Abstract
Background
The morbidity and treatment costs associated with skin and soft tissue infections (SSTIs) are high. Linezolid and vancomycin are antibiotics that are commonly used in treating skin and soft‐tissue infections, specifically those infections due to methicillin‐resistant Staphylococcus aureus (MRSA).
Objectives
To compare the effects and safety of linezolid and vancomycin for treating people with SSTIs.
Search methods
For this first update of this review we conducted searches of the following databases: Cochrane Wounds Group Specialised Register (searched 24 March 2015; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; and EBSCO CINAHL. We also contacted manufacturers for details of unpublished and ongoing trials. We scrutinised citations within all obtained trials and major review articles to identify any additional trials.
Selection criteria
We included all randomised controlled trials (RCTs) comparing linezolid with vancomycin in the treatment of SSTIs.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias and extracted data. The primary outcomes were clinical cure, microbiological cure, and SSTI‐related and treatment‐related mortality. We performed subgroup analyses according to age, and whether the infection was due to MRSA.
Main results
No new trials were identified for this first update. We included nine RCTs (3144 participants). Linezolid was associated with a significantly better clinical (RR 1.09, 95% CI 1.03 to 1.16) and microbiological cure rate in adults (RR 1.08, 95% CI 1.01 to 1.16). For those infections due to MRSA, linezolid was significantly more effective than vancomycin in clinical (RR 1.09, 95% CI 1.03 to 1.17) and microbiological cure rates (RR 1.17, 95% CI 1.04 to 1.32). No RCT reported SSTI‐related and treatment‐related mortality. There was no significant difference in all‐cause mortality between linezolid and vancomycin (RR 1.44, 95% CI 0.75 to 2.80). There were fewer incidents of red man syndrome (RR 0.04, 95% CI 0.01 to 0.29), pruritus (RR 0.36, 95% CI 0.17 to 0.75) and rash (RR 0.27, 95% CI 0.12 to 0.58) in the linezolid group compared with vancomycin, however, more people reported thrombocytopenia (RR 13.06, 95% CI 1.72 to 99.22), and nausea (RR 2.45, 95% CI 1.52 to 3.94) when treated with linezolid. It seems, from the available data, that length of stay in hospital was shorter for those in the linezolid group than the vancomycin group. The daily cost of outpatient therapy was less with oral linezolid than with intravenous vancomycin. Although inpatient treatment with linezolid cost more than inpatient treatment with vancomycin per day, the median length of hospital stay was three days shorter with linezolid. Thus, total hospital charges per patient were less with linezolid treatment than with vancomycin treatment.
Authors' conclusions
Linezolid seems to be more effective than vancomycin for treating people with SSTIs, including SSTIs caused by MRSA. The available evidence is at high risk of bias and is based on studies that were supported by the pharmaceutical company that makes linezolid. Further well‐designed, independently‐funded, RCTs are needed to confirm the available evidence.
Keywords: Adult; Humans; Anti‐Bacterial Agents; Anti‐Bacterial Agents/adverse effects; Anti‐Bacterial Agents/therapeutic use; Drug Eruptions; Drug Eruptions/etiology; Length of Stay; Linezolid; Linezolid/adverse effects; Linezolid/therapeutic use; Pruritus; Pruritus/chemically induced; Randomized Controlled Trials as Topic; Skin Diseases, Bacterial; Skin Diseases, Bacterial/drug therapy; Soft Tissue Infections; Soft Tissue Infections/drug therapy; Thrombocytopenia; Thrombocytopenia/chemically induced; Vancomycin; Vancomycin/adverse effects; Vancomycin/therapeutic use
Plain language summary
Antibiotic drugs for treating skin and soft tissue infections
Skin and soft tissue infections such as impetigo, abscesses, ulcers, and surgical site infections are common infections of the skin. For serious skin and soft tissue infections involving the deeper tissues, the death rate and treatment costs are high. Linezolid and vancomycin are antibiotics that are effective in treating skin and soft tissue infections, particularly infections caused by bacteria that have developed resistance to some antibiotics. This review identified nine RCTs, with a total of 3144 participants, and compared treatment with linezolid against treatment with vancomycin for skin and soft tissue infections. No new trials were identified for this first update. Linezolid was found to be more effective than vancomycin for treating these infections. There were fewer skin complications in the group that were treated with linezolid. There were no differences between the two groups in the number of reported deaths, and those treated with linezolid had shorter lengths of hospital stay than those treated with vancomycin. The daily cost of outpatient therapy was less with oral linezolid than with intravenous vancomycin, although for inpatient treatment, linezolid was more expensive than vancomycin. Well‐designed trials will be required in future to confirm these results, as the trials from which these conclusions were drawn were of poor methodological quality, at high risk of bias, and were funded by the pharmaceutical company that makes linezolid.
Background
Please see the Glossary of terms in Appendix 1 for additional information and definitions.
Description of the condition
Skin and soft tissue infections (SSTIs) are common infections of the epidermis, dermis, or subcutaneous tissue and characterised by induration (hardening), erythema (redness), warmth and pain or tenderness and range from mild, self‐limiting furunculosis (boils) to life‐threatening necrotising fasciitis (Stevens 2005). SSTIs include:
Impetigo.
Abscesses, cellulitis (infection just under the skin), and erysipelas (skin infection).
Necrotising skin and soft‐tissue infections.
Infections following animal and human bites.
Soft‐tissue infections following animal contact.
Surgical site infections.
Infections in people whose immune systems are compromised.
Infections resulting from treatment (i.e. iatrogenic) (e.g. postoperative wounds).
The Food and Drugs Administration (FDA) classifies SSTIs as either "uncomplicated" or "complicated". Uncomplicated SSTIs are superficial infections and simple surgical incisions amenable to treatment with antibiotics, e.g. simple abscesses, carbuncles, impetigo lesions, furuncles (boils), and cellulitis. Complicated SSTIs are infections involving the deeper tissues, such as subcutaneous tissue, fascia, and skeletal muscle, or SSTIs in patients with co‐morbidities such as diabetes mellitus, HIV, and other immunocompromised states (FDA 1998). SSTIs are caused by a wide variety of organisms, most of which are Gram‐positive (i.e. are stained by a particular biological dye). The SENTRY Antimicrobial Surveillance Program has been monitoring SSTIs in more than 70 medical centres in North America, Europe, Latin America, and the Asia‐Pacific region since 1997. Their report, which presents data over a seven year period (1998 to 2004), ranks SSTIs by frequency of pathogen as follows: Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Enterococcus species, Klebsiella species, Enterobacter species, β‐haemolytic streptococci, coagulase‐negative staphylococci, Proteus mirabilis, and Acinetobacter species (Fritsche 2007). The complications of SSTIs, particularly those caused by Staphylococcus aureus, may cause bacteraemia (bacteria in the blood) and induce focal points of infection, such as bacterial endocarditis (the lining of the heart), osteomyelitis (bones), brain abscesses, brain meningitis, and/or lung abscesses (Eisenstein 2008), at a distance from the original infection.
The morbidity and treatment costs associated with SSTIs are high, and treatment has become more complex recently due to the increasing prevalence of multidrug‐resistant pathogens (Moet 2006). During the past decade the prevalence of antibiotic resistance among Gram‐positive cocci (bacteria) ‐ particularly Staphylococcus aureus ‐ has increased sharply. A considerable variation in the methicillin‐resistant Staphylococcus aureus (MRSA) rate was noted between countries and continents. According to the SENTRY report, the MRSA infection rate of all infections was 35.9 per cent in North America, compared with 29.4 per cent in Latin America and 22.8 per cent in Europe. The MRSA rate of all infections varied considerably among the European countries too, ranging from 0.8 per cent in Sweden to 50 per cent in Portugal (Fritsche 2007). Variability in MRSA infection rate was also apparent in Latin America, where Mexico had 50 per cent of all infections, Chile 38 per cent, Brazil 29 per cent, Argentina 28 per cent, and Columbia and Venezuela combined had 3 per cent (Moet 2006). Antibiotic resistance increases the length of stay in hospital, costs of treatment and the mortality rate. A review of the epidemiology of severe Staphylococcus aureus infections in Europe reports that the overall seven‐day case fatality rate was 19 per cent (Lamagni 2008). A US study reported that patients with MRSA‐infected surgical sites had a three times greater 90‐day mortality rate and a greater duration of hospitalisation after infection (median additional days 5; P value less than 0.001) than patients infected by methicillin‐sensitiveStaphylococcus aureus (MSSA). Median hospital charges were USD 92,363 for patients with MRSA surgical site infections compared with USD 52,791 for patients with MSSA infections (Engemann 2003).
Description of the intervention
The treatment of uncomplicated SSTIs and complicated SSTIs differs, with different clinical outcomes. Uncomplicated SSTIs are usually treated locally with, or without, antibiotics, whereas most complicated SSTIs require hospitalisation, treatment with intravenous antibiotics, and possibly surgical intervention (Eron 2003). Choice of the initial antibiotic is crucial for patients with complicated SSTIs. It has been demonstrated that correct use of antibiotics is associated with lower morbidity and mortality in patients who have an infection (Bouza 2004).
The antibiotics commonly used for the treatment of SSTIs caused by Gram‐positive cocci are beta‐lactams (including semisynthetic penicillins and cephalosporins), clindamycin, vancomycin, and linezolid (Fung 2003). Beta‐lactam antibiotics remain the mainstay of treatment for suspected streptococcal and MMSA infections. In proven penicillin‐sensitive infection, use of benzyl penicillin remains appropriate. Clindamycin can be administered in combination with beta‐lactam antibiotics for rapidly‐progressing infections, such as severe streptococcal infections, where beta‐lactam antibiotics alone are less effective. During the past decade the prevalence of antibiotic resistance among Gram‐positive cocci (e.g. MRSA) has increased sharply. Vancomycin has been the mainstay of therapy in MRSA infections and for patients who are intolerant of or allergic to the beta‐lactams. Linezolid is a novel oxazolidinone agent for use against staphylococci and enterococci, with a spectrum of activity against Gram‐positive bacteria similar to that of vancomycin so linezolid and vancomycin are often compared.
How the intervention might work
Mechanism of action
Vancomycin is a traditional antibiotic for the treatment of Gram‐positive cocci, especially MRSA, which acts by inhibiting proper cell wall synthesis in Gram‐positive bacteria. Due to the different mechanism by which Gram‐negative bacteria produce their cell walls, and the various factors related to entering the outer membrane of Gram‐negative organisms, vancomycin is not active against Gram‐negative bacteria (except some non‐gonococcal species of Neisseria).
Linezolid, the first member of the oxazolidinone class of antibiotics to be approved by the FDA, is indicated for the treatment of SSTIs caused by methicillin‐sensitive or methicillin‐resistant S aureus, or vancomycin‐resistant enterococci and other susceptible micro‐organisms (Fung 2001). Linezolid has a unique mechanism of action. It stops the growth and reproduction of bacteria by disrupting translation of messenger RNA into proteins in the ribosome. Linezolid selectively binds to the 50S ribosomal unit and inhibits initiation of complex protein synthesis (Wilson 2008). This unique mechanism has not been seen in any other antibiotic agents, thus, cross‐resistance of linezolid has not been observed. One of the advantages of linezolid is its high bioavailability when given by mouth (close to 100 per cent). This means that people receiving intravenous linezolid may be switched to oral linezolid as soon as their condition allows it, whereas vancomycin can only be given intravenously (Moellering 1999).
Resistance
During recent years, the decreasing susceptibility of some bacteria to traditional antibiotics has been a significant problem in treating SSTIs. The increasing incidence of infections caused by resistant Gram‐positive cocci has led to a sharp increase in the use of vancomycin (Pallares 1993). As a result, the emergence of vancomycin‐resistant strains of enterococci and staphylococci has been widely observed in the last few years. Between the years 1998 to 2004 vancomycin‐resistant enterococci increased in Europe to 4.1 per cent, and in North America to 6.2 per cent (Fritsche 2007)
The resistance of Gram‐positive bacteria to linezolid has not been noted to the same extent. Linezolid‐resistant Staphylococcus aureus was first isolated in 2001 (Tsiodras 2001). The seventh year of the Zyvox Annual Appraisal of Potency and Spectrum Program (2008) monitored the in vitro activities of linezolid and comparator agents tested against Gram‐positive pathogens in Latin America, Europe, Canada, and the Asia‐Pacific region. Overall resistance to linezolid in 23 countries was only 0.13 per cent across all monitored Gram‐positive pathogens. Oxazolidinone‐resistant strains continued to be identified in several nations (Brazil, China, France, Germany, Italy, and Sweden) and among three prevalent pathogen groups (S aureus, coagulase‐negative staphylococci, and enterococci) (Jones 2009).
Adverse reactions
The common adverse reactions indicated for vancomycin are nephrotoxicity (damage to kidneys) and ototoxicity (damage to ears) (Finch 2005). These adverse reactions were both side‐effects of early, impure versions of vancomycin (Levine 2006). Later trials, that used purer forms of vancomycin, found that, while renal toxicity (kidney damage) is an infrequent adverse effect, it is accentuated by the presence of aminoglycosides (Finch 2005). Erythroderma (red man syndrome) may also occur. This syndrome is an allergic reaction characterised by the flushing of the upper body, with itching due to histamine release (Sivagnanam 2003).
When used for short periods, linezolid is a relatively safe drug. Long‐term use of linezolid has been associated with bone marrow suppression, which is characterised particularly by thrombocytopenia (low blood platelet count). Thrombocytopenia appears to be the only adverse effect that occurs significantly more frequently with linezolid than with glycopeptides or beta‐lactams (Falagas 2008).
Why it is important to do this review
Several studies have compared linezolid with vancomycin; the outcomes were inconsistent. Other reviews have reported that linezolid is more effective than vancomycin in the treatment of SSTIs caused by Gram‐positive bacteria or MRSA (Beibei 2010; Bounthavong 2010; Falagas 2008). The outcome from another systematic review (Dodds 2009), however, showed that there is no statistically significant difference between linezolid with vancomycin. It disagreed with the conclusions of the other three reviews. Until now, there has been no Cochrane systematic review to summarise the evidence for the beneficial and adverse effects of linezolid compared with vancomycin in people with SSTIs.
Objectives
To compare the effects of linezolid and vancomycin for treating skin and soft tissue infections.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) that compared linezolid with vancomycin in the treatment of skin and soft tissue infections.
