, 2018. doi: 10.1161/ATVBAHA.118.311689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sag CM, Schnelle M, Zhang J, Murdoch CE, Kossmann S, Protti A, Santos CXC, Sawyer G, Zhang X, Mongue-Din H, Richards DA, Brewer AC, Prysyazhna O, Maier LS, Wenzel P, Eaton PJ, Shah AM. Distinct regulatory effects of myeloid cell and endothelial cell NAPDH oxidase 2 on blood pressure. Circulation 135: 2163–2177, 2017. doi: 10.1161/CIRCULATIONAHA.116.023877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jin YJ, Chennupati R, Li R, Liang G, Wang S, Iring A, Graumann J, Wettschureck N, Offermanns S. Protein kinase N2 mediates flow-induced endothelial NOS activation and vascular tone regulation. J Clin Invest 131: e145734, 2021. doi: 10.1172/JCI145734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Breikaa RM, Denman K, Ueyama Y, McCallinhart PE, Khan AQ, Agarwal G, Trask AJ, Garg V, Lilly B. Loss of Jagged1 in mature endothelial cells causes vascular dysfunction with alterations in smooth muscle phenotypes. Vascul Pharmacol 145: 107087, 2022. doi: 10.1016/j.vph.2022.107087. [DOI] [PubMed] [Google Scholar]
- 45. Hamzaoui M, Groussard D, Nezam D, Djerada Z, Lamy G, Tardif V, Dumesnil A, Renet S, Brunel V, Peters DJM, Chevalier L, Hanoy M, Mulder P, Richard V, Bellien J, Guerrot D. Endothelium-specific deficiency of polycystin-1 promotes hypertension and cardiovascular disorders. Hypertension 79: 2542–2551, 2022. doi: 10.1161/HYPERTENSIONAHA.122.19057. [DOI] [PubMed] [Google Scholar]
- 46. Keijer J, Li M, Speakman JR. What is the best housing temperature to translate mouse experiments to humans? Mol Metab 25: 168–176, 2019. doi: 10.1016/j.molmet.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Loo PLP, Mol JA, Koolhaas JM, van Zutphen BFM, Baumans V. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72: 675–683, 2001. doi: 10.1016/S0031-9384(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 48. Ader DN, Johnson SB, Huang SW, Riley WJ. Group size, cage shelf level, and emotionality in non-obese diabetic mice: impact on onset and incidence of IDDM. Psychosom Med 53: 313–321, 1991. doi: 10.1097/00006842-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 49. Hutchinson EK, Avery AC, Vandewoude S. Environmental enrichment during rearing alters corticosterone levels, thymocyte numbers, and aggression in female BALB/c mice. J Am Assoc Lab Anim Sci 51: 18–24, 2012. [PMC free article] [PubMed] [Google Scholar]
- 50. Whitaker J, Moy SS, Godfrey V, Nielsen J, Bellinger D, Bradfield JB. Effects of cage size and enrichment on reproductive performance and behavior in C57BL/6Tac mice. Lab Anim 38: 24–34, 2009. doi: 10.1038/laban0109-24. [DOI] [PubMed] [Google Scholar]
- 51. Edwards MR, Dai R, Heid B, Cecere TE, Khan D, Mu Q, Cowan C, Luo XM, Ahmed SA. Commercial rodent diets differentially regulate autoimmune glomerulonephritis, epigenetics and microbiota in MRL/lpr mice. Int Immunol 29: 263–276, 2017. doi: 10.1093/intimm/dxx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yellon SM, Tran LT. Photoperiod, reproduction, and immunity in select strains of inbred mice. J Biol Rhythms 17: 65–75 2002. doi: 10.1177/074873002129002348. [DOI] [PubMed] [Google Scholar]
- 53. Melin VE, Potineni H, Hunt P, Griswold J, Siems B, Werre SR, Hrubec TC. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod Toxicol 50: 163–170, 2014. doi: 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hrubec TC, Melin VE, Shea CS, Ferguson EE, Garofola C, Repine CM, Chapman TW, Patel HR, Razvi RM, Sugrue JE, Potineni H, Magnin-Bissel G, Hunt PA. Ambient and dosed exposure to quaternary ammonium disinfectants causes neural tube defects in rodents. Birth Defects Res 109: 1166–1178, 2017. doi: 10.1002/bdr2.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hershey JD, Gifford JJ, Zizza LJ, Pavlenko DA, Wagner GC, Miller S. Effects of various cleaning agents on the performance of mice in behavioral assays of anxiety. J Am Assoc Lab Anim Sci 57: 335–339, 2018. doi: 10.30802/AALAS-JAALAS-17-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sciurba JD, Chlipala GE, Green SJ, Delaney MA, Fortman JD, Purcell JE. Evaluation of effects of laboratory disinfectants on mouse gut microbiota. Comp Med 71: 492–501, 2021. doi: 10.30802/AALAS-CM-21-000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: https://doi.org/10.6084/m9.figshare.22311202.v1.
Supplemental Video 2: https://doi.org/10.6084/m9.figshare.22311925.v1.
Data Availability Statement
Data will be made available upon reasonable request.