Types of participants
We included people of any age or gender presenting with skin and soft tissue infections (e.g. cellulitis, erysipelas, furuncles, simple abscesses, wound infections, and deeper infections such as necrotising fasciitis, myositis (inflammation of muscles), and gas gangrene).
Types of interventions
Any dose of linezolid or vancomycin, by any route.
We intended to present comparisons as follows: 1. Linezolid compared with vancomycin alone. 2. Linezolid plus co‐interventions compared with vancomycin plus co‐interventions.
Co‐interventions might include other antibiotics for use against Gram‐negative bacteria, or other routine medications and surgical interventions, as long as participants in the different trial arms had equal access to such co‐interventions.
Types of outcome measures
Primary outcomes
1. Clinical cure (resolution of symptoms and signs) and microbiological cure (eradication of bacteria on wound culture).
Proportion of patients or infections healed. We defined healing as either the resolution of all clinical signs and symptoms of infection as assessed by laboratory test or defined by trialists, or microbiological cure (i.e. eradication of MRSA on wound culture).
2. SSTI‐related and treatment‐related mortality.
Secondary outcomes
Adverse events.
Duration of hospital stay.
Duration of treatment.
Costs.
Search methods for identification of studies
Electronic searches
For this first update of this review we searched the following electronic databases to find reports of relevant RCTs:
The Cochrane Wounds Group Specialised Register (searched 24 March 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 2);
Ovid MEDLINE (1950 to March Week 4 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, March 23 2015);
Ovid EMBASE (1980 to March 23 2015);
EBSCO CINAHL (1982 to March 24 2012).
The following search strategy was used in CENTRAL and modified appropriately for other databases: #1 MeSH descriptor Oxazolidinones explode all trees #2 MeSH descriptor Oxazolone explode all trees #3 (linezolid* or oxazolone*):ti,ab,kw #4 MeSH descriptor Glycopeptides explode all trees #5 (vancomycin* or glycopeptide*):ti,ab,kw #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Soft Tissue Infections explode all trees #8 MeSH descriptor Staphylococcal Skin Infections explode all trees #9 MeSH descriptor Cellulitis explode all trees #10 MeSH descriptor Erysipelas explode all trees #11 MeSH descriptor Furunculosis explode all trees #12 MeSH descriptor Abscess explode all trees #13 MeSH descriptor Wound Infection explode all trees #14 MeSH descriptor Fasciitis, Necrotizing explode all trees #15 MeSH descriptor Myositis explode all trees #16 MeSH descriptor Gas Gangrene explode all trees #17 (soft NEXT tissue NEXT infection* or skin NEXT infection*):ti,ab,kw #18 (cellulitis or erysipelas or furuncul* or abscess* or absess* or "necrotizing fasciitis" or myositis or "gas gangrene"):ti,ab,kw #19 (wound* NEAR/2 infect*):ti,ab,kw #20 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 (#6 AND #20)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). There were no restrictions with respect to language, date of publication or study setting.
Searching other resources
We also contacted Pharmacia, Pfizer Pharmaceuticals in China for details of unpublished and ongoing trials. We scrutinised citations within all identified trials and major review articles to identify any additional trials.
Data collection and analysis
Selection of studies
Two review authors (YJ and YM) independently scanned the title, abstract and keywords of every record retrieved to determine which studies required further assessment. Full articles were retrieved when the information given in the titles, abstracts and keywords suggested the possibility that:
The study compared linezolid and vancomycin (with or without co‐interventions).
The study had a prospective design.
If, after scanning the titles and abstracts, there was any doubt regarding these criteria, we retrieved the full article for clarification. We resolved disagreement by discussion with a third review author (BD), where necessary.
Data extraction and management
Two review authors (YJ and YM) independently extracted data using a standard data extraction form specifically adapted for this review. The data extraction form included the following details:
General information: whether the paper was published or unpublished, title, authors, country of study, contact address, year of study, language of publication, year of publication, sponsor or funding organisation, setting.
Methodological details: including criteria for risk of bias assessment (see below).
Intervention: descriptions of dose, route, and timing of linezolid and vancomycin, with descriptions of dose, route, and timing of co‐medication(s).
Participants: inclusion and exclusion criteria, total number recruited and numbers in comparison groups, sex, age, baseline characteristics, withdrawals and losses to follow‐up with reasons and descriptions, subgroups.
Outcomes: clinical cure, microbiological cure, SSTI‐related and treatment‐related mortality, adverse events, duration of treatment, duration of hospitalisation and costs.
If information regarding data was unclear, we attempted to contact the authors of the original study reports to provide further details. When more than one publication related to the same study, we extracted data from all relevant publications, but did not duplicate the data.
Assessment of risk of bias in included studies
Two review authors (YJ andYM) independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011a). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. baseline imbalances) (see Appendix 3 for details of criteria on which the judgements were based). Blinding and completeness of reporting of outcome data were assessed for each outcome separately. We completed a risk of bias table for each eligible study. We discussed any disagreements amongst all review authors to achieve a consensus.
We presented the assessment of risk of bias using a 'risk of bias summary figure', which presents all of the judgments in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
Measures of treatment effect
Dichotomous data
We presented dichotomous outcomes (e.g. clinical cure, microbiological cure, adverse events, mortality) as risk ratios (RR) with corresponding 95% confidence intervals (CI). For statistically significant effects (primary outcomes), we calculated number the needed to treat (NNT) from the risk difference (RD).
Continuous data
We presented continuous data (e.g. duration of hospitalisation, duration of treatment, and costs) as mean differences (MD) with corresponding 95% CI.
Unit of analysis issues
Comparisons that randomise or allocate clusters (e.g. clinics) but do not account for clustering during analysis have potential unit of analysis errors resulting in artificially low P values and over‐narrow confidence intervals. We decided to attempt to re‐analyse studies with potential unit of analysis errors by calculating effective sample sizes, where possible (Higgins 2011b). If a comparison was re‐analysed, then the P value was to be quoted and annotated as "re‐analysed". If this was not possible, we would have reported only the point estimate (Donner 2001). If trials included multiple intervention groups receiving a complex intervention as defined above, we would have split the shared control group into two or more groups with smaller sample sizes, depending on the number of interventions studied, and included two or more comparisons (Higgins 2011b).
Dealing with missing data
When data were missing from the trial reports, we attempted to contact the trial authors to request these values. If this was not successful, we conducted intention‐to‐treat (ITT) analysis for all dichotomous outcomes (e.g. clinical cure, microbiological cure, adverse events, mortality). We analysed data on an endpoint basis for continuous outcomes (e.g. duration of hospital stay, duration of treatment, and costs), including only those participants for whom a final data point measurement was obtained (available case analysis). If the standard deviation (SD) was missing, and when the standard error (SE) was available, we imputed the SD from the SE using the formula SD = SE x N‐2 (Higgins 2011b).
Assessment of heterogeneity
Population, methodology, intervention and outcome measures for each study were assessed for clinical heterogeneity to see if pooling of results was feasible. Assessment for heterogeneity was carried out using the chi‐squared test, with significance set at P value less than 0.1. In addition I2 was used to estimate the total variation due to heterogeneity across studies (Higgins 2003). Values of I2 less than 25 per cent were regarded as representing low heterogeneity, and we would then use a fixed‐effect model for meta‐analysis. Values of I2 between 25 and 75 per cent were considered to represent moderate levels of heterogeneity, and we then used a random‐effects model. Values of I2 higher than 75 per cent indicated high levels of heterogeneity, in which case we did not undertake meta‐analysis.
Assessment of reporting biases
If enough studies were identified, funnel plot analysis would have been performed to check for publication bias.
Data synthesis
We pooled results following assessment for statistical heterogeneity as described above. Statistical analysis was performed in accordance with the guidelines for statistical analysis in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We conducted a narrative review of eligible studies if there was only one trial or the I2 statistic was above 75%. A fixed‐effect method would be used where there were too few studies (less than three) to inform the distribution of heterogeneity.
Subgroup analysis and investigation of heterogeneity
To assess whether the treatment effect is modified by clinical and demographic variables, we undertook subgroup analyses as follows:
Children (younger than 18 years) and adults (18 years or older).
Uncomplicated SSTIs and complicated SSTIs.
MRSA subset.
Sensitivity analysis
If a sufficient number of trials were found, sensitivity analysis would be done to assess the robustness of the results as follows:
Exclusion of studies with inadequate concealment of allocation.
Exclusion of studies in which outcome evaluation was not blinded.
Results
Description of studies
See the "Characteristics of included studies" and "Characteristics of excluded studies" tables.
Results of the search
In total, we retrieved 670 articles through searching electronic databases; 11 articles were excluded because they were duplicates, and 659 were excluded after reading the abstracts and applying our inclusion criteria. The full text of the remaining 31 articles was reviewed, and 18 met the inclusion criteria for the review. Of these, thirteen articles were multiple publications, in which nine articles relate to four trials (Lin 2008; Stevens 2002; Weigelt 2005; Yogev 2003). Other five papers (Itani 2010; Jaksic 2006; Kohno 2007;Sharpe 2005; Wilcox 2009) were not multiple publications and referred to one trial each. Finally nine RCTs were included in the review (Figure 1). No new trials were identified for this first update.
1.
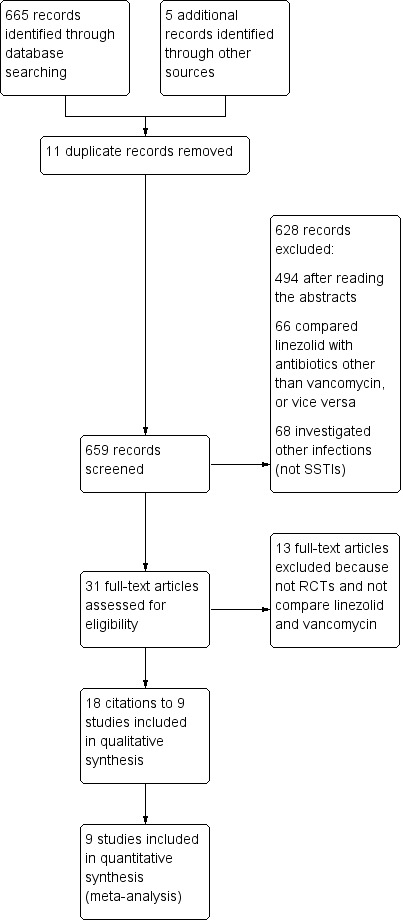
Study flow diagram.
Included studies
Nine RCTs were included in the review (Itani 2010; Jaksic 2006; Kohno 2007; Lin 2008; Sharpe 2005; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003). Four of these nine RCTs evaluated SSTIs as the only type of infection (Itani 2010; Sharpe 2005; Weigelt 2005; Yogev 2003); four evaluated mixed infections and included types of infection other than SSTIs, such as bacteraemia of unknown source, pneumonia, and urinary tract infections (Jaksic 2006; Kohno 2007; Lin 2008; Stevens 2002); and one evaluated central venous, pulmonary artery, or arterial catheter‐related infection with a subset of SSTIs (Wilcox 2009).
Characteristics of studies
All RCTs were parallel‐group studies: two trials were randomised in a 2:1 ratio (Kohno 2007; Yogev 2003); one was a single‐centre RCT located in the USA (Sharpe 2005), another was a multicentre RCT located in Japan (Kohno 2007); and a third was a multicentre RCT located in China (Lin 2008). The remaining six RCTs were multinational studies (Itani 2010; Jaksic 2006; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003). The duration of follow‐up ranged from 28 to 42 days.
Characteristics of patients
A total of 4496 participants were randomised, 3114 of whom had SSTIs. The subgroup data for SSTIs were obtained from trial reports; all were complicated SSTIs. Four RCTs reported the types of SSTIs (Itani 2010; Sharpe 2005; Weigelt 2005; Yogev 2003): abscess and infected skin ulcer was the most common infection (39.7%), followed by cellulitis (35.6%) and surgical wound infection (24.7%). Five trials enrolled people with MRSA infections (Itani 2010; Kohno 2007; Sharpe 2005; Stevens 2002; Weigelt 2005), while four trials enrolled people with Gram‐positive bacterial infection (Jaksic 2006; Lin 2008; Wilcox 2009; Yogev 2003).
One RCT included children younger than 12 years old, with a mean age of 3.25 years (Yogev 2003). Five RCTs included adults (aged 18 years or over) with a mean age of 59.7 years (Itani 2010; Kohno 2007; Lin 2008; Sharpe 2005; Weigelt 2005). The remaining three RCTs included mixed populations over the age of 13 years, with a mean age of 54.4 years (Jaksic 2006; Stevens 2002; Wilcox 2009).
All RCTs included both males and females. A larger proportion of males was recruited, and they constituted 58.9% of all randomised patients. Only one RCT recruited more females than males, at 67% (Sharpe 2005).
Study treatment details
The doses of each drug were similar in all trials. In the RCTs with participants 13 years old or older, 600 mg linezolid was given as either an intravenous injection (IV) or orally every 12 hours whilst 1000 mg vancomycin was given IV every 12 hours except one RCT(Itani 2010) in which patients were randomised to receive oral or intravenous linezolid 600 mg every 12 hours, or intravenous vancomycin 15 mg/kg mg every 12 hours with dose adjustment as necessary, based on trough levels and creatinine clearance. The route of linezolid administration was IV in two RCTs (Jaksic 2006; Lin 2008); IV followed by oral administration in six RCTs (Itani 2010; Kohno 2007; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003); and oral in only one RCT (Sharpe 2005). One RCT that enrolled participants under the age of 12 years (Yogev 2003), gave participants 10 mg/kg IV linezolid every eight hours or 10 to 15 mg/kg vancomycin every six to 24 hours according to age‐dosing guidelines. People with concomitant presumed Gram‐negative or mixed infections were treated with appropriate regimens, mainly aztreonam and aminoglycoside. The two groups had equal access to such co‐interventions.
Excluded studies
Eight studies were excluded after assessment of the full‐text. Reasons for exclusion were:
Not a RCT(Joseph 2007; Bal 2013 )
Were cost‐effectiveness analysis (Bounthavong 2009; Hau 2002; Kalil 2006; Lipsky 2011; Schurmann 2009; Janis 2014)
Were a comment (Kalil 2006) or a review (McKinnon 2007)
Were health economics analysis (Patanwala 2007;)
Were post‐hoc pooled data analysis (Puzniak 2014; Puzniak 2013)
Not compare linezolid vs. vancomycin (Bhavnani 2015)
Risk of bias in included studies
Risk of bias assessments are detailed in the Characteristics of included studies table and are represented by Figure 2 and Figure 3. In general, all trials were at high risk of bias.
2.
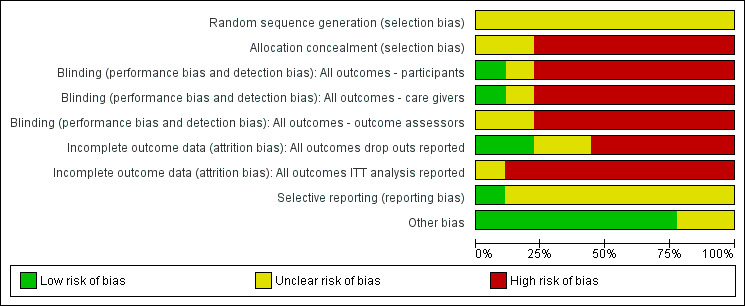
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
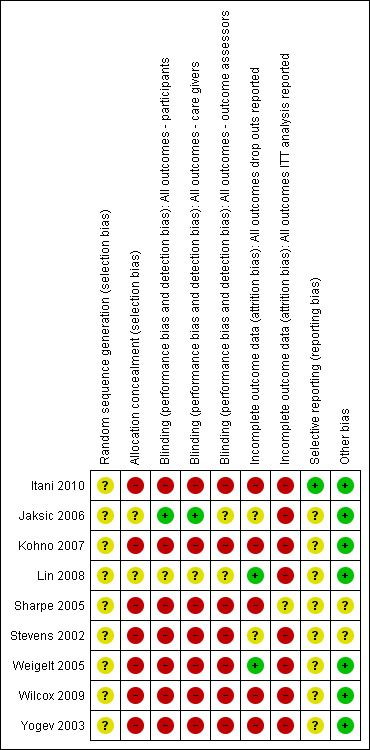
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Sequence generation was unclear in all included studies.
Allocation concealment
Allocation concealment was unclear in two RCTs (Jaksic 2006; Lin 2008). The treatment allocation was not concealed in the remaining seven studies (Itani 2010; Kohno 2007; Sharpe 2005; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003).
Overall there was high risk of selection bias.
Blinding
Participants
The participants were blinded in Jaksic 2006 for all reported outcomes. Lin 2008 only reported that the study was "double‐blind", but did not report details about who was blinded. The other seven RCTs were not blinded (Itani 2010; Kohno 2007; Sharpe 2005; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003).
Care givers
The care givers were blinded in Jaksic 2006 for all reported outcomes. Lin 2008 only reported that the study was "double‐blind", but did not report details about who was blinded.
Outcomes assessor
Two RCTs did not report whether outcome assessors were blinded (Jaksic 2006; Lin 2008). The outcome assessors in the other seven RCTs were not blinded (Itani 2010; Kohno 2007; Sharpe 2005; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003).
Overall, the only two trials that reported being "double‐blind' were small, and contributed to two primary analyses. Most of the trials did not undertake blinding, and so are at high risk of performance and detection bias.
Incomplete outcome data
Two RCTs (Itani 2010; Sharpe 2005) did not undertake ITT analyses. Seven RCTs (Jaksic 2006; Kohno 2007; Lin 2008; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003) reported that an ITT analysis was performed. After looking at the full text of the potential seven trials and comparing the data reported at the beginning of randomisation with the data included in the analysis, however, we found that there were discrepancies between baseline data and final analysis which indicated that ITT had not been performed. So we assumed that none of the included trials undertook ITT analyses. Clinical cure and microbiological cure were reported by all studies, other outcomes were reported by some of the trials. We contact the authors requesting these data, but, to date, have received no reply.
Clinical and microbiological cure
All RCTs reported clinical and microbiological cure. Two trials, in which incomplete outcome data were adequately addressed, were judged to be at low risk of bias (Lin 2008; Weigelt 2005). Another two studies reported dropouts only for MRSA infections, but it was not clear how many dropouts were from the SSTIs subset, so we judged the trials to be at unclear risk of bias (Jaksic 2006; Stevens 2002). The remaining five RCTs did not report reasons or the numbers of dropouts, and were judged to be at high risk of bias.
SSTI‐related and treatment‐related mortality
No RCT reported SSTI‐related and treatment‐related mortality. Five RCTs reported all‐cause mortality (Itani 2010; Jaksic 2006; Weigelt 2005; Wilcox 2009; Yogev 2003). Of these, three trials reported mortality in SSTI patients (Itani 2010; Weigelt 2005; Yogev 2003), while the other two reported mortality in mixed populations (Jaksic 2006; Wilcox 2009). Mortality data relating to the SSTI groups could not be extracted.
Adverse events
Three RCTs evaluated drug‐related adverse events in SSTI participants (Itani 2010; Weigelt 2005; Yogev 2003); one RCT did not report adverse events (Sharpe 2005); while the remaining five RCTs reported adverse events of mixed infection types such as bacteraemia, pneumonia, and urinary tract infections (Kohno 2007; Lin 2008; Jaksic 2006; Stevens 2002; Wilcox 2009). We contacted the study authors for data relating to the SSTI subsets, but have not yet received a response.
Duration of hospital stay
Four RCTs reported the duration of hospital stay (Itani 2010; Sharpe 2005; Stevens 2002; Weigelt 2005), reported as the mean number of days and P value.
Duration of treatment
Seven RCTs reported the duration of treatment (Itani 2010; Jaksic 2006; Kohno 2007; Lin 2008; Stevens 2002; Weigelt 2005; Wilcox 2009), but only two RCTs specifically reported the results for the subsets of SSTI patients (Itani 2010; Weigelt 2005).
Costs
Two trials reported the treatment cost (Sharpe 2005; Weigelt 2005), but Sharpe 2005 reported only the mean cost and did not report the variance (SD).
Conclusion
Overall, two of the nine RCTs adequately addressed incomplete outcome data, and had a low dropout rate (Lin 2008; Weigelt 2005). In addition, although some of the included studies reported that they undertook ITT analyses, this was not confirmed by the data presented and, therefore, we have judged them to be at high risk of attrition bias.
Selective reporting
We found the protocol for one RCT (Itani 2010); all of the primary and secondary outcomes pre‐specified in the protocol were subsequently reported, and, accordingly, the trial was judged to be at low risk of bias for this domain. We searched, but did not find the protocols for the other included trials, and so the remaining eight RCTs were judged to be at unclear risk of bias.
Other potential sources of bias
There were baseline imbalances in two trials: in Stevens 2002, participants in the linezolid group were significantly older than those in the vancomycin group (63.9 versus 59.8 years; P value 0.0157), while in Sharpe 2005, patients in the linezolid group were significantly younger than those in the vancomycin group (66 versus 76 years). These two trials were judged to be at an high risk of bias for this domain.
Effects of interventions
Linezolid compared with vancomycin (nine RCTs)
Primary outcomes
Clinical cure
Eight RCTs that reported outcomes in adult or mixed populations (Itani 2010; Jaksic 2006; Kohno 2007; Lin 2008; Sharpe 2005; Stevens 2002; Weigelt 2005; Wilcox 2009), and one RCT that reported outcomes in children (Yogev 2003), were included for this outcome. In total, 3114 participants with SSTIs were randomised in nine RCTs. We conducted ITT analysis for all randomised participants. We coded indeterminate outcomes and missing data as "no cure". Pooling of the nine trials demonstrated a statistically significant difference in cure rate of SSTIs in favour of linezolid (RR 1.09, 95% CI 1.03 to 1.16; I2 = 13%; Analysis 1.1). The NNT was 20.
1.1. Analysis.
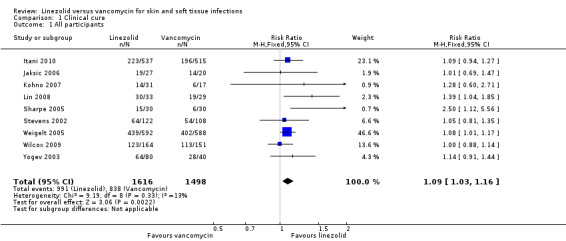
Comparison 1 Clinical cure, Outcome 1 All participants.
Microbiological cure
The meta‐analysis to evaluate the microbiological cure rate included 2014 SSTI participants from all nine trials with a positive culture at baseline. More SSTIs achieved microbiological cure when treated with linezolid than with vancomycin (RR 1.08, 95% CI 1.01 to 1.16; I2 = 42%; Analysis 2.1).The NNT was 20.
2.1. Analysis.
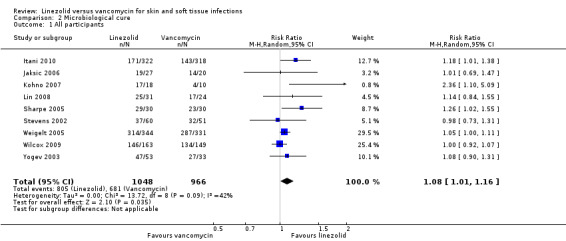
Comparison 2 Microbiological cure, Outcome 1 All participants.
SSTI‐related and treatment‐related mortality
No RCT reported SSTI‐related or treatment‐related mortality. Three RCTs (2352 participants) reported all‐cause mortality of SSTI patients (Itani 2010; Weigelt 2005; Yogev 2003), and found there was no significant difference in all‐cause mortality between linezolid and vancomycin (RR 1.44, 95% CI 0.75 to 2.80; I2 = 0%; Analysis 3.1). Although two RCTs (1331 participants) reported mortality data in hospitalised, febrile adults with cancer and proven, or suspected, Gram‐positive bacterial infection (Jaksic 2006); and catheter‐related infection (Wilcox 2009), the data were mixed, and mortality data due to SSTIs could not be extracted. We contacted the trial authors to obtain separate mortality data for patients with SSTIs, but have not received any response. The remaining four RCTs did not report mortality data.
3.1. Analysis.
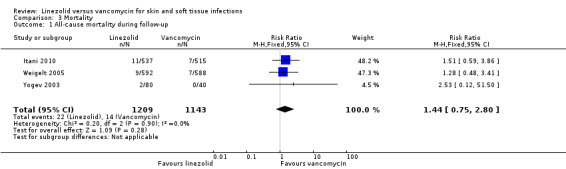
Comparison 3 Mortality, Outcome 1 All‐cause mortality during follow‐up.
Secondary outcomes
Adverse events
One trial (726 participants) reported adverse events including events related, or unrelated, to treatment (Wilcox 2009). Seven trials (3710 participants) reported data on treatment‐related adverse events (Itani 2010; Jaksic 2006; Kohno 2007; Lin 2008; Stevens 2002; Weigelt 2005; Yogev 2003). One trial (60 participants) did not mention adverse events (Sharpe 2005).
Three RCTs evaluated adverse events in SSTI participants and were included in the meta‐analysis (Itani 2010; Weigelt 2005; Yogev 2003), while the other five RCTs reported data on adverse effects for a variety of infection types, such as bacteraemia, pneumonia, and urinary tract infections, so that data relating to SSTIs could not be extracted (Kohno 2007; Lin 2008; Jaksic 2006; Stevens 2002; Wilcox 2009).
Fewer people in the linezolid group had red man syndrome (two RCTs, 1172 patients; RR 0.04, 95% CI 0.01 to 0.29; I2 = 0%; Analysis 4.3), pruritus (three RCTs, 2352 patients; RR 0.36, 95% CI 0.17 to 0.75; I2 = 1%; Analysis 4.4), and rash (three RCTs, 2352 patients; RR 0.27, 95% CI 0.12 to 0.58; I2 = 6%; Analysis 4.5), when compared with vancomycin. More people in the linezolid group had thrombocytopenia (two RCTs, 1300 patients; RR 13.06, 95% CI 1.72 to 99.22; I2 = 31%; Analysis 4.6), and nausea (two RCTs, 2232 patients; RR 2.45, 95% CI 1.52 to 3.94; I2 = 0%; Analysis 4.8). No differences were reported for anaemia (two RCTs,1300 patients; RR 0.73, 95% CI 0.33 to 1.62; I2 = 0%; Analysis 4.1), diarrhoea (three RCTs, 2352 patients; RR 1.78, 95% CI 0.82 to 3.88; I2= 59%; Analysis 4.2), headache (two RCTs,2232 patients; RR 1.23, 95% CI 0.59 to 2.61; I2= 59%; Analysis 4.7), or vomiting (two RCTs, 2232 patients; RR 2.20, 95% CI 0.96 to 5.04; I2 = 0%; Analysis 4.9).
4.3. Analysis.
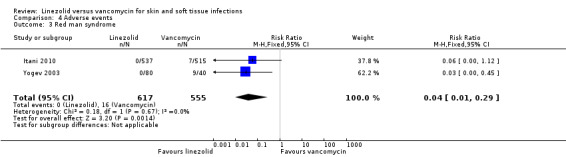
Comparison 4 Adverse events, Outcome 3 Red man syndrome.
4.4. Analysis.
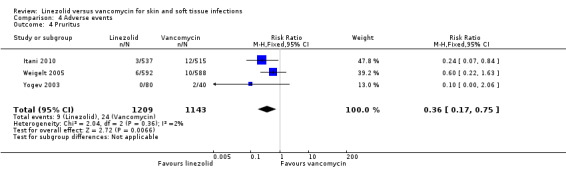
Comparison 4 Adverse events, Outcome 4 Pruritus.
4.5. Analysis.
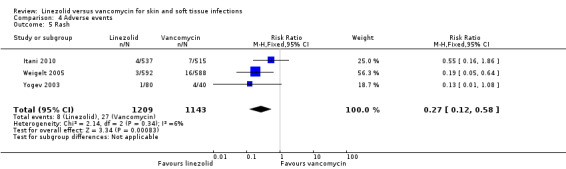
Comparison 4 Adverse events, Outcome 5 Rash.
4.6. Analysis.
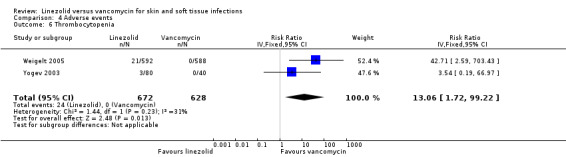
Comparison 4 Adverse events, Outcome 6 Thrombocytopenia.
4.8. Analysis.
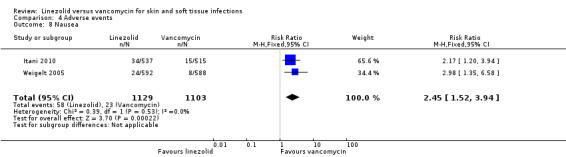
Comparison 4 Adverse events, Outcome 8 Nausea.
4.1. Analysis.
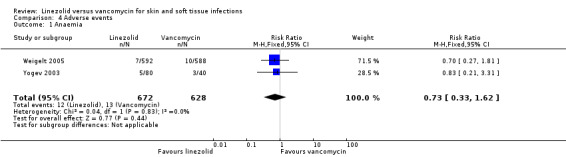
Comparison 4 Adverse events, Outcome 1 Anaemia.
4.2. Analysis.
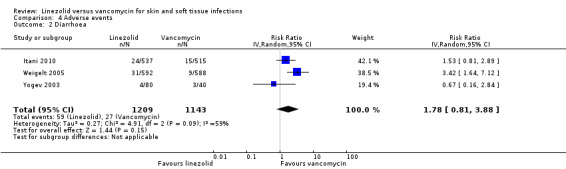
Comparison 4 Adverse events, Outcome 2 Diarrhoea.
4.7. Analysis.
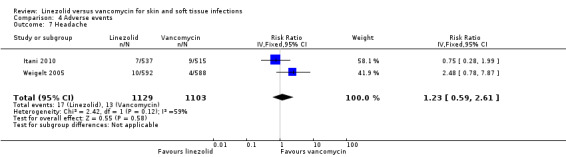
Comparison 4 Adverse events, Outcome 7 Headache.
4.9. Analysis.
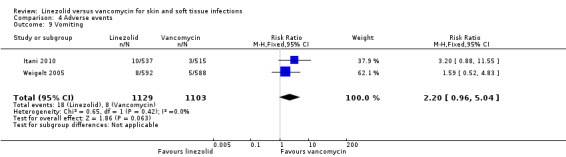
Comparison 4 Adverse events, Outcome 9 Vomiting.
Duration of hospital stay
Four RCTs (2522 participants) reported the duration of hospital stay (Itani 2010; Sharpe 2005; Stevens 2002; Weigelt 2005), but reported only the mean days and P value. Itani 2010 showed that in the linezolid group, the median and mean lengths of stay (LOS) were 5.0 and 7.7 days, respectively, compared with 7.0 and 8.9 days, respectively, in the vancomycin group (P value 0.016). Sharpe 2005 reported that the median LOS was three days shorter with linezolid (P value 0.003). Stevens 2002 reported that LOS was five days shorter for the linezolid group than the vancomycin group (9 versus 14 days, P value 0.052) among patients with SSTI. Weigelt 2005 found that the mean all cause total LOS was significantly shorter in the linezolid arm (7.4 versus 9.8 days, P value less than 0.0001).
Duration of treatment
Seven RCTs (4316 participants) reported the treatment duration (Itani 2010; Jaksic 2006; Kohno 2007; Lin 2008; Stevens 2002; Weigelt 2005; Wilcox 2009), but only two reported results for the subset of SSTI patients (Itani 2010; Weigelt 2005). Itani 2010 reported only the mean days and P value. The mean duration of intravenous therapy was reported as being significantly shorter in the linezolid group than the vancomycin group (5.3 versus 9.8 days; P value 0.001). For Weigelt 2005, however, the mean treatment duration was longer for the linezolid group than for the vancomycin group (MD 0.90 days, 95% CI 0.32 to 1.48; Analysis 5.1).
5.1. Analysis.
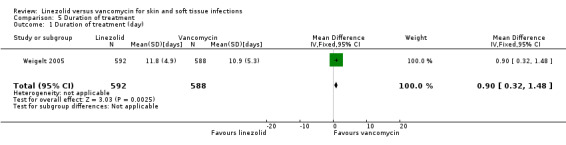
Comparison 5 Duration of treatment, Outcome 1 Duration of treatment (day).
Costs
Two RCTs (1240 participants) reported the treatment cost (Sharpe 2005; Weigelt 2005), but Sharpe 2005 did not report the SD or SE.The daily cost of outpatient therapy was USD 97 less with oral linezolid than with intravenous vancomycin (USD 103 versus USD 200, P value less than 0.001). Medication charges per day for inpatient linezolid treatment were USD 117 more than those for inpatient vancomycin (USD 277 versus USD 160, P value 0.069). However, the median length of hospital stay was three days shorter with linezolid (P value 0.003). Thus, with linezolid treatment, an average of USD 6438 in total hospital charges per patient was avoided (Sharpe 2005). Weigelt 2005 found that mean cost (plus or minus SD) for treatIing with linezolid was less than for vancomycin; that is, USD 4865 plus or minus USD 4367 compared with USD 5738 plus or minus USD 5190, respectively (P value 0.017).
Subgroup analyses
1. Children (under 18 years) and adults (18 years or over)
There was only one study (120 participants) in children (Yogev 2003), though five RCTs (2402 participants) included adults (18 years or over) with SSTI (Itani 2010; Kohno 2007; Lin 2008; Sharpe 2005; Weigelt 2005). The remaining three RCTs (592 participants) included mixed populations (13 years and over) with SSTI (Jaksic 2006; Stevens 2002; Wilcox 2009). In children, there was no statistically significant difference for either clinical cure (RR 1.14; 95% CI 0.91 to 1.44) or microbiological cure (RR 1.08; 95% CI 0.90 to 1.31). In adults (18 years and over), there was a statistically significant difference in favour of linezolid for both clinical cure (RR 1.16; 95% CI 1.02 to 1.32; I2 = 42%; Analysis 1.2) and microbiological cure (RR 1.17; 95% CI 1.02 to 1.34; I2 = 61%; Analysis 2.2). There were insufficient data to undertake subgroup analyses for other outcomes.
1.2. Analysis.
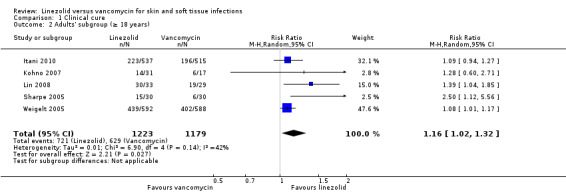
Comparison 1 Clinical cure, Outcome 2 Adults' subgroup (≥ 18 years).
2.2. Analysis.
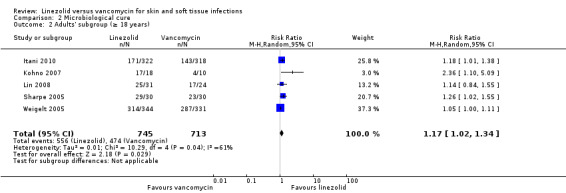
Comparison 2 Microbiological cure, Outcome 2 Adults' subgroup (≥ 18 years).
2. Uncomplicated SSTIs and complicated SSTIs
No trial reported uncomplicated SSTIs. This meant that there were insufficient data to permit this subgroup analysis.
3. MRSA subset
Five RCTs (2570 participants) enrolled people with MRSA infections (Itani 2010; Kohno 2007; Sharpe 2005; Stevens 2002; Weigelt 2005). One RCT enrolled people with Gram‐positive bacterial infection, but reported data of MRSA as a subset (89 participants) (Wilcox 2009). Thus, six RCTs reported the clinical and microbiological cure rate of MRSA infections. Clinical cure was evaluated in the people who were suspected or proven to have MRSA infections (2659 participants), while microbiological cure rate was evaluated for the people who had a positive MRSA culture at baseline (1289 participants). The results showed that linezolid achieved both a significantly better clinical cure rate (RR 1.09, 95% CI 1.03 to 1.17; I2 = 0%; Analysis 1.3), and microbiological cure rate (RR 1.17, 95% CI 1.04 to 1.32; I2 = 46%; Analysis 2.3), than vancomycin. There were insufficient data to do subgroup analyses for other outcomes.
1.3. Analysis.
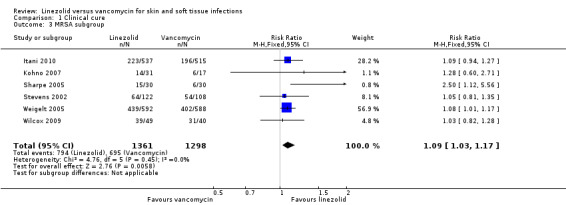
Comparison 1 Clinical cure, Outcome 3 MRSA subgroup.
2.3. Analysis.
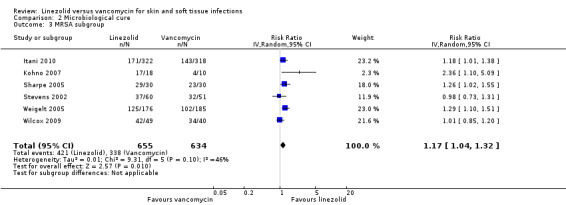
Comparison 2 Microbiological cure, Outcome 3 MRSA subgroup.
Sensitivity analysis
1. Exclusion of studies with inadequate concealment of allocation
Allocation concealment was unclear in all included RCTs and, therefore, this analysis was not undertaken.
2. Exclusion of studies in which outcome evaluation was not blinded
Only two of the nine included trials had a blinded design (Jaksic 2006; Lin 2008). After removal of the studies in which outcome evaluation was not blinded, there was no difference in treatment success for clinical cure for patients (RR 1.22, 95% CI 0.97 to 1.53), or for microbiological cure for patients (RR 1.08, 95% CI 0.85 to 1.38). There was no blinding for any other outcome for the RCTs, so there were insufficient data to perform the planned sensitivity analyses.
Discussion
Summary of main results
This review evaluated the effects and safety of linezolid compared with vancomycin for the treatment of people with skin and soft tissue infections (SSTIs). Of the 26 studies initially identified, nine (with 18 citations) were included in the review.
In summary, the included RCTs were of poor methodological quality, with high risk of bias, which weakens the confidence that can be placed on the individual and pooled results. The results of our review suggest that linezolid was more effective than vancomycin for treatment of SSTIs. Linezolid treatment was associated with a better clinical cure rate (RR 1.09, 95% CI 1.03 to 1.16), and better microbiological cure rate (RR 1.08, 95% CI 1.01 to 1.16). There was no significant difference in mortality between linezolid and vancomycin (RR 1.44, 95% CI 0.75 to 2.80). The linezolid group had a lower incidence of red man syndrome (RR 0.04, 95% CI 0.01 to 0.29), pruritus (RR 0.36, 95% CI 0.17 to 0.75), and rash (RR 0.27, 95% CI 0.12 to 0.58) than vancomycin. The linezolid group, however, had a greater incidence of thrombocytopenia (RR 13.06, 95% CI 1.72 to 99.22), and nausea (RR 2.45, 95% CI 1.52 to 3.94). We undertook subgroup analyses in people who had MRSA infections and found that linezolid was more effective than vancomycin in achieving a clinical cure (RR 1.09, 95% CI 1.03 to 1.17) and a microbiological cure (RR 1.17, 95% CI 1.04 to 1.32). We also undertook subgroup analyses in children (under 18 years of age) and adults (18 years and over). In children, there was no statistically significant difference for clinical cure and microbiological cure (one RCT Yogev 2003). In adults (18 years and over), there was a statistically significant difference in favour of linezolid for both clinical cure (RR 1.16; 95% CI 1.02 to 1.32) and microbiological cure (RR 1.17; 95% CI 1.02 to 1.34).The lengths of stay in hospital were shorter for the linezolid group than the vancomycin group. The daily cost of outpatient therapy was less with oral linezolid than with intravenous vancomycin. Although inpatient treatment with linezolid cost more than inpatient treatment with vancomycin per day, the median length of hospital stay was three days shorter with linezolid. Thus, total hospital charges per patient was less with linezolid treatment than with vancomycin treatment.
Overall completeness and applicability of evidence
The outcomes reported in the trials focused on clinical and microbiological cure. Economics of health, such as duration of hospital stay, treatment duration and cost were reported in only a few RCTs. Four RCTs reported the duration of hospital stay (Itani 2010; Sharpe 2005; Stevens 2002; Weigelt 2005), but they only reported the mean days and P value. Only two RCTs reported results for subsets of SSTI patients (Itani 2010; Weigelt 2005). The duration of intravenous treatment was shorter for patients treated with linezolid in Itani 2010, however, it was longer in Weigelt 2005. Two trials reported the treatment cost (Sharpe 2005; Weigelt 2005).
No RCT reported SSTI‐related and treatment‐related mortality, and only three reported all‐cause mortality and adverse effects for the subset of SSTI patients (Itani 2010; Weigelt 2005; Yogev 2003). Other RCTs reported the outcome for a pooled population of patients with mixed infection types, but, as we know, potential differences in mortality and adverse effects may exist within these different infection types. Unless individual subsets of patients were investigated, this result could not reflect mortality or adverse events specifically for SSTIs.
Six of the nine included trials were multinational studies (Itani 2010; Jaksic 2006; Stevens 2002; Weigelt 2005; Wilcox 2009; Yogev 2003); the remaining three were located in the USA (Sharpe 2005), Japan (Kohno 2007), and China (Lin 2008). Participants came from diverse cultural and geographic backgrounds, were of all ages, and both sexes were represented, therefore, this review is representative of different racial and regional groups.
Quality of the evidence
In general, all the trials were of poor methodological quality. Firstly, although all studies stated that randomisation was used, none mentioned the method of randomisation. Treatment allocation was not concealed in seven of the trials and it was unclear whether it had been in the remaining two RCTs. The lack of disclosure of the method of randomisation and the lack of allocation concealment in all included trials is concerning. Treatment effect may be overestimated by 30 to 40 per cent when allocation concealment is absent (Schulz 1995). Secondly, only two of the nine RCTs (with small sample sizes) had a blinded design (Jaksic 2006; Lin 2008), and lack of blinding might cause an overestimation of treatment effects.Thirdly, seven of the nine RCTs were either unclear about, or did not report dropout data, and none of them undertook an ITT analysis. The omission or non‐reporting of these items may lead to, or indicate, attrition bias that may contribute to false positive or negative findings. Finally, all the trials were funded by the pharmaceutical company that produces linezolid. It should be noted that, while the pharmaceutical companies need to support research in an ethical manner, pharmaceutical funding may introduce publication bias and, in this case, might overestimate the effects of linezolid.
Potential biases in the review process
1. We could not perform a funnel plot to investigate publication bias in this systematic review due to the small numbers of trials. Thus, one of our concerns is the potential for publication bias. The pharmaceutical company that produces linezolid funded all nine of the included trials in this review, which might mean that positive results are more likely to be reported than negative ones. Previously an association between positive results and publication has been demonstrated (Dickersin 1990), which may have identified a potential source of bias that, in this case, could have led to an overestimation of the treatment effect.
2. All RCTs excluded patients with severe SSTIs, such as necrotising fasciitis, gas gangrene, and infected burns, therefore, the effects of linezolid or vancomycin for such severe infections could not be estimated.
3. We undertook subgroup analysis for MRSA‐infected SSTIs. Two potential biases need to be considered: firstly, when a person presents with a SSTI, the identity of the infecting pathogen is usually not known. Whilst, for the outcome of clinical cure, it was suspected that the participants had MRSA‐infected SSTIs, MRSA can only be identified after culture. Secondly, although testing for microbiological eradication may provide definitive confirmation that the MRSA SSTI has been resolved, use of this endpoint is confounded by people who have natural colonisation of MRSA. This may be a potential source of bias, as it could have led to an underestimate of the treatment effect. Hence, clinicians should consider both the clinical and microbiological cure as indicators of treatment response.
4. The heterogeneity in this review was somewhat high for five outcomes. Possible reasons for this could include the lack of concealment of allocation, failure to perform an intention‐to‐treat analysis, and underlying patient characteristics such as different types of SSTIs and age.
5. After removal of the trials in which outcome assessment was not blinded, there were only two trials (Jaksic 2006; Lin 2008). Sensitivity analyses showed no difference in treatment success for clinical or microbiological cure. This may be due to the small sample sizes of the trials included (109 participants).
Agreements and disagreements with other studies or reviews
Our results are in agreement with some studies. Worldwide clinical trials with linezolid have demonstrated that linezolid has significantly better clinical and microbiological cure rates than vancomycin in people with pneumonia (Kollef 2004; Wunderink 2003). There also are some reviews which have favoured linezolid: one meta‐analysis evaluated the clinical and microbiological outcomes of linezolid compared with vancomycin in MRSA complicated SSTIs (Bounthavong 2010): it reported that resolution of infection favoured the use of linezolid over vancomycin (OR 1.41; 95% CI: 1.03 to 1.95), and that microbiological eradication in MRSA patients consistently favoured the use of linezolid (OR 2.90; 95% CI: 1.90 to 4.41). Another meta‐analysis also demonstrated that linezolid was more effective than vancomycin (OR 1.40, 95% CI 1.01 to 1.95) in people with SSTIs (Beibei 2010). For adverse effects, both reviews found that people treated with linezolid had higher proportions of thrombocytopenia, and a higher proportion of people treated with vancomycin had renal insufficiency. No difference in mortality was reported between the two antibiotics. Nonetheless, these reviews have limitations: they assessed mortality and adverse effects using mixed data, whilst data due to SSTIs could not be extracted. As we know, the mortality and side effects of SSTIs may not be the same as with other infections. For example, the mortality for bacteraemia is higher than for SSTIs. Secondly, some RCTs we identified and included in this review were not included in the earlier reviews (Itani 2010; Jaksic 2006; Sharpe 2005).
Despite this, our results are inconsistent with some other reviews. One review compared the effects of linezolid with vancomycin for the treatment of MRSA SSTIs in hospital inpatients (Dodds 2009). This review included four trials with a total of 174 participants for clinical outcomes and 439 participants for microbiological outcomes, respectively (Sharpe 2005; Stevens 2002; Weigelt 2005; Yogev 2003). There was no significant effect for clinical outcomes (RR 0.34; 95% CI 0.04 to 2.89) or microbiological outcomes (RR 0.55; 95% CI 0.30 to 1.01). Another review compared linezolid with vancomycin (Shorr 2005), and concluded that linezolid was not inferior to vancomycin in patients with secondary S aureus bacteraemia (five RCTs; 144 adults with S aureus bacteraemia). These two reviews were small, which may be a possible cause of these inconsistencies.
Authors' conclusions
Implications for practice.
The poor quality evidence contained within this systematic review shows that linezolid seems to be more effective than vancomycin for the treatment of patients with SSTIs and SSTIs caused by MRSA. The lengths of stay in hospital were shorter, and the costs of treatment were lower, for people in the linezolid group compared to the vancomycin group. Fewer people in the linezolid group suffered from red man syndrome, pruritus and rash compared with vancomycin, but more people in the linezolid group suffered from thrombocytopenia and nausea. In spite of these results, the evidence may be limited by potential biases, so further evidence from higher quality trials is necessary before definite conclusions can be drawn.
Implications for research.
Further well‐designed and reported randomised controlled trials are needed to confirm the available evidence. The following features should be addressed in future studies:
Detailed reporting of the methods of randomisation and allocation concealment.
Application and clear description of blinding.
Adverse events critically assessed by standardised reporting and RCTs with larger samples.
Appropriate outcomes: mortality, cure rate, duration of hospital stay, duration of treatment, and cost‐effectiveness of the treatment.
Pharmaceutical companies producing the drugs being assessed should not fund these trials.
Reporting of the methods and results of the trials using relevant reporting standards.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2015 | New citation required but conclusions have not changed | First update. New search. No new trials. Conclusions unchanged |
Acknowledgements
We would like to thank the following peer referees for their helpful comments on the review: Julie Bruce, Susan O’Meara, Ruth Foxlee, Jane Burch, Elmer Villanueva, Beryl de Souza, Brian Gilchrist, Patricia Davies, Shirley Manknell. Sally Bell‐Syer is especially acknowledged for language assistance, and Nicola Thomis for her contributions to the glossary. We also would like to acknowledge the copy editor, Elizabeth Royle, for edits to our draft.
Appendices
Appendix 1. Glossary
| Terms | Interpretation | Abbreviations |
| Abscesses | Abscesses are localised or walled‐off accumulations of pus. They are caused by infections and can occur anywhere within the body. | |
| β‐lactams | β‐lactam antibiotics are a broad class of antibiotics that share a similar molecular structure. | |
| Bacteraemia | Bacteria infecting the blood. | |
| Bioavailability | The rate at which a drug is absorbed by the body. | |
| Carbuncles | Similar to an abscess, a carbuncle is a collection of infected hair follicles, often with multiple openings, and filled with pus and dead tissue. Carbuncles are caused by bacteria. | |
| Cellulitis | A bacterial skin infection characterized by redness, swelling, and a feeling of heat or tenderness around the affected area. | |
| Clinical cure | The resolution of all signs and symptoms of infections. | |
| Complicated skin and soft tissue infection | An infection involving the deeper tissues of the body, including muscles and fat layers. Alternatively, SSTIs in patients with other illnesses such as diabetes or HIV. | cSSTI |
| Defervescence | The subsidence of fever. | |
| Endocarditis | An inflammation of the valves and internal lining of the heart. | |
| Erysipelas | A bacterial skin infection characterized by redness, swelling, sores and a feeling of heat or tenderness around the affected area. Erysipelas is more superficial than cellulitis. | |
| Escherichia coli | A bacterium that belongs to the Enterobacteriaceae family. | |
| Fascia | A layer of fibrous, connective tissue that often surrounds muscles, blood vessels and nerves. | |
| Furuncles | Often called a boil, a furuncle is a collection of pus in the skin. Furuncles often appear in areas of friction such as underneath the belt, the fronts of the thighs, buttocks, groin, and armpits. | |
| Gas gangrene | A bacterial infection that causes tissues to die, and gas to be produced within the tissues of the body. | |
| Glycopeptides | A class of antibiotic. | |
| Gram‐negative bacteria | One of two distinct types of bacteria. Gram‐negative bacteria do not turn purple when stained with a special dye. This is due to the structure of their cell walls. | |
| Gram‐positive bacteria | One of two distinct types of bacteria. Gram‐positive bacteria turn purple when stained with a special dye. This is due to the structure of their cell walls. | |
| Hypoderm/hypodermis | Tissue under the skin. | |
| Iatrogenic | Illness caused by medical examination or treatment. | |
| Impetigo | A common and highly contagious bacterial infection that causes blisters on the skin. | |
| Meningitis | An inflammation of the membranes that surround the brain and the spinal cord. | |
| Metastatic | The spread of a disease from one part of the body to another, non‐adjacent part. | |
| Methicillin‐resistant Staphylococcus aureus | A strain of bacterium that has become resistant to the antibiotics commonly used to treat ordinary infections, particularly methicillin. | MRSA |
| Methicillin‐sensitive Staphylococcus aureus | A strain of bacterium that is sensitive to the commonly used antibiotic, methicillin. | MSSA |
| Microbiological cure | Eradication of bacteria in a wound; assessed by means of laboratory test or wound culture (a swab taken from the wound). | |
| Necrotising skin and soft‐tissue infections | A rare, but very severe, type of bacterial infection that can destroy the muscles, skin, and underlying tissue. ‘Necrotising’ refers to something that causes tissue death. | |
| Nephrotoxicity | The poisonous effect of some substances on the kidneys. | |
| Neutropenia | A deficiency of white blood cells in the body. | |
| Nosocomial | Originating or taking place in a hospital, acquired in a hospital, especially in reference to an infection. | |
| Osteomyelitis | An infection in a bone. | |
| Ototoxicity | Damage to the ears caused by a toxin. | |
| Oxazolidinone | A type of antibiotic. | |
| Parenterally | A way of introducing substances such as nutrients, or medication, by a non‐oral route, for example by injection. | |
| Pruritus | Itching. | |
| Pseudomonas aeruginosa | A type of bacterium often found in soil or ground water. It can cause illness and infection in humans. | |
| Red man syndrome or erythroderma | An allergic reaction characterized by reddening of the upper body and itching. | |
| Skin and soft tissue infections | Infections involving layers of the skin and the soft tissues beneath. | SSTIs |
| Staphylococcus aureus | A type of bacterium that lives on the skin and sometimes in nasal passages. It is the most common cause of skin and soft tissue infections. | |
| Test of cure | Evaluation of the healing of skin and soft tissue infections after treatment. | TOC |
| Thrombocytopenia | A disorder that causes a decrease of platelets in blood. Platelets help the blood to clot. | |
| Toxicity | Toxicity refers to the ability of a substance to cause harmful effects in the body. | |
| Vancomycin‐resistant enterococci | Bacteria that have developed resistance to many antibiotics, especially vancomycin. | VRE |
Appendix 2. Search strategies for Ovid Medline, Ovid Embase and EBSCO CINAHL
Ovid Medline
1 exp Oxazolidinones/ (3454) 2 exp Oxazolone/ (471) 3 (linezolid$ or oxazolone$).ti,ab. (3313) 4 or/1‐3 (5205) 5 exp Glycopeptides/ (24374) 6 (vancomycin$ or glycopeptide$).ti,ab. (14989) 7 or/5‐6 (32269) 8 exp Soft Tissue Infections/ (1969) 9 exp Staphylococcal Skin Infections/ (2085) 10 exp Cellulitis/ (2621) 11 exp Erysipelas/ (360) 12 exp Furunculosis/ (298) 13 exp Abscess/ (17532) 14 exp Wound Infection/ (15200) 15 exp Fasciitis, Necrotizing/ (1891) 16 exp Myositis/ (6975) 17 exp Gas Gangrene/ (356) 18 (soft tissue infection$ or skin infection$).ti,ab. (4702) 19 (cellulitis or erysipelas or furuncul$ or abscess$ or absess$ or necrotizing fasciitis or myositis or gas gangrene or (wound$ adj2 infect$)).ti,ab. (44369) 20 or/8‐19 (69951) 21 4 and 7 and 20 (216) 22 randomized controlled trial.pt. (247475) 23 controlled clinical trial.pt. (40136) 24 randomized.ab. (201843) 25 placebo.ab. (93559) 26 clinical trials as topic.sh. (80952) 27 randomly.ab. (138890) 28 trial.ti. (75242) 29 or/22‐28 (558737) 30 Animals/ (2530681) 31 Humans/ (7027945) 32 30 not 31 (1649878) 33 29 not 32 (508211) 34 21 and 33 (43)
Ovid Embase
1 exp Oxazolidinone Derivative/ (2611) 2 exp Oxazolone/ (956) 3 exp Linezolid/ (10231) 4 (linezolid$ or oxazolone$).ti,ab. (5207) 5 or/1‐4 (13224) 6 exp Vancomycin/ (43322) 7 exp Vancomycin Derivative/ (216) 8 exp Glycopeptide/ (5164) 9 (vancomycin$ or glycopeptide$).ti,ab. (22171) 10 or/6‐9 (51486) 11 exp Soft Tissue Infection/ (5340) 12 exp Skin Infection/ (77395) 13 exp Cellulitis/ (8532) 14 exp Erysipelas/ (1406) 15 exp Furunculosis/ (857) 16 exp Abscess/ (41568) 17 exp Wound Infection/ (19807) 18 exp Necrotizing Fasciitis/ (3450) 19 exp Myositis/ (15627) 20 exp Gas Gangrene/ (680) 21 (soft tissue infection$ or skin infection$).ti,ab. (7434) 22 (cellulitis or erysipelas or furuncul$ or abscess$ or absess$ or necrotizing fasciitis or myositis or gas gangrene or (wound$ adj2 infect$)).ti,ab. (65393) 23 or/11‐22 (178863) 24 5 and 10 and 23 (1789) 25 Clinical trial/ (715292) 26 Randomized controlled trials/ (29861) 27 Random Allocation/ (51197) 28 Single‐Blind Method/ (15897) 29 Double‐Blind Method/ (87219) 30 Cross‐Over Studies/ (32445) 31 Placebos/ (169756) 32 Randomi?ed controlled trial$.tw. (82914) 33 RCT.tw. (10982) 34 Random allocation.tw. (931) 35 Randomly allocated.tw. (14603) 36 Allocated randomly.tw. (1227) 37 (allocated adj2 random).tw. (266) 38 Single blind$.tw. (9897) 39 Double blind$.tw. (92147) 40 ((treble or triple) adj blind$).tw. (248) 41 Placebo$.tw. (140349) 42 Prospective Studies/ (206934) 43 or/25‐42 (1077729) 44 Case study/ (16788) 45 Case report.tw. (170882) 46 Abstract report/ or letter/ (519805) 47 or/44‐46 (703087) 48 43 not 47 (1048538) 49 animal/ (730814) 50 human/ (8821758) 51 49 not 50 (489053) 52 48 not 51 (1026150) 53 24 and 52 (546)
EBSCO CINAHL
S17 S16 and S4 and S1 S16 S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 S15 wound* N2 infection* S14 cellulitis or erysipelas or furuncul* or abscess* or absess* or necrotizing fasciitis or myositis or gas gangrene S13 soft tissue infection* or skin infection* S12 (MH "Gas Gangrene") S11 (MH "Myositis") S10 (MH "Fasciitis, Necrotizing") S9 (MH "Wound Infection+") S8 (MH "Abscess+") S7 (MH "Furunculosis") S6 (MH "Cellulitis") S5 (MH "Soft Tissue Infections") S4 (S3 or S2) S3 glycopeptide* S2 (MH "Vancomycin") S1 linezolid* or oxazolone* or oxazolidinone*
Appendix 3. Assessment of risk of bias in included studies
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, because allocation based on one of the following or an equivalent method: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to have introduced bias.
Unclear
Either of the following:
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers, or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were pre‐specified.
High risk of bias
Any one of the following:
Not all of the study's pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists (e.g. baseline imbalances); or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Clinical cure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All participants | 9 | 3114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.16] |
| 2 Adults' subgroup (≥ 18 years) | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| 3 MRSA subgroup | 6 | 2659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.17] |
Comparison 2. Microbiological cure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All participants | 9 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.16] |
| 2 Adults' subgroup (≥ 18 years) | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.34] |
| 3 MRSA subgroup | 6 | 1289 | Risk Ratio (IV, Random, 95% CI) | 1.17 [1.04, 1.32] |
Comparison 3. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality during follow‐up | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.75, 2.80] |
Comparison 4. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia | 2 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| 2 Diarrhoea | 3 | 2352 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.81, 3.88] |
| 3 Red man syndrome | 2 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.29] |
| 4 Pruritus | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| 5 Rash | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.58] |
| 6 Thrombocytopenia | 2 | 1300 | Risk Ratio (IV, Fixed, 95% CI) | 13.06 [1.72, 99.22] |
| 7 Headache | 2 | 2232 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.59, 2.61] |
| 8 Nausea | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.52, 3.94] |
| 9 Vomiting | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.96, 5.04] |
Comparison 5. Duration of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of treatment (day) | 1 | 1180 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.32, 1.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Itani 2010.
| Methods | Open‐label, multicentred, randomised study. | |
| Participants | Location: 102 centres in the USA, Eastern and Western Europe, Latin America, South Africa, Malaysia, and Singapore. Time frame: October 2004‐July 2007. Inclusion criteria: patients ≥18 years, with MRSA‐infected cSSTIs. Patient numbers: 1052 randomised. 640 were confirmed as MRSA positive, 322 in linezolid group, and 318 in vancomycin group. Average age: Linezolid group: 49.7 years, Vancomycin group: 49.4 years. Male:female ratio: Linezolid group: 305:232, Vancomycin group: 315:200. | |
| Interventions | Linezolid group (n = 537): 600 mg IV linezolid every 12 h; could be switched to oral at any time at investigator's discretion. Vancomycin group (n = 515): IV 15 mg/kg vancomycin every 12 h with dose adjustment as necessary, based on trough levels and creatinine clearance. Treatment duration: 7–14 days for both groups. | |
| Outcomes | Clinical outcomes: the number of cures. Microbiological outcomes: measured by pathogen eradication rate. Safety: treatment‐related adverse events. Mortality: number of deaths in each group; investigator considered cause of death to be unrelated to the study drug. Length of hospital stay. Duration of intravenous therapy. | |
| Notes | Aztreonam (or other antibiotic known to be inactive against Gram‐positive organisms/MRSA) and metronidazole were permitted to treat suspected Gram‐negative pathogens and anaerobic pathogens, respectively. Quote: "This study was funded by Pfizer, Inc; editorial support was provided by Elizabeth Melby Wells and Jean Turner of PAREXEL, Stamford, CT, and was funded by Pfizer, Inc; Kamal Itani has been a consultant and speaker for Pfizer; Matthew Dryden has been a member of advisory boards and has been a speaker for Pfizer, Wyeth, Bayer, and Jansen Cilag; Helen Bhattacharyya, Mark Kunkel, and Alice Baruch are employees of Pfizer; John Weigelt is a consultant to Pfizer, Ortho McNeil, and Schering‐Plough." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: the method of sequence generation was not reported in the trial. |
| Allocation concealment (selection bias) | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | High risk | Dropouts: Linezolid group: 93/322 (28.9%); Vancomycin group: 108/318 (33.9%). Comment: the reasons for dropping‐out were not reported, and the rate of dropout was high. We think this level of losses would have affected the outcome. |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 1052 randomised, 640 included in analysis. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Low risk | Quote: "study was conducted between October 2004 and July 2007 (Clinical Trials gov: no. NCT00087490)". Comment: the study protocol was available and all of the study's pre‐specified (primary and secondary) outcomes that were of interest in the review were reported in the pre‐specified way. |
| Other bias | Low risk | No other biases identified. |
Jaksic 2006.
| Methods | Randomised, multicentred, multinational, double‐blind study. | |
| Participants | Location: 58 sites in Australia, Austria, Belgium, Croatia, France, Germany, Greece, Italy, Poland, Russia, Slovenia, South Africa, Spain, and Switzerland. Time frame: November 2000‐May 2002. Inclusion criteria: patients ≥ 13 years, hospitalised febrile adults with cancer and proven, or suspected, Gram‐positive bacterial infection. Patient numbers: 605 randomised, 47 had SSTIs. Average age: Linezolid group: 47.2 years, Vancomycin group : 48.1 years (for all study participants). Male:female ratio: Linezolid group: 129:125, Vancomycin group: 161:140 (for all study participants). | |
| Interventions | Linezolid group (n = 304, 27 SSTIs): linezolid: 600 mg IV every 12 h. Vancomycin group (n = 301, 20 SSTIs): vancomycin: 1 g IV every 12 h. Treatment duration: median length of therapy 10 days for both groups. Follow‐up duration: 10–28 days for both groups. | |
| Outcomes | Clinical outcomes: clinical success was defined as cure (defervescence (abatement of fever) and resolution of signs and symptoms of infection), or improvement (defervescence and improvement of signs and symptoms of infection). Defervescence was defined as maximum oral temperature of ≤ 37.5 oC or axillary temperature of ≤ 36.7 oC on 3 consecutive days. Failure was defined as persistence, or progression, of clinical signs and symptoms of infection, or development of new findings. An indeterminate outcome was defined as an inability to make an assessment. Microbiological outcomes: assessed as success (documented eradication, presumed eradication, or colonisation), failure (documented persistence, presumed persistence, or superinfection), indeterminate, or missing. Safety: drug‐related adverse events. Mortality: mortality rates at 16 days after completion of therapy. | |
| Notes | SSTIs as a subgroup of Gram‐positive bacterial infection. Quote: "B.J., G.M., and J.P.‐O. received research grants from Pfizer. C.S.H., L.B.L, and K.J.T. are employed by Pfizer." Comment: the study was supported by a grant from Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly assigned in a 1:1 ratio". Comment: the method of sequence generation was not reported in the trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: did not report whether allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Quote: "To maintain blinding, a research pharmacist prepared study medications; an unblinded co investigator monitored vancomycin or serum creatinine levels in accordance with local practice". Comment: participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | Low risk | Quote: "To maintain blinding, a research pharmacist prepared study medications; an unblinded co investigator monitored vancomycin or serum creatinine levels in accordance with local practice". Comment: the care‐giver was blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Unclear risk | Quote: "an unblinded co investigator monitored vancomycin or serum creatinine levels in accordance with local practice". Comment: knowledge of the intervention was unlikely to cause bias of vancomycin or serum creatinine levels, but the trial report did not state whether the assessor of signs and symptoms of infection was blinded. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | Unclear risk | Comment: dropouts reported for all study participants, but not clear for SSTIs. dropouts: Linezolid group: 53/304 (17.4%); Vancomycin group: 64/301 (21.2%). |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 605 randomised, 488 included in analysis for all study participants. 47 randomised, 38 included in analysis for SSTIs. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and the trial authors did not report whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Kohno 2007.
| Methods | Open‐label, comparator‐controlled, multicentred study, 2:1 ratio randomised. | |
| Participants | Location: 84 sites in Japan. Time frame: October 2001‐January 2004. Inclusion criteria: patients > 20 years, with confirmed, or suspected, MRSA‐related pneumonia, cSSTI or sepsis. Patient numbers: 151 randomised, 48 had MSRA SSTIs. Average age: Linezolid group: 68.4 years, Vancomycin group: 67.5 years (for all study participants). Male:female ratio: Linezolid group: 70:30, Vancomycin group: 36:15 (for all study participants). | |
| Interventions | Linezolid group (n = 100, 31 SSTIs): linezolid 600 mg IV every 12 h, could be switched to oral after a minimum of 3 days. Vancomycin group (n = 51, 17 SSTIs): vancomycin 1 g IV every 12 h. Treatment duration: 7–28 days for both groups. | |
| Outcomes | Clinical outcomes: the success rate was defined as the number of cures and improvements divided by the number of cures, improvements and failures. "Cured" defined as resolution of the clinical signs and symptoms of infection when compared with baseline; "improved" defined as improvement in 2 or more, but not all, clinical signs and symptoms of infection when compared with baseline; "failed" defined as persistence or progression of baseline clinical signs and symptoms of infection; and "indeterminate" defined as unable to assess. Microbiological outcomes: microbiological eradication rates. Safety: treatment‐related adverse events. | |
| Notes | Patients could receive aztreonam or gentamicin (or other aminoglycosides with no activity against the isolated MRSA) for Gram‐negative coverage. Quote: "This study was sponsored by Pfizer Inc. Editorial support was provided by Philip Matthews at PAREXEL and was funded by Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: the method of sequence generation in the trial was not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | High risk | Only reported the number of dropouts, but not the reasons. These figures were not clear for SSTIs. |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 151 randomised, 92 were included in analysis for all study participants. 48 randomised, 28 were included in analysis for SSTIs. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and the trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Lin 2008.
| Methods | Randomised, double‐blind, multicentred study. | |
| Participants | Location: 7 sites in China. Time frame: April 2001‐March 2005. Inclusion criteria: hospitalised patients aged 18–75 years with known, or suspected, infection due to Gram‐positive bacteria, including pneumonia and cSSTI. Patient numbers: 142 randomised, 62 had cSSTI. Average age: Linezolid group: 56.3 years, Vancomycin group: 59.6 years (for all study participants). Male:female ratio: Linezolid group: 46:25, Vancomycin group: 42:29 (for all study participants). | |
| Interventions | Linezolid group ( n = 71, 33 SSTIs): linezolid 600 mg IV every 12 h. Vancomycin group (n = 71, 29 SSTIs): vancomycin 1 g IV every 12 h if aged ≤ 60 years, or 0.75 g if aged > 60 years. Treatment duration: 7–21 days for both groups. | |
| Outcomes | Clinical outcomes: "cured" defined as complete resolution of 4 areas identified at baseline as abnormal: (i) signs; (ii) symptoms; (iii) haematology and chemistry; and (iv) microbiology; "marked improvement" defined as resolution of 3/4 areas; "improved" defined as resolution of at least 2 areas; "failed"defined as persistence or progression of baseline. Microbiological outcomes: measured by pathogen eradication rate. Safety: drug‐related adverse events. | |
| Notes | Concomitant use of aztreonam was permitted in patients with documented mixed Gram‐positive and Gram‐negative organisms. Quote: "This study was sponsored by Pfizer Inc. Editorial support was provided by Jean Turner and Elizabeth Melby Wells of PAREXEL (Stamford, CT) and was funded by Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: the method of sequence generation in the trial was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no report of whether allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Unclear risk | Quote: "This Phase 3, randomised, double‐blind, comparator controlled, multicentre study". Comment: reported to be double‐blind, but no specific details provided about who was blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | Unclear risk | Quote: "This Phase 3, randomised, double‐blind, comparator controlled, multicentre study" Comment: reported to be double‐blind, but no specific details provided about who was blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Unclear risk | Quote: "This Phase 3, randomised, double‐blind, comparator controlled, multicentre study" Comment: reported to be double‐blind, but no specific details provided about who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | Low risk | dropouts were adequately addressed. dropouts(for all study participants): Linezolid group: 12/71 (16.9%); Vancomycin group: 14/71 (19.7%). |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 142 randomised, 121 included in analysis for all study participants. 62 randomised, 59 were included in analysis for SSTIs. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and the trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Sharpe 2005.
| Methods | Single‐centred, open‐label randomised study. | |
| Participants | Location: USA. Unknown time frame. Inclusion criteria: patients ≥18 years, with proven MRSA‐related cSSTIs requiring surgical intervention. Patient numbers: 60 randomised. Average age: Linezolid group: 66 years, Vancomycin group: 76 years. Male:female ratio: Linezolid group: 10:20, Vancomycin group: 10:20. | |
| Interventions | Linezolid group (n = 30): linezolid 600 mg orally every 12 h. Vancomycin group (n = 30): vancomycin 1 g IV every 12 h. Treatment duration: the median length of therapy was 10 days for both groups. | |
| Outcomes | Clinical outcomes: clinical cure defined as temperature normalization; presence of granulation or wound healing; resolution of pain; and decreased or resolved erythema, oedema, induration, and colour. Ulceration could persist, but lesions must appear noninfected to be defined as clinically cured. Clinical improvement defined as moderate resolution of 2 or more clinical symptoms. Clinical failure defined as persistence or progression of baseline signs and symptoms, development of new symptoms consistent with Gram‐positive infection, or inability to complete the study because of adverse events. Microbiological outcomes: microbiological eradication documented by culture or presumed because of an absence of clinical symptoms. Microbiological persistence documented by presence of 1 or more of the original infecting organisms on the culture test for cure. Microbiological recurrence defined as presence on final culture of an original infecting organism whose eradication had been either documented or presumed at the end of therapy. Hospitalisation duration: median length of hospital stay. Cost: total hospital charges per patient; the daily cost of outpatient therapy. | |
| Notes | All patients received perioperative cefazolin while awaiting culture results. Patients could receive up to 48 h of topical or systemic antibiotics before randomisation. Quote: "Supported by an unrestricted educational grant from Pfizer Inc." Comment: this study was supported by Pfizer, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty patients were randomised". Comment: the method of sequence generation used in the trial was not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | High risk | Comment: data concerning dropouts were not reported. The trial paper only reported the percentage cured. We calculated the number of clinical cures from this percentage. |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | Unclear risk | Comment: did not report whether ITT was undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not report whether the published reports included all expected outcomes. |
| Other bias | Unclear risk | Comment: there was baseline imbalance as the group of patients who received linezolid were significantly younger than those who received vancomycin (66 vs 76 years), this is unlikely to be clinically significant. |
Stevens 2002.
| Methods | Open‐label, multicentred, randomised phase III clinical trial. | |
| Participants | Location: 104 sites in North America, Europe, Latin America and Asia. Time frame: July 1998‐July 1999. Inclusion criteria: patients ≥ 13 years, hospitalised with presumed MRSA infection. Patient numbers: 460 randomised, 230 had SSTIs. Average age: Linezolid group: 63.9 years, Vancomycin group: 59.8 years (for all study participants). Male:female ratio: Linezolid group: 143:97, Vancomycin group: 131:89 (for all study participants). | |
| Interventions | Linezolid group (n = 240, 122 SSTI): linezolid 600 mg IV twice daily, which could be changed to oral with clinical improvement. Vancomycin group (n = 220, 108 SSTI): vancomycin 1 g IV twice daily. Treatment duration: 7–14 days for both groups. | |
| Outcomes | Clinical outcomes: used 4 possible clinical outcomes: "cure," "treatment failure," "indeterminate," or "missing." "Cure" defined as resolution of baseline clinical signs and symptoms of infection after ≥ 5 days and ≥ 10 doses of treatment. "Treatment failure" assigned if there was persistence or progression of signs and symptoms of infection after ≥ 2 days and ≥ 4 doses of treatment, or if there was no clinical assessment at end of therapy and test‐of‐cure. "Indeterminate" assigned if there was clinical improvement, or cure, at end of therapy but no test‐of‐cure assessment, or if there was cure after receipt of < 5 days or < 10 doses of study medication. "Missing" was assigned if < 2 days or < 4 doses of treatment were received.
Microbiological outcomes: 4 possible microbiological outcomes: "success," "treatment failure," "indeterminate," or "missing." "Success" defined as documented or presumed eradication of all pathogens present at baseline or colonization. "Treatment failure" defined as documented or presumed persistence of 1 pathogen present at baseline, superinfection, or reinfection. "Indeterminate" assigned if the clinical outcome at test‐of‐cure visit was indeterminate or missing. "Missing" assigned if there were no microbiological data from the test‐of‐cure visit.
Safety: drug‐related adverse events. Length of stay. |
|
| Notes | SSTIs were a subset of MRSA infection. The trial also included other infection types such as bacteraemia, pneumonia, and urinary‐tract infections.
Gram‐negative coverage was allowed. Quote: "D.L.S. has received funding from Pharmacia, Pfizer Pharmaceuticals, and Wyeth‐Ayerst for investigator‐initiated research proposals". Comment: DLS was the lead author of the study report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Hospitalized patients were randomised". Comment: the method of sequence generation used in the trial was not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | Unclear risk | Quote: "Overall, 78 (32.5%) of 240 patients in the linezolid group and 69 (31.4%) of 220 in the vancomycin group discontinued treatment. The most common reasons for discontinuation of study medication were as follows: no methicillin‐resistant pathogen detected at baseline (13.3% of patients [32/240] in the linezolid group vs 17.3% of patients [38/220] in the vancomycin group) . . ." Comment: dropouts reported for all MRSA infections, but not clear for SSTIs. |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 460 randomised, 361 included in analysis for all study participants. 230 randomised, 186 included in analysis for SSTIs. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not report whether the published reports included all expected outcomes |
| Other bias | Unclear risk | Quote: "patients who received linezolid were significantly older than those who received vancomycin (63.9 vs 59.8 yrs p = 0.0157)". Comment: baseline imbalance reported, this is unlikely to be clinically significant, therefore, judged to be at unclear risk of bias. |
Weigelt 2005.
| Methods | Randomised, open‐label, multicentred study. | |
| Participants | Location: Asia Pacific, South America, North America, Europe and New Zealand. Timeframe: October 2002‐March 2003. Inclusion criteria: suspected or proven MRSA complicated SSTIs requiring hospitalisation. Patient numbers: 1200 randomised; 20 of these were randomised but never received treatment (8 never received linezolid and 12 never received vancomycin). Average age: 52 years in both groups. Male:female ratio: Linezolid group: 375:217, Vancomycin group: 363:225. | |
| Interventions | Linezolid group (n = 592): linezolid 600 mg every 12 h, IV or oral. Vancomycin group (n = 588): vancomycin 1 g IV every 12 h. Treatment duration: 4–21 days for both groups. | |
| Outcomes | Clinical outcomes: patients counted as (i) "cured" if complete resolution of all pre‐therapy clinical signs and symptoms of infection (e.g. body temperature and white blood cell count) was achieved; (ii) "improved" if, at the end of treatment, 2 or more (but not all) of the pre‐therapy clinical signs and symptoms of CSSTI were resolved; (iii) "failed" if they exhibited persistence or progression of baseline clinical signs and symptoms of infection, development of new clinical findings consistent with active infection, or an inability to complete the study because of adverse events; and (iv) "indeterminate" if extenuating circumstances precluded classification to one of the above‐described categories, usually because of missed appointments.
Microbiological outcomes: were categorised as: (i) "success" if had documented or presumed eradication of the pathogen present at baseline; (ii) "failure" if had documented or presumed persistence of pathogen present at baseline; (iii) "indeterminate" if pathogen data were indeterminate; or (iv) "missing" if the pathogen data were missing. Mortality: the number of death in each group. Cause of death was judged by the investigator to be unrelated to the study drug. Duration of treatment. Safety: drug‐related adverse events. Length of stay. Cost. |
|
| Notes | If MSSA was found, patients were switched to an appropriate antibiotic. Concomitant use of aztreonam or other antibiotics for Gram‐negative organisms was permitted. Quote: "J. Weigelt, D. Stevens, K. Itani, W. Lau, and M. Dryden have conducted research on behalf of Pfizer and have been on the Pfizer speakers' bureau. C. Knirsch is an employee of Pfizer, Inc." Comment: this study was supported by Pfizer, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: the method of sequence generation used in the trial was not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | Low risk | dropouts were adequately addressed. dropouts: Linezolid group: 46/592 (7.8%); Vancomycin group: 66/588 (11.2%). |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 1200 randomised, 930 included in analysis. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Wilcox 2009.
| Methods | Open‐label, multicentred, randomised study. | |
| Participants | Location: 100 centres in Europe, USA, Latin America and Asia. Time frame: May 2002‐May 2005. Inclusion criteria: patients ≥ 13 years, with a central venous, pulmonary artery, or arterial catheter in place for 13 days and suspected catheter‐related infection. Patient numbers: 726 randomised, 315 had cSSTI. Average age: Linezolid group: 53.7 years, Vancomycin group: 53.8 years; (for all study participants). Male:female ratio: Linezolid group: 202:161, Vancomycin group: 210:153 (for all study participants). | |
| Interventions | Linezolid group (n = 363, 164 SSTIs): linezolid 600 mg IV every 12 h; could be switched to oral. Vancomycin group (n = 363, 151 SSTIs): vancomycin 1 g IV every 12 h. Duration: 7–28 days for both groups. | |
| Outcomes | Clinical outcomes: assessed as "success" (cure with resolution of signs and symptoms or, at end of treatment only, improvement
with moderate resolution of signs and symptoms and no additional antibiotic treatment); or "failure" (persistence or progression of clinical signs and symptoms or new clinical findings of infection). Microbiological outcomes: assessed as "success" (documented or presumed eradication based on clinical outcome) or "failure" (documented or presumed persistence based on clinical failure and either missing microbiologic outcome or use of non‐study antibiotic because of lack of efficacy). Safety: all adverse events. All cause mortality: 1‐2 weeks after treatment. |
|
| Notes | For methicillin‐susceptible pathogens, vancomycin could be switched to oxacillin 2 g IV, or dicloxacillin 500 mg orally, each given every 6 h. Concomitant therapy allowed on the basis of susceptibility and local practice. Quote: "M.H.W. has received honoraria for consultancy work, financial support to attend meetings, and research funding from Astra‐Zeneca, Bayer, Cerexa, Genzyme, Nabriva, Pfizer, Targanta, Vicuron" Comment: M.H.W was the lead author of the study report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: the method of sequence generation used in the trial was not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | High risk | dropouts (for all study participants): Linezolid group: 177/363 (48.8%); Vancomycin group: 179/363 (49.3%). Comments: reasons for dropouts were reported, but the levels were very high. In addition, the dropout rate was not clear for SSTIs, therefore, this was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 726 randomised, 422 included in analysis for all study participants. 315 randomised, 296 included in analysis for SSTIs. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not stated whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Yogev 2003.
| Methods | Open label, randomised, multicentred study, randomised in a 2:1 ratio. | |
| Participants | Location: 59 sites throughout USA, Mexico and South America. Time frame: February 2001‐December 2001. Inclusion criteria: hospitalised patients ≤ 12 years, with pneumonia, SSTIs, catheter‐related bacteraemia, or bacteraemia of unknown source because of resistant Gram‐positive pathogen. This paper only addressed the cSSTIs outcome. Patient numbers: 120 randomised. Average age: Linezolid group: 3.48 years, Vancomycin group: 3.03 years. Male:female ratio: Linezolid group: 46:34, Vancomycin group: 23:17. | |
| Interventions | Linezolid group (n = 80): linezolid 10 mg/kg IV every 8 h; could be switched to oral after at least 3 days. Vancomycin group (n = 40): vancomycin 10–15 mg/kg IV every 6–24 h; duration: 10–28 days. Treatment duration: 7–14 days for both groups. | |
| Outcomes | Clinical outcomes: resolution of the signs associated with the cSSI, including lesion size, tenderness, erythema, swelling, induration, fluctuance, heat/localized warmth or discharge (purulent or nonpurulent). Microbiological outcomes: individual pathogen eradication rates. Safety: drug‐related adverse events. All cause mortality: the number of death in each group. | |
| Notes | SSTIs as a subset of study Kaplan 2003.
Vancomycin patients could be switched to an alternative antibiotic if non‐MRSA pathogen isolated. Gram‐negative coverage was allowed. Quote: "From the Children's Memorial Hospital, Chicago . . . and Pharmacia Corp.". Comment: the study was funded by Pharmacia Corporation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised in a 2:1 ratio". Comment: the method of sequence generation used in the trial was not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ care givers | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes drop outs reported | High risk | Comment: data relating to dropouts were not reported. |
| Incomplete outcome data (attrition bias) All outcomes ITT analysis reported | High risk | 120 randomised, 108 included in analysis for all study participants. Comment: ITT was not undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Abbreviations
≥ = equal to or greater than > = greater than ≤ = equal to or less than < = less than cSSTI = complicated skin and soft tissue infection h = hour(s) IV = intravenously ITT = intention‐to‐treat analysis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bal 2013 | Not a RCT. |
| Bhavnani 2015 | Not compare linezolid vs. vancomycin. |
| Bounthavong 2009 | Cost‐effectiveness analysis, not a RCT. |
| Hau 2002 | Cost‐effectiveness analysis, not a RCT. |
| Janis 2014 | Cost‐effectiveness analysis, not a RCT. |
| Joseph 2007 | Not a RCT. |
| Kalil 2006 | A comment, not a RCT. |
| Lipsky 2011 | A review; pooled data from three prospective clinical trials. |
| McKinnon 2007 | Health economics analysis, not a RCT. |
| Patanwala 2007 | Cost‐effectiveness analysis, not a RCT. |
| Puzniak 2013 | Post‐hoc pooled data analysis, not a RCT |
| Puzniak 2014 | Post‐hot pooled data analysis, not a RCT |
| Schurmann 2009 | Cost‐effectiveness analysis, not an RCT. |
Abbreviation
RCT = randomised controlled trial
Differences between protocol and review
Under "Types of outcome measures/Primary outcomes" in the sentence "Proportion of patients or infections healed: healing is defined as either the resolution of all clinical signs and symptoms of infection, as assessed by laboratory test or as defined by trialists", we changed to, "clinical cure (resolution of symptoms and signs) and microbiological cure (eradication of bacteria on wound culture)." We think the meaning of the two statements is the same, however, "clinical/microbiological cure" is more concise and helpful than the original wording when used throughout the text of the review.
In the section "Subgroup analysis and investigation of heterogeneity", we added:"3. MRSA subset", because MRSA is important for SSTIs. The morbidity and treatment costs associated with MRSA‐infected SSTIs are higher than for other pathogen infections, so we added this subgroup analysis.
We added some information in the "Background" section in line with comments from peer referees.
We added some information in the "Methods/Dealing with missing data" section, in line with comments from peer referees.
Contributions of authors
Yue Jirong: conceived the review question; co‐ordinated the protocol development; completed the first draft and edited the review. Dong Birong: developed the review and advised on the review, co‐ordinated the review development and performed part of writing and editing of the review, approved the final version of the review prior to submission and is guarantor. Yang Ming: developed and edited the review and made an intellectual contribution to it. Chen Xiaomei: conceived the review question, edited the review and made an intellectual contribution to it. Wu Taixiang: developed the review, advised and performed part of writing and editing of the review, and made an methodological contribution. Liu Guanjian: advised and performed part of writing and editing of the review, and made a statistical contribution.
Contributions of editorial base
Nicky Cullum: edited the review; advised on methodology, interpretation and review content. Julie Bruce, Editor: approved the final review prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited and copy edited the review. Ruth Foxlee: designed the search strategy and edited the search methods section.
Sources of support
Internal sources
Chinese Cochrane Center, China.
External sources
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Yue Jirong: none known. Dong Birong: none known. Yang Ming: none known. Chen Xiaomei: none known. Wu Taixiang: none known. Liu Guanjian: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Itani 2010 {published data only}
- Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft‐tissue infections proven to be caused by methicillin‐resistant Staphylococcus aureus. American Journal of Surgery 2010;199(6):804‐16. [DOI] [PubMed] [Google Scholar]
Jaksic 2006 {published data only}
- Jaksic B, Martinelli G, Perez‐Oteyza J, Hartman CS, Leonard LB, Tack KJ. Efficacy and safety of linezolid compared with vancomycin in a randomized, double‐blind study of febrile neutropenic patients with cancer. Clinical Infectious Diseases 2006;42(5):597‐607. [DOI] [PubMed] [Google Scholar]
Kohno 2007 {published data only}
- Kohno S, Yamaguchi K, Aikawa N, Sumiyama Y, Odagiri S, Aoki N, et al. Linezolid versus vancomycin for the treatment of infections caused by methicillin‐resistant Staphylococcus aureus in Japan. Journal of Antimicrobial Chemotherapy 2007;60(6):1361‐9. [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin DF, Wu JF, Zhang YY, Zheng JC, Miao JZ, Zheng LY, et al. A randomized, double‐blinded, controlled, multicenter clinical trial of linezolid versus vancomycin in the treatment of gram positive bacterial infection. Chinese Journal of Infection and Chemotherapy 2009;9(1):10‐7. [Google Scholar]
- Lin Df, Zhang YY, Wu JF, Wang F, Zheng JC, Miao JZ, et al. Linezolid for the treatment of infections caused by Gram‐positive pathogens in China. International Journal of Antimicrobial Agents 2008;32(3):241‐9. [DOI] [PubMed] [Google Scholar]
Sharpe 2005 {published data only}
- Sharpe JN, Shively EH, Polk HC. Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA‐complicated, lower‐extremity skin and soft‐tissue infections caused by methicillin‐resistant Staphylococcus aureus. American Journal of Surgery 2005;189(4):425‐8. [DOI] [PubMed] [Google Scholar]
Stevens 2002 {published data only}
- Li JZ, Willke RJ, Rittenhouse BE. Effect of linezolid versus vancomycin on length of hospital stay in patients with complicated skin and soft tissue infections caused by known or suspected methicillin‐resistant staphylococci: results from a randomized clinical trial. Surgical Infections 2003;4(1):57‐70. [DOI] [PubMed] [Google Scholar]
- Li Z, Willke RJ, Pinto LA, Rittenhouse BE, Rybak MJ, Pleil AM, et al. Comparison of length of hospital stay for patients with known or suspected methicillin‐resistant Staphylococcus species infections treated with linezolid or vancomycin: a randomized, multicenter trial. Pharmacotherapy 2001;21(3):263‐74. [DOI] [PubMed] [Google Scholar]
- Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infections. Clinical Infectious Diseases 2002;34(11):1481‐90. [DOI] [PubMed] [Google Scholar]
Weigelt 2005 {published data only}
- Itani KM, Weigelt J, Li JZ, Duttagupta S. Linezolid reduces length of stay and duration of intravenous treatment compared with vancomycin for complicated skin and soft tissue infections due to suspected or proven methicillin‐resistant Staphylococcus aureus (MRSA). International Journal of Antimicrobial Agents 2005;26(6):442‐8. [DOI] [PubMed] [Google Scholar]
- McCollum M, Sorensen SV, Liu LZ. A comparison of costs and hospital length of stay associated with intravenous/oral linezolid or intravenous vancomycin treatment of complicated skin and soft‐tissue infections caused by suspected or confirmed methicillin‐resistant Staphylococcus aureus in elderly US patients. Clinical Therapeutics 2007;29(3):469‐77. [DOI] [PubMed] [Google Scholar]
- McKinnon PS, Sorensen SV, Liu LZ, Itani KM. Impact of linezolid on economic outcomes and determinants of cost in a clinical trial evaluating patients with MRSA complicated skin and soft‐tissue infections. Annals of pharmacotherapy 2006;40(6):1017‐23. [DOI] [PubMed] [Google Scholar]
- Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C, et al. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrobial Agents and Chemotherapy 2005;49(6):2260‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt J, Kaafarani HM, Itani KM, Swanson RN. Linezolid eradicates MRSA better than vancomycin from surgical‐site infections. American Journal of Surgery 2004;188(6):760‐6. [DOI] [PubMed] [Google Scholar]
Wilcox 2009 {published data only}
- Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ljzerman MM. Complicated skin and skin‐structure infections and catheter‐related bloodstream infections: Noninferiority of linezolid in a phase 3 study. Clinical Infectious Diseases 2009;48(2):203‐12. [DOI] [PubMed] [Google Scholar]
Yogev 2003 {published data only}
- Deville JG, Adler S, Azimi PH, Jantausch BA, Morfin MR, Beltran S, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant gram‐positive infections in neonates. Pediatric Infectious Disease Journal 2003;22(9 suppl):S158‐63. [DOI] [PubMed] [Google Scholar]
- Kaplan SL, Afghani B, Lopez P, Wu E, Fleishaker D, Edge‐Padbury B, et al. Linezolid for the treatment of methicillin‐resistant Staphylococcus aureus infections in children. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S178‐85. [DOI] [PubMed] [Google Scholar]
- Yogev R, Patterson LE, Kaplan SL, Adler S, Morfin MR, Martin A, et al. Linezolid for the treatment of complicated skin and skin structure infections in children. Pediatric Infectious Disease Journal 2003;22(9 SUPPL):S172‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bal 2013 {published data only}
Bhavnani 2015 {published data only}
- Bhavnani SM, Hammel JP, Wart SA, Rubino CM, Reynolds DK, Forrest A, et al. Pharmacokinetic‐pharmacodynamic analysis for efficacy of ceftaroline fosamil in patients with acute bacterial skin and skin structure infections. Antimicrobial agents and chemotherapy 2015; Vol. 59, issue 1:372‐80. [DOI] [PMC free article] [PubMed]
Bounthavong 2009 {published data only}
- Bounthavong M, Hsu DI, Okamoto MP. Cost‐effectiveness analysis of linezolid vs. vancomycin in treating methicillin‐resistant Staphylococcus aureus complicated skin and soft tissue infections using a decision analytic model. International Journal of Clinical Practice 2009;63(3):376‐86. [DOI] [PubMed] [Google Scholar]
Hau 2002 {published data only}
- Hau T. Efficacy and safety of linezolid in the treatment of skin and soft tissue infections. European Journal of Clinical Microbiology and Infectious Diseases 2002;21(7):491‐8. [DOI] [PubMed] [Google Scholar]
Janis 2014 {published data only}
Joseph 2007 {published data only}
- Joseph WS. Commentary on Linezolid eradicates MRSA better than vancomycin from surgical‐site infections. Foot and Ankle Quarterly‐‐The Seminar Journal 2007;19(1):26‐8. [Google Scholar]
Kalil 2006 {published data only}
- Kalil AC, Puumala S, Stoner J. Is linezolid superior to vancomycin for complicated skin and soft tissue infections due to methicillin‐resistant Staphylococcus aureus? comment. Antimicrobial Agents and Chemotherapy 2006;50(5):1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipsky 2011 {published data only}
- Lipsky BA, Itani KM, Weigelt JA, Joseph W, Paap CM, Reisman A, et al. The role of diabetes mellitus in the treatment of skin and skin structure infections caused by methicillin‐resistant Staphylococcus aureus: results from three randomized controlled trials. International Journal of Infectious Diseases 2011;15(2):e140‐6. [DOI] [PubMed] [Google Scholar]
McKinnon 2007 {published data only}
- McKinnon PS, Carter CT, Girase PG, Liu LZ, Carmeli Y. Health economics. The economic effect of oral linezolid versus intravenous vancomycin in the outpatient setting: the payer perspective. Managed Care Interface 2007;20(1):23‐34. [PubMed] [Google Scholar]
Patanwala 2007 {published data only}
- Patanwala AE, Erstad BL, Nix DE. Cost‐effectiveness of linezolid and vancomycin in the treatment of surgical site infections. Current Medical Research and Opinion 2007;23(1):185‐93. [DOI] [PubMed] [Google Scholar]
Puzniak 2013 {published data only}
Puzniak 2014 {published data only}
- Puzniak LA, Capitano B, Biswas P, Lodise TP. Impact of patient characteristics and infection type on clinical outcomes of patients who received linezolid or vancomycin for complicated skin and skin structure infections caused by methicillin‐resistant Staphylococcus aureus: A pooled data analysis. Diagnostic microbiology and infectious disease 2014; Vol. 78, issue 3:295‐301. [DOI] [PubMed]
Schurmann 2009 {published data only}
- Schurmann D, Sorensen SV, Cock E, Duttagupta S, Resch A. Cost‐effectiveness of linezolid versus vancomycin for hospitalised patients with complicated skin and soft‐tissue infections in Germany. European Journal of Health Economics 2009;10(1):65‐79. [DOI] [PubMed] [Google Scholar]
Additional references
Beibei 2010
- Beibei L, Yun C, Mengli C, Nan B, Xuhong Y, Rui W. Linezolid versus vancomycin for the treatment of Gram‐positive bacterial infections: meta‐analysis of randomised controlled trials. International Journal of Antimicrobial Agents 2010;35(1):3‐12. [DOI] [PubMed] [Google Scholar]
Bounthavong 2010
- Bounthavong M, Hsu DI. Efficacy and safety of linezolid in methicillin‐resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infection (cSSTI): A meta‐analysis. Current Medical Research and Opinion 2010;26(2):407‐21. [DOI] [PubMed] [Google Scholar]
Bouza 2004
- Bouza E, Sousa D, Muñoz P, Rodríguez‐Créixems M, Fron C, Lechuz JG. Bloodstream infections: a trial of the impact of different methods of reporting positive blood culture results. Journal of Infectious Diseases 2004;39(8):1161‐9. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of theCochrane Statistical Methods Group and the CochraneBias Methods Group (Editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dickersin 1990
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990;263(10):1385. [PubMed] [Google Scholar]
Dodds 2009
- Dodds TJ, Hawke CI. Linezolid versus vancomycin for MRSA skin and soft tissue infections (systematic review and meta‐analysis). ANZ Journal of Surgery 2009;79(9):629‐35. [DOI] [PubMed] [Google Scholar]
Donner 2001
- Donner A, Piaggio G, Villar J. Statistical methods for the meta analysis of cluster randomization trials. Statistical Methods in Medical Research 2001;10(5):325‐38. [DOI] [PubMed] [Google Scholar]
Eisenstein 2008
- Eisenstein BI. Treatment challenges in the management of complicated skin and soft‐tissue infections. Clinical Microbiology and Infection 2008;14(Suppl 2):17‐25. [DOI] [PubMed] [Google Scholar]
Engemann 2003
- Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clinical Infectious Diseases 2003;36(5):592‐8. [DOI] [PubMed] [Google Scholar]
Eron 2003
- Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Managing skin and soft tissue infections: expert panel recommendations on key decision points. Journal of Antimicrobial Chemotherapy 2003;52(Suppl 1):i3‐i17. [DOI] [PubMed] [Google Scholar]
Falagas 2008
- Falagas ME, Siempos II, Vardakas KZ. Linezolid versus glycopeptide or beta‐lactam for treatment of Gram‐positive bacterial infections: meta‐analysis of randomised controlled trials. Lancet Infectious Diseases 2008;8(1):53‐66. [DOI] [PubMed] [Google Scholar]
FDA 1998
- FDA. Uncomplicated and complicated skin and skin structure infections‐‐developing antimicrobial drugs for treatment. Guidance for Industry: Center for Drug Evaluation and Research (CDER). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf 1998.
Finch 2005
- Finch RG, Eliopoulos GM. Safety and efficacy of glycopeptide antibiotics. Journal of Antimicrobial Chemotherapy 2005;55(Suppl 2):ii5‐ii13. [DOI] [PubMed] [Google Scholar]
Fritsche 2007
- Fritsche TR, Sader HS, Jones RN. Potency and spectrum of garenoxacin tested against an international collection of skin and soft tissue infection pathogens: report from the SENTRY antimicrobial surveillance program (1999‐2004). Diagnostic Microbiology and Infectious Disease 2007;58(1):19‐26. [DOI] [PubMed] [Google Scholar]
Fung 2001
- Fung HB, Kirschenbaum HL, Ojofeitimi BO. Linezolid: an oxazolidinone antimicrobial agent. Clinical Therapeutics 2001;23(3):356‐91. [DOI] [PubMed] [Google Scholar]
Fung 2003
- Fung HB, Chang JY, Kuczynski S. A practical guide to the treatment of complicated skin and soft tissue infections. Drugs 2003;63(14):1459‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 2009
- Jones RN, Ross JE, Bell JM, Utsuki U, Fumiaki I, Kobayashi I, et al. Zyvox(R) Annual Appraisal of Potency and Spectrum program: linezolid surveillance program results for 2008. Diagnostic microbiology and infectious disease 2009;65(4):404‐13. [DOI] [PubMed] [Google Scholar]
Kollef 2004
- Kollef MH, Rello J, Cammarata SK, Croos‐Dabrera RV, Wunderink RG. Clinical cure and survival in Gram‐positive ventilator‐associated pneumonia: retrospective analysis of two double‐blind studies comparing linezolid with vancomycin. Intensive Care Medicine 2004;30(3):388‐94. [DOI] [PubMed] [Google Scholar]
Lamagni 2008
- Lamagni TL, Darenberg J, Luca‐Harari B, Siljander T, Efstratiou A, Henriques‐Normark B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. Journal of Clinical Microbiology 2008;46(7):2359‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levine 2006
- Levine DP. Vancomycin: a history. Clinical Infectious Diseases 2006;42(Supplement 1):S5. [DOI] [PubMed] [Google Scholar]
Moellering 1999
- Moellering RC Jr. A novel antimicrobial agent joins the battle against resistant bacteria. Annals of Internal Medicine 1999;130(2):155‐7. [DOI] [PubMed] [Google Scholar]
Moet 2006
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998‐2004). Diagnostic Microbiology and Infectious Disease 2007;57(1):7‐13. [DOI] [PubMed] [Google Scholar]
Pallares 1993
- Pallares R, Dick R, Wenzel RP, Adams JR, Nettleman MD. Trends in antimicrobial utilization at a tertiary teaching hospital during a15‐year period (1978–1992). Infection Control and Hospital Epidemiology 1993;14(7):376–82. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shorr 2005
- Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. Journal of Antimicrobial Chemotherapy 2005;56(5):923. [DOI] [PubMed] [Google Scholar]
SIGN 2008
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 27 May 2008).
Sivagnanam 2003
- Sivagnanam S, Deleu D. Red man syndrome. Critical Care 2003;7(2):119‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2005
- Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clinical Infectious Diseases 2005;41(10):1373‐406. [DOI] [PubMed] [Google Scholar]
Tsiodras 2001
- Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 2001;358(9277):207‐8. [DOI] [PubMed] [Google Scholar]
Wilson 2008
- Wilson DN, Schluenzen F, Harms JM, Starosta AL, Connell SR, Fucini P. The oxazolidinone antibiotics perturb the ribosomal peptidyl‐transferase center and effect tRNA positioning. Proceedings of the National Academy of Sciences 2008;105(36):13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wunderink 2003
- Wunderink RG, Rello J, Cammarata S, Croos‐Dabrera RV, KollefMH. Linezolid vs vancomycin: analysis of two double‐blind studies of patients with methicillin‐resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003;124(5):1789‐97. [PubMed] [Google Scholar]